Abstract
Background
The Wilms' tumor suppressor gene, wt1, encodes a zinc-finger protein, WT1, that functions as a transcription regulator. Previous studies have suggested a contradictory role of WT1 in breast cancer development.
Materials and Methods
MCF10AT3B cells, a cell line derived from a xenograft model of progressive human proliferative breast disease, were used to study WT1 function in early development of breast cancer. A stable cell line was established from MCF10AT3B cells that ectopically expressed the Wilms’ tumor suppressor, WT1. Western blot analysis, in vitro and in vivo growth assays were used to study the effects of constitutive WT1 expression on malignant progression of MCF10AT3B cells.
Results
WT1 expression had a profound effect on several aspects of the cell cycle machinery and inhibited estrogen-stimulated and non-stimulated cell growth in vitro. In nude mice, WT1 expression strongly suppressed estrogen-stimulated tumorigenesis of neoplastigenic MCF10AT3B cells.
Conclusion
WT1 plays an important role in maintaining normal growth of mammary epithelial cells and dysregulated WT1 expression may contribute to breast cancer development.
Keywords: WT1, tumor suppressor, MCF10AT3B cells, proliferative breast disease, breast cancer
The Wilms’ tumor susceptibility gene, wt1, at chromosome locus 11p13 (1–3) encodes a C2-H2-type zinc-finger protein, WT1. The protein has a predicted molecular weight of 52 to 54 kDa, depending on the presence or absence of two alternatively spliced exons (4).
The first report of an association between WT1 and breast cancer was from Silberstein et al. (5). Using immunohistochemistry staining, this group demonstrated WT1 expression in myoepithelial cells in the normal breast duct. However, reduced WT1 staining was seen in 60% of breast tumors, suggesting a correlation between the loss of WT1 expression and mammary carcinogenesis. Laux et al. (6) and Huang et al. (7) used methylation-sensitive restriction endonucleases to demonstrate aberrant methylation of the WT1 promoter and first intron in breast cancer samples, further suggesting that down-regulation of WT1 is involved in the carcinogenesis of breast cancer.
Previously, we reported that constitutive expression of WT1 reverts the transformed phenotypes of MDA-MB-231 breast cancer cells (8), supporting the view that WT1 functions as a tumor suppressor in breast cancer tumorigenesis. However, Loeb et al. (9), using more sensitive RT-PCR assays, demonstrated that WT1 was strongly expressed in primary carcinoma but not in normal breast epithelium, leading these authors to conclude that WT1 may not have a tumor suppressor role in the tumorigenesis of breast cancer. In addition, Miyoshi et al. (10) correlated high levels of WT1 expression with poor prognosis in breast cancer patients. Zapata-Benavides et al. (11) reported that introduction of WT1 anti-sense oligos into breast cancer cells led to growth inhibition, suggesting a growth-promoting role of WT1. Therefore, the exact function of WT1 in the tumorigenesis of breast cancer remains controversial.
Human breast cancer evolves in a progressive stepwise manner from benign hyperplasia through atypical hyperplasia to carcinoma in situ (CIS) and eventually to invasive carcinomas with the potential to metastasize. In the human female breast, a spectrum of histopathological changes has been characterized as proliferative breast disease. The progression of proliferative breast disease has been associated with increased risk for the development of invasive carcinoma (12). Thus, the study of proliferative breast disease is likely to provide important information about the molecular and cellular events in the early development of breast cancer. The human cell line MCF10A originated from spontaneous immortalization of non-malignant breast epithelium (13). MCF10A cells transfected with T24 Ha-ras mutant (designated as MCF10AT) acquired the ability for xenograft growth in the dorsal flank of nude mice. The MCF10AT3B cell line was established from a hyperplastic lesion formed by the third generation transplant in nude mice. When xenografted into nude mice, the MCF10AT3B cells form atypical hyperplasia, CIS and invasive carcinoma (14). Long-term transplant of MCF10AT3B cells in nude mice produces lesions of various morphological types and grades, resembling the morphological characteristics of progressive human proliferative breast disease as well as in situ and invasive carcinoma (13, 14).
Previously, most studies of WT1 function were performed in established breast cancer cell lines. The biological function of WT1 in early development of breast cancer has not been studied. Here, we investigated the role of WT1 in MCF10AT3B cells to test whether constitutive expression of WT1 influences malignant progression of these cells in vitro and in vivo.
Materials and Methods
Cell culture and establishment of stable cell lines
MCF10AT3B cells were obtained from Karmanos Cancer Institute (Detroit, MI, USA) and maintained at 37°C in a 10% CO2 atmosphere in Dulbecco’s Modified Eagle’s Medium (DMEM)/F12 medium supplemented with 5% horse serum, L-glutamine (2 mM), penicillin (100 U/ml), streptomycin (100 µg/ml), hydrocortisone (0.5 µg/ml), insulin (10 µg/ml), epithelial growth factor (EGF, 2 ng/ml), cholera toxin (0.1 µg/ml) and fungizone (0.5 µg/ml). Cells were plated at a density of 1×105 cells per 60-mm dish and transfected 24 hours later with an HA-tagged WT1 expression vector driven by the cytomagalovirus (CMV) promoter in the mammalian expression vector pCB6+ as described elsewhere (15), using the FuGene6 transfection reagent (Roche Applied Sciences, Indianapolis, IN, USA). The empty expression vector was also transfected into cells to serve as a control. Forty-eight hours after transfection, the cells were re-plated and selected with 500 µg/ml of G418 (Invitrogen Corporation, Carlsbad, CA, USA) for two weeks. The medium was changed every three days until colonies appeared. More than 20 individual clones were then pooled and expanded to confirm expression of WT1 by Western blot analysis. MCF10AT3B cells transfected with the empty expression vector were used as a control.
To examine cell growth, cells maintained in DMEM/F12 medium and 5% horse serum were seeded at 1×103 cells per well in a 24-well plate and counted every day using a hemacytometer. Three wells were examined for each time point.
For estrogen-stimulated cell growth assays, cells maintained in phenol red-free DMEM/F12 medium plus 2.5% charcoal-dextran stripped fetal calf serum (HyClone, Logan, UT, USA) for three days were treated with 1 nM of 17β-estradiol (E2), or ethanol vehicle as a control. Antiestrogens tamoxifen (1 µM, Sigma, St. Louis, MO, USA) or fulvestrant (ICI 182,780, 1 µM, Sigma, St. Louis, MO, USA) respectively were included in experiments. The cells were seeded at 1×103 cells per well in a 6-well dish and counted after 12 days using a hemacytometer. Three wells were used for each experiment and experiments were repeated three times.
Western blot analysis
Cells were washed with phosphate buffered saline (PBS) and lysed with lysis buffer [50 mM Tris-HCl pH 8.0, 150 mM NaCl, 0.25 mM EDTA pH 8.0, pH8.0, 0.1% sodium dodecyl sulfate (SDS), 1% Triton® X-100, 50 mM NaF and protease inhibitor cocktail from Sigma, St. Louis, MO, USA]. After adjustment to the same total protein content, cell lysates were analyzed by Western blot analysis. Twenty micrograms of cell lysate were boiled for 5 minutes in SDS gel loading buffer and separated on a 12% or 10% SDS-PAGE gel. After electrophoresis, the proteins were transferred to a PVDF membrane (Bio-Rad Laboratories, Hercules, CA, USA). The membranes were probed with different primary antibodies against p16 (N-20), p19 (M-167), p21 (F-5), p27 (C-19), cyclin A (BF683), cyclin B1 (GNS1), cyclin D1 (HD11) and cyclin E1 (C-19) (Santa Cruz Biotechnology, Santa Cruz, CA, USA), HA-tag (Upstate Biotechnology, Charlottesville, VA, USA) and affinity-purified rabbit polyclonal anti-ER-α36 antibody generated as a custom service from Pacific Immunology Corp. Ramona, CA, USA (15). The membranes were then incubated with appropriate HRP-conjugated secondary antibodies, and visualized with enhanced chemiluminescence (ECL) detection reagents (Amersham Pharmacia Biotech., Piscataway, NJ, USA). The same membranes were stripped and reprobed with an antibody against β-actin (I-19) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) to confirm equal loading.
Tumor formation assay in nude mice
For tumor formation assay in nude mice, forty-eight female athymic nude (nu/nu) mice were purchased fromCharles River Laboratories (Wilmington, MA, USA). All mice were ovariectomized and a 60-day release pellet containing 1.7 mg of 17β-estradiol (E2β; Innovative Research of America, Sarasota, FL, USA) was placed under the skin of twenty-four mice. As controls, placebo pellets (Innovative Research of America) were implanted into the remaining twenty-four mice. One week before the injection, the culture medium of both cell lines, TG3B/vector and TG3B/WT1 was switched to phenol red–free improved minimum essential medium (IMEM) plus 5% charcoal-dextran-stripped fetal calf serum. Five days after implantation of estrogen or placebo pellets, 1×107 cells of the WT1-transfected (TG3B/WT1) or empty vector-transfected MCF10AT3B (TG3B/vector) cells suspended in 200 µl of sterile Matrigel (BD Biosciences, San Jose, CA, USA) were subcutaneously injected into the mammary fat pad of ovariectomized mice. There are four groups of mice, TG3B/vector−E2, TG3B/vector+E2, TG3B/WT1−E2 and TG3B/WT1+E2.
Animals were monitored weekly, beginning at two weeks after the injection, to check the rate of tumor growth by palpation weekly. Pellets were replaced at seven weeks. After 10 weeks, all mice were sacrificed, tissues from the injection sites were dissected and lesions were weighed with portions of each fixed in neutral buffered formalin and embedded in paraffin for histological examination. Histological grading of lesions was performed as described elsewhere (13,16,17). The categories were as follows: G0. no lesions were formed; G1. mild hyperplasia; G2. moderate hyperplasia; G3. atypical hyperplasia; G4. carcinoma in situ (CIS); G5. invasive carcinoma. Each lesion was graded according to the most advanced morphological pattern observed within it.
Results
Constitutive WT1 expression suppresses growth of MCF10AT3B cells in the presence and absence of estrogen
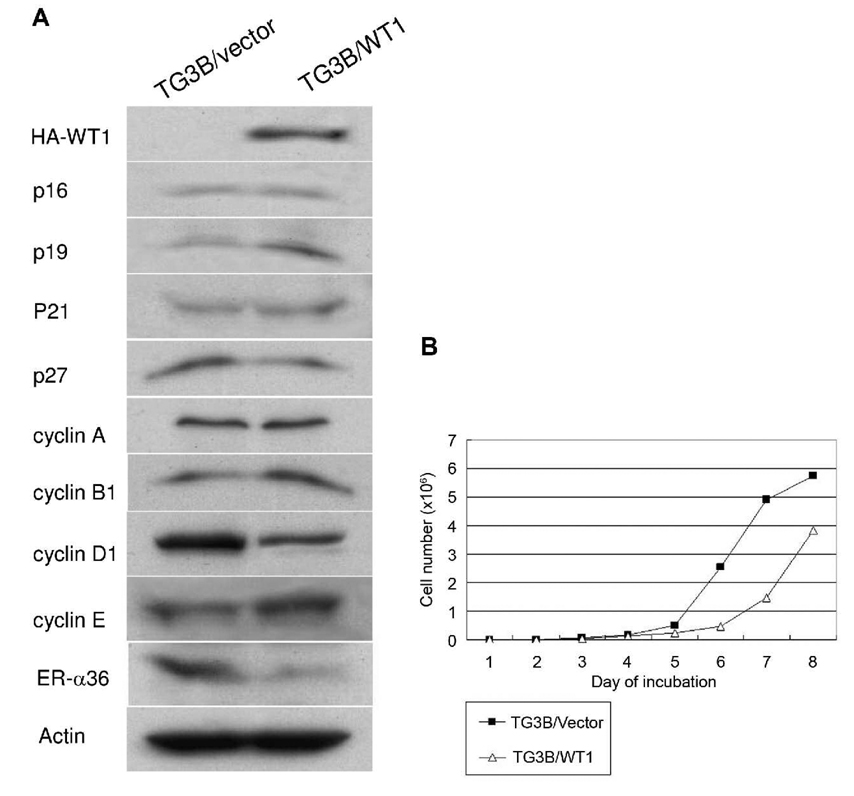
To investigate the effects of constitutive WT1 expression on early development of breast cancer, we established a stable cell line that expresses exogenous WT1 from MCF10AT3B, a cell line derived from a xenograft model of progressive human proliferative disease (13, 14). We established a cell line (TG3B/WT1) from a mixture of more than 20 transfectants that expressed high levels of recombinant WT1. A cell line pooled from a mixture of cells transfected with the empty expression vector (TG3B/vector) was also generated. Both cell lines were examined with Western blot analysis using an antibody against the HA tag. WT1 is highly expressed in TG3B/WT1 cells compared with the control TG3B/vector cells (Figure 1A), indicating we established a cell line that highly and constitutively expresses recombinant WT1. We then examined the expression of cell cycle regulatory proteins in these two cell lines. The cell cycle protein p19ARF expression was increased in TG3B/WT1 cells compared to TG3B/vector cells, while expression of p16INK4, p21CIP1 and p27KIP1 were essentially without any change. The expression of the important cell cycle regulator, cyclin D1 was dramatically reduced in TG3B/WT1 cells compared with the control TG3B/vector cells. However, expression levels of cyclin A and B1 were similar in these two cell lines, while cyclin E expression was slightly increased in TG3B/WT1 cells. These results demonstrated that constitutive WT1 expression had a profound impact on the expression of important cell cycle regulators in pre-malignant mammary epithelial cells.
Figure 1. WT1 expression influences expression of cell cycle regulators and inhibits the growth of MCF10AT3B cells.
A. Western blot analysis of the cell lysates from WT1-transfected TG3B/WT1 cells and control TG3B/vector cells transfected with empty expression vector using different primary antibodies as described in Materials and Methods. B. Growth rate of TG3B/WT1 cells and TG3B/vector cells. The cells were seeded at 1×103 cells per well in a 24 well plate and counted every day for eight days. Three wells were used for each experiment, which were repeated three times. Data presented are means of three experiments and standard deviations (SD) for each point were less than 10%.
Previously, it was reported that expression of estrogen receptor-α (ER-α) is activated in cells derived from the MCF10AT xenograft model (18, 19). In our study, ER-α expression was not detected using Western blot analysis in MCF10AT3B cells (data not shown). However, ER-α36, a novel variant of ER-α, was highly expressed in TG3B/vector cells but down-regulated in TG3B/WT1 cells.
To examine the influence of constitutive WT1 expression on cell growth, the TG3B/WT1 cells and TG3B/vector cells were maintained under optimal growth condition and the growth rate of each cell line was determined by counting the number of cells every day. TG3B/WT1 cells exhibited a two-fold lower growth rate than the control TG3B/vector cells (Figure 1B). This result indicates that the high levels of WT1 expression reduce the growth rate of pre-malignant MCF10AT3B cells, consistent with the function of WT1 as a potent growth inhibitor.
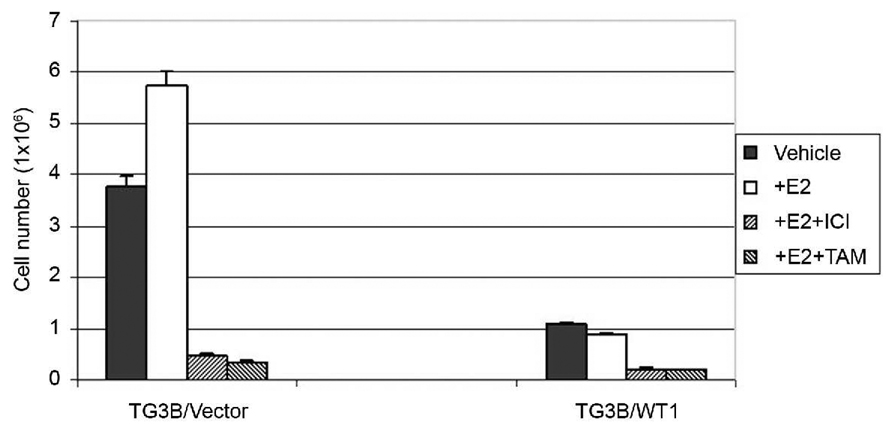
It was reported that estrogen signaling stimulates malignant progression of the cells derived from the MCF10AT model system (18, 19). To test whether constitutive expression of WT1 also influences estrogen-stimulated cell growth in MCF10AT3B cells, TG3B/vector and TG3B/WT1 cells were treated with 1 nM of 17β-estradiol (E2) or 12 days and cell number was then counted. Estrogen treatment weakly stimulated growth of the control TG3B/vector cells, consistent with the previous reports that MCF10AT cell variants respond to mitogenic estrogen signaling (18,19). However, constitutive expression of WT1 strongly inhibited estrogen-dependent cell proliferation at a similar efficiency as antiestrogens ICI 186, 780 and tamoxifen (Figure 2).
Figure 2. WT1 expression suppresses estrogen-stimulated cell growth in MCF10AT3B cells.
