Abstract
Osteopontin (Opn) is a broadly expressed pleiotropic cytokine and has been shown to play an important role in various autoimmune diseases including multiple sclerosis (MS) and its animal model experimental autoimmune encephalomyelitis (EAE). It is reported that Opn exacerbates EAE by skewing T cell differentiation towards IFN-γ producing Th1 cells. Opn expression in dendritic cells (DCs) and its role in IL-17 induction from T cells during EAE or MS is unknown. We found that during EAE Opn expression is elevated in DCs both in the periphery and in the central nervous system. There was increased expression of Opn receptor on T cells and Opn induced IL-17 production by CD4+ T cells via the β3 integrin receptor and Opn inhibited IL-10 production via the CD44 receptor. Furthermore, anti-Opn treatment reduced clinical severity of EAE by reducing IL-17 production. Anti-Opn was also effective in reducing clinical severity of EAE when given after the appearance of clinical symptoms. Analogous to EAE, in subjects with MS we found increased expression of Opn in DCs and increased expression of the Opn receptors CD44, β3 and αv on T cells. Furthermore, Opn stimulated CD4+ T cells from MS patients produced significantly higher amounts of IL-17. Our results demonstrate a role for DC produced Opn both in EAE and MS that is linked to the production of IL-17.
Keywords: Dendritic cells, T cells, EAE/MS and Osteopontin
Introduction
Osteopontin (Opn) is a pleiotropic protein that is broadly expressed and participates in wide range of biological processes, including bone remodeling, inflammation, cancer and immunity to infectious disease (1, 2). In addition, Opn has been linked to many autoimmune disease conditions such as multiple sclerosis (MS), rheumatoid arthritis, inflammatory bowel disease, systemic lypus erythematosus, type 1 diabetes and autoimmune myocarditis (3–8). The importance of Opn has been demonstrated in experimental autoimmune encephalomyelitis (EAE), a commonly studied animal model for MS. Mice deficient for Opn (Spp1−/−, also known as Opn−/−) show milder EAE without disease exacerbation or progression compared to WT mice (3, 9). Patients with MS have been shown to have elevated levels of Opn in their serum and plasma (4, 10). Many biological functions of Opn could influence disease progression in MS and EAE including enhanced survival of activated T cells in the central nervous system (CNS) (11, 12); increased IL-12 production by macrophages (13) and enhanced secretion of IFN-γ by T cells (3, 9). However, the relationship of Opn to IL-17 and the innate immune system in MS and EAE is unknown.
In EAE it was initially thought that CD4+ T cells mediating autoimmunity had a Th1 phenotype characterized by the production of IFN-γ (14). However IFN-γ deficient mice show enhanced EAE (15). Evidence now indicates that T cells critical for EAE are characterized by the production of IL-17. In addition, IL-17 expression has been detected in the target tissue in human autoimmune diseases including MS, rheumatoid arthritis and psoriasis (16) and increased production of IL-17 in MS patients has been observed (17). IL-17-deficient animals develop EAE with delayed onset and reduced severity (18) and anti-IL-17 antibody prevents chemokine expression in the brain and the subsequent development of EAE (19). Furthermore, T cell infiltration and inflammation in the brain in EAE may occur only when Th17 cells outnumber Th1 cells and trigger IL-17 expression (20).
Dendritic cells (DCs) play a pivotal role in orchestrating the immune response. The activation status and cytokine secretion profile of DCs control both activation and tolerization of immune responses against self and non-self antigens (21). Given this we investigated DC produced Opn both in EAE and MS and the role of Opn in inducing IL-17 from T cells. Here we report that Opn expression is highly increased in DCs both in EAE and MS and that Opn induces IL-17 production from T cells via specific Opn receptors on T cells.
Materials and Methods
Mice
C57BL/6 WT, Opn−/−, CD44−/− and Itgb3−/− mice were purchased from the Jackson Laboratory (Bar Harbor, ME). MOG specific TCR transgenic mice - 2D2 were obtained from Dr. Vijay Kuchroo (Harvard University). All mice were 6–8 weeks old at the beginning of experiments. Animals were maintained in a specific pathogen-free condition in the animal facility of Harvard Institutes of Medicine. All experiments were in accordance with guidelines from the committee on animals at Harvard Medical School.
Induction and evaluation of EAE
Mice were injected subcutaneously in both flanks with 100 μg of MOG35–55 peptide (MEVGWYRSPFSRVVHLYRNGK) dissolved in PBS emulsified in an equal volume complete Freund’s adjuvant- CFA (Difco) supplemented with 5mg/ml Mycobacterium tuberculosis H37Ra and injected twice intravenously with 200 ng of pertussis toxin (List Biological Laboratories) administered on the day of immunization and 48 h later. Clinical assessment of EAE was performed daily after disease induction according to the following criteria: 0, no disease; 1, tail paralysis; 2, hindlimb weakness or partial paralysis; 3, complete hindlimb paralysis; 4, forelimb and hindlimb paralysis; 5, moribund state. Mean clinical scores on separate days were calculated by adding scores of individual mice and dividing total number of mice in each group, including mice not develops signs of EAE.
Anti-Opn treatment
Mice (n =8) received 30μg of affinity purified polyclonal antibody to Opn (R&D systems, AF-808) or goat IgG control intraperitoneally on day 5, 7 and 9 post immunization. For EAE reversal, anti-Opn was administered when a clinical score of ≥1.5 was observed.
RNA isolation, cDNA synthesis and real-time PCR
Total RNA was isolated from cell pellets using RNA easy Mini Kit (QIAGEN). RNA was stored at −80°C. First strand cDNA synthesis was performed for each RNA sample from 0.5–1 μg of total RNA using Taqman reverse transcription reagents. cDNA was amplified using sequence specific primers (for Opn, Opn receptors, transcription factors and indicated cytokines) and real-time PCR mix (Applied Biosystems) on ABI7500 cycler. GAPDH gene was used as an endogenous control to normalize for differences in the amount of total RNA in each sample. All values were expressed as fold increase or decrease relative to the expression of GAPDH.
Flow cytometry analysis
Cells were resuspended in PBS containing 1% BSA and 0.1% sodium azide and incubated with fluorochrome conjugated antibodies or isotype control antibodies for 30 minutes on ice. Antibodies against CD4, CD11c, OPN-receptors CD44, CD51, β3 were obtained from BD Biosciences. Antibody to α4-integrin was obtained from- Abcam. Antibody to α9-integrin was purchased from R & D systems and β5-integrin from Ebiosciences. For intracellular staining we used the BD Cytofix/Cytoperm Fixation/Permeabilization kit (BD Biosciences). Briefly, cells were stimulated for 5 hours in the presence of PMA, ionomycin and protein transport inhibitor (golgistop- from BD Biosciences). Cells were then fixed using the fixative buffer and permeabilized using the saponin based permeabilization buffer. Antibodies used were the FITC conjugated mouse anti-human IFN-gamma (clone B27 from BD Biosciences) and the Alexa Fluor 647 conjugated mouse anti-human IL-17A (clone eBio64DEC17 from eBioscience). Samples were acquired on FACSCalibur or LSR II flow cytometers. Data analysis was done using the FloJo software.
Proliferative responses of T cell and cytokine analysis
Spleens or draining lymph nodes (inguinal regions) were harvested and pooled at onset (score = 0.5–1 on days 7–9) and peak of disease (score = 2.5–3 at day 12–16) from EAE mice and single-cell suspensions were prepared. Cells were cultured at 0.5million/well in 96-well U-bottom plates with a range of concentrations of MOG35–55 peptide or anti-CD3 antibodies in RPMI 1640 medium supplemented with 10% FCS. Plates were pulsed after 60h of culture with [3H] thymidine at 1μCi/well for the final 18h, harvested and assayed for proliferation. Mean incorporation of thymidine in DNA was measured in triplicate wells and is indicated as counts per minute (cpm). For ELISA, supernatants were harvested at 60 h of culture. The concentrations of indicated cytokines were measured by quantitative capture ELISA according to the guidelines of the manufacturers (BD Biosciences).
Cell culture
CD4+ T and CD8+ T cells were cultured with plate coated anti-CD3 and anti-CD28 mAb (0.3 μg/ml) in the presence or absence of 1 μg/ml of mouse rOpn. For blocking experiments, purified monoclonal antibodies to mouse CD44, β1-integrin and β3-integrin (BD Biosciences) were added at 5μg/ml concentration. For DC- T cell cocultures- total CD4+ T cells isolated from MOG specific TCR transgenic mice (2D2) were cocultured with CD11c+ DC isolated from naïve and MOG35-55 immunized (day 10 post-immunization) WT and Opn−/− mice. Cocultures were performed at a 1:3 DC/T-cell ratio in U-bottom 96-well plates. For the last 16 h, cells were pulsed with 1 μCi of thymidine (H3) and assayed for proliferation as indicated above. Assays were performed in triplicate. Supernatants from parallel cultures were harvested 60 h after initiation of cultures. Indicated cytokines in the supernatants were assayed by ELISA kits (BD Biosciences).
Western blot analysis
Whole cell extracts (WCE) were prepared using Roche complete lysis buffer and protease inhibitor tablets. 20μg WCE was resolved on 10% acrylamide gels (Invitrogen) and transferred onto nitrocellulose membrane. Membranes were blocked at 5% non-fat milk powder at room temperature. For Opn expression membranes were then incubated with 0.2μg/ml anti-Opn antibody (R & D Systems) for 1h at room temperature. Expression was visualized using secondary HRP antibody and SuperSignal substrate kit from Pierce. For α-tubulin expression nitrocellulose membranes were stripped and reprobed with antibody to α-tubulin.
Preparation and evaluation of CNS cells
Animals were perfused with cold PBS. Brains and spinal cords were dissected and incubated in 2.5mg/ml colleganase D and 1mg/ml DNAseI for 30 minutes at 37°C. Single-cell suspensions were prepared by passing through 70μm strainer. Cells were washed in RPMI 1640 medium and mononuclear cells were isolated using a discontinuous Percoll gradient (Pharmacia, Piscataway, NJ). Cells were washed twice and CD11c+ cells were isolated from this suspension by magnetic separation using microbeads (Miltenyi Biotec). The resultant CD11c+ DCs were greater than 97% pure.
Human Subjects
Peripheral blood was obtained after informed consent from healthy subjects (n=25) and MS patients (n=25) according to the Institutional Ethics Review Board Protocols. Healthy donors had an average age = 35+/−6yrs which was the same age range for MS patients. All patients were seen at Partners MS Center at Brigham and Women’s Hospital. MS patients consisted of untreated relapsing-remitting patients that had not received steroids in the 6 months prior to blood drawing, or beta-interferon in the 10 months prior to blood drawing. None of the patients had been treated with glatiramer acetate.
Human DC and T cell Isolation and stimulation
PBMCs were isolated by Ficoll (Pharmacia LKB Biotechnology) gradient centrifugation. mDCs from both healthy controls and MS patients were directly isolated from the blood using CD11c beads (Miltenyi Biotec) and were determined to be >97% CD11c+ by flow cytometry. T cells for OPN stimulation were enriched (purity>95%) by immunomagnetic negative-depletion using T-cell separation kit II and MACS columns (Miltenyi Biotec) according to manufacturer’s instructions. CD4+ T cells were cultured with plate-bound anti-CD3 and anti-CD28 mAb (0.3μg/ml) in the presence or absence of 1 μg/ml of human rOPN. For Th17 polarization experiments, naïve CD4+ T cells were seeded at a density of 1.5× 106 cells per ml in 24-well plates with coated with anti-CD3 and anti-CD28 (1μg/ml). IL-1β (50 ng/ml), IL-6 (50 ng/ml), TGF-β1 (2 ng/ml) was added at day 0 and was maintained throughout the experiment. Cell free culture supernatants were collected on day 5 for intracellular cytokine staining. For the expression of Opn expression, myeloid DCs (purity, >95%) were isolated directly from the blood using CD11c beads (Miltenyi Biotec), RNA was prepared and OPN expression was determined by real-time PCR as described above. CD4+ T cells were isolated as described above and analyzed for Opn receptor expression by real-time PCR. The purity of the resulting T cell preparations was greater than 97%.
Statistical Analysis
Statistical analysis was performed using the unpaired t test. A value of P < 0.05 was considered significant. Data are presented as mean S.E.M. For EAE, groups were compared using linear regression analysis. The difference between the two groups was highly significant (P<0.0001).
Results
Elevated Opn expression in DCs from EAE mice
Since DCs are the primary antigen presenting cells in regulating immune response and Opn has a profound influence on inflammatory conditions we investigated whether DCs express Opn during EAE. Opn expression was analyzed at disease onset and peak stages of the disease in CD11c+ DCs (spleen, lymph node (LN) and CNS) from C57BL/6 mice immunized for EAE with MOG35-55 peptide. We found that Opn mRNA expression was markedly increased in CD11c+ DCs at the peak of disease both in spleen, LN and CNS (Fig. 1A). Purified CD11c+ DCs from spleen, LN and CNS were then analyzed for Opn protein expression by Western blot (Fig. 1B). Opn protein expression in splenic CD11c+ DCs was slightly increased at onset and markedly increased at peak stage of disease. In LN DCs Opn expression was observed only at peak of disease. CNS DCs also showed Opn expression at peak of disease (Fig. 1B). By flow cytometry we found that the number of CNS infiltrating DCs increased progressively with disease stage (Fig. 1C). Note, there were insufficient numbers of DCs in naïve animals and at disease onset stage to measure protein expression in the CNS. These results indicate that Opn expression is elevated at both the mRNA and protein levels in DCs during the course of EAE and thus could impact on immune responses both in the periphery and CNS.
Figure 1.
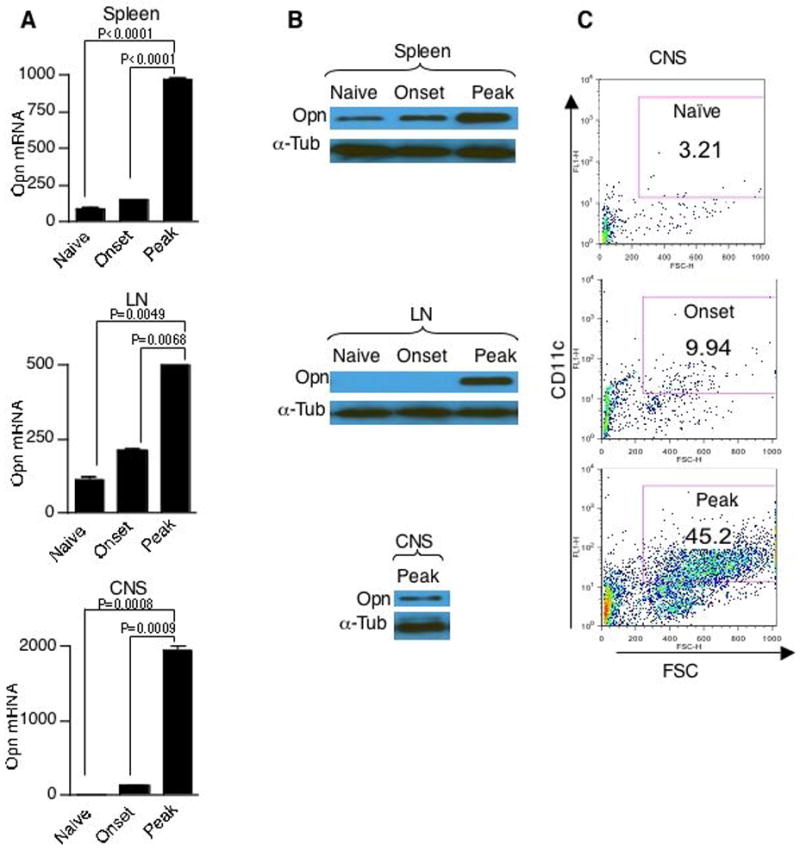
Elevated Opn expression in DCs during EAE. Increased Opn mRNA and protein expression from CD11c+ DCs in EAE mice. (A) Opn mRNA was determined by quantitative real-time PCR analysis in CD11c+ DCs from spleen, LN and CNS from naïve, onset (EAE score 0.5–1, n=5–6 per group) and peak (EAE score 3, n=5–6 per group) C57BL/6 mice. Expression of Opn was normalized to GAPDH. (B) Opn protein expression was determined by Western blot analysis using anti-Opn antibodies. 20 μg of whole cell extract from CD11c+ DCs from indicated organs and timepoints were resolved on a 10% acrylamide gel. α-tubulin was used for loading control. (n=5–6 per group) (C) Increased infiltration of CD11c+ DCs in the CNS during disease- was assayed during naïve, onset and peak state of EAE. The mononuclear fraction from CNS was stained with antibodies to CD11c. Data are representative of three independent experiments.
DC expressed Opn induces IL-17 from T cells
Polarization of helper T cells to Th1, Th2 or Th17 phenotypes is a critical feature of cell-mediated immunity and is influenced by production of cytokines by DCs. Since we observed elevated Opn expression in DCs during EAE we next investigated whether DC derived Opn had a role in pathogenic T helper function specifically, T cell production of IL-17 and IFN-γ. For this we induced EAE in WT and Opn−/− mice with MOG35-55 and at day 10 post immunization isolated splenic CD11c+ DCs. Naïve splenic DCs were used as controls. We cocultured naïve DC and DCs derived from immunized mice from WT and Opn−/− mice with MOG specific TCR transgenic CD4+ T cells and measured MOG35-55 antigen-specific proliferation (Fig. 2A). T cells cultured with DCs from Opn−/− mice showed reduced proliferative responses compared with T cells cultured with WT DCs. When cytokine secretion was quantified, significant differences were observed between T cells cultured with DCs from Opn−/− mice compared to WT controls. In Opn−/− mice, there was less IL-17 and IFN-γ production, and increased IL-10 production by T cells (Fig. 2B–D). It has been shown that other DC secreted molecules (IL-6 and IL-1β) appear to synergize with IL-23 in the induction of IL-17 by CD4+ T cells (22). We thus tested whether the expression of these factors are altered in DC derived from Opn-deficient animals. No significant differences in the expression of IL-1β, IL-6 and IL-23 were observed between WT and Opn deficient DCs (Fig. 2E). We then tested for the contribution of DC expressed Opn on IL-17 production from T cells. We found that T cells from both WT and Opn-deficient mice produced less IL-17 when they were cultured with Opn-deficient DCs. However, coculture of WT-DC with T cells from WT and Opn-deficient mice showed significant increase in IL-17 production demonstrating that Opn expression in DC modulates IL-17 production by T cells (Fig. 2F).
Figure 2.
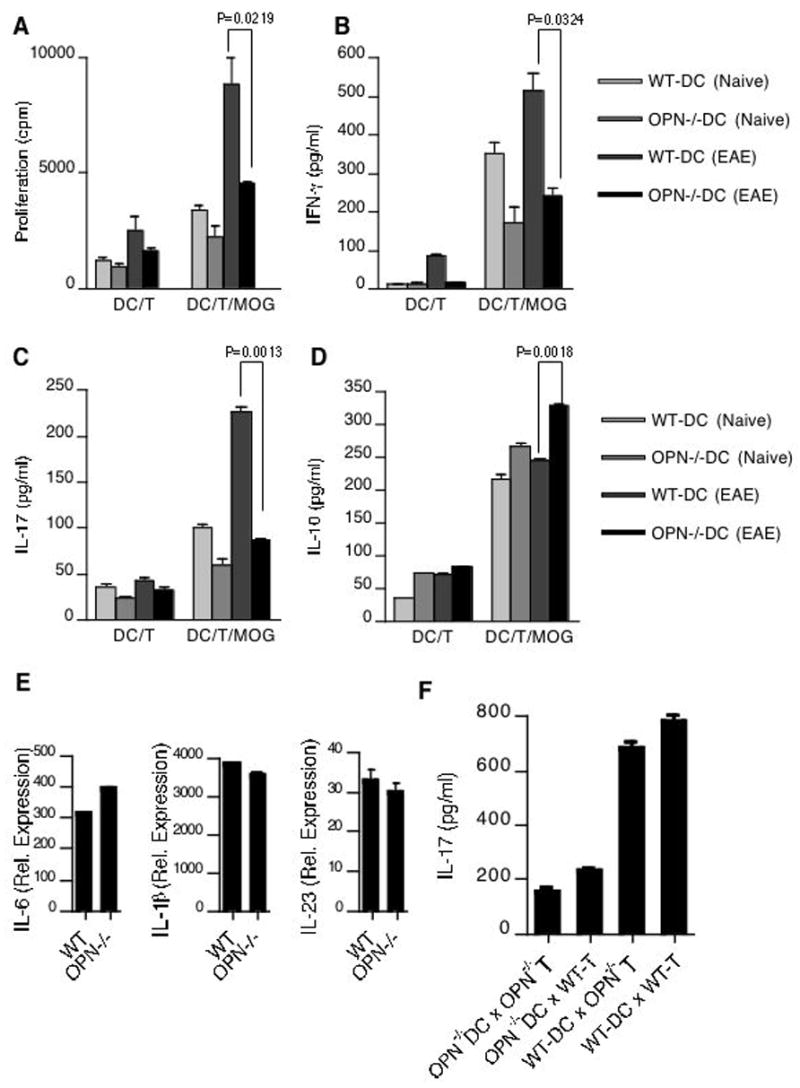
Opn production from DC induces IL-17 production from T cells. Total CD4+ T cells isolated from 2D2 mice were cocultured with CD11c+ DC isolated from naïve and MOG35-55 immunized WT and Opn−/− mice. (A) In the last 16h, cells were pulsed with thymidine and assayed for proliferation (cpm). Error bars represent s.e.m. between triplicates. Supernatants from parallel cultures were harvested 60 h after initiation of cultures and assayed by ELISA for (B) IFN-γ (C) IL-17 and (D) IL-10. (E) Splenic DCs isolated from WT and Opn−/− mice were analyzed by real-time RT-PCR for the expression of IL6, IL-1β and IL-23. (F) DC expressed Opn induces IL-17 production from T cells. Total CD4+ T cells isolated from WT and Opn−/− mice were cocultured with CD11c+ DCs isolated from WT and Opn−/− mice. Supernatants from cultures were harvested 72 h after initiation of cultures and assayed by ELISA for IL-17.
To directly demonstrate the effect of Opn on T cells we stimulated CD4+ T cells with antibodies to CD3 and CD28 with or without mouse recombinant Opn (rOPN) and measured cytokine production. We found that Opn greatly increased the amounts of IL-17 and IFN-γ production and suppressed IL-10 production (Fig. 3A–C). RORγt is a transcription factor required for generation of Th17 cells (23). To test if Opn activated RORγt, CD4+ T cells were activated with anti-CD3 and anti-CD28 with or without rOPN. Addition of rOPN increased the expression of both RORγt and T-bet, a Th1 specifying transcription factor (24) (Fig. 3D and E). We then tested CD8+ T cells and also found that Opn stimulation induced significantly higher amounts of IFN-γ and IL-17 from CD8+ T cells while inhibiting IL-10 production (Fig. 3F–H).
Figure 3.
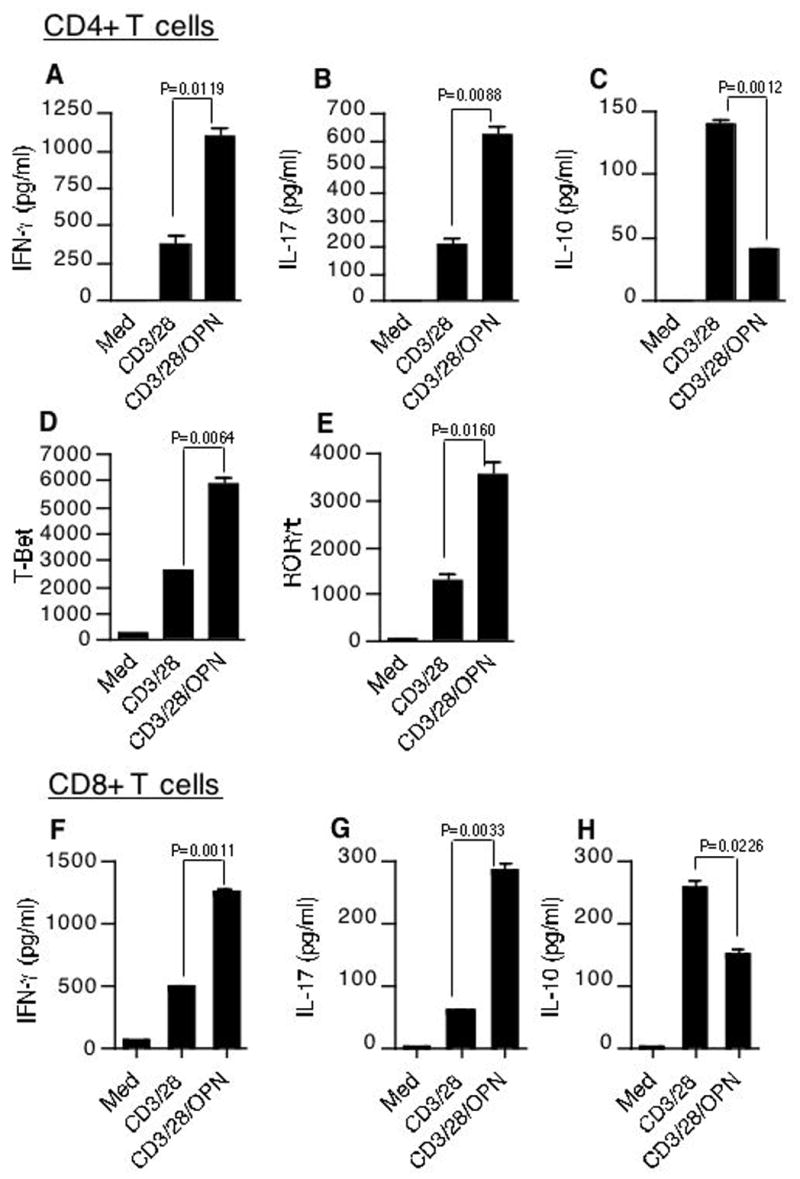
Differential regulation of IL-17, IFN-γ and IL-10 by Opn. CD4+ T cells were activated with anti-CD3 and anti-CD28 mAb (0.3 μg/ml) in the presence or absence of 1 μg/ml of mouse rOPN. Supernatants from cultures were harvested 60 h after initiation of cultures and analyzed for cytokines (A) IL-17 (B) IFN-γ (C) IL-10. Similarly stimulated cells were harvested 24hrs post stimulation for mRNA and analyzed for (D) T-bet and (E) RORγT by quantitative real-time PCR. Data are representative of 3 independent experiments. (F–H) Opn induces IL-17 and IFN-γ while inhibiting IL-10 from CD8+ T cells. CD8+ T cells were activated with anti-CD3 and anti-CD28 mAb (0.3 μg/ml) in the presence or absence of 1 μg/ml of mouse rOPN. Supernatants from cultures were harvested 60 h after initiation of cultures and analyzed for cytokines (F) IL-17 (G) IFN-γ (H) IL-10.
Opn induced IL-17 production by CD4+ T cells is mediated through β3 integrin receptor
Opn interacts with a variety of cell surface receptors, including the integrins αvβ3, αvβ1, αvβ5, α4β4 and α9β1as well as CD44. Binding of Opn to these cell surface receptors stimulates cell adhesion, chemotactic migration and specific signaling (1, 2). We thus asked which of these receptors was required for the affect of Opn on T cell cytokine production. For this we first examined the expression of Opn receptors during EAE. Surface expression of Opn receptors was analyzed on CD4+ T cells from spleen, draining inguinal lymph nodes, blood and CNS at peak of disease. Both CNS and peripheral blood CD4+ T cells showed a marked increase in surface expression of CD44, αV, β3 and β1. The CNS also showed an increase in expression of the common chain αV/CD51 (Fig. 4A). No significant difference in expression of these integrins was observed in T cells from spleen and inguinal lymph nodes (data not shown).
Figure 4.
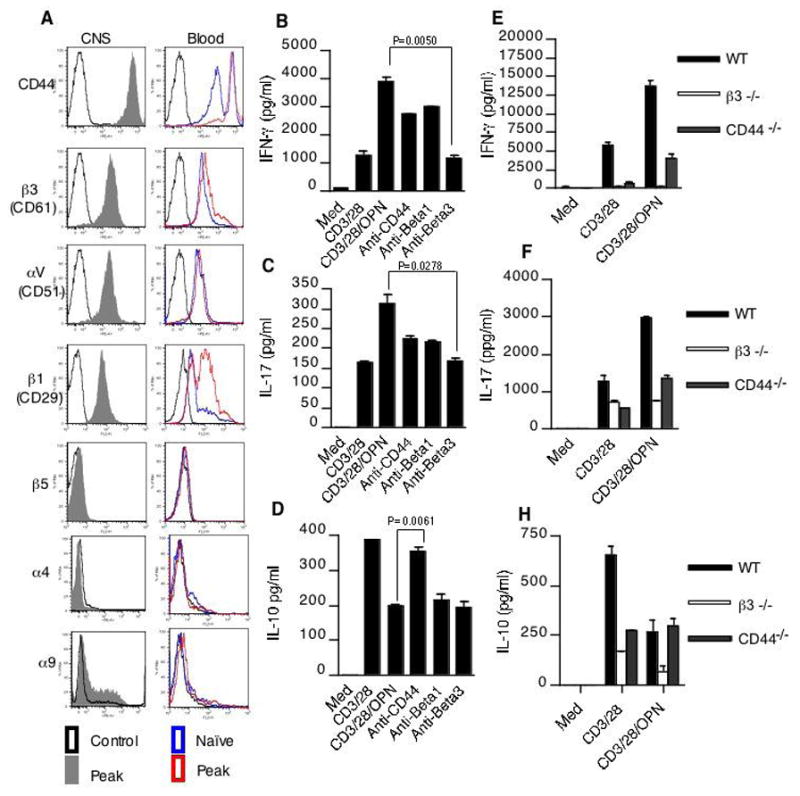
Regulation of T cell cytokine expression by distinct Opn receptors. (A) Opn receptor expression on T cells from CNS and peripheral blood of naïve mice and mice with peak EAE. Cells were stained with fluorochrome conjugated CD4 and indicated integrin receptors. CD4+ T cells were gated and analyzed for expression of Opn receptors. (B–D) Opn induces secretion of IFN-γ and IL-17 while inhibiting IL-10 production from T cells. CD4+ T cells were activated with anti-CD3 and anti-CD28 mAb (0.3 μg/ml) in the presence or absence of 1 μg/ml of mouse rOPN. Blocking antibodies to integrin β3, CD44 or integrin β1 were added at 5μg/ml. Supernatants from cultures were harvested 60 h and analyzed for IFN-γ, IL-17 and IL-10. (E–G) T cells from WT, Itgb3−/− and CD44−/− mice were activated with anti-CD3 and anti-CD28 mAb (0.3 μg/ml) in the presence or absence of 1 μg/ml of mouse rOPN. Culture supernatants were harvested 60h later were analyzed for IFN-γ, IL-17 and IL-10. Data are representative of 2–4 independent experiments.
Since the Opn receptors CD44, αV, β3 and β1 were selectively increased on T cells from EAE mice, we investigated which receptor was responsible for the regulation of T cell cytokine production by Opn. For this CD4+ T cells were stimulated with anti-CD3 and anti-CD28 with or without rOPN in the presence of blocking antibodies to these receptors. Induction of IFN-γ and IL-17 was inhibited by a blocking antibody to the integrin β3 subunit (Fig. 4B and C). This was confirmed using β3-integrin deficient mice (Itgb3−/− mice), and T cells from these mice were unresponsive to Opn stimulation (Figure 4E and F). In contrast to IL-17 and IFN-γ, inhibition of IL-10 was blocked by antibody to CD44 (Fig. 4D). Consistent with this, Opn did not inhibit IL-10 responses in T cells from CD44−/− mice (Fig. 4G). Thus far our experiments suggest that production of Opn by DC is an essential proximal event that potentiates T cell production of IL-17 and IFN-γ through β3-integrin engagement and diminished the IL-10 response through CD44 engagement. These effects create a cytokine milieu that favors CNS autoimmunity.
Anti-Opn treatment reduces the clinical severity of EAE
Although Opn has been shown previously to play an important role in EAE, suppression of EAE by anti-opn antibody has not been demonstrated. We thus investigated whether anti-Opn antibody could affect the clinical severity of EAE. We administered 30μg of anti-Opn antibody or isotype (IgG) matched control antibody to mice on the fifth, seventh and ninth day after MOG immunization. As shown in Fig. 5A clinical disease in anti-Opn treated mice was markedly reduced. In order to investigate whether anti-Opn treatment affected antigen-specific recall responses we isolated spleen and lymph nodes of MOG-immunized mice treated with anti-Opn or isotype control antibody and stimulated them in vitro with MOG peptide. We found that T cells from these mice had a diminished MOG specific proliferative response, reduced IFN-γ and IL-17 secretion and increased IL-10 production (Fig. 5B–E). Thus, anti-Opn antibody suppresses EAE in association with an increase in IL-10 and decrease in IL-17 and IFN-γ. In addition, consistent with our data and with the dramatic inhibitory effect on the clinical EAE symptoms by anti-Opn, we found that anti-Opn treatment prevented up-regulation of proinflammatory cytokines (IL-17 and IFN-γ) while inducing IL-10 transcripts in CD4+ T cells isolated from the CNS of the protected mice (Fig. 5F). In the clinical setting of MS, therapeutic intervention is often started after the onset of the symptoms. Therefore, it is important to investigate whether a treatment regimen, which is effective EAE prevention, can also reverse established disease. Thus we tested the efficacy of anti-Opn treatment of EAE after the onset of clinical symptoms. We found that treatment with anti-Opn after the onset of EAE (score of ≥1.5) on days 16, 17, 18 and 19 (after initial immunization with MOG peptide) resulted in a rapid clinical recovery from EAE (Fig. 5G).
Figure 5.
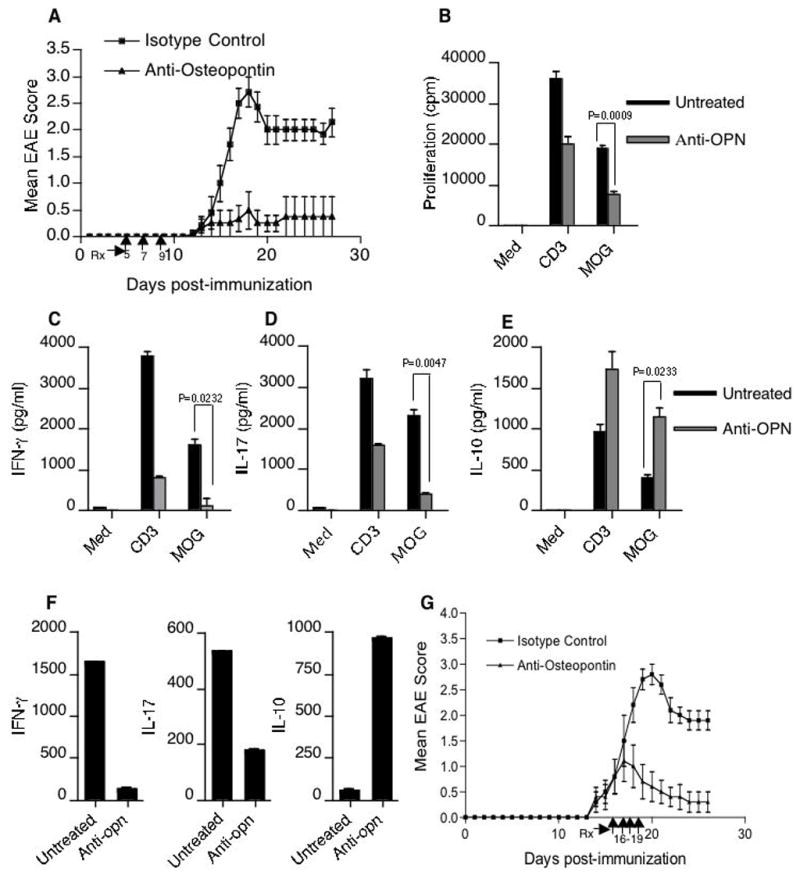
Anti-Osteopontin suppresses EAE. (A) Mean clinical scores of EAE mice treated with anti-Opn antibody or isotype control (n = 8 per group) on days 5, 7 and 9 post immunization (indicated by arrows on X-axis) (P < 0.0001) (B) Spleen cells from anti-Opn and control mice were activated in vitro MOG35-55 (20 μg/ml) for 60h. In the last 16h, cells were pulsed with thymidine and proliferation was represented as cpm. Data represent the s.e.m+/− of triplicate assays. (C–E) Cell-free supernatants from the above culture conditions were harvested at 60h and assayed for indicated cytokines. (F) CNS-T cells isolated from mice treated with anti-Opn or control antibody (n = 10 mice per group) were analyzed by real-time RT-PCR for the expression of IL-17, IFN-γ and IL-10. (G) Mice were treated with anti-Opn after onset of EAE (score of ≥1.5) on days 16, 17, 18 and 19 (post immunization) resulted in a rapid clinical recovery from EAE.
Opn induces IL-17 production from Human CD4+ T cells
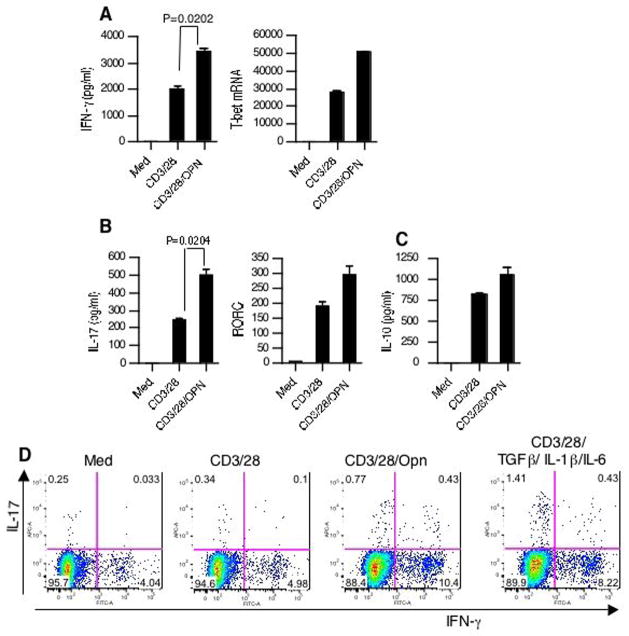
We then investigated whether rOPN increased IFN-γ, IL-17 and decreased IL-10 in human CD4+ T cells. Similar to mice, in normal human subjects rOPN increased IFN-γ and IL-17 and this correlated with an increase in their transcription factors (Fig. 6A and B). However, contrary to what we observed in mice, Opn stimulation did not lead to a decrease in IL-10 levels from human T cells (Fig. 6C). In order to determine whether the increased IL-17 production was related to increased number of IL-17+ cells we performed intracellular cytokine analysis. As shown in Fig. 6D, the increase in IL-17 was secondary to increase in the number of IL-17+ cells, not due to a per cell increase in IL-17 synthesis. Regarding IFN-γ, the increase observed with Opn consisted of both an increase in double positive and an increase in discrete single positive populations. It has been recently shown that TGF-β in combination with proinflammatory cytokines (IL-1β and IL-6) is capable of driving IL-17 secretion from naïve T cells (25). We thus compared the effectiveness of Opn vs Th17 polarizing conditions to induce IL-17 production from CD4+ T cells and found that the combination of TGF-β with proinflammatory cytokines (IL-6 and IL-1β) were more effective than Opn in inducing Th17 differentiation (Fig. 6D).
Figure 6.
Opn induces IL-17 production from human CD4+ T cells. (A–C) Effect of Opn on T cell cytokine and transcription factors in healthy controls. Total CD4+ T cells from HC were stimulated with anti-CD3/CD28 in the presence or absence of rOPN (1μg/ml). Supernatants (at 60h) were harvested and assayed for indicated cytokines by ELISA. Parallel cultures were analyzed for real-time PCR analysis for human (A) T-bet and (B) RORC. (D) Opn stimulation of CD4+ T cells leads to increased expression of IL-17+ and IFN-γ+ T cells. Naïve CD4+ T cells from HC were stimulated with anti-CD3/CD28 in the presence or absence of rOPN (1μg/ml). Cells were also stimulated under the indicated Th17 polarizing conditions. Intracellular cytokine staining was performed after 5 days of culture.
Investigation of Opn in DCs and T cells in subjects with MS
Although we have demonstrated an important role for Opn in DC and T cells in EAE, it is unknown whether similar abnormalities exist in MS. It has been reported that there are elevated levels of Opn in serum and plasma of MS patients (4). We isolated CD11c+ DC from PBMCs of untreated relapsing remitting MS patients and compared them with control DCs derived from healthy individuals. There was virtually no Opn expression in healthy controls, whereas Opn expression was markedly increased in DCs from MS patients (Fig. 7A). In our EAE studies we found increased expression of Opn receptors on T cells. We thus asked whether there was increased expression of Opn receptors on CD4+ T cells from MS patients. As shown in Fig. 7B, we found that the expression of the Opn receptors CD44, β3-integrin and α v (CD51) were increased on CD4+ T cells from MS patients.
Figure 7.
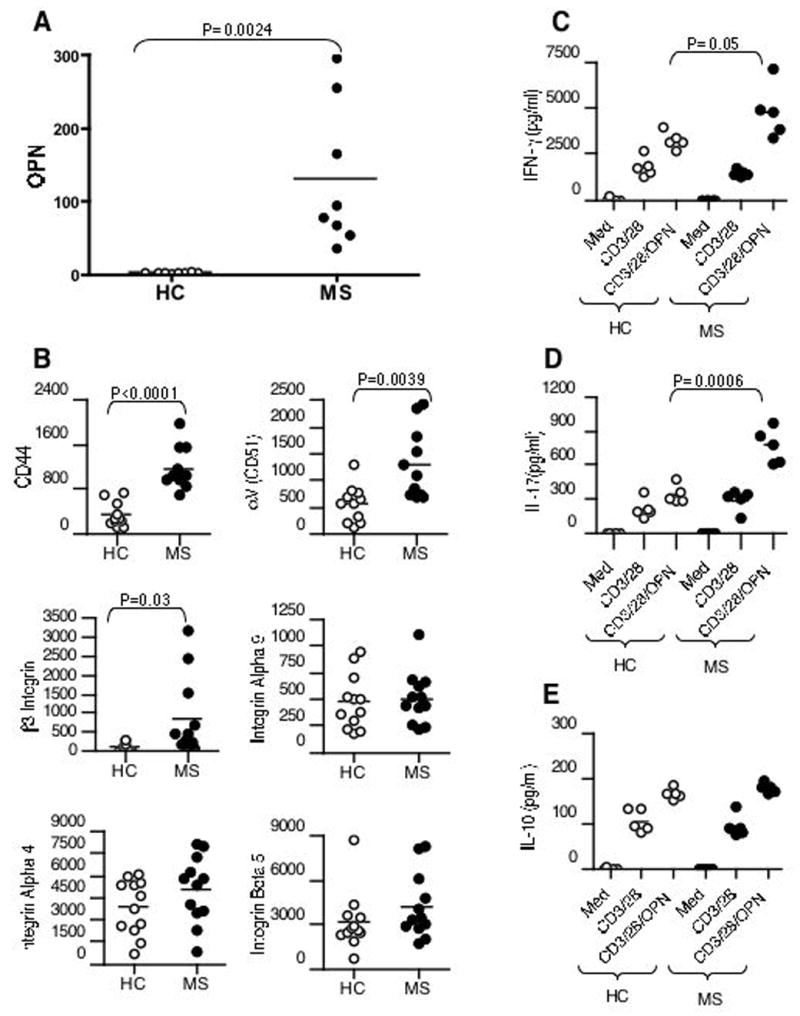
Investigation of Opn in DCs and T cells in subjects with MS. (A) Elevated Opn in DC from MS patients. RNA from peripheral blood CD11c+ cells of MS patients (n=8) and healthy controls (HC) (n=8) was analyzed by real-time PCR and normalized to GAPDH. (B) Opn receptor expression is increased in patients with MS. Total CD4+ T cells isolated from MS patients (n=12) and HC (n=12) were analyzed for Opn receptor expression by real-time PCR and normalized to GAPDH. (C–E) CD4+ T cells from controls (n=5) or MS patients (n=5) were stimulated with anti-CD3/CD28 in the presence or absence of rOPN (1mg/ml). Supernatants were harvested and assayed for indicated cytokines by ELISA. All statistical analyses herein were performed using the unpaired t test. A value of P < 0.05 was considered significant.
As described above, CD4+ T cells from MS patients have increased β3 and CD44 Opn receptors on their surface. We thus hypothesized that CD4+ T cells from MS subjects would have heightened responses to exogenous Opn. To test this, we stimulated purified CD4+ T cells from MS patients with plate-bound anti-CD3 and anti-CD28 in the presence or absence of human rOPN. We found a significant increase in IL-17 production by CD4+ T cells from MS patients versus controls (Fig. 7D). An increase in IFN-γ was also observed in MS patients versus controls though not as marked (Fig. 7C). No difference in IL-10 production was observed between MS versus controls (Fig. 7E). Taken together, these results demonstrate that there are abnormalities in Opn in MS patients that are analogous to what we observed in the EAE disease model.
Discussion
Opn is a proinflammatory cytokine associated with several autoimmune diseases including MS. It is classified as a Th1 cytokine because of its ability to enhance the production of IFN-γ from T cells and IL-12 production from macrophages (13). While IFN-γ secreting Th1 cells have been considered the disease-inducing CD4+ T lymphocyte subset in EAE, recent studies have shown that Th17 cells play a central role in initiation and pathogenesis (26, 27). The role of Opn on IL-17 production from T cells and its expression by DCs are unknown. We show that DCs have increased expression of Opn during EAE, which drives myelin antigen specific IL-17 and IFN-γ production from T cells. It is well known that the cytokine profile of CD4+ T lymphocytes is dictated by the ability of antigen-presenting cells (such as DCs) to secrete either IL-12, favoring Th1 responses, or the combination of transforming growth factor- beta 1 (TGF-β1) and IL-6, favoring a Th17 phenotype (28. In addition DC secreted IL-10 has been shown to induce IL-10 producing Tr1 cells (29). Furthermore, DC expressed Opn has been shown to alter the B cell function during HIV infection (30) and Opn exposed DCs induce IFN-γ production from human T cells(31). Thus Opn production by DCs can alter effector T or B cell function. It has been shown that myeloid DCs (CD11c+) accumulate at sites of chronic inflammation (32). We show here that Opn is abundantly produced by CD11c+ DCs from animals with EAE and patients with MS. Thus, in these conditions DC produced Opn may be an important pathway that drives pathogenic autoimmune responses by amplifying IL-17 production from T cells. However, other cell types that express Opn including macrophages, glial cells, endothelial cells and neurons cannot be excluded in this process (12).
It is known that Opn interacts with a variety of cell surface receptors, including CD44 and αv (β1, β3, β5), α4β1, and α9β1 integrins. The role these receptors play on T cell responses is unknown. We found that during EAE Opn receptors αv, β1, β3 and CD44 are increased on the T cells and are involved in differential regulation of T cell cytokine production. We found that IFN-γ and IL-17 production is mediated through β3-integrin receptors, where as Opn dampens the IL-10 response via CD44 engagement. Thus, binding of Opn to its integrin receptor β3 would be expected to perpetuate Th1 and Th17 mediated inflammation in vivo. In contrast to the induction of IFN-γ and IL-17, Opn dampens the T cell IL-10 response through CD44 engagement, which might then lead to further amplify inflammation through the up-regulation of Th1 and Th17 cytokines.
A central question related to our findings in the EAE model is the degree to which these changes are also observed in subjects with MS. We found that DCs from MS patients show a marked increase in Opn levels compared to healthy controls. Furthermore, our cellular studies examining the role of Opn in MS patients revealed an increased expression of specific Opn receptors αv, β3-integrins and CD44. Others have observed increased expression of αV in active MS lesions in macrophages and endothelial cells (33). Consistent with the increased expression of these receptors on T cells from MS patients, we found an increased production of IL-17 by MS T cells stimulated in vitro with Opn. One of the differences between the effect of Opn on T cells in mice versus human is that in mice Opn leads to a decrease in IL-10 secretion whereas in humans, there is no effect. Consistent with this, we did not observe a difference between MS and controls in the production of IL-10 following in vitro stimulation by anti-CD3 and anti-CD28 in the presence of exogenous Opn. Given the ameliorating effect of anti-Opn treatment in EAE in mice and our findings in MS patients, anti-Opn antibodies may be an attractive candidate for therapy. In summary, we have identified Opn as a key mediator that may serve to perpetuate and amplify the inflammatory process in MS. Our findings related to expression of Opn in DCs, surface receptor on T cells and the relationship between Opn and IL-17 have implications not only for MS but in other autoimmune diseases as well.
Acknowledgments
The authors thank David Anderson for his helpful discussions.
This work was supported by the National Institutes of Health Grants (NS038037, AI043458 and NS23132) and the Nancy Davis Foundation. A.M. was supported by the NRSA fellowship (grant number F32AI075761) from the National Institute Of Allergy and Infectious Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute Of Allergy and Infectious Diseases or the National Institutes of Health.
Footnotes
Competing financial interest Authors declare that they have no competing financial interests.
References
- 1.Denhardt DT, Noda M, O’Regan AW, Pavlin D, Berman JS. Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. The Journal of clinical investigation. 2001;107:1055–1061. doi: 10.1172/JCI12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gravallese EM. Osteopontin: a bridge between bone and the immune system. The Journal of clinical investigation. 2003;112:147–149. doi: 10.1172/JCI19190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chabas D, Baranzini SE, Mitchell D, Bernard CC, Rittling SR, Denhardt DT, Sobel RA, Lock C, Karpuj M, Pedotti R, Heller R, Oksenberg JR, Steinman L. The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science (New York, NY. 2001;294:1731–1735. doi: 10.1126/science.1062960. [DOI] [PubMed] [Google Scholar]
- 4.Comabella M, Pericot I, Goertsches R, Nos C, Castillo M, Blas Navarro J, Rio J, Montalban X. Plasma osteopontin levels in multiple sclerosis. Journal of neuroimmunology. 2005;158:231–239. doi: 10.1016/j.jneuroim.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Wong CK, Lit LC, Tam LS, Li EK, Lam CW. Elevation of plasma osteopontin concentration is correlated with disease activity in patients with systemic lupus erythematosus. Rheumatology (Oxford, England) 2005;44:602–606. doi: 10.1093/rheumatology/keh558. [DOI] [PubMed] [Google Scholar]
- 6.Sato T, Nakai T, Tamura N, Okamoto S, Matsuoka K, Sakuraba A, Fukushima T, Uede T, Hibi T. Osteopontin/Eta-1 upregulated in Crohn’s disease regulates the Th1 immune response. Gut. 2005;54:1254–1262. doi: 10.1136/gut.2004.048298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aspord C, Rome S, Thivolet C. Early events in islets and pancreatic lymph nodes in autoimmune diabetes. Journal of autoimmunity. 2004;23:27–35. doi: 10.1016/j.jaut.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Shin T, Ahn M, Kim H, Kim HM, Matsumoto Y. Increased expression of osteopontin in the heart tissue of Lewis rats with experimental autoimmune myocarditis. The Journal of veterinary medical science / the Japanese Society of Veterinary Science. 2006;68:379–382. doi: 10.1292/jvms.68.379. [DOI] [PubMed] [Google Scholar]
- 9.Jansson M, Panoutsakopoulou V, Baker J, Klein L, Cantor H. Cutting edge: Attenuated experimental autoimmune encephalomyelitis in eta-1/osteopontin-deficient mice. J Immunol. 2002;168:2096–2099. doi: 10.4049/jimmunol.168.5.2096. [DOI] [PubMed] [Google Scholar]
- 10.Vogt MH, Floris S, Killestein J, Knol DL, Smits M, Barkhof F, Polman CