Abstract
Anti-apoptotic Bcl-2 family proteins such as Bcl-2 and Bcl-XL have been recently validated as targets for the discovery of novel anti-cancer agents. We previously reported that racemic (+/−) Apogossypol, a semi-synthetic compound derived from the natural product Gossypol, binds and inhibits Bcl-2 and Bcl-XL in vitro and in cell. Given that (+) and (−) Gossypol display different proapoptotic activities, here we report on the synthesis of (+) and (−) Apogossypol and the evaluation of their in vitro and cellular activity.
Keywords: Apogossypol atropisomers, Gossypol, Apoptosis, Bcl-2, Bcl-xL
1. Introduction
Programmed cell death plays an essential role in normal tissue homeostasis, ensuring a proper balance of cell production and cell loss. Defects in the regulation of programmed cell death promote tumorgenesis, and also contribute significantly to chemoresistance [1, 2]. The Bcl-2 (B-cell lymphocyte/leukemia-2) family of proteins is central for the regulation of apoptosis [3–5]. Overexpression of antiapoptotic Bcl-2-family proteins, such as Bcl-2, Bcl-XL, Mcl-1, Bfl-1, Bcl-W and Bcl-B, occurs in many human cancers and leukemias and therefore these proteins are very attractive targets for the development of novel anticancer agents [6–9]. Bcl-2 family proteins also have pro-apoptotic members such as Bak, Bax, Bad, Bim or Bid. Anti-apoptotic and pro-apoptotic Bcl-2 family proteins dimerize and negate each other’s function [10]. Structural studies have elucidated a hydrophobic crevice on the surface of anti-apoptotic proteins that binds the BH3 dimerization domain of proapoptotic family members. Thus, molecules that mimic proapoptotic BH3 domain will displace anti-apoptotic proteins hence inducing apoptosis.
We and others have reported that the natural product (+/−) Gossypol is a potent inhibitor of Bcl-2, Bcl-XL and Mcl-1, functioning as a BH3 mimic [11–13]. Naturally, Gossypol is composed of the (+) and (−) enantiomers (atropisomers) due to hindered rotation along the single C-C bond between the two substituted naphthalene groups. Given that (+/−) Gossypol has toxicity problems likely due to two reactive aldehyde groups, we selected (+/−) Apogossypol, a derivative that lacks two aldehyde groups, but that retains most of anti-Bcl-2 properties in vitro and against cancer cells [14]. Recently, we further compared the efficacy and toxicity in mice of (+/−) Gossypol and Apogossypol. Our preclinical in vivo data supports the hypothesis that (+/−) Apogossypol has superior efficacy and markedly reduced toxicity compared to (+/−) Gossypol [15]. We also evaluated the single-dose pharmacokinetic characteristics of (+/−) Apogossypol in mice, revealing superior blood concentrations over time compared to (+/−) Gossypol, due to slower clearance of the compound [16]. These observations indicate that further development of (+/−) Apogossypol for cancer therapy is warranted.
Here we first prepared the individual isomers of Apogossypol and we further investigated their activity in vitro and in cell, given that similar studies with Gossypol revealed a marked differential activity for the isolated enantiomers [17]. Indeed, (−) Gossypol and not its natural racemic mixture, is currently under clinical evaluation [18]. The synthesis of (+) Apogossypol has been reported by Seshadri et al. However, no details on optical rotation or HPLC separation were provided [19]. The synthesis of (−) Apogossypol has not been reported. In this current work we focused our attention on preparing and evaluating activities of (+) and (−) atropisomers of Apogossypol.
2. Material and methods
2.1. Preparation of Apogossypol enantiomers
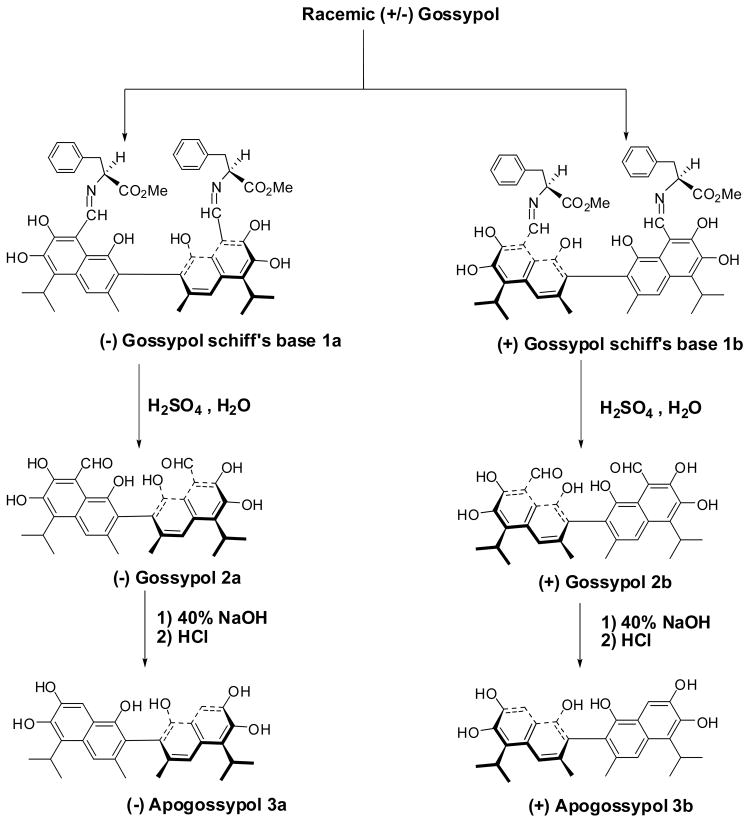
Racemic (+/−) gossypol acetic acid (5.0 g, Yixin Pharmaceutical Co.) was dissolved in 120 ml of diethyl ether and washed with water (2 × 100 ml) to remove acetic acid [20]. The ether layer was dried over MgSO4 followed by removal of the solvent under vacuum to give gossypol as yellow brown solid. L-Phenylalanine methyl ester hydrochloride (13.8 g, Sigma Aldrich) was dissolved into 200 ml of CH2Cl2 and then washed with saturated NaHCO3 solution (2 × 50 ml) to remove hydrochloride. The CH2Cl2 layer was dried over MgSO4 followed by removal of the solvent under vacuum to give pure L-Phenylalanine methyl ester as oil. Racemic gossypol (4.47 g) was dissolved in 120 ml of CH2Cl2. To the solution, L-Phenylalanine methyl ester (9.79 g) in CH2Cl2 (100 ml) and isopropanol (4.5 ml) were added. The reaction mixture was stirred at 20 °C for 10 hours in the dark. TLC indicated completion of the reaction. Flash chromatography on silica purification gave Gossypol Schiff’s bases 1a and 1b separately. The resolved Gossypol Schiff’s base (1a or 1b, 1.43 g) was dissolved in diethyl ether (50 ml) and acetic acid (12 ml) at 0 °C. Concentrated H2SO4 (1.6 ml) and distilled H2O (3.2 ml) were added and the reaction mixture was stirred at 0 °C in the dark for 12 hours and TLC indicated completion of the reaction. Aqueous saturated NaHCO3 solution was added and the ether layer was washed with H2O (3 × 10 ml), aqueous saturated NaCl (10 ml) and dried over MgSO4. Filtration and evaporation of the ether gave the corresponding (−) Gossypol 2a or (+) Gossypol 2b. The resolved Gossypol atropisomer (2a or 2b, 0.180 g) in 2.0 ml of 40% sodium hydroxide was heated under nitrogen at 90 °C for 1.5 hours in the dark. The resulting solution was cooled with an ice bath under nitrogen and HCl (6 M, 6 ml) was added slowly under nitrogen. Ascorbic acid (80 mg, 0.45 mmol, FW 176.13) was added in one portion followed immediately by more hydrochloric acid (9 M, 1 ml) and distilled H2O (50 ml). A straw colored emulsion was evenly divided by weight in two centrifuge tubes and centrifuge. The supernatant was decanted and the precipitation in each tube was re-suspended and washed with 50 ml of water four times. After washing, the suspensions were freeze-dried for 48 hours under dark to obtain corresponding (−) Apogossypol (compound 3a) or (+) Apogossypol (compound 3b) atropoisomer as a straw colored powder. The racemic (+/−) Apogossypol is made from the racemic (+/−) Gossypol using same method as (−) Apogossypol.
The enantiomeric purity of 3a and 3b was analyzed using a normal phase chiral column on a HPLC from Water Corp. The column is a Whelk-O2 10 μm 250 × 4.6 mm chiral column from Regis Technologies Inc. Results were analyzed using the Breeze software. Mobile phase A was 0.1% acetic acid in 2-propanol and mobile phase B is 0.1% acetic acid in hexane. Flow rate was 2 ml/min. The run duration was 37 min. The separation was done using constant 8% A and 92% B in 25 min followed by 12 min at 100% A to wash column. The compounds 3a and 3b are also analyzed by optical rotation, 1H-NMR and high resolution mass spectrometry.
2.2. Molecular modeling
Molecular modeling studies were conducted on a Linux workstation and a 64 3.2-GHz CPUs Linux cluster. Docking studies were performed using the crystal structure of Bcl-xL in complex with BAK-derived peptide [21], Protein Data Bank code 1BXL. The docked structures of (+) and (−) Apogossypol in the peptide binding pocket were obtained by ChemScore [22] as the scoring function in the GOLD [23] docking program. The protein surface was prepared with the program MOLCAD [24] in Sybyl (Tripos, St. Louis).