TG3B/vector and TG3B/WT1 cells maintained in phenol red-free DMEM/F12 medium containing 2.5% charcoal/dextran-stripped fetal calf serum were seeded at a density of 1x×103 cells per well in a 6-well dish and treated with 1 nM 17β-estradiol (+E2), or ethanol vehicle as controls (vehicle). After incubation with E2 for 12 days, cells were trypsinized and counted. Antiestrogen, 1 µM of tamoxifen (TAM) or ICI 182, 780 (ICI) was included in control experiments, respectively. Three wells were used for each experiment, which were repeated three times. SD for each point is also shown.
WT1 expression inhibits malignant progression of neoplastigenic MCF10AT3B cells
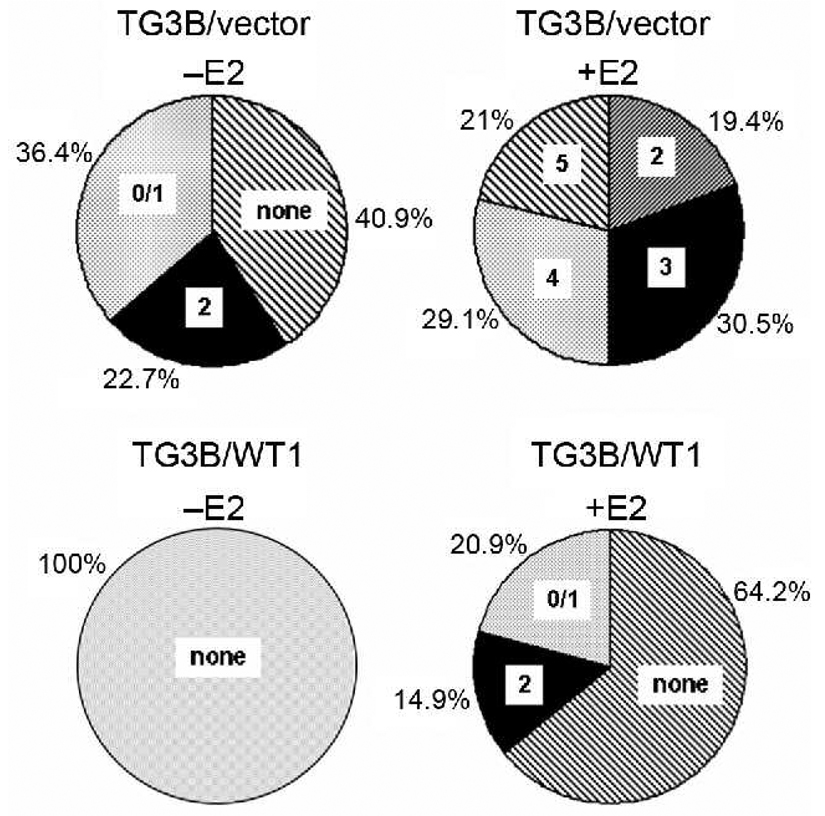
We then tested whether constitutive WT1 expression inhibits estrogen-stimulated progression of the preneoplastic MCF10AT3B cells in nude mice. Ovariectomized female nude mice supplemented with or without estrogen pellets were injected with control TG3B/vector and TG3B/WT1 cells in the mammary fatty pad. In the absence of estrogen pellets, TG3B/vector cells formed a few small lesions displaying histological characteristic of G0/1 or G2 after 10 weeks, which could be totally inhibited by WT1 expression (Figure 3). In the presence of estrogen pellets, control TG3B/vector cells formed more and relatively large lesions with more advanced grade of tumors (Figure 3), consistent with the previous reports that estrogen signaling stimulates malignant progression of the cells derived from the MCF10AT series (18, 19). However, WT1 expression strongly suppressed estrogen-stimulated progression of the preneoplastic MCF10AT3B cells; WT1 expression strongly reduced the frequency of lesion formation, the size of lesions and the degree of dysplasia of MCF10AT3B cells in nude mice (Figure 3).
Figure 3. Frequency of lesions with different grades formed by TG3B/vector and TG3B/WT1 cells injected in control and estrogen-supplemented nude mice.
Cells (1×107) suspended in 200 µl of Matrigel were subcutaneously injected into the mammary fat pad of ovariectomized mice supplemented with placebo (−E2) or E2 pellets (+E2) and maintained for 10 weeks. Tissues from the injection sites were processed and scored as described in Materials and Methods.
Discussion
We used neoplastigenic mammary epithelial cells MCF10AT3B as a model system to study the effects of WT1 expression on the early development of breast cancer. Our results from both in vitro and in vivo assays demonstrated that constitutive WT1 expression strongly inhibits estrogen-dependent and-independent proliferation and tumorigenesis of MCF10AT3B cells. There was a good correlation between the inhibition of cell proliferation in vitro and the inhibition of tumor progression in vivo. These data demonstrated that WT1 functions as a potent tumor suppressor which might prevent initiation and progression of breast cancer.
Recently, Zapata-Benavides et al. (11) reported that the expression levels of WT1 were correlated with cell proliferation of several lines of breast cancer cells and down-regulation of WT1 expression using liposome-incorporated WT1 antisense oligonucleotides led to growth inhibition of breast cancer cells. They concluded that WT1 contributes to breast cancer malignancy by promoting breast cancer cell proliferation. These data conflict with our results that forced expression of WT1 inhibits cell proliferation and abolishes tumorigenecity in neoplastigenic MCF10AT3B cells. These conflicting results may be explained by the different isoforms of WT1 studied. The WT1 gene undergoes alternative splicing at two different positions; a 51-bp sequence encoded by exon 5 (Ex5) and a 9-bp sequence that results from the use of an alternative 5’ splice junction in exon 9 leads to the inclusion of a tripeptide (Lys-Thr-Ser, KTS) between zinc-fingers 3 and 4 (4). It was reported recently that induced expression of the (−Ex5/−KTS) WT1 isoform, the same isoform we used in this study, resulted in a G2-phase cell cycle arrest and a slow cell growth, whereas expression of the (+Ex5/+KTS) WT1 isoform had no effect on cell growth but induced an epithelial-mesenchymal transition (20). Different isoforms of WT could function differently either to promote or to inhibit malignant growth of breast cancer cells.
In this study, we also found that expression of cyclin-dependent kinase (CDK) inhibitory protein p19ARF was increased in WT-expressing cells TG3B/WT1 whereas expression of other CDK inhibitors including p16INK4, p21CIP1, and p27KIP1 was unchanged. Previously, it was reported that p21CIP1 is one of the target genes that is activated by WT1 (21), suggesting that the mechanism by which WT1 regulates cell cycle progression is dependent on cell context. WT1 may use a mechanism other than p21CIP1 induction in mammary epithelial cells to inhibit cell proliferation. Our data would suggest that the CDK inhibitor, p19ARF, plays an important role in growth inhibitory activity of WT1 in mammary epithelial cells.
In WT1-expressing TG3B/WT1 cells, cyclin D1 expression was dramatically reduced while cyclin E expression was slightly increased compared to that of control TG3B/vector cells. WT1 expression did not substantially affect the expression levels of two other cyclins, cyclin A and B1. Cyclin D-CDK4/6 and cyclin E-CDK2 are key factors in the G1 checkpoint to promote transition into the S-phase. Ectopic expression of cyclin D1 and E accelerates the G1/S transition. Our results indicate an opposite expression pattern of these two proteins in asynchronous cell populations. It has been reported that cyclin D and E may control two different rate-limiting events (22). The cyclin E protein level has been shown to peak at the G1/S boundary and to decline as the S-phase progresses (23) by degradation via the ubiquitin-proteasome system (24). The down-regulated expression of cyclin D may arrest cells at a specific checkpoint in the G1/S boundary, which accumulates cyclin E-expressing cells at that specific point. These cells thus exhibited increased levels of cyclin E expression. These data provide supporting evidence for the notion that the event of cyclin D1 regulation by WT1 is a rate-limiting factor for the S-phase progression of the cell cycle in MCF10AT3B cells.
Furthermore, it has been reported that MCF10AT cells develop into atypical hyperplasia, carcinoma in situ, or invasive carcinoma at a much greater frequency and in a relatively short time when stimulated with estrogens (20), indicating that mitogenic estrogen signaling directly promotes neoplastic progression of MCF10AT cells. In this study, we found that WT1 expression strongly inhibited estrogen-stimulated malignant progression of MCF10AT3B cells, indicating that WT1 is involved in the regulation of estrogen-stimulated cell growth.
