Abstract
Natural Killer T (NKT) cells generally recognize lipid-antigens presented in the context of the MHC class I-like molecule CD1d. CD1d-restricted NKT cells consist of two broad subsets: Type I, which express an invariant T cell receptor (TCR) and type II, which utilize diverse TCR gene segments. A major type II NKT subset has been shown to recognize a self-glycolipid, sulfatide. Both subsets play important roles in autoimmune diseases, tumor surveillance, and infectious diseases. While type I NKT cells protect from tumor growth by enhancing tumor surveillance, type II NKT cells may suppress anti-tumor immune responses. In a murine autoimmune hepatitis model, type I NKT cells contribute to pathogenesis, whereas activation of sulfatide-reactive type II NKT cells protects from disease. Sulfatide-mediated activation of type II NKT cells results in modification of dendritic cells and induction of anergy in type I NKT cells. Elucidation of this novel pathway of cross-regulation among NKT cell subsets will provide tools for intervention in autoimmune diseases and for designing strategies for effective anti-tumor immunity.
Keywords: NKT cells, Cancer, Tumor, Autoimmunity, Sulfatide, CD1d, Cross-regulation, Anergy
Natural Killer T (NKT) cells bridging innate and adaptive immunity have biological features of natural killer cells and conventional T cells. NKT cells express the markers NK1.1 or CD56/CD161 as well as the T cell receptor (TCR) (Bendelac et al., 2007; Gumperz et al., 2002; MacDonald, 1995; Prussin and Foster, 1997). Most NKT cells recognize antigens presented by the MHC class I-like and β2-microglobulin-associated molecule CD1d (Bendelac et al., 1994; Brigl and Brenner, 2004; Ohteki and MacDonald, 1994). The CD1 proteins can be classified into three groups (Barral and Brenner, 2007): Group 1 comprises CD1a, CD1b and CD1c; group 2 consists of CD1d and group 3 comprises CD1e. Humans and many other mammalian species express all CD1 isoforms (CD1a-e), while mice express only CD1d (Barral and Brenner, 2007; Brigl and Brenner, 2004; Godfrey et al., 2008). Interestingly, CD1d is highly conserved among the species and between humans and mice (Brossay et al., 1998). While CD1a-c are involved in presenting (mostly microbial) lipid antigens to conventional T cells, CD1d presents lipids, glycolipids and lipoproteins, which can be of self or foreign origin, to NKT cells (Barral and Brenner, 2007). CD1e is involved in intracellular lipid trafficking, as it lacks a transmembrane domain and is exclusively found in intracellular compartments (De Libero and Mori, 2006). CD1d is expressed on dendritic cells, macrophages (including the liver resident Kupffer cells), subsets of B cells, thymocytes, hepatocytes (Bendelac et al., 2007) and tumor cells (Fais et al., 2004; Fiedler et al., 2002; Metelitsa et al., 2003). Hence these cells are also potentially capable of presenting lipid antigens to NKT cells.
CD1d-restricted NKT cells can be broadly categorized into two groups: Type I and type II (see figure 1). Type I NKT cells are also named invariant NKT (iNKT), because they express an invariant TCR encoded by the Vα14Jα18 gene segment paired with one of a limited number of Vβ chains (mainly Vβ8.2, Vβ7 or Vβ2) in mice (Godfrey and Kronenberg, 2004; Kronenberg and Gapin, 2002) and, correspondingly, by the Vα24Jα18 gene segments and the Vβ11 chain, in humans (Dellabona et al., 1994; Porcelli et al., 1993). Stimulation of type I NKT cells with the superantigen-like marine sponge-derived glycolipid αgalactosylceramide (αGalCer) results in a cytokine burst, TCR downregulation, and apoptosis (Crowe et al., 2003; Kawano et al., 1997; Wilson et al., 2003). Depending on their context and the antigen involved in their stimulation, they may express different cytokine secretion profiles, either a Th1-type, secreting IFN-γ and TNF-α, a Th2-type, secreting IL-4 and IL-13 (Godfrey and Kronenberg, 2004), or a combination of the two. Additionally, both the self-glycolipid isoglobotrihexosylceramide (iGb3) and bacterial-derived lipids are recognized by these cells (Kawano et al., 1997; Kinjo et al., 2005; Mattner et al., 2005; Zhou et al., 2004). Type I NKT cells can be identified using αGalCer/CD1d-tetrameric reagents. Type II NKT cells have variable TCR V-gene rearrangements that are distinct from those used by type I NKT cells (Behar et al., 1999; Cardell et al., 1995; Chiu et al., 1999). A major type II NKT cell subset recognizes the self-glycolipid 3-sulfated galactosylceramide, called sulfatide, and can be identified by sulfatide/CD1d-tetramers (Jahng et al., 2004).
Figure 1.
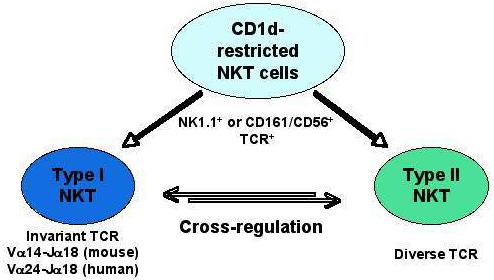
A broad category of NKT cell subsets
In this review, we will focus on the role of both type I and type II NKT cell subsets in autoimmunity and anti-tumor immunity. We will also discuss a novel mechanism of immune regulation resulting in anergy in type I NKT cells following activation of sulfatide-reactive type II NKT cells and how this pathway can be exploited to manipulate immune responses against tumors and in autoimmune disease.
Type I NKT cells in autoimmunity and in anti-tumor immunity
In autoimmune disease models, type I NKT cells have been shown to play either a potentiating or a protective role, depending on the disease, the administration of an activating stimulus (αGalCer or its analogs), and the resulting cytokine secretion profile (Th1 vs. Th2). Thus in the absence of type I NKT cells a milder course of disease has been found in a murine allergen-induced airway hypersensitivity model for asthma (Akbari et al., 2003) and in Concanavalin A (ConA)-induced hepatitis, a model for autoimmune hepatitis (Kaneko et al., 2000; Takeda et al., 2000). Notably, adoptive transfer of type I NKT cells restores disease susceptibility in these models, and in both cases these cells have been shown to produce Th2 cytokines (Akbari et al., 2003; Kaneko et al., 2000; Takeda et al., 2000), suggesting their involvement in pathogenesis. Consistently, a high frequency of type I NKT cells secreting Th2 cytokines has been detected in the lungs of human bronchial asthma patients (Akbari et al., 2006). Protective or regulatory role for type I NKT cells has also been suggested in diabetes-prone NOD mice (Godfrey et al., 1997; Gombert et al., 1996). Following adoptive transfer of NKT cells into NOD mice, it is the Th2 cytokines which are responsible for protection from diabetes, (Hammond et al., 1998). Conversely, a skewing of type I NKT cells towards Th1 has been shown to correlate with the manifestation of type I diabetes in human (Wilson et al., 1998), although a protective role for type I NKT cells in human type I diabetes remains controversial (Lee et al., 2002).
Several studies have used administration of the foreign glycolipid αGalCer to activate type I NKT cells in order to determine their effect in disease models. It has to be kept in mind, that the immune consequences following activation by αGalCer may not represent a physiological event, since αGalCer is not a mammalian ligand and in many ways acts as a superantigen for type I NKT cells. In some disease models, stimulation of type I NKT cells results in disease exacerbation, while in others protection from disease is observed. The role of the cytokine profile following stimulation is inconsistent, as in some cases Th2 cytokines are found to be pathogenic, although they are protective in most models. In experimental autoimmune encephalomyelitis (EAE), a murine model for multiple sclerosis, activation of type I NKT cells with αGalCer modulates the course of disease, depending on the MHC haplotype and on the timing of activation (Jahng et al., 2001; Singh et al., 2001). Coimmunization with αGalCer and disease-inducing myelin antigen potentiates disease in B10.PL mice, mediated by Th1 cytokine secretion, while it prevents EAE in C57BL/6 mice via Th2 cytokines (Jahng et al., 2001). Prior immunization (before induction of disease), however, prevents EAE in both strains, mediated by Th2 cytokines (Jahng et al., 2001). In dextran sulfate sodium-induced cholangitis, a murine model for primary sclerosing cholangitis, single administration of αGalCer results in disease exacerbation, involving Th1 cytokines, whereas repetitive stimulation is protective, skewing the cytokine profile towards Th2 (Numata et al., 2005). This could be explained by observations of Parekh et al., that repeated stimulation with αGalCer is followed by long-term anergy in type I NKT cells, which become unable to proliferate and produce IFN-γ, but which retain the ability to secrete IL-4 (Parekh et al., 2005). Protection from autoimmune disease following stimulation of type I NKT cells has further been observed following αGalCer administration in NOD mice (Hong et al., 2001; Sharif et al., 2001), although it is not clear whether protection is mediated by Th2 cytokines. Administration of OCH, a synthetic analog of αGalCer, results in Th2 cytokine release from type I NKT cells and protection from both EAE and collagen-induced arthritis (Chiba et al., 2004; Miyamoto et al., 2001).
Disease exacerbation after stimulation with αGalCer occurs in allergen-induced airway hypersensitivity (Meyer et al., 2006) and in the early stage of autoimmune cholangitis, resembling primary biliary cirrhosis (PBC) in the dominant-negative TGF-β receptor II mouse model (Chuang et al., 2008). The first involves Th2 cytokine secretion, whereas the later is associated with Th1 cytokine production. Of note, in human PBC, a role for type I NKT cells has been suggested, since their frequency is increased in livers of PBC patients, while they are decreased in the peripheral blood (Kita et al., 2002).
Type I NKT cells exert an anti-tumor effect following their activation with αGalCer (Kawano et al., 1997; Kobayashi et al., 1995; Morita et al., 1995; Motoki et al., 1995). Thus activated type I NKT cells induced rejection of several experimental tumor lines, including carcinoma, sarcoma, melanoma and thymoma (Hayakawa et al., 2004; Kawano et al., 1997; Smyth et al., 2002). Protection without exogenous stimulation has been found in the murine methylcholanthrene-induced sarcoma model (Smyth et al., 2000). Furthermore, adoptive transfer of NKT cells into type I NKT-deficient mice protects animals from sarcomas (Crowe et al., 2002).
Examinations of the mechanism involved in type I NKT cell-mediated tumor surveillance has revealed a pivotal role for IFN-γ, subsequently enhancing NK cell and cytotoxic CD8+T cell activity against the tumor (Berzofsky and Terabe, 2008). Recently, an anti-tumor role for type I NKT cells has also been confirmed in a murine B cell lymphoma model (Renukaradhya et al., 2008). This protection was dependent on CD1d expression by the tumor, involved IFN-γ and IL-12 secretion and reduction of CD11b+Gr1+ myeloid suppressor cells capable of suppressing anti-tumor immunosurveillance. Anti-tumor immunity is further enforced by DC maturation and activation following stimulation of type I NKT cells with αGalCer in vivo (Fujii et al., 2003). Thus activated DC produce IL-12, upregulate costimulatory molecules, and induce a more effective adaptive immune response mediated by conventional class II and class I MHC-restricted CD4+ and CD8+ T cells, respectively (Fujii et al., 2003). NKT and NK responses are also enhanced following intravenous injection of αGalCer–loaded tumor cells, resulting in tumor cell lysis by NK cells, subsequent uptake of αGalCer by DC and its presentation in the context of CD1d, leading to DC maturation and prolonged adaptive immunity (Fujii et al., 2007). Consistently, αGalCer-pulsed DC have been shown to expand and activate human type I NKT cells and induce their IFN-γ secretion in vitro (van der Vliet et al., 2003).
Collectively, these findings led to to the design of a few clinical trials in cancer patients, using either soluble αGalCer (Crul et al., 2002; Giaccone et al., 2002), in vitro-expanded αGalCer-pulsed dendritic cells (DCs) (Chang et al., 2005; Ishikawa et al., 2005; Nieda et al., 2004; Okai et al., 2002; Uchida et al., 2008) or adoptively transferred autologous NKT cells activated in vitro with αGalCer and IL-2 (Motohashi et al., 2006). Although biological effects like expansion of type I NKT cells and increased IFN-γ production were observed in the patients, none of the clinical trials so far have shown significant efficacy in terms of a partial or complete remission of tumors. This may relate to the small number of type I NKT cells in humans and insufficiency of their expansion to reach the tumor environment. For detailed information on type I NKT cells, please see comprehensive reviews on this topic by Godfrey et al..
Type II NKT cells in autoimmunity and in anti-tumor immunity
Although type II NKT cells have not been as extensively studied as type I NKT cells, these cells have been shown to play an important role in a number of autoimmune diseases. A majority of reports have revealed a potential protective role for type II NKT cells in autoimmunity. Thus, overexpression of a type II NKT cell subset, described as Vα3.2+Vβ9+, resulted in the protection from development of autoimmune diabetes in transgenic NOD mice (Duarte et al., 2004). Importantly, activation of a major subset of type II NKT cells by administration of the self-glycolipid sulfatide prevents EAE (Jahng et al., 2004) and Con A-induced hepatitis (Halder et al., 2007), murine models for multiple sclerosis and autoimmune hepatitis, respectively. It may be significant that during EAE, sulfatide-reactive type II NKT cells are increased several-fold in central nervous tissue, but not type I NKT cells. Furthermore, it has been shown in human active multiple sclerosis patients that NKT cells, secreting INF-γ, are significantly more frequent than in normal individuals (Shamshiev et al., 1999). In patients with autoimmune hepatitis and multiple sclerosis, the prevalence of anti-sulfatide antibodies has been demonstrated (Ilyas et al., 2003; Toda et al., 1990). Sulfatide-mediated protection from autoimmunity involves regulation of type I NKT cells, inhibition of effector functions of conventional T cells and modification of DC functions (Halder et al., 2007). We propose that anergized type I NKT cells behave like regulatory T cells and further control autoimmunity and anti-tumor immunity, as discussed below. Interestingly, a pathogenic role for type II NKT cells, involving an atypical Th2 response, has been suggested in ulcerative colitis, since these cells are present among the lamina propria T cell population from patients with ulcerative colitis, secreting high levels of IL-13 (Fuss et al., 2004).
It appears that, in contrast to the role of type I NKT cells enhancing anti-tumor responses, type II NKT cells are able to suppress tumor immunosurveillance in some tumor models (Ambrosino et al., 2007; Terabe et al., 2005). Thus, CD1d-deficient mice, lacking both type I and type II NKT cells, display enhanced anti-tumor immunity resulting in reduced tumor growth, whereas tumors in wild type mice and Jα18−/−mice, lacking only type I NKT cells, developed equally (Terabe et al., 2005). These findings indicated a suppressive role for type II NKT cells in tumor surveillance and are consistent with an earlier observation that indicated enhanced anti-tumor immunity in CD1d−/−mice vs. Jα18−/−mice by CpG oligodeoxynucleotides (Sfondrini et al., 2002). A significant increase in tumor growth following stimulation of type II NKT cells with sulfatide further indicates cross-regulation of type I NKT cells by type II NKT cells and suppression of tumor immunosurveillance (Ambrosino et al., 2007). The suppression of anti-tumor immunity by type II NKT cells appears to be associated with elevated levels of the anti-inflammatory cytokines IL-13 and TGF-β as well as CD11b+Gr1+ myeloid-derived suppressor cells (Renukaradhya et al., 2008; Terabe et al., 2003). Notably, a subset of type II NKT cells has been identified in humans with malignant disease: Chang et al. found a higher frequency of type II NKT cells reactive to lysophosphatidylcholine species and skewed towards IL-13 secretion in the plasma of multiple myeloma patients (Chang et al., 2008).
Cross-regulation among NKT cell subsets
In three different experimental models, including anti-tumor immune responses, autoimmune hepatitis, and in immunity against parasites, opposing roles for type I vs. type II NKT cells have been suggested. In murine infection with Trypanosoma cruzi, causing Chagas’ disease in humans, CD1d−/− and wild-type mice exhibited milder disease in comparison to Jα18−/−mice that developed more severe disease and died more frequently (Duthie et al., 2005). Thus, type II NKT cells seem to play a pathogenic role, involving Th1 cytokines, in this disease, while type I NKT cells might counteract by down-regulating the response. In contrast, in murine acute schistosomiasis, type I NKT cells contribute to Th1 responses and type II NKT cells might promote Th2 responses (Mallevaey et al., 2007). We have demonstrated the opposing roles for type I and type II NKT cells in autoimmunity as well as in anti-tumor immunity (Ambrosino et al., 2007; Halder et al., 2007). Thus, anergy induction in type I NKT cells occurs following activation of sulfatide-reactive type II NKT cells, resulting in subsequent protection from autoimmune disease (Halder et al., 2007). Since adoptive transfer of purified dendritic cells from sulfatide-treated animals into naïve recipients can induce anergy in type I NKT cells and prevent disease, these findings reveal a novel immune-regulatory axis in which sulfatide-reactive type II NKT cells are able to regulate type I NKT cells by modulating the function of dendritic cells (Figure 2). Currently, we are investigating the role of distinct DC subsets (e.g. plasmacytoid vs. myeloid) in this immune regulatory mechanism.
Figure 2.
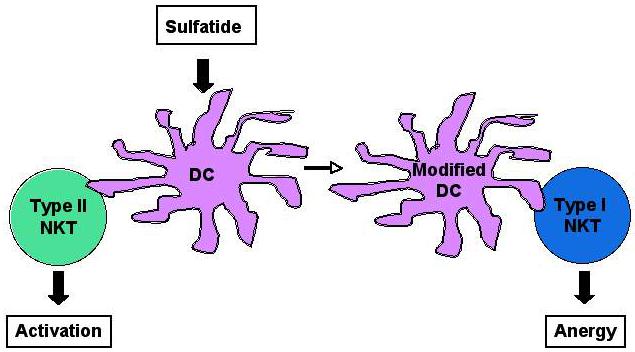
A model for the type II NKT cell-mediated regulation of type I NKT cells: Following activation of sulfatide-reactive type II NKT cells, dendritic cells (DC) are modified and mediate anergy in type I NKT cells.
Summary and Perspectives
NKT cells appear to play an important role in autoimmune diseases, tumor surveillance, infectious diseases and several other pathological conditions. It is likely that involvement of different NKT subsets and their cytokine secretion profile (Th1-like vs. Th2-like) will account for their differential role in various disease settings. Interestingly, type I and type II NKT cell subsets seem to have opposing roles and functionally cross-regulate each other in experimental settings of autoimmunity, anti-tumor immunity and in responses to parasitic infections (Ambrosino et al., 2007; Duthie et al., 2005; Halder et al., 2007; Mallevaey et al., 2007). While type I NKT cells predominate in mice, type II NKT cells predominate in humans. Characterization of distinct type II NKT cell subsets and their self-lipid ligands will be important in understanding important biological and physiological roles of these cells in health and disease. Furthermore, a detailed understanding of the mechanisms involved in cross-regulation of type I and type II NKT cells subsets will have crucial implication for manipulating the outcome of immune responses against cancer, autoimmune and infectious diseases. Since the CD1d-dependent antigen-recognition pathway is highly conserved from mice to humans, findings in experimental models can be relatively easily translated to the clinical setting.
Acknowledgments
Contract grant sponsor: The National Institutes of Health, Contract grant number: R01 CA 100660
Contract grant sponsor: German Research Foundation, Contract grant number: AR 645/1-1
REFERENCES
- Akbari O, Faul JL, Hoyte EG, Berry GJ, Wahlstrom J, Kronenberg M, DeKruyff RH, Umetsu DT. CD4+ invariant T-cell-receptor+ natural killer T cells in bronchial asthma. N Engl J Med. 2006;354(11):1117–1129. doi: 10.1056/NEJMoa053614. [DOI] [PubMed] [Google Scholar]
- Akbari O, Stock P, Meyer E, Kronenberg M, Sidobre S, Nakayama T, Taniguchi M, Grusby MJ, DeKruyff RH, Umetsu DT. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med. 2003;9(5):582–588. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- Ambrosino E, Terabe M, Halder RC, Peng J, Takaku S, Miyake S, Yamamura T, Kumar V, Berzofsky JA. Cross-regulation between type I and type II NKT cells in regulating tumor immunity: a new immunoregulatory axis. J Immunol. 2007;179(8):5126–5136. doi: 10.4049/jimmunol.179.8.5126. [DOI] [PubMed] [Google Scholar]
- Barral DC, Brenner MB. CD1 antigen presentation: how it works. Nat Rev Immunol. 2007;7(12):929–941. doi: 10.1038/nri2191. [DOI] [PubMed] [Google Scholar]
- Behar SM, Podrebarac TA, Roy CJ, Wang CR, Brenner MB. Diverse TCRs recognize murine CD1. J Immunol. 1999;162(1):161–167. [PubMed] [Google Scholar]
- Bendelac A, Killeen N, Littman DR, Schwartz RH. A subset of CD4+ thymocytes selected by MHC class I molecules. Science. 1994;263(5154):1774–1778. doi: 10.1126/science.7907820. [DOI] [PubMed] [Google Scholar]
- Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- Berzofsky JA, Terabe M. NKT cells in tumor immunity: opposing subsets define a new immunoregulatory axis. J Immunol. 2008;180(6):3627–3635. doi: 10.4049/jimmunol.180.6.3627. [DOI] [PubMed] [Google Scholar]
- Brigl M, Brenner MB. CD1: antigen presentation and T cell function. Annu Rev Immunol. 2004;22:817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- Brossay L, Chioda M, Burdin N, Koezuka Y, Casorati G, Dellabona P, Kronenberg M. CD1d-mediated recognition of an alpha-galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J Exp Med. 1998;188(8):1521–1528. doi: 10.1084/jem.188.8.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardell S, Tangri S, Chan S, Kronenberg M, Benoist C, Mathis D. CD1-restricted CD4+ T cells in major histocompatibility complex class II-deficient mice. J Exp Med. 1995;182(4):993–1004. doi: 10.1084/jem.182.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang DH, Deng H, Matthews P, Krasovsky J, Ragupathi G, Spisek R, Mazumder A, Vesole DH, Jagannath S, Dhodapkar MV. Inflammation-associated lysophospholipids as ligands for CD1d-restricted T cells in human cancer. Blood. 2008;112(4):1308–1316. doi: 10.1182/blood-2008-04-149831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang DH, Osman K, Connolly J, Kukreja A, Krasovsky J, Pack M, Hutchinson A, Geller M, Liu N, Annable R, Shay J, Kirchhoff K, Nishi N, Ando Y, Hayashi K, Hassoun H, Steinman RM, Dhodapkar MV. Sustained expansion of NKT cells and antigen-specific T cells after injection of alpha-galactosyl-ceramide loaded mature dendritic cells in cancer patients. J Exp Med. 2005;201(9):1503–1517. doi: 10.1084/jem.20042592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba A, Oki S, Miyamoto K, Hashimoto H, Yamamura T, Miyake S. Suppression of collagen-induced arthritis by natural killer T cell activation with OCH, a sphingosine-truncated analog of alpha-galactosylceramide. Arthritis Rheum. 2004;50(1):305–313. doi: 10.1002/art.11489. [DOI] [PubMed] [Google Scholar]
- Chiu YH, Jayawardena J, Weiss A, Lee D, Park SH, Dautry-Varsat A, Bendelac A. Distinct subsets of CD1d-restricted T cells recognize self-antigens loaded in different cellular compartments. J Exp Med. 1999;189(1):103–110. doi: 10.1084/jem.189.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang YH, Lian ZX, Yang GX, Shu SA, Moritoki Y, Ridgway WM, Ansari AA, Kronenberg M, Flavell RA, Gao B, Gershwin ME. Natural killer T cells exacerbate liver injury in a transforming growth factor beta receptor II dominant-negative mouse model of primary biliary cirrhosis. Hepatology. 2008;47(2):571–580. doi: 10.1002/hep.22052. [DOI] [PubMed] [Google Scholar]
- Crowe NY, Smyth MJ, Godfrey DI. A critical role for natural killer T cells in immunosurveillance of methylcholanthrene-induced sarcomas. J Exp Med. 2002;196(1):119–127. doi: 10.1084/jem.20020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe NY, Uldrich AP, Kyparissoudis K, Hammond KJ, Hayakawa Y, Sidobre S, Keating R, Kronenberg M, Smyth MJ, Godfrey DI. Glycolipid antigen drives rapid expansion and sustained cytokine production by NK T cells. J Immunol. 2003;171(8):4020–4027. doi: 10.4049/jimmunol.171.8.4020. [DOI] [PubMed] [Google Scholar]
- Crul M, Mathot RA, Giaccone G, Punt CA, Rosing H, Hillebrand MX, Ando Y, Nishi N, Tanaka H, Schellens JM, Beijnen JH. Population pharmacokinetics of the novel anticancer agent KRN7000. Cancer Chemother Pharmacol. 2002;49(4):287–293. doi: 10.1007/s00280-001-0413-3. [DOI] [PubMed] [Google Scholar]
- De Libero G, Mori L. Mechanisms of lipid-antigen generation and presentation to T cells. Trends Immunol. 2006;27(10):485–492. doi: 10.1016/j.it.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Dellabona P, Padovan E, Casorati G, Brockhaus M, Lanzavecchia A. An invariant V alpha 24-J alpha Q/V beta 11 T cell receptor is expressed in all individuals by clonally expanded CD4-8- T cells. J Exp Med. 1994;180(3):1171–1176. doi: 10.1084/jem.180.3.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte N, Stenstrom M, Campino S, Bergman ML, Lundholm M, Holmberg D, Cardell SL. Prevention of diabetes in nonobese diabetic mice mediated by CD1d-restricted nonclassical NKT cells. J Immunol. 2004;173(5):3112–3118. doi: 10.4049/jimmunol.173.5.3112. [DOI] [PubMed] [Google Scholar]
- Duthie MS, Kahn M, White M, Kapur RP, Kahn SJ. Critical proinflammatory and anti-inflammatory functions of different subsets of CD1d-restricted natural killer T cells during Trypanosoma cruzi infection. Infect Immun. 2005;73(1):181–192. doi: 10.1128/IAI.73.1.181-192.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fais F, Morabito F, Stelitano C, Callea V, Zanardi S, Scudeletti M, Varese P, Ciccone E, Grossi CE. CD1d is expressed on B-chronic lymphocytic leukemia cells and mediates alpha-galactosylceramide presentation to natural killer T lymphocytes. Int J Cancer. 2004;109(3):402–411. doi: 10.1002/ijc.11723. [DOI] [PubMed] [Google Scholar]
- Fiedler T, Walter W, Reichert TE, Maeurer MJ. Regulation of CD1d expression by murine tumor cells: escape from immunosurveillance or alternate target molecules? Int J Cancer. 2002;98(3):389–397. doi: 10.1002/ijc.10141. [DOI] [PubMed] [Google Scholar]
- Fujii S, Shimizu K, Hemmi H, Steinman RM. Innate Valpha14(+) natural killer T cells mature dendritic cells, leading to strong adaptive immunity. Immunol Rev. 2007;220:183–198. doi: 10.1111/j.1600-065X.2007.00561.x. [DOI] [PubMed] [Google Scholar]
- Fujii S, Shimizu K, Smith C, Bonifaz L, Steinman RM. Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J Exp Med. 2003;198(2):267–279. doi: 10.1084/jem.20030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuss IJ, Heller F, Boirivant M, Leon F, Yoshida M, Fichtner-Feigl S, Yang Z, Exley M, Kitani A, Blumberg RS, Mannon P, Strober W. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. J Clin Invest. 2004;113(10):1490–1497. doi: 10.1172/JCI19836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaccone G, Punt CJ, Ando Y, Ruijter R, Nishi N, Peters M, von Blomberg BM, Scheper RJ, van der Vliet HJ, van den Eertwegh AJ, Roelvink M, Beijnen J, Zwierzina H, Pinedo HM. A phase I study of the natural killer T-cell ligand alpha-galactosylceramide (KRN7000) in patients with solid tumors. Clin Cancer Res. 2002;8(12):3702–3709. [PubMed] [Google Scholar]
- Godfrey DI, Kinder SJ, Silvera P, Baxter AG. Flow cytometric study of T cell development in NOD mice reveals a deficiency in alphabetaTCR+CDR-CD8-thymocytes. J Autoimmun. 1997;10(3):279–285. doi: 10.1006/jaut.1997.0129. [DOI] [PubMed] [Google Scholar]
- Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest. 2004;114(10):1379–1388. doi: 10.1172/JCI23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey DI, Rossjohn J, McCluskey J. The fidelity, occasional promiscuity, and versatility of T cell receptor recognition. Immunity. 2008;28(3):304–314. doi: 10.1016/j.immuni.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Gombert JM, Herbelin A, Tancrede-Bohin E, Dy M, Carnaud C, Bach JF. Early quantitative and functional deficiency of NK1+-like thymocytes in the NOD mouse. Eur J Immunol. 1996;26(12):2989–2998. doi: 10.1002/eji.1830261226. [DOI] [PubMed] [Google Scholar]
- Gumperz JE, Miyake S, Yamamura T, Brenner MB. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J Exp Med. 2002;195(5):625–636. doi: 10.1084/jem.20011786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder RC, Aguilera C, Maricic I, Kumar V. Type II NKT cell-mediated anergy induction in type I NKT cells prevents inflammatory liver disease. J Clin Invest. 2007;117(8):2302–2312. doi: 10.1172/JCI31602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond KJ, Poulton LD, Palmisano LJ, Silveira PA, Godfrey DI, Baxter AG. alpha/beta-T cell receptor (TCR)+CD4-CD8- (NKT) thymocytes prevent insulin-dependent diabetes mellitus in nonobese diabetic (NOD)/Lt mice by the influence of interleukin (IL)-4 and/or IL-10. J Exp Med. 1998;187(7):1047–1056. doi: 10.1084/jem.187.7.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa Y, Godfrey DI, Smyth MJ. Alpha-galactosylceramide: potential immunomodulatory activity and future application. Curr Med Chem. 2004;11(2):241–252. doi: 10.2174/0929867043456115. [DOI] [PubMed] [Google Scholar]
- Hong S, Wilson MT, Serizawa I, Wu L, Singh N, Naidenko OV, Miura T, Haba T, Scherer DC, Wei J, Kronenberg M, Koezuka Y, Van Kaer L. The natural killer T-cell ligand alpha-galactosylceramide prevents autoimmune diabetes in non-obese diabetic mice. Nat Med. 2001;7(9):1052–1056. doi: 10.1038/nm0901-1052. [DOI] [PubMed] [Google Scholar]
- Ilyas AA, Chen ZW, Cook SD. Antibodies to sulfatide in cerebrospinal fluid of patients with multiple sclerosis. J Neuroimmunol. 2003;139(1-2):76–80. doi: 10.1016/s0165-5728(03)00131-0. [DOI] [PubMed] [Google Scholar]
- Ishikawa A, Motohashi S, Ishikawa E, Fuchida H, Higashino K, Otsuji M, Iizasa T, Nakayama T, Taniguchi M, Fujisawa T. A phase I study of alpha-galactosylceramide (KRN7000)-pulsed dendritic cells in patients with advanced and recurrent non-small cell lung cancer. Clin Cancer Res. 2005;11(5):1910–1917. doi: 10.1158/1078-0432.CCR-04-1453. [DOI] [PubMed] [Google Scholar]
- Jahng A, Maricic I, Aguilera C, Cardell S, Halder RC, Kumar V. Prevention of autoimmunity by targeting a distinct, noninvariant CD1d-reactive T cell population reactive to sulfatide. J Exp Med. 2004;199(7):947–957. doi: 10.1084/jem.20031389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahng AW, Maricic I, Pedersen B, Burdin N, Naidenko O, Kronenberg M, Koezuka Y, Kumar V. Activation of natural killer T cells potentiates or prevents experimental autoimmune encephalomyelitis. J Exp Med. 2001;194(12):1789–1799. doi: 10.1084/jem.194.12.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko Y, Harada M, Kawano T, Yamashita M, Shibata Y, Gejyo F, Nakayama T, Taniguchi M. Augmentation of Valpha14 NKT cell-mediated cytotoxicity by interleukin 4 in an autocrine mechanism resulting in the development of concanavalin A-induced hepatitis. J Exp Med. 2000;191(1):105–114. doi: 10.1084/jem.191.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278(5343):1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- Kinjo Y, Wu D, Kim G, Xing GW, Poles MA, Ho DD, Tsuji M, Kawahara K, Wong CH, Kronenberg M. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434(7032):520–525. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- Kita H, Naidenko OV, Kronenberg M, Ansari AA, Rogers P, He XS, Koning F, Mikayama T, Van De Water J, Coppel RL, Kaplan M, Gershwin ME. Quantitation and phenotypic analysis of natural killer T cells in primary biliary cirrhosis using a human CD1d tetramer. Gastroenterology. 2002;123(4):1031–1043. doi: 10.1053/gast.2002.36020. [DOI] [PubMed] [Google Scholar]
- Kobayashi E, Motoki K, Uchida T, Fukushima H, Koezuka Y. KRN7000, a novel immunomodulator, and its antitumor activities. Oncol Res. 1995;7(10-11):529–534. [PubMed] [Google Scholar]
- Kronenberg M, Gapin L. The unconventional lifestyle of NKT cells. Nat Rev Immunol. 2002;2(8):557–568. doi: 10.1038/nri854. [DOI] [PubMed] [Google Scholar]
- Lee PT, Putnam A, Benlagha K, Teyton L, Gottlieb PA, Bendelac A. Testing the NKT cell hypothesis of human IDDM pathogenesis. J Clin Invest. 2002;110(6):793–800. doi: 10.1172/JCI15832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald HR. NK1.1+ T cell receptor-alpha/beta+ cells: new clues to their origin, specificity, and function. J Exp Med. 1995;182(3):633–638. doi: 10.1084/jem.182.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallevaey T, Fontaine J, Breuilh L, Paget C, Castro-Keller A, Vendeville C, Capron M, Leite-de-Moraes M, Trottein F, Faveeuw C. Invariant and noninvariant natural killer T cells exert opposite regulatory functions on the immune response during murine schistosomiasis. Infect Immun. 2007;75(5):2171–2180. doi: 10.1128/IAI.01178-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattner J, Debord KL, Ismail N, Goff RD, Cantu C, 3rd, Zhou D, Saint-Mezard P, Wang V, Gao Y, Yin N, Hoebe K, Schneewind O, Walker D, Beutler B, Teyton L, Savage PB, Bendelac A. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434(7032):525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- Metelitsa LS, Weinberg KI, Emanuel PD, Seeger RC. Expression of CD1d by myelomonocytic leukemias provides a target for cytotoxic NKT cells. Leukemia. 2003;17(6):1068–1077. doi: 10.1038/sj.leu.2402943. [DOI] [PubMed] [Google Scholar]
- Meyer EH, Goya S, Akbari O, Berry GJ, Savage PB, Kronenberg M, Nakayama T, DeKruyff RH, Umetsu DT. Glycolipid activation of invariant T cell receptor+ NK T cells is sufficient to induce airway hyperreactivity independent of conventional CD4+ T cells. Proc Natl Acad Sci U S A. 2006;103(8):2782–2787. doi: 10.1073/pnas.0510282103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 2001;413(6855):531–534. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- Morita M, Motoki K, Akimoto K, Natori T, Sakai T, Sawa E, Yamaji K, Koezuka Y, Kobayashi E, Fukushima H. Structure-activity relationship of alpha-galactosylceramides against B16-bearing mice. J Med Chem. 1995;38(12):2176–2187. doi: 10.1021/jm00012a018. [DOI] [PubMed] [Google Scholar]
- Motohashi S, Ishikawa A, Ishikawa E, Otsuji M, Iizasa T, Hanaoka H, Shimizu N, Horiguchi S, Okamoto Y, Fujii S, Taniguchi M, Fujisawa T, Nakayama T. A phase I study of in vitro expanded natural killer T cells in patients with advanced and recurrent non-small cell lung cancer. Clin Cancer Res. 2006;12(20 Pt 1):6079–6086. doi: 10.1158/1078-0432.CCR-06-0114. [DOI] [PubMed] [Google Scholar]
- Motoki K, Morita M, Kobayashi E, Uchida T, Akimoto K, Fukushima H, Koezuka Y. Immunostimulatory and antitumor activities of monoglycosylceramides having various sugar moieties. Biol Pharm Bull. 1995;18(11):1487–1491. doi: 10.1248/bpb.18.1487. [DOI] [PubMed] [Google Scholar]
- Nieda M, Okai M, Tazbirkova A, Lin H, Yamaura A, Ide K, Abraham R, Juji T, Macfarlane DJ, Nicol AJ. Therapeutic activation of Valpha24+Vbeta11+ NKT cells in human subjects results in highly coordinated secondary activation of acquired and innate immunity. Blood. 2004;103(2):383–389. doi: 10.1182/blood-2003-04-1155. [DOI] [PubMed] [Google Scholar]
- Numata Y, Tazuma S, Ueno Y, Nishioka T, Hyogo H, Chayama K. Therapeutic effect of repeated natural killer T cell stimulation in mouse cholangitis complicated by colitis. Dig Dis Sci. 2005;50(10):1844–1851. doi: 10.1007/s10620-005-2949-2. [DOI] [PubMed] [Google Scholar]
- Ohteki T, MacDonald HR. Major histocompatibility complex class I related molecules control the development of CD4+8- and CD4-8- subsets of natural killer 1.1+ T cell receptor-alpha/beta+ cells in the liver of mice. J Exp Med. 1994;180(2):699–704. doi: 10.1084/jem.180.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okai M, Nieda M, Tazbirkova A, Horley D, Kikuchi A, Durrant S, Takahashi T, Boyd A, Abraham R, Yagita H, Juji T, Nicol A. Human peripheral blood Valpha24+ Vbeta11+ NKT cells expand following administration of alpha-galactosylceramide-pulsed dendritic cells. Vox Sang. 2002;83(3):250–253. doi: 10.1046/j.1423-0410.2002.00217.x. [DOI] [PubMed] [Google Scholar]
- Parekh VV, Wilson MT, Olivares-Villagomez D, Singh AK, Wu L, Wang CR, Joyce S, Van Kaer L. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J Clin Invest. 2005;115(9):2572–2583. doi: 10.1172/JCI24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcelli S, Yockey CE, Brenner MB, Balk SP. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4-8- alpha/beta T cells demonstrates preferential use of several V beta genes and an invariant TCR alpha chain. J Exp Med. 1993;178(1):1–16. doi: 10.1084/jem.178.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prussin C, Foster B. TCR V alpha 24 and V beta 11 coexpression defines a human NK1 T cell analog containing a unique Th0 subpopulation. J Immunol. 1997;159(12):5862–5870. [PubMed] [Google Scholar]
- Renukaradhya GJ, Khan MA, Vieira M, Du W, Gervay-Hague J, Brutkiewicz RR. Type I NKT cells protect (and type II NKT cells suppress) the host's innate antitumor immune response to a B-cell lymphoma. Blood. 2008;111(12):5637–5645. doi: 10.1182/blood-2007-05-092866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfondrini L, Besusso D, Zoia MT, Rodolfo M, Invernizzi AM, Taniguchi M, Nakayama T, Colombo MP, Menard S, Balsari A. Absence of the CD1 molecule upregulates antitumor activity induced by CpG oligodeoxynucleotides in mice. J Immunol. 2002;169(1):151–158. doi: 10.4049/jimmunol.169.1.151. [DOI] [PubMed] [Google Scholar]
- Shamshiev A, Donda A, Carena I, Mori L, Kappos L, De Libero G. Self glycolipids as T-cell autoantigens. Eur J Immunol. 1999;29(5):1667–75. doi: 10.1002/(SICI)1521-4141(199905)29:05<1667::AID-IMMU1667>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Sharif S, Arreaza GA, Zucker P, Mi QS, Sondhi J, Naidenko OV, Kronenberg M, Koezuka Y, Delovitch TL, Gombert JM, Leite-De-Moraes M, Gouarin C, Zhu R, Hameg A, Nakayama T, Taniguchi M, Lepault F, Lehuen A, Bach JF, Herbelin A. Activation of natural killer T cells by alpha-galactosylceramide treatment prevents the onset and recurrence of autoimmune Type 1 diabetes. Nat Med. 2001;7(9):1057–1062. doi: 10.1038/nm0901-1057. [DOI] [PubMed] [Google Scholar]
- Singh AK, Wilson MT, Hong S, Olivares-Villagomez D, Du C, Stanic AK, Joyce S, Sriram S, Koezuka Y, Van Kaer L. Natural killer T cell activation protects mice against experimental autoimmune encephalomyelitis. J Exp Med. 2001;194(12):1801–1811. doi: 10.1084/jem.194.12.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth MJ, Crowe NY, Hayakawa Y, Takeda K, Yagita H, Godfrey DI. NKT cells - conductors of tumor immunity? Curr Opin Immunol. 2002;14(2):165–171. doi: 10.1016/s0952-7915(02)00316-3. [DOI] [PubMed] [Google Scholar]
- Smyth MJ, Thia KY, Street SE, Cretney E, Trapani JA, Taniguchi M, Kawano T, Pelikan SB, Crowe NY, Godfrey DI. Differential tumor surveillance by natural killer (NK) and NKT cells. J Exp Med. 2000;191(4):661–668. doi: 10.1084/jem.191.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Hayakawa Y, Van Kaer L, Matsuda H, Yagita H, Okumura K. Critical contribution of liver natural killer T cells to a murine model of hepatitis. Proc Natl Acad Sci U S A. 2000;97(10):5498–5503. doi: 10.1073/pnas.040566697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terabe M, Matsui S, Park JM, Mamura M, Noben-Trauth N, Donaldson DD, Chen W, Wahl SM, Ledbetter S, Pratt B, Letterio JJ, Paul WE, Berzofsky JA. Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J Exp Med. 2003;198(11):1741–1752. doi: 10.1084/jem.20022227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terabe M, Swann J, Ambrosino E, Sinha P, Takaku S, Hayakawa Y, Godfrey DI, Ostrand-Rosenberg S, Smyth MJ, Berzofsky JA. A nonclassical non-Valpha14Jalpha18 CD1d-restricted (type II) NKT cell is sufficient for down-regulation of tumor immunosurveillance. J Exp Med. 2005;202(12):1627–1633. doi: 10.1084/jem.20051381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda G, Ikeda Y, Kashiwagi M, Iwamori M, Oka H. Hepatocyte plasma membrane glycosphingolipid reactive with sera from patients with autoimmune chronic active hepatitis: its identification as sulfatide. Hepatology. 1990;12(4 Pt 1):664–670. doi: 10.1002/hep.1840120408. [DOI] [PubMed] [Google Scholar]
- Uchida T, Horiguchi S, Tanaka Y, Yamamoto H, Kunii N, Motohashi S, Taniguchi M, Nakayama T, Okamoto Y. Phase I study of alpha-galactosylceramide-pulsed antigen presenting cells administration to the nasal submucosa in unresectable or recurrent head and neck cancer. Cancer Immunol Immunother. 2008;57(3):337–345. doi: 10.1007/s00262-007-0373-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vliet HJ, Molling JW, Nishi N, Masterson AJ, Kolgen W, Porcelli SA, van den Eertwegh AJ, von Blomberg BM, Pinedo HM, Giaccone G, Scheper RJ. Polarization of Valpha24+ Vbeta11+ natural killer T cells of healthy volunteers and cancer patients using alpha-galactosylceramide-loaded and environmentally instructed dendritic cells. Cancer Res. 2003;63(14):4101–4106. [PubMed] [Google Scholar]
- Wilson MT, Johansson C, Olivares-Villagomez D, Singh AK, Stanic AK, Wang CR, Joyce S, Wick MJ, Van Kaer L. The response of natural killer T cells to glycolipid antigens is characterized by surface receptor down-modulation and expansion. Proc Natl Acad Sci U S A. 2003;100(19):10913–10918. doi: 10.1073/pnas.1833166100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SB, Kent SC, Patton KT, Orban T, Jackson RA, Exley M, Porcelli S, Schatz DA, Atkinson MA, Balk SP, Strominger JL, Hafler DA. Extreme Th1 bias of invariant Valpha24JalphaQ T cells in type 1 diabetes. Nature. 1998;391(6663):177–181. doi: 10.1038/34419. [DOI] [PubMed] [Google Scholar]
- Zhou D, Mattner J, Cantu C, 3rd, Schrantz N, Yin N, Gao Y, Sagiv Y, Hudspeth K, Wu YP, Yamashita T, Teneberg S, Wang D, Proia RL, Levery SB, Savage PB, Teyton L, Bendelac A. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306(5702):1786–1789. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]