2.3. NMR experiments
NMR-based binding assays have been conducted by acquiring 1H, 1D experiments with 500 μL solution of Bcl-xL at 50 μM concentration, recorded in absence and presence of added compounds, each at 200 μM concentration. The binding mode has been characterized by recording [15N, 1H]-HSQC experiments with 500 μL solution of uniformly 15N-labeled Bcl-xL (125 μM concentration) in absence and presence of added compounds, each at 500 μM concentration. 15N and unlabeled Bcl-xL samples were prepared and purified as described previously [25]. All experiments were performed with a 600 MHz spectrometer Bruker Avance 600 equipped with four rf channels and z-axis pulse-field gradients.
2.4. Fluorescence polarization assays (FPA)
FPAs were performed with a fluorescein-labeled Bak peptide (FITC-Ahx-PSSTMGQVGRQLAIIGDDINRRYDS) using an LJL Analyst HT (Molecular Devices Co., Sunnyvale, CA). Dilution buffer for all stocks and samples was 50 mM phosphate buffer (pH 7.4). A series of 2-fold dilutions of Apogossypol were prepared, i.e., 20 μM, 10, μM down to 0.1 μM in dilution buffer. To each tube was added a solution containing 100 nM of Bcl-XL or Mcl-1 proteins and 10 nM fluoresceinated peptide. The tubes were incubated for 5 min at room temperature, and 100 μl each of reaction mixture was transferred to 96-well black PS, HE Microplate (LJL Biosystems Co.). All assays were performed in quadruplicate, with blank wells receiving no compounds. Then, the plate was read for total intensity and polarization (in mP units) was measured. Controls included dose-responses measurements in absence of the proteins, to assess any interactions between the compounds and the FITC-BH3 peptide. Approximate Ki values were obtained according to the following equation: Ki = IC50/(1+ [Bcl-XL]/Kd), where Kd is the dissociation constant for the FITC-BH3 peptide derived from a titration of Bcl-XL or Mcl-1 in the same FP assay.
2.5. Cell viability assay
Two lymphoma cell lines, RS11846 and DOHH2, were cultured in RPMI 1640 medium (Mediatech Inc., Herndon, VA 20171) containing 10% fetal bovine serum (Mediatech Inc., Herndon, VA 20171) and Penicillin/Streptomycin (Mediatech Inc., Herndon, VA 20171). Cells were cultured with various concentrations of racemic, (−) and (+) Apogossypol for 1 – 2 days. The percentage of viable cells was determined by Annexin V- and propidium iodide (PI)-labeling, using an Apoptosis Detection kit (BioVision Inc.), and analyzing stained cells by flow cytometry (FACSort; Bectin-Dickinson, Inc.; Mountain View, CA). Cells that were annexin-V-negative and PI-negative were considered viable.
3. Results
3.1. Resolution of Apogossypol enantiomers
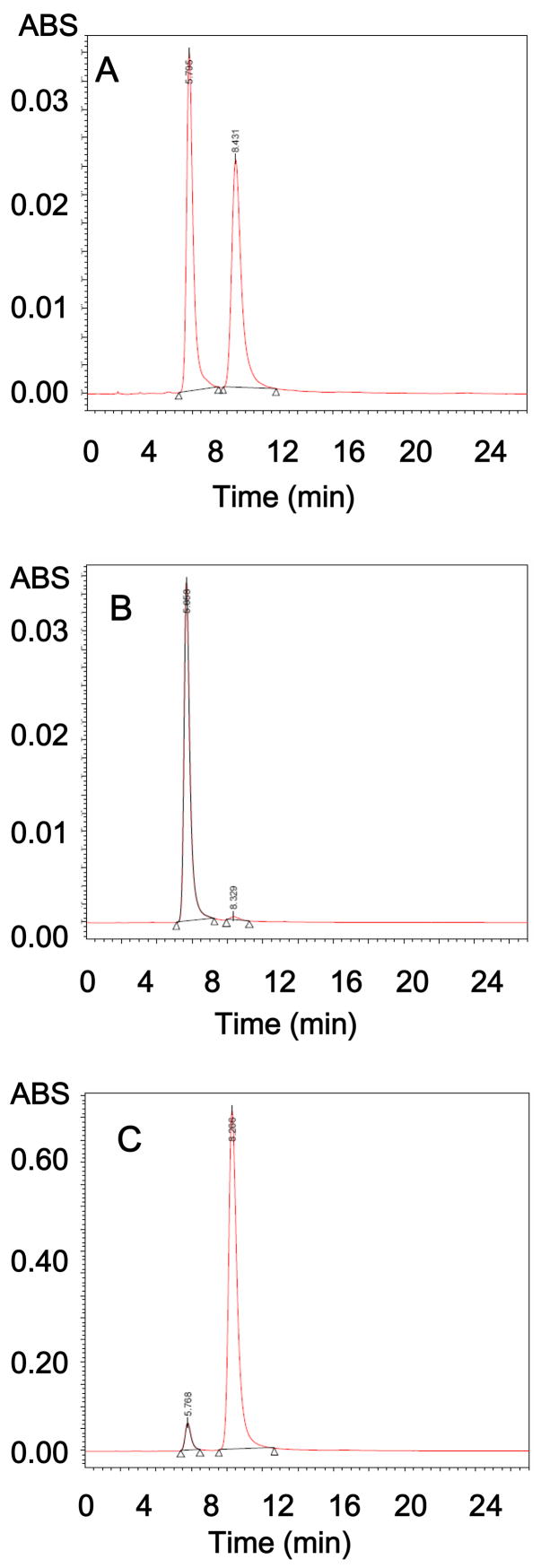
The optical rotation (α) of (+) and (−) Gossypol, expressed as + 348° and – 352° respectively, and 1H NMR data (600 MHz, CDCl3 : δ1.55 (d, 12H, J=5.5 Hz); 2.16 (s, 6H); 3.94 (m, 2H); 5.80 (s, 2H); 6.44 (s, 2H); 7.79 (s, 2H); 11.15 (s, 2H); 15.19 (s, 2H)) are in excellent agreement with published physical data for (+) and (−) gossypol [26]. The optical rotation of compounds 3a and 3b are −92.5° and + 95.0° respectively. In comparison, optical rotation of racemic Apogossypol is + 2.0°. Since there are no optical rotation data for (+) and (−) Apogossypol in literature as comparison, it’s necessary to perform further HPLC analysis to verify the enantiomeric purity of the isolated (−) and (+) Apogossypol compounds. Different chiral columns under different HPLC conditions were screened and finally the racemic Apogossypol was well resolved by the normal phase chiral column Whelk-O2 10 μm 250 × 4.6 mm chiral column (Regis Technologies Inc.). With this column, the retention times of (−) and (+) Apogossypol are 5.7 and 8.4 min, respectively, using 8% 2-propanol and 92% hexane. The enantiomeric purity of the synthetically isolated isomers, compounds 3a and 3b, using same method, showed 98.4% purity for (−) Apogossypol and 94.9% purity for (+) Apogossypol respectively (Figure 2). The compound 3b has same 1H-NMR spectra as 3a (600 MHz, CDCl3: δ1.55 (d, 12H, J=5.5 Hz); 2.12 (s, 6H); 3.88 (m, 2H); 5.13 (s, 2H); 5.27 (s, 2H); 5.93 (s, 2H); 7.50 (s, 2H); 7.61 (s, 2H)). Both compounds 3a and 3b are 99% chemically pure based on HPLC (Figure 2). The exact calculated mass of compound 3a (C28H30O6) is 462.2042, which compares very well with the observed (M+H) value of 463.2108. The exact calculated mass of 3b (C28H30O6) is 462.2042, again comparing very well with the observed (M+H) value of 463.2110.
Figure 2.
Normal phase chiral HPLC of Apogossypol isomers (A) HPLC of racemic Apogossypol (B) HPLC of the (−) Apogossypol (C) HPLC of the (+) Apogossypol.
3.2 Results of molecular modeling
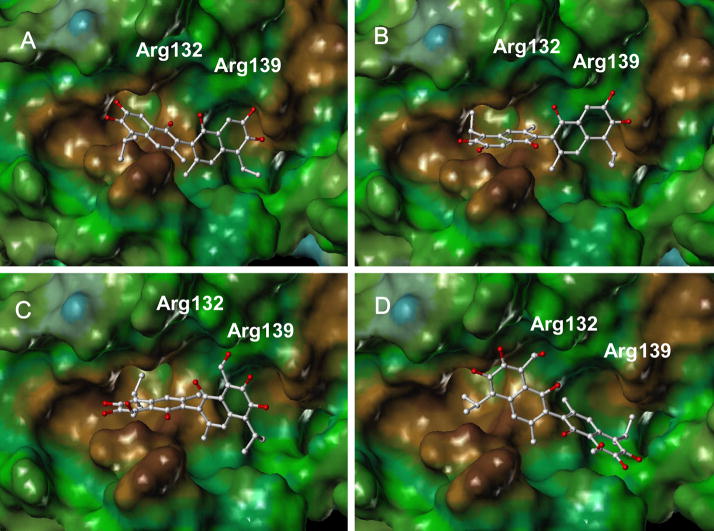
To gain further insights on the binding mode of Apogossypol enantiomers to Bcl-xL, we performed molecular docking studies using Gold [23] and the resulting models were visualized with MOLCAD [24] and Sybyl (TRIPOS, St. Louis). For these studies, we used the experimentally derived conformation of Bcl-xL when in complex with a Bak-peptide (Protein Data Bank code 1BXL [21]). We found that (−) and (+) Apogossypol can adopt similar binding modes [Figure 3A and 3B] and their docking scores are close. The overall positioning of (−) and (+) Apogossypol in the binding groove of Bcl-xL are almost identical. In comparison, the positioning of (−) and (+) Gossypol enantiomers [Fig. 3C and 3D] [27] in the binding pocket of Bcl-xL are quite different, due to the more anisotropic shape of the molecule for the presence of the two aldehyde functionalities. These docking studies would suggest that while a differential activity may be observed between the atropisomers of Gossypol, this may not be anticipated for the isolated isomers of Apogossypol.
Figure 3.
Molecular modeling studies. Stereo views of the docked structures of (−) Apogossypol (A), (+)Apogossypol (B), (−) Gossypol (C) and (+) Gossypol in the peptide binding pocket of the Bcl-xL.
3.3 1D and 2D NMR-based binding assays
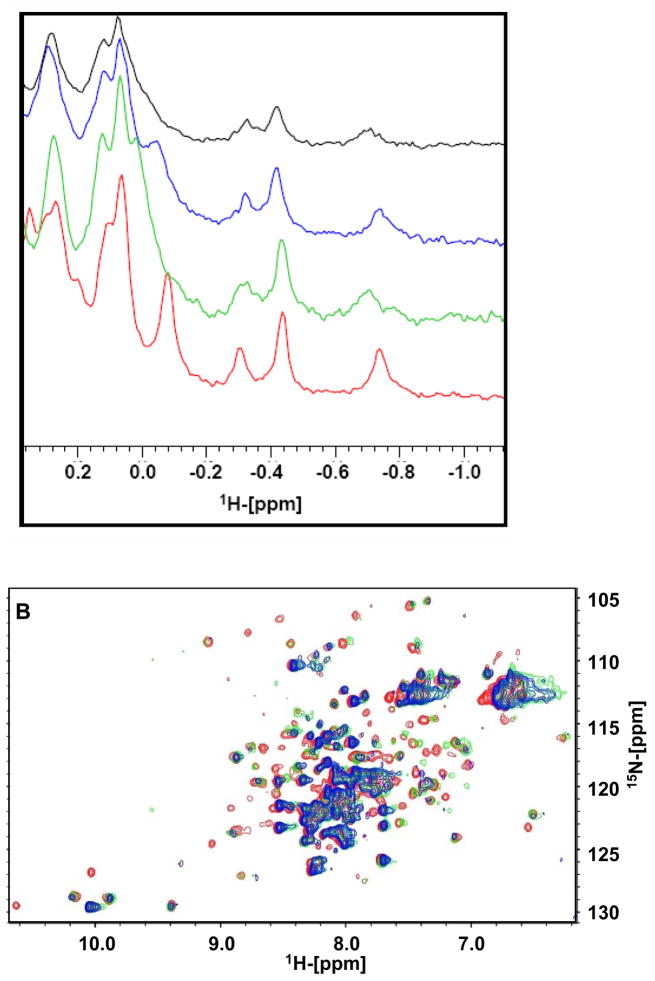
In order to test the ability of Apogossypol isomers to bind to Bcl-xL in vitro, we employed NMR-based binding assays (Fig. 4A and 4B). A sample of Bcl-xL was prepared and 1D 1H-NMR spectra were collected in absence and presence of the isolated Apogossypol isomers. By observing the aliphatic region of the spectra, binding could be readily detected due to chemical shift changes in active site methyl groups of Ile, Leu, Thr, Val or Ala (region between −0.8 and 0.3 ppm Fig. 4A). Both (−) and (+) Apogossypol induce significant chemical shift changes in 1D 1H-NMR spectra indicative of binding to Bcl-xL. Chemical shift changes in 1H-NMR spectrum of (−) and (+) Apogossypol have some small difference but, in general, they both induce a similar chemical shift changes pattern as that of racemic Apogossypol. In oder to confirm the results from 1D 1H-NMR binding assay, we also produced uniformly 15N-labeled Bcl-xL protein and measured 2D [15N,1H]-HSQC correlation spectra in absence and presence of the two isolated isomers and racemic Apogossypol (Figure 4B). Both (−) and (+) Apogossypol display strong binding with Bcl-xL, as qualitatively evaluated by the nature of the shifts at the ligand/protein ratio of 4:1, and there are only some small difference between spectra of Bcl-xL in complex with (−) and (+) Apogossypol, indicating that the two isomers can adopt a similar binding mode. These data confirm the findings from molecular docking studies that suggested a possible similar binding pose for the isolated atropisomers of Apogossypol in the BH3 binding groove of Bcl-xL.
Figure 4.
NMR binding studies. (A) Aliphatic region of the 1H-NMR spectrum of Bcl-xL (in red) and Bcl-xL (50 μM) in presence of (−) Apogossypol (200 μM, green), (+) Apogossypol (200 μM, blue) and racemic Apogossypol (200 μM, black). (B) Superposition of [15N, 1H]-TROSY spectra of free Bcl-xL (125 μM, red) after addition of (−) Apogossypol (green, 500 μM) and (+) Apogossypol (blue,500 μM).
3.4 Fluorescence polarization assays (FPA)
To further evaluate the inhibitory properties of Apogossypol isomers for Bcl-xL, we employed a competitive fluorescence polarization assay (FPA), in which the displacement of a fluorescein-labeled BH3 peptide is monitored upon titration of compound. (−), (+) and racemic Apogossypol displace fluorescein-labeled BH3 peptide from Bcl-xL with a Ki of 1.2, 1.7 and 3.3 μM respectively (Figure 5A). Since recent studies have demonstrated that Mcl-1 plays an essential role in apoptosis resistance of cancer cells and has become a highly attractive anticancer drug target, we also evaluate the inhibitory properties of Apogossypol isomers for Mcl-1 using same competitive fluorescence polarization assay. (−), (+) and racemic Apogossypol bind to Mcl-1 with a Ki of 1.4, 1.7 and 3.1 μM respectively (Figure 5B). Our FPA studies demonstrated that (−) and (+) Apogossypol display similar binding activity and that they are both slightly more active than racemic Apogossypol.
Figure 5.
Fluorescence polarization-based competitive binding assay analysis of Apogossypol isomers.
3.5 Results of cell viability assay
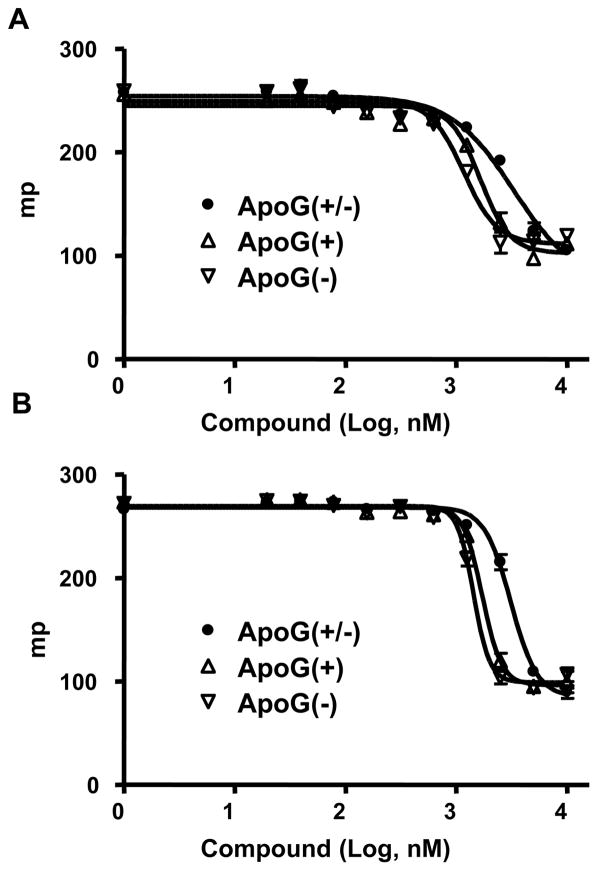
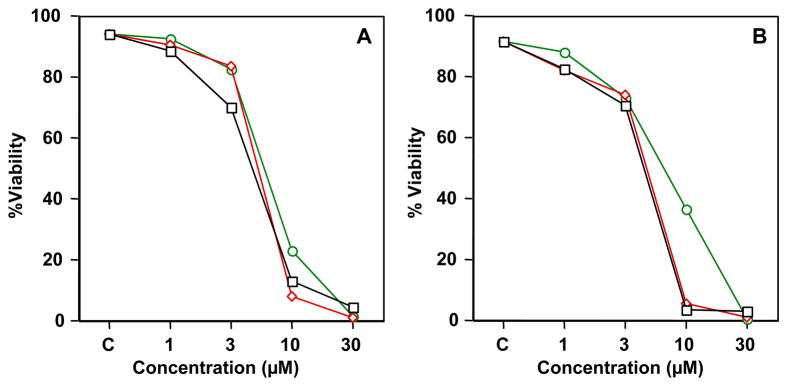
To evaluate the cellular pro-apoptotic activity of the isolated Apogossypol isomers, we studied RS11846 and DOHH2 lymphoma cell lines. The effect of (−), (+) and racemic Apogossypol on lymphoma cell viability was evaluated by staining with Annexin V-FITC/propidium iodide (PI), follwed by flow-cytometry analysis. (−), (+) and racemic Apogossypol induced apoptosis of RS11846 and DOHH2 lymphoma cell lines in a dose-dependent manner (Figure 6). Apoptosis activities of (−) and (+) Apogossypol are close and they are both slightly stronger than racemic Apogossypol. These results are in agreement with our fluorescence polarization assay data.
Figure 6.
Cell-based assays. (A) Effects of (−) Apogossypol (black squares), (+) Apogossypol (red diamonds), and racemic Apogossypol (green circles) on cell viability of the RS11846 lymphoma cell line. (B) Effects of (−) Apogossypol (black squares), (+) Apogossypol (red diamonds), and racemic Apogossypol (green circles) on cell viability of the DOHH2 lymphoma cell line.
4. Conclusions
Both (−) and (+) Apogossypol display similar in vitro binding and displacement properties and cellular activity as racemic Apogossypol. In comparison, Gossypol enantiomers have remarkable stereo-selective cytotoxic difference in several tumor cell lines. Due to the presence of two aldehyde groups, Gossypol enationmers are more anisotropic in shape than Apogossypol enantiomers. In fact, the optical rotation difference between (+) and (−) Gossypol enantiomers are 700°. In comparison, the optical rotation difference between (+) and (−) Apogossypol are only 187.5°. Molecular modeling studies also suggest that the overall positioning of both Apogossypol enantiomers in Bcl-xL is silimar, but the overall positions of Gossypol enantiomes in Bcl-xL are quite different. As a consequence, cellular activity of the isolated atropisomers of Apogossypol is similar and only slightly better than what was observed with racemic Apogossypol. Because racemic Apogossypol is more easily synthesized than the isolated isomers, further development of racemic Apogossypol for cancer therapy is prefered.
Figure 1.
Preparation of apogossypol enantiomers
Acknowledgments
We thank NIH (Grant U01 AI061139) and Coronado Biosciences (CSRA #08-02) for financial support and the NCI-RAID program for technical and scientific support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Reed JC. Dysregulation of apoptosis in cancer. J Clin Oncol. 1999;17:2941–2953. doi: 10.1200/JCO.1999.17.9.2941. [DOI] [PubMed] [Google Scholar]
- 2.Reed JC. Apoptosis-based therapies. Nat Rev Drug Discov. 2002;1:111–121. doi: 10.1038/nrd726. [DOI] [PubMed] [Google Scholar]
- 3.Reed JC. Molecular biology of chronic lymphocytic leukemia: implications for therapy. Semin Hematol. 1998;35:3–13. [PubMed] [Google Scholar]
- 4.Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 5.Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 6.Wang JL, Liu D, Zhang ZJ, Shan S, Han X. Structure-based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells. Proc Natl Acad Sci USA. 2000;97:7124–7129. doi: 10.1073/pnas.97.13.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Degterev A, Lugovskoy A, Cardone M, Mulley B, Wagner G. Identification of small-molecule inhibitors of interaction between the BH3 domain and Bcl-xL. Nat Cell Biol. 2001;3:173–182. doi: 10.1038/35055085. [DOI] [PubMed] [Google Scholar]
- 8.Reed JC. Oncogene. 1998;17:3225–3236. doi: 10.1038/sj.onc.1202591. [DOI] [PubMed] [Google Scholar]
- 9.Reed JC. Bcl-2 family proteins: Strategies for overcoming chemoresistance in cancer. Adv Pharmacol. 1997;41:501–553. doi: 10.1016/s1054-3589(08)61070-4. [DOI] [PubMed] [Google Scholar]
- 10.Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108:153–164. doi: 10.1016/s0092-8674(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 11.Kitada S, Leone M, Sareth S, Zhai D, Reed JC, Pellecchia M. Discovery, characterization, and structure-activ-ity relationships studies of proapoptotic polyphenols targeting B-cell lymphocyte/leukemia-2 proteins. J Med Chem. 2003;46:4259–4264. doi: 10.1021/jm030190z. [DOI] [PubMed] [Google Scholar]
- 12.Zhang M, Liu H, Guo R, Ling Y, Wu X, Li B, Roller PP, Wang S, Yang D. Molecular mechanism of gossypol-induced cell growth inhibition and cell death of HT-29 human colon carcinoma cells. Biochem Pharmacol. 2003;66:93–103. doi: 10.1016/s0006-2952(03)00248-x. [DOI] [PubMed] [Google Scholar]
- 13.Wang G, Nikolovska-Coleska Z, Yang CY, Wang R, Tang G, Guo J, Shangary S, Qiu S, Gao W, Yang D, Meagher J, Stuckey J, Krajewski K, Jiang S, Roller PP, Abaan HO, Tomita Y, Wang S. Structure-based design of potent small-molecule inhibitors of anti-apoptotic Bcl-2 proteins. J Med Chem. 2006;49:6139–6142. doi: 10.1021/jm060460o. [DOI] [PubMed] [Google Scholar]
- 14.Becattini B, Kitada S, Leone M, Monosov E, Chandler S, Zhai D, Kipps TJ, Reed JC, Pellecchia M. Rational design and real time in-cell detection of the proapoptotic activity of a novel compound targeting Bcl-xL. Chem Biol. 2004;11:389–395. doi: 10.1016/j.chembiol.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 15.Kitada S, Kress CL, Krajewska M, Jia L, Pellecchia M, Reed JC. Bcl-2 antagonist ApoGossypol (NSC736630) displays single-agent activity in Bcl-2 transgenic mice and has superior efficacy with less toxicity compared to Gossypol (NSC19048) Blood. 2008 doi: 10.1182/blood-2007-09-113647. Accepted, In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coward L, Gorman G, Noker P, Kerstner-Wood C, Pellecchia M, Reed JC. Quantitative determination of apogossypol, a pro-apoptotic analog of gossypol, in mouse plasma using LC/MS/MS. J Pharm Biomed Anal. 2006;42:581–586. doi: 10.1016/j.jpba.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 17.Qiu J, Levin LR, Buck J, Reidenberg MM. Different pathways of cell killing by gossypol enantiomers. Exp Biol Med (Maywood) 2002;227:398–401. doi: 10.1177/153537020222700605. [DOI] [PubMed] [Google Scholar]
- 18.Saleh M, Pitot H, Hartung J, Holmlund J, LoBuglio A, Forero A, Phase A. I trial of AT-101, an orally bioavailable inhibitor of Bcl-2, in patients with advanced malignancies. Presented at the 2005 AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics: Discovery, Biology, and Clinical Applications; November 14–18, 2005; Philadelphia, Pennsylvania. p. Abstract C89. [Google Scholar]
- 19.Seshadri TR, Sharma NN. An efficient method for the preparation of (+)-apogossypol. Indian J Chem Sect B. 1977;15B(11):1069–1070. [Google Scholar]
- 20.Shelley MD, Hartley L, Fish RG, Groundwater P, Morgan JJG, Mort D, Mason M, Evans A. Stereo-specific cytotoxic effects of gossypol enantiomers and gossypolone in tumor cell lines. Cancer Lett. 1999;135:171–180. doi: 10.1016/s0304-3835(98)00302-4. [DOI] [PubMed] [Google Scholar]
- 21.Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M, Yoon HS, Shuker SB, Chang BS, Minn AJ, Thompson CB, Fesik SW. Science. 1997;275:983–986. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- 22.Eldridge MD, Murray CW, Auton TR, Paolini GV, Mee RP. J Comput-Aided Mol Des. 1997;11:425–445. doi: 10.1023/a:1007996124545. [DOI] [PubMed] [Google Scholar]
- 23.Jones G, Willett P, Glen RC, Leach AR, Taylor R. Development and Validation of a Genetic Algorithm for Flexible Docking. J Mol Biol. 1997;267:727–748. doi: 10.1006/jmbi.1996.0897. [DOI] [PubMed] [Google Scholar]
- 24.Waldherr-Teschner M, Henn Ch, Vollhardt H, Reiling S, Brickmann J. Texture Mapping: A New Tool for Molecular Graphics. J Mol Graphics. 1994;12:89–105. doi: 10.1016/0263-7855(94)80074-x. [DOI] [PubMed] [Google Scholar]
- 25.Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M, Yoon HS, Shuker SB, Chang BS, Minn AJ, Thompson CB, Fesik SW. Science. 1997;275:983–986. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- 26.Brezezinski B, Olejnik J, Paszycs S. 1H NMR studies of gossypol and its complexes with some organic compounds. J Mol Struct. 1990;220:261–268. [Google Scholar]
- 27.Dowd MK, Stevens ED. The (−)-gossypol-2,4-pentanedione (1:2) inclusion complex. J Chem Crystallogr. 2004;34:559–564. [Google Scholar]