Recently, we have identified and cloned a 36-kDa novel isoform of estrogen receptor α (ER-α36) that is generated from a promoter located in the first intron of the original 66-kDa ER-α (ER-α66) gene (15). ER-α36 lacks both transcriptional activation domains (AF-1 and AF-2) and localizes mainly on the plasma membrane and in the cytoplasm (15). ER-α36 functions differently from previously discovered estrogen receptors by mediating membrane-initiated estrogen signaling that strongly stimulates cell proliferation (15). Thus, ER-α36 is a novel and important player of mitogenic estrogen signaling in mammary epithelial cells. In this study, we observed that expression levels of ER-α36 were dramatically reduced in TG3B/WT1 cells compared to control TG3B/vector cells, indicating that ER-α36 is a WT1 target gene down-regulated by WT1. We recently cloned and characterized the promoter region of ER-α36 gene and confirmed that there are two WT1-binding sequences located in the promoter region of ER-α36 gene. It was previously reported that estrogen treatment induces WT1 expression in ER-positive breast cancer cells (11), suggesting WT1 is involved in the regulation of mitogenic estrogen signaling. This together with our data suggest that it is possible that WT1 may function as a feedback regulator of estrogen signaling by directly suppressing ER-α36 expression. Future study of ER-α36 function and its regulation by WT1 in mammary epithelial cells will provide important information about the molecular mechanisms underlying estrogen-stimulated early development of human breast cancer.
Acknowledgements
We thank Dr. Hong Deng for his help with histology and scoring of lesions. Grant support: NIH grant DK070016 (Z.Y. Wang) and the Nebraska Tobacco Settlement Biomedical Research Program Award (LB-595) to (Z.Y. Wang).
References
- 1.Bonetta L, Kuehn SE, Huang A, Law DJ, Kalikin LM, Koi M, Reeve AE, Brownstein BH, Yéger H, Williams BRG, Feingberg AP. Wilms’ tumor locus on 11p13 defined by multiple CpG island associated transcripts. Science. 1990;250:994–997. doi: 10.1126/science.2173146. [DOI] [PubMed] [Google Scholar]
- 2.Call KM, Glaser T, Ito CY, Buckler AJ, Pelletier J, Haber DA, Rose EA, Kral A, Yeger H, Lewis WH, Jones C, Housman DE. Isolation and characterization of a zinc finger polypeptide gene at the human chromosome 11 Wilms’ tumor locus. Cell. 1990;60:509–520. doi: 10.1016/0092-8674(90)90601-a. [DOI] [PubMed] [Google Scholar]
- 3.Gessler M, Poutska A, Cavenee W, Neve RL, Orkin SH, Bruns GA. Homozygouse deletion in Wilms’ tumors of a zinger finger gene identified by chromosome jumping. Nature (London) 1990;343:774–778. doi: 10.1038/343774a0. [DOI] [PubMed] [Google Scholar]
- 4.Haber DA, Sohn L, Buckler AJ, Pelletier J, Call KM, Housman DE. Alternative splicing and genomic structure of the Wilms’ tumor gene. Proc Natl Acad Sci USA. 1991;88:9618–9622. doi: 10.1073/pnas.88.21.9618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silberstein GB, Van Horn K, Strickland P, Roberts CT, Jr, Daniel CW. Altered expression of the WT1 Wilms tumor suppressor gene in human breast. Proc Natl Acad Sci USA. 1997;94:8132–8137. doi: 10.1073/pnas.94.15.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laux DE, Curran EM, Welshons WV, Lubahn DB, Huang TH. Hypermethylation of the Wilms’ tumor suppressor gene CpG island in human breast carcinomas. Breast Cancer Res Treat. 1999;56:35–43. doi: 10.1023/a:1006222803788. [DOI] [PubMed] [Google Scholar]
- 7.Huang TH, Laux DE, Hamlin BC, Tran P, Tran H, Lubahn DB. Identification of DNA methylation markers for human breast carcinomas using the methylation-sensitive restriction fingerprinting technique. Cancer Res. 1997;57:1030–1034. [PubMed] [Google Scholar]
- 8.Zhang TF, Yu SQ, Guan LS, Wang ZY. Inhibition of breast cancer cell growth by the Wilms’ tumor suppressor WT1 is associated with a destabilization of β-Catenin. Anticancer Res. 2003;3:35785–35874. [PubMed] [Google Scholar]
- 9.Loeb DM, Evron E, Patel CB, Sharma PM, Niranjan B, Buluwela L, Weitzman SA, Korz D, Sukumar S. Wilms tumor suppressor (WT1) is expressed in primary breast tumors despite tumor-specific promoter methylation. Cancer Res. 2001;76:921–925. [PubMed] [Google Scholar]
- 10.Miyoshi Y, Ando A, Egawa C, Taguchi T, Tamaki Y, Tamaki H, Sugiyama H, Noguchi S. High expression of Wilms' tumor suppressor gene predicts poor prognosis in breast cancer patients. Clin Cancer Res. 2002;8:1167–1171. [PubMed] [Google Scholar]
- 11.Zapata-Benavides P, Tuna M, Lopez-Berestein G, Tari AM. Downregulation of Wilms’ tumor 1 protein inhibits breast cancer proliferation. Biochem Biophys Res Commun. 2002;295:784–790. doi: 10.1016/s0006-291x(02)00751-9. [DOI] [PubMed] [Google Scholar]
- 12.Page DL, Dupont WD. Anatomic markers of human premalignancy and risk of breast cancer. Cancer. 1990;66:1326–1335. doi: 10.1002/1097-0142(19900915)66:14+<1326::aid-cncr2820661405>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 13.Dawaon PJ, Wolman SR, Tait L, Heppner GH, Miller FR. MCF10AT: A model for the evolution of cancer from proliferative breast disease. Am J Pathl. 1996;148:313–319. [PMC free article] [PubMed] [Google Scholar]
- 14.Miller FR, Soule HD, Tait L, Pauley RJ, Wolman SR, Dawson PJ, Heppner GH. Xenograft model of progressive human proliferative breast disease. J Natl Cancer Inst. 1993;85:1725–1732. doi: 10.1093/jnci/85.21.1725. [DOI] [PubMed] [Google Scholar]
- 15.Wang ZY, Zhang XT, Shen P, Loggie BW, Chang YC, Deuel TF. A variant of estrogen receptor-α, hER-α36: transduction of estrogen and anti-estrogen dependent membrane-initiated mitogenic signaling. Proc Natl Acad Sci USA. 2006;103:9063–9068. doi: 10.1073/pnas.0603339103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Page DL, Anderson TJ. Diagnostic Histopathology of the Breast. Edinburgh, UK: Churchill Livingstone; 1987. pp. 120–145. [Google Scholar]
- 17.Page DL, Dupont WD, Rogers LW, Rados MS. Atypical hyperplastic lesions of the female breast: along-term follow-up study. Cancer. 1985;55:2698–2708. doi: 10.1002/1097-0142(19850601)55:11<2698::aid-cncr2820551127>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 18.Shekhar MPV, Chen ML, Werdell J, Heppner GH, Miller FR, Christman JK. Transcriptional activation of functional endogenous estrogen receptor gene expression in MCF10AT cells: a model for early breast cancer. Int J Oncol. 1998;13:907–915. doi: 10.3892/ijo.13.5.907. [DOI] [PubMed] [Google Scholar]
- 19.Shekhar MPV, Nangia-Makker P, Wolman SR, Tait L, Heppner GH, Visscher DW. Direct action of estrogen on sequence of progression of human preneoplastic breast disease. Am J of Pathl. 1998;152:1129–1132. [PMC free article] [PubMed] [Google Scholar]
- 20.Burwell EA, McCarty GP, Simpson LA, Thompson KA, Loeb DM. Isoforms of Wilms’ tumor suppressor gene (WT1) have distinct effects on mammary epithelial cells. Oncogene. 2006;26:3423–3430. doi: 10.1038/sj.onc.1210127. [DOI] [PubMed] [Google Scholar]
- 21.Englert C, Maheswaran SA, Garvin J, Kreidberg J, Haber DA. Induction of p21 by the Wilms’ tumor suppressor gene WT1. Cancer Res. 1997;57:1429–1434. [PubMed] [Google Scholar]
- 22.Resnitzky D, Reed SI. Different roles for cyclins D1 and E in regulation of the G1-to-S transition. Mol Cell Biol. 1995;15:3463–3469. doi: 10.1128/mcb.15.7.3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koff A, Giordano A, Desai D, Yamashita K, Harper JW, Elledge SJ, Nishimoto T, Morgan DO, Franza BR, Roberts JM. Formation and activation of a cyclin E-cdk2 complex during the G1 phase of the human cell cycle. Science. 1992;257:1689–1694. doi: 10.1126/science.1388288. [DOI] [PubMed] [Google Scholar]
- 24.Clurman BE, Sheaff RJ, Thress K, Groudine M, Roberts JM. Turnover of cyclin E by the ubiquitinproteasome pathway is regulated by cdk2 binding and cyclin phosphorylation. Genes Dev. 1996;10:1979–1990. doi: 10.1101/gad.10.16.1979. [DOI] [PubMed] [Google Scholar]