Abstract
Ciliary neurotrophic factor (CNTF) is expressed by glial cells at multiple levels of the magnocellular neurosecretory system (MNS). CNTF is present in astrocytes in the hypothalamic supraoptic nucleus (SON) as well as in perivascular cells in the neurohypophysis, and a several fold increase in CNTF immunoreactivity occurs in the SON following either axotomy of magnocellular neurons or during axonal sprouting by intact magnocellular neurons. CNTF also promotes survival and stimulates process outgrowth from magnocellular neurons in vitro. While these findings suggest that CNTF may act as a growth factor in support of neuronal plasticity in the MNS, little is known regarding possible expression of receptors for CNTF in the MNS. We have therefore used immunocytochemistry and in situ hybridization to examine the expression of CNTF receptor alpha (CNTFRα) in the rat MNS. Robust immunoreactivity for CNTFRα was observed associated with oxytocinergic and vasopressinergic neurons distributed throughout the SON. Astrocytes located within the ventral glial lamina (VGL) of the SON were also immunoreactive for CNTFRα. Robust hybridization of an anti-sense [35S]-cRNA probe to CNTFRα mRNA was observed throughout the SON, while binding of a control sense probe was much lower. Grains were found clustered predominantly over neuronal somata, indicative of expression by magnocellular neurons within the SON. We next examined changes in expression of CNTFRα mRNA by magnocellular neurons 7 days following unilateral transection of the hypothalamo-neurohypophysial tract. The level of CNTFRα mRNA was increased 32% (compared to age-matched intact controls; p<.05) in magnocellular neurons in the SON contralateral to the lesion, which are undergoing extensive collateral axonal sprouting, but was unchanged in axotomized magnocellular neurons in the SON ipsilateral to the lesion. These findings suggest that CNTF produced by MNS glia and acting via CNTFRα may exert neurotrophic effects on magnocellular neurons.
Introduction
The magnocellular neurosecretory system (MNS) is comprised of the vasopressin (VP) and oxytocin (OT) producing neurons of the supraoptic (SON) and paraventricular nuclei (PVN) and their axonal projections to the neural lobe of the pituitary gland (NL). The MNS has long been recognized as a model system for the study of neuron-glial interactions (reviewed in Miyata and Hatton, 2002), and the MNS is highly unusual among CNS systems in maintaining the ability to regenerate axons following injury in adult animals (Billenstein and Leveque, 1955; Moll, 1957; Adams et al., 1969; Beck et al., 1969; Raisman, 1973; Antunes et al., 1980; Silverman and Zimmerman, 1982). We have demonstrated previously that, in addition to regenerating severed axons, intact adult magnocellular neurons are also capable of undergoing extensive collateral sprouting in response to a hypothalamic lesion. The unilateral lesion severs the contralateral hypothalamo-neurohypophysial tract resulting in a 42% reduction in terminal density in the NL (Watt and Paden, 1991). This robust sprouting response restores the axon density of the NL to normal values within 30 days post-denervation and is dependent upon a sustained increase in neurosecretory and metabolic activity by the sprouting neurons (Paden et al., 1995; Watt et al., 1999).
The dramatic restorative capabilities of the MNS are presumably dependent upon the actions of multiple growth factors, and synthesis of several such factors and/or their receptors has been demonstrated with the MNS including interleukin-1β (Watt and Hobbs, 1999), the p75 neurotrophin receptor (Watt and Paden, 2001), and IGF-I (Zhou et al., 1999). In addition, recent studies have implicated CNTF as playing a significant role in MNS plasticity. Survival of magnocellular VP and OT neurons was significantly increased in explants of the SON or PVN cultured in the presence of exogenous CNTF, and increased process outgrowth originating from the explants was observed (Vutskits et al., 1998; Rusnak et al., 2002; Rusnak et al., 2003). These reports led us to examine potential sources of endogenous CNTF within the MNS. Immunoreactive CNTF was localized to astrocytes within the SON and perivascular cells within the NL, and a dramatic and sustained increase in CNTF immunoreactivity occurred in the SON following a unilateral lesion of the MNS (Watt et al., 2006). The largest increase in CNTF was observed in the axotomized SON, but a significant increase also occurred in the contralateral SON concomitant with axonal sprouting by uninjured magnocellular neurons.
Ciliary neurotrophic factor receptor alpha (CNTFRα) is expressed in the MNS (MacLennan, et al., 1996). However, the specific neuronal and/or glial phenotypes in the SON or NL which express the receptor and regulation of CNTFRα expression have yet to be defined. Determining which cell types express CNTFRα is important for understanding how CNTF may act to promote cell survival and sprouting. For example, the greater resiliency of oxytocin vs vasopressin neurons to axotomy may be related to cell type-specific differences in CNTFRα expression levels. Likewise, expression of CNTFRα by a specific neuron may be related to that phenotypes sprouting efficacy.
The molecular mechanisms which control regeneration following injury are not well understood, but include activation of distinct signal transduction pathways. Principle among them for CNTF involves binding with CNTFRα and recruitment of the LIFR/gp-130 receptor complex. Neurons respond to CNTF via activation of STAT pathways associated with the CNTFRα receptor complex. Activation of neuronal STAT3 has also been correlated directly with neuronal survival and sprouting (Xu et al., 2001). Thus, it is clear that CNTFRα activation is involved with regulation of neuronal and astrocytic responses to injury and the induction of restorative functions in neurons following injury.
In order to further examine the role of endogenous CNTF as a survival and/or growth factor in the MNS we have employed immunocytochemistry and in situ hybridization to determine the cellular localization of CNTFRα and its mRNA within the SON and to examine potential changes in expression of the receptor during injury-induced axonal plasticity.
Methods
Tissue preparation and immunocytochemical analysis
Male Sprague-Dawley rats were purchased from Charles River and housed in the University of North Dakota Biomedical Research Facility an AAALAC accredited facility, under a 12L:12D light cycle with ad lib access to lab chow and tap water throughout the investigations. Experimental protocols utilized in these studies followed the guidelines in the NIH Guide for the Care and Use of Laboratory Animals and were approved by the UND Institutional Animal Care and Use Committee.
For immunocytochemical analysis, all animals were perfused intracardially with saline for two minutes under deep isoflurane (Abbott Laboratories) anesthesia and then perfused for 20 minutes with a modified Nakane’s periodate-lysine-paraformaldehyde (PLP) fixative prepared immediately before use (Moffet and Paden, 1994). For dual fluorescence labeling with goat anti-rat CNTFRα (R & D Systems, 1:15) and rabbit anti-vasopressin (Pennisula Labs, 1:40,000), rabbit anti-oxytocin (Pennisula labs 1:15,000) or rabbit anti-GFAP (1:1000; Dako) all incubation steps were performed at 4°C and separated by a minimum of three 10 minute PBS/0.3% triton washes. Sections were initially treated with a blocking buffer containing 4% normal horse serum//0.3% Triton X-100 in PBS for 1 hr to reduce nonspecific staining. The sections were then incubated for 48 hours at 4°C in a cocktail of primary antibodies in blocking buffer. Sections were then incubated sequentially in biotinylated horse anti-goat IgG (1:500 in blocking buffer; Jackson ImmunoResearch) followed sequentially by either fluoroscein or Texas red-conjugated anti-rabbit IgG and then streptaviden-fluoroscein or Texas red (1:1000 in PBS; Jackson ImmunoResearch). The sections were then washed repeatedly in PBS/0.3% triton, and coverslipped using Vectashield mounting medium (Vector). All samples were examined and images obtained using a Zeiss Meta confocal microscope. The slides were prepared for page-reproduction using Adobe Photoshop (v.6.0).
Specificity Controls for Immunocytochemistry
Three control groups were established. Omission of each individual primary antibody revealed negative staining in sections representing all experimental groups. Likewise, omission of the appropriate secondary antibodies yielded negative staining for individual primaries. Goat anti CNTF (1:100) preabsorbed with a 10 molar excess of purified rat recombinant CNTFRα (R&D Systems) for 24 hrs at 4°C eliminated immunolabeling of the goat anti CNTFRα primary antibody.
Preparation of CNTFRα cRNA probes
The [35S]-labeled cRNA probes for rat CNTFRα were made using a cDNA template made from mRNA isolated from whole rat brain. Using standard PCR methods, two oligonucleotide primers were designed to amplify a 524 nucleotide region (nucleotides 144 to 668; accesion NM_001003929) of the CNTFRα cDNA. T7 or SP6 promoter sites were incorporated into the primer design by adding a 32 nucleotide sequence to the 5′ end of the DNA primers that defined the rat CNTFRα sequence. The T7 promoter site was added to the 5′ end of the down stream primer and the SP6 promoter site was added to the 5′ end of the upstream primer, allowing us to construct a cDNA that was used as a template to synthesize both an anti-sense cRNA probe by using T7 polymerase and a sense (negative control) cRNA probe by using SP6 polymerase. The cDNA template was sequenced by the DNA Sequencing Facility at Washington State University and found to match the published sequence for rat CNTFRα (accession NM_001003929; nucleotides 144 to 668). [35S]-labeled sense and anti-sense cRNA probes were then produced using standard techniques as described by Promega Life Sciences, Madison, WI.
Tissue Preparation and In Situ Hybridization Histochemistry
Male Sprague-Dawley rats were purchased from Charles River Laboratories and housed in the Montana State University Animal Resource Center, an AAALAC accredited facility, under a 12L:12D light cycle with ad lib access to lab chow and tap water. Animals were 45–50 days of age when a unilateral lesion of the hypothalamo-neurohypophysial tract was performed using a wire knife as previously described (Watt and Paden, 1991; Watt et al, 2006). Complete unilateral transection of the hypothalamo-neurohypophysial tract was verified histologically in each animal. Experimental protocols utilized in these studies followed the guidelines in the NIH Guide for the Care and Use of Laboratory Animals and were approved by the MSU Institutional Animal Care and Use Committee.
Rats receiving hypothalamic lesions and intact age-matched controls were decapitated while under deep isoflurane anesthesia. The brain was rapidly removed and frozen in isopentane chilled in dry ice, then briefly stored at −80°C until sectioned. Serial 20 μm cryosections were mounted on Superfrost slides (Fisher) and stored at −80°C until use. The procedures for 35S autoradiography have been described in detail (Giulian et al., 1991). Briefly, tissues were immersion-fixed for 30 min in buffered 4% paraformaldehyde and then deproteinated with proteinase K (0.1 mu;g/ml) for 15 min at 37°C in preparation for in situ hybridization using the 35S-labeled sense and antisense cRNA probes described above. Slides were then rinsed in nanopure water, washed in 0.1 M triethanolamine, pH 8.0, for 1 min, rinsed in 2xSSC and dehydrated in graded alcohols. The 35S-labeled probe was then added to the hybridization buffer containing 50% formamide (Amresco) and 20 mM DTT and denatured at 65°C for 5 min. The probe was then applied to each slide at a concentration of 2×106 cpm/slide and incubated overnight at 55°C in a hydration chamber. Following hybridization, the slides were rinsed in 2xSSC, treated with RNase A (100 μg/ml) for 30 min at 37°C, followed by a 1 hr incubation in 2xSSC at 65°C. Slides were then dehydrated in a graded series of alcohols, air-dried at room temperature, and dipped in Kodak NTB2 emulsion. After a 28 day exposure, the emulsion was developed in Kodak D19, fixed, counterstained with cresyl violet, dehydrated and coverslipped using Permount (Sigma).
Quantification of Relative Autoradiographic Signal Strength
All slides were coded, placed in random order, and analyzed blind using the MCID M4 image analysis system (Imaging Research). The SON was outlined using brightfield optics at 20x magnification excluding the VGL region, then refocused on the plane of emulsion and the grains enhanced using a target detection algorithm subroutine before measuring the proportional area of the SON occupied by grains. The right and left SON from each of 6 intact and 6 seven day post-lesion (L7) rats were analyzed, with 2–3 sense and 3–7 antisense sections measured for each animal. SON were measured bilaterally with the sense signal subtracted independently for each subject to calculate proportional area of each SON occupied by grains, then the mean proportional area occupied by grains was calculated for each side of each subject.
Results
CNTFRα immunoreactivity is localized in oxytocin and vasopressin neurons in the SON as well as associated astrocytes
We performed immunocytochemical analysis of CNTFR localization to identify cell phenotypes that express the CNTFRα within the PVN and SON and to determine if expression was altered in response to unilateral lesion and/or during initiation of collateral axonal sprouting by magnocellular neurons. We observed robust immunoreactivity for CNTFRα on neuronal somata distributed throughout the SON (Fig. 1A–F), suggesting that virtually all magnocellular neurons in the SON express the protein. Dual-label immunocytochemistry confirmed the presence of CNTFRα immunoreactivity associated with both oxytocinergic (Fig. 1, A–C) and vasopressinergic neurons (Fig. 1D–F). In contrast, immunoreactivity for CNTFRα was never observed in the PVN in control or lesioned animals (not shown).
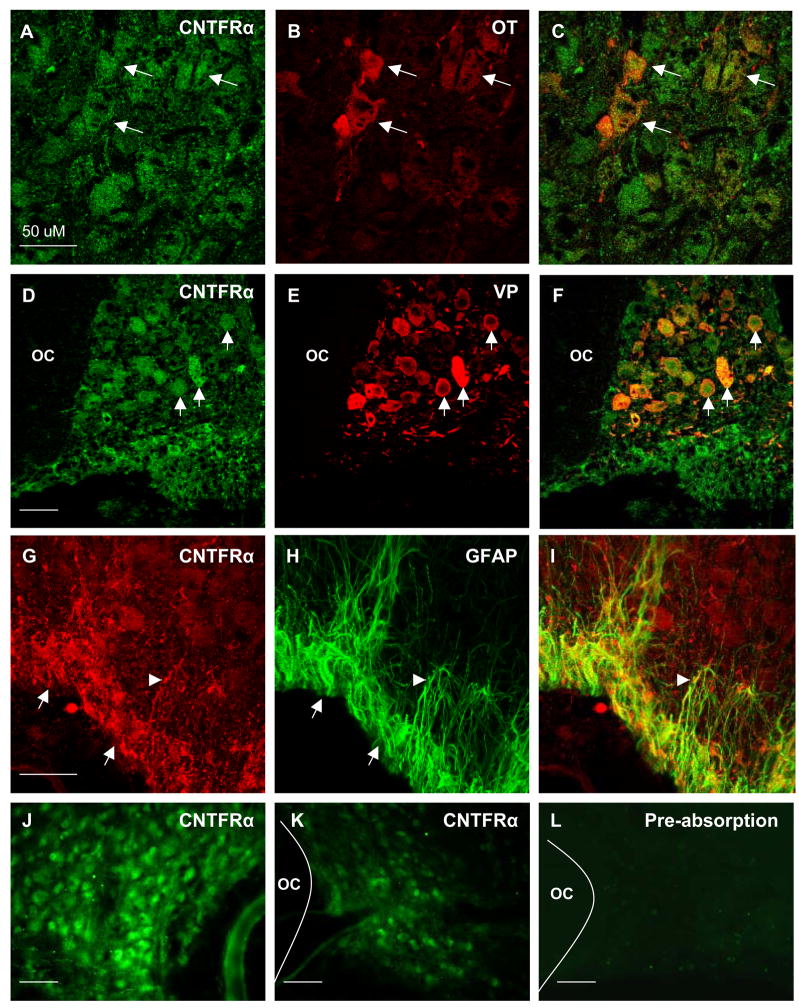
Figure 1.
CNTFR-ir was observed in magnocellular neurons and astrocytes throughout the SON. (A–C) Dual fluorescent colocalization of anti-CNTFRα (A), with anti-oxytocin (B), revealed extensive colocalization in oxytocin-ir neurons (C, arrows). Likewise, dual fluorescent colocalization of anti-CNTFRα (D), with anti-vasopressin (E), revealed complete colocalization in all vasopressinergic neuron soma (F, arrows). In addition, CNTFRα-ir (G, arrows) was prevalent in GFAP-ir astrocyte cell bodies within the VGL (H). Close examination revealed CNTFRα-ir astrocyte cell processes originating from the VGL extending into the magnocellular region of the SON (arrowheads). As shown in panels J and K, the observed differences in mRNA levels (see Figure 2) are also reflected in the relative level of immunoreactivity observed when comparing the contralateral intact sprouting SON (J) to the lesioned SON (K) of the same animal at 7 days post lesion. (L) Preabsorption controls revealed that a 10 fold excess of purified rat recombinant CNTFRα completely eliminated immunoreactivity. Negative omission controls revealed the same (not shown). OC = optic chiasm, Magnification bars = 50μM.
We also observed CNTFRα-ir associated with small cells located in the ventral glial lamina (VGL) of the SON as well as with cellular processes projecting dorsally into the body of the SON from the VGL (Fig. 1, G–I), consistent with expression of the protein by astrocytes. Dual-localization pairing anti-CNTFRα with anti-GFAP confirmed that these cells were astrocytes. CNTFRα immunoreactivity was never observed on astrocytes outside the SON except in the immediate vicinity of the lesion tract. Preabsorption of the anti-CNTFRα antibody with a 10M excess of purified rat recombinant CNTFRα resulted in complete and replicable loss of immunoreactivity (Fig. 1, L).
CNTFRα mRNA is expressed by magnocellular neurons and levels of CNTFRα mRNA are increased in the during collateral axonal sprouting
We performed in situ hybridization to confirm expression of CNTFRα mRNA within the PVN and SON and to determine if expression was altered in response to axotomy and/or during initiation of collateral axonal sprouting by magnocellular neurons. Hybridization of the anti-sense cRNA probe was observed within the PVN with the highest densities located in the lateral magnocellular division and medial magnocellular subnuclei (Armstrong et al., 1980)(Fig. 2A–C). Grain densities in the juxtaposed parvocellular divisions of the PVN were notably lower than the magnocellular subnuclei. Hybridization of the anti-sense cRNA probe was also evident within the SON of intact control animals (Fig. 2D), while binding of a control sense probe was much lower (Fig. 2E). Grains were clustered over neuronal somata, consistent with the distribution of CNTFRα-ir, providing further evidence for expression by magnocellular neurons.
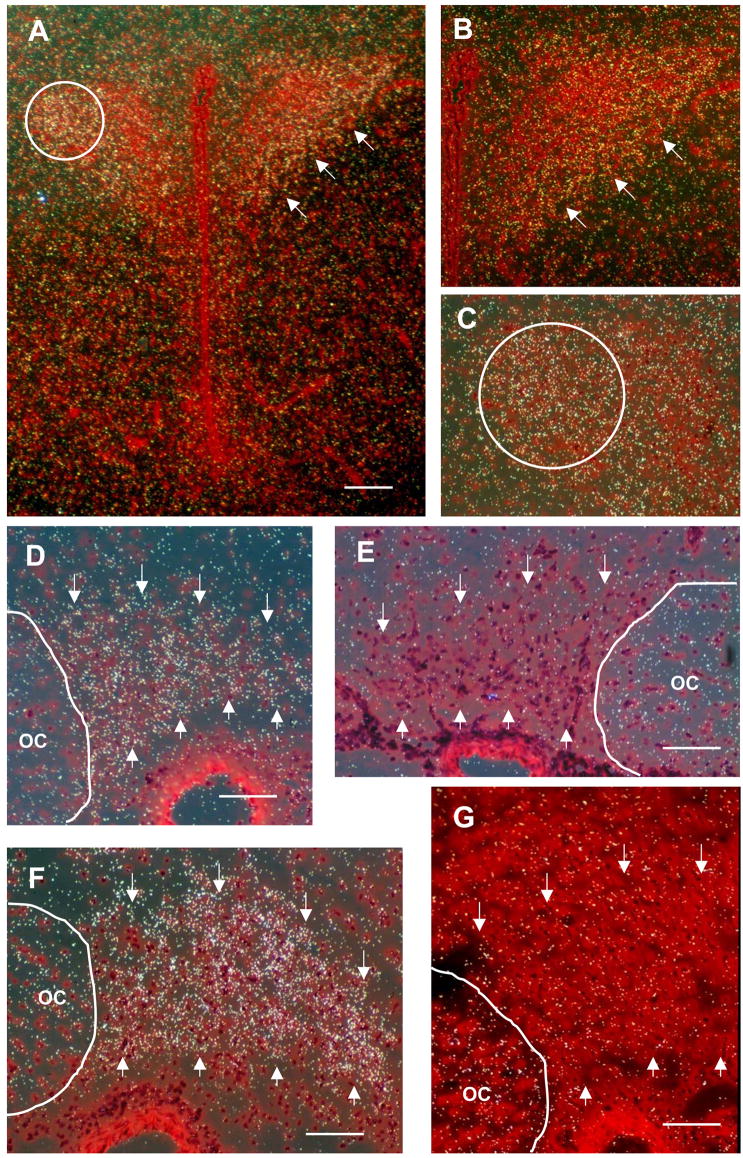
Figure 2.
In situ hybridization revealed that CNTFRα message was expressed in magnocellular divisions of the PVN and in magnocellular neurons in the SON. (A) This low magnification image of the intact (non-lesion control) PVN shows dense grain distributions located predominantly in the lateral magnocellular division (PVL, circle) and the medial magnocellular division (PVM, arrows) of the PVN. Higher magnification images from the same section shown in (A) further illustrate the distribution of grains in the PVL (B) and PVM regions (C, circle). Note the relatively low grain densities in juxtaposed parvocellular regions of the PVN and the surrounding neuropil of the hypothalamus. (D) The SON of intact non-lesion controls, outlined with arrows, also showed significantly higher grain densities than the surrounding neuropil indicative of high levels of endogenous receptor expression. (E) Sense strand control section from the same animal as in D showing grain densities comparable to background levels. (F) Grain densities within the sprouting SON contralateral to the lesion are significantly higher at 7 days post-lesion ( p< 0.05) than in intact control SON. (G) Sense strand control from the same 7 day post-lesion animal shown in F. OC = optic chiasm. Magnification bars: A = 200 μM, D–G = 100 μM.
We next examined changes in expression of CNTFRα mRNA by magnocellular neurons 7 days following unilateral transection of the hypothalamo-neurohypophysial tract. SON neurons ipsilateral to the hypothalamic knife cut are axotomized by this lesion, while uninjured neurons in the contralateral SON undergo extensive collateral sprouting of their axons in the NL (Watt et al, 1991; 1999). We observed an increase in hybridization intensity confined to the contralateral SON at 7 days post-lesion (compare Figures 2 D, F). Measurement of the proportional area of the SON covered by grains of emulsion (excluding the VGL, and corrected for nonspecific binding) confirmed that the level of CNTFRα mRNA was increased 32% (p<.05) in magnocellular neurons in the SON contralateral to the lesion (sprouting neurons) but was unchanged in the SON ipsilateral to the lesion (axotomized neurons) compared to the level in age-matched intact controls (Table 1). As indicated in Figure 1 panels J and K, the differences in mRNA levels are further reflected in the relative level of immunoreactivity observed when comparing the contralateral intact sprouting SON (Fig. 1, J) to the lesioned SON (Fig. 1, K) of the same animal at 7 days post lesion.
Table 1.
Quantitative proportional area analysis of the SON occupied by grains revealed a significant increase in grain area in the contralateral sprouting SON by 7 days post lesion compared to intact and lesioned SON values. The proportional area of grain distribution in the lesioned SON was not different from normal intact control SON.
| Relative CNTFRα mRNA Levels in the SON | |
|---|---|
| Exp. Group | Proportional Area (%) of SON Occupied by Grains Mean ± SEM |
| Intact control (n=12)a | 5.39 ± 0.42 |
| 7 day post-axotomy (n=6) | 5.37 ± 0.66 |
| 7 day sprouting (n=6) | 7.11 ± 0.58b |
Left and right SON measured in 6 rats
Students two-tailed t=2.37 vs intact, p<.05
Discussion
Results of our immunocytochemical and in situ hybridization studies show a high level of expression of CNTFRα mRNA and protein in magnocellular neurons, as well as prominent protein expression in astrocytes within the VGL, confirming and extending previous reports of CNTFRα expression in the SON (MacLennan et al., 1996; Lee et al.,1997). Considered together with an earlier report of CNTF expression by astrocytes in the VGL and perivascular cells in the NL (Watt et al., 2006), these data indicate that CNTF may act in a dual role as an important autocrine glial factor and paracrine neuronal growth factor in the MNS.
CNTF plays an important role as a survival factor across a variety of CNS neuronal phenotypes (Larkfors et al., 1994; Magal et al., 1993; Burnham et al., 1994; Sendtner et al., 1990; Clatterbuck et al., 1993) including magnocellular neurons of the SON and PVN in vitro (Vutskits et al., 1998; Rusnak et al.,2002,2003). CNTF also promotes axonal sprouting by magnocellular neurons in vitro (Vutskits et al.,1998) and motor neurons in vivo (Siegel et al., 2000; Kwon and Gurney, 1994; Gurney et al., 1992). The later observation is particularly striking given our finding that uninjured magnocellular neurons upregulate levels of CNTFRα mRNA simultaneously with increased expression of CNTF by SON astrocytes (Watt et al., 2006). These coordinated events coincide with the onset of axonal sprouting (Watt and Paden, 1991; Paden et al., 1995). A similar coordinated upregulation of CNTF and CNTFRα has also been observed in the deafferented outer molecular layer of the hippocampus between 3 and 10 days following entorhinal cortex ablation (Lee et al., 1997), a period during which prolific terminal sprouting is known to occur within the deafferented outer molecular layer (Steward et al., 1988).
Of particular interest is the observed upregulation of CNTFRα message by intact magnocellular neurons (contralateral to the lesion) undergoing collateral sprouting at 7 days post-lesion when upregulation is not present in the axotomized magnocellular neurons, in spite of the significant increase in CNTF protein levels seen in both the lesioned SON and contralateral sprouting SON. We propose that increased activity of intact magnocellular neurons undergoing collateral sprouting (Watt et al., 1999) promotes CNTFRα upregulation, making neurons more responsive to trophic support. Activity-induced upregulation of CNTFRα provides a potential mechanism to explain the observation that increased neuronal activity induced by chronic KCL infusion into the SON has a significant neuroprotective effect on axotomized magnocellular neurons concomitant with increased neurite outgrowth (Shahar et al., 2004).
Expression of CNTFRα by MNS neurons that are in intimate contact with astrocyte processes immunoreactive for CNTF suggests a paracrine mechanism of activity. However, CNTF lacks the N-terminal hydrophobic signal sequence necessary for transport across a lipid bilayer as would be expected if it followed the classic secretion route. Nevertheless, other proteins lacking identified signal sequences have been shown to be released by alternative secretory pathways (Kandel et al., 1991; Cooper and Barondes, 1990; Rubartelli et al., 1990; Rubartelli and Sitia, 1991) including IL-1β (Siders and Mizel, 1995; Giri et al., 1985). Consistent with these observations, CNTF has been found in the culture media of non-injured rat astrocytes following treatment of the cells with IL-1β in vitro (Kamiguchi et al., 1995). Indeed, there is strong evidence for the expression and secretion of IL-1β by magnocellular neurons under conditions of heightened neurosecretory activity (Watt and Hobbs, 1999; Huitinga et al., 2000; Breder et al., 1988; Yasin et al., 1994; Tringali et al., 1997) and direct osmotic stimulation of the SON in vivo (Summy-Long et al., 2006). Taken together, these observations raise the intriguing possibility that release of endogenous IL-1β from MNS neurons may stimulate secretion of CNTF from astrocytes within the neurosecretory system.
Although a comparatively low hybridization signal was observed in the VGL region of the SON, our immunocytochemical analysis demonstrated constitutive expression of CNTFRα protein by astrocytes in the VGL, the same glial population that has previously been shown to be the source of CNTF (Watt et al., 2006). These results indicate that CNTF may play an autocrine as well as a paracrine role in the SON. Although CNTFRα expression by astrocytes in rat CNS is generally considered to be below the level of detection in normal brain, astrocyte expression levels will increase significantly following injury in vivo (Guthrie et al., 1997; Rudge et el., 1994) indicative of their capacity to respond to CNTF protein. Endogenous expression of the CNTFRα by astrocytes is further supported by studies demonstrating that acute infusion of recombinant rat CNTF into the rat cortex will activate directly astrocytes resulting in cellular hypertrophy (Hudgins and Levison, 1998) and increased expression of GFAP (Kahn et al., 1997). Thus, CNTF could be important in mediating the coordinated activity-dependent neural-glial morphological plasticity that has been so extensively documented in this system (Miyata and Hatton, 2002).
Magnocellular vasopressinergic neurons in PVN explants have also been shown to respond to exogenously applied CNTF in vitro (Vutskits et al., 1998). Thus, it is surprising that we were unable to detect CNTF immunoreactivity in the PVN in a previous study (Watt et al., 2006). These data suggest the possibility that neurons in the PVN may respond to CNTF following its retrograde transport from the NL, in analogy to the mechanism by which CNTF acts to promote survival and axonal sprouting by peripheral motor neurons following injury (Curtis et al., 1993; Kirsch et al,. 2003). The presence of CNTF immunoreactivity (Watt et al., 2006) and mRNA in the NL (Vutskits et al., 1998) is consistent with this hypothesis, which also leads to the prediction that magnocellular neurons in the PVN should express CNTFRα. While CNTFRα message was clearly present in magnocellular regions of the PVN, we did not observe immunoreactive CNTFRα protein. This discrepancy suggests either that the level of expression of CNTFRα protein is below the limit of detection by immunocytochemistry within the PVN, or that the receptor is localized within PVN neurons in a manner that masks it from binding of the primary antisera.
In summary, our results confirm and extend the reports of MacLennon et al., (1996) and demonstrate the constitutive expression of CNTFRα message and protein in the SON. Furthermore, we demonstrate that receptor levels are increased significantly by intact magnocellular neurons contralateral to the injury during a period when these same neurons are actively increasing their axon terminal density within the NL. These data, together with our earlier report of increased CNTF expression by astrocytes within the contralateral SON provide support for the hypothesis that CNTF acts as an endogenous sprouting factor in the magnocellular neuroendocrine system.
Acknowledgments
These studies were supported by NIH R01 NS32507 to CMP; NIH P20 RR-16455-01 and NIH RO3-MH64171-01 to JAW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Adams JH, Daniel PM, Prichard MMI. Degeneration and regeneration of hypothalamic nerve fibers in the neurohypophysis after pituitary stalk section in the ferret. J Comp Neurol. 1969;135:121–144. doi: 10.1002/cne.901350202. [DOI] [PubMed] [Google Scholar]
- Alonzi T, Middleton G, Wyatt S, Buchman V, Betz UAK, Muller W, Musiani P, Poli V, Davies AM. Role of STAT3 and PI 3-kinase/Akt in mediating the survival actions of cytokines on sensory neurons. Mol Cell Neurosci. 2001;18:270–282. doi: 10.1006/mcne.2001.1018. [DOI] [PubMed] [Google Scholar]
- Antunes JL, Louis KM, Huang S, Zimmerman E. Section of the pituitary stalk in the rhesus monkey: morphological and endocrine observations. Ann Neurol. 1980;8:308–316. doi: 10.1002/ana.410080315. [DOI] [PubMed] [Google Scholar]
- Armstrong WE, Warach S, Hatton GI, McNeill TH. Subnuclei in the hypothalamic paraventricular nucleus: A cytoarchitectural, horseradish peroxidase and immunocytochemical analysis. Neuroscience. 1980;5:1931–1958. doi: 10.1016/0306-4522(80)90040-8. [DOI] [PubMed] [Google Scholar]
- Beck E, Daniel PM, Prichard ML. Regeneration of hypothalamic nerve fibers in the goat. Neuroendocrinology. 1969;5:161–182. doi: 10.1159/000121858. [DOI] [PubMed] [Google Scholar]
- Billenstein DC, Leveque TF. The reorganization of the neurohypophysial stalk following hypophysectomy in the rat. Endocrinology. 1955;56:704–717. doi: 10.1210/endo-56-6-704. [DOI] [PubMed] [Google Scholar]
- Breder CD, Dinarello CA, Saper CB. Interleukin-1 immunoreactive innervation of the human hypothalamus. Science. 1988;240:321–323. doi: 10.1126/science.3258444. [DOI] [PubMed] [Google Scholar]
- Clatterbuck RE, Price DL, Koliatos VE. Ciliary neurotrophic factor prevents retrograde neuronal death in the adult central nervous system. Proc Natl Acad Sci USA. 1993;90:2222–2226. doi: 10.1073/pnas.90.6.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper DNV, Barondes SH. Evidence for export of a muscle lectin from cytosol to extracellular matrix and for a novel secretory mechanism. J Cell Biol. 1990;110:681–691. doi: 10.1083/jcb.110.5.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri JG, Lomedico PT, Mizel SB. Studies on the synthesis and secretion of interleukin 1.I A 33,000 molecular weight precursor for interleukin 1. J Immunol. 1985;134:343–349. [PubMed] [Google Scholar]
- Giulian D, Johnson B, Krebs JF, George JK, Tapscott M. Microglial mitogens are produced in the developing and injured mammalian brain. J Cell Biol. 1991;112:323–333. doi: 10.1083/jcb.112.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney ME, Yamamoto H, Kwon Y. Induction of motor neuron sprouting by ciliary neurotrophic factor and basic fibroblast growth factor. J Neurosci. 1992;12:3241–3247. doi: 10.1523/JNEUROSCI.12-08-03241.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie KM, Woods AG, Nguyen T, Gall CM. Astroglial ciliary neurotrophic factor mRNA expression is increased in fields of axonal sprouting in deafferented hippocampus. J Comp Neurol. 1997;386:137–148. [PubMed] [Google Scholar]
- Hudgins SN, Levison SW. Ciliary neurotrophic factor stimulates astroglial hypertrophy in vivo and in vitro. Exp Neurol. 1998;150:171–182. doi: 10.1006/exnr.1997.6735. [DOI] [PubMed] [Google Scholar]
- Huitinga I, van der Cammen M, Salm L, Erkut Z, Van Dam AM, Tilders F, Swaab D. IL-1β immunoreactive neurons in the human hypothalamus: reduced numbers in multiple sclerosis. J Neuroimmunol. 2000;7:8–20. doi: 10.1016/s0165-5728(00)00248-4. [DOI] [PubMed] [Google Scholar]
- Kahn MA, Ellison JA, Chang RP, Speight GJ, de Vellis J. CNTF induces GFAP in an S-100α brain cell population: the pattern of CNTF-αR suggests an indirect mode of action. Brain Res Dev Brain Res. 1997;98:221–233. doi: 10.1016/s0165-3806(96)00180-0. [DOI] [PubMed] [Google Scholar]
- Kamiguchi H, Yoshida K, Sagoh M, Sasaki H, Inaba M, Wakamoto H, Otani M, Toya S. Release of ciliary neurotrophic factor from cultured astocytes and its modulation by cytokines. Neurochem Res. 1995;20:1187–1193. doi: 10.1007/BF00995382. [DOI] [PubMed] [Google Scholar]
- Kandel J, Bossy-Wetzel E, Radvanyi F, Klagsbrun M, Folkman J, Hanahan D. Neovascularization is associated with a switch to the export of bFGF in the multistep development of fibrosarcoma. Cell. 1991;66:1095–1104. doi: 10.1016/0092-8674(91)90033-u. [DOI] [PubMed] [Google Scholar]
- Kwon YW, Gurney ME. Systemic injections of ciliary neurotrophic factor induce sprouting by adult motor neurons. NeuroReport. 1994;5:789–792. doi: 10.1097/00001756-199403000-00013. [DOI] [PubMed] [Google Scholar]
- Lee MY, Deller T, Kirsch M, Frotscher M, Hofmann HD. Differential regulation of ciliary neurotrophic factor (CNTF) and CNTF receptor alpha expression in astrocytes and neurons of the fascia dentata after entorhinal cortex lesion. J Neurosci. 1997;17:1137–1146. doi: 10.1523/JNEUROSCI.17-03-01137.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summy-Long JY, Hu A, Pruss X, Chen X, Phillips TM. Response of interleukin-1β in the magnocellular system to salt-loading. J Neuroendocrinol. 2006;18:926–937. doi: 10.1111/j.1365-2826.2006.01490.x. [DOI] [PubMed] [Google Scholar]
- MacLennan AJ, Vinson EN, Marks L, McLaurin DL, Pfeifer M, Lee N. Immunohistochemical localization of ciliary neurotrophic factor receptor alpha expression in the rat nervous system. J Neurosci. 1996;16:621–630. doi: 10.1523/JNEUROSCI.16-02-00621.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata S, Hatton GI. Activity-related, dynamic neuron–glial interactions in the hypothalamo-neurohypophysial system. Micro Res Tech. 2002;56:143–157. doi: 10.1002/jemt.10012. [DOI] [PubMed] [Google Scholar]
- Moffet CW, Paden CM. Microglia in the rat neurohypophysis increase expression of class I major histocompatibility antigens following central nervous system injury. J Immunol. 1994;50:139–151. doi: 10.1016/0165-5728(94)90040-x. [DOI] [PubMed] [Google Scholar]
- Moll J. Regeneration of the supraoptico-hypophysial and paraventriculo-hypophysial tracts in the hypophysectomized rat. Z Zellforsch. 1957;46:686–709. doi: 10.1007/BF00339372. [DOI] [PubMed] [Google Scholar]
- Paden CM, Zhou X, Watt JA, Burton R, Pickett J, Oblinger MM. Coordinated upregulation of α1-and βII-tubulin mRNAs during collateral axonal sprouting of central peptidergic neurons. J Neurosci Res. 1995;42:402–412. doi: 10.1002/jnr.490420315. [DOI] [PubMed] [Google Scholar]
- Raisman G. Electron microscopic studies of the development of new neurohemal contacts in the median eminence of the rat after hypophysectomy. Brain Res. 1973;55:245–261. doi: 10.1016/0006-8993(73)90294-1. [DOI] [PubMed] [Google Scholar]
- Rubartelli A, Cozzolino F, Talio M, Sitia R. A novel secretory pathway for interleukin 1β, a protein lacking a signal sequence. EMBO J. 1990;9:1503–1510. doi: 10.1002/j.1460-2075.1990.tb08268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubartelli A, Sitia R. Interleukin 1β and thioredoxin are secreted through a novel pathway of secretion. Biochem Soc Trans. 1991;19:255–259. doi: 10.1042/bst0190255. [DOI] [PubMed] [Google Scholar]
- Rudge JS, Li Y, Pasnikowski EM, Mattsson K, Pan L, Yancopoulos GD, Wiegand SJ, Lindsay RM, Ip NY. Neurotrophic factor receptors and their signal transduction capabilities in rat astrocytes. Eur J Neurosci. 1994;6:693–705. doi: 10.1111/j.1460-9568.1994.tb00981.x. [DOI] [PubMed] [Google Scholar]
- Rusnak M, House SB, Arima H, Gainer H. Ciliary neurotrophic factor increases the survival of magnocellular vasopressin and oxytocin neurons in rat supraoptic nucleus in organotypic cultures. Micro Res Tech. 2002;56:101–112. doi: 10.1002/jemt.10015. [DOI] [PubMed] [Google Scholar]
- Rusnak M, House SB, Gainer H. Long term effects of ciliary neurotrophic factor on the survival of vasopressin magnocellular neurons in the rat supraoptic nucleus in vitro. J Neuroendocrinol. 2003;10:933–939. doi: 10.1046/j.1365-2826.2003.01080.x. [DOI] [PubMed] [Google Scholar]
- Sendtner M, Kreutzberg GW, Thoenen H. Ciliary neurotrophic factor prevents the degeneration of motorneurons after axotomy. Nature. 1990;345:440–441. doi: 10.1038/345440a0. [DOI] [PubMed] [Google Scholar]
- Shahar T, House SB, Gainer H. Neural activity protects hypothalamic magnocellular neurons against axotomy-induced programmed cell death. J Neurosci. 2004;24:6553–6562. doi: 10.1523/JNEUROSCI.0886-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siders WM, Mizel SB. Interleukin-1β secretion. A possible multistep process that is regulated in a cell type-specific manner. J Biol Chem. 1995;270:16258–15264. doi: 10.1074/jbc.270.27.16258. [DOI] [PubMed] [Google Scholar]
- Siegel SG, Patton B, English AW. Ciliary neurotrophic factor is required for motoneuron sprouting. Exp Neurol. 2000;166:205–212. doi: 10.1006/exnr.2000.7528. [DOI] [PubMed] [Google Scholar]
- Silverman AJ, Zimmerman EA. Adrenalectomy increases sprouting in a peptidergic neurosecretory system. Neuroscience. 1982;7:2705–2714. doi: 10.1016/0306-4522(82)90094-x. [DOI] [PubMed] [Google Scholar]
- Tringali G, Mirtella A, Mancuso C, Guerriero G, Preziosi P, Navarra P. The release of immunoreactive interleukin-1β from rat hypothalamic explants is modulated by neurotransmitters and corticotropin-releasing hormone. Pharm Res. 1997;36:269–273. doi: 10.1006/phrs.1997.0235. [DOI] [PubMed] [Google Scholar]
- Vutskits L, Bartanusz V, Schultz MF, Kiss JZ. Magnocellular vasopressinergic neurons in explant cultures are rescued from cell death by ciliary neurotrophic factor and leukemia inhibitory factor. Neuroscience. 1998;87:571–582. doi: 10.1016/s0306-4522(98)00177-8. [DOI] [PubMed] [Google Scholar]
- Watt JA, Paden CM. Compensatory sprouting of uninjured magnocellular neurosecretory axons in the rat neural lobe following unilateral hypothalamic lesion. Exp Neurol. 1991;111:9–24. doi: 10.1016/0014-4886(91)90046-f. [DOI] [PubMed] [Google Scholar]
- Watt JA, Bone S, Pressler M, Cranston HJ, Paden CM. Ciliary neurotrophic factor is expressed in the magnocellular neurosecretory system of the rat in vivo: evidence for injury- and activity-induced upregulation. Exp Neurol. 2006;197:206–214. doi: 10.1016/j.expneurol.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Watt JA, Moffet CW, Zhou X, Short S, Herman JP, Paden CM. Central peptidergic neurons are hyperactive during collateral sprouting and inhibition of activity suppresses sprouting. J Neurosci. 1999;19:1586–1598. doi: 10.1523/JNEUROSCI.19-05-01586.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt JA, Hobbs NK. Interleukin-1β immunoreactivity in identified neurons of the rat magnocellular neurosecretory system: evidence for activity-dependent release. J Neurosci Res. 2000;60:478–489. doi: 10.1002/(SICI)1097-4547(20000515)60:4<478::AID-JNR6>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Watt JA, Paden CM. Upregulation of the p75 low affinity neurotrophin receptor on active phagocytes during axonal degeneration in the rat neural lobe. Cell Tissue Res. 2001;303:81–91. doi: 10.1007/s004410000295. [DOI] [PubMed] [Google Scholar]
- Yasin SA, Costa A, Forsling ML, Grossman A. Interleukin-1 beta and interleukin-6 stimulate neurohypophysial hormone release in vitro. J Neuroendocrinol. 1994;6:179–184. doi: 10.1111/j.1365-2826.1994.tb00570.x. [DOI] [PubMed] [Google Scholar]
- Zhou X, Herman JP, Paden CM. Evidence that IGF-1 acts as an autocrine/paracrine growth factor in the magnocellular neurosecretory system: neuronal synthesis and induction of axonal sprouting. Exp Neurol. 1999;159:419–432. doi: 10.1006/exnr.1999.7189. [DOI] [PubMed] [Google Scholar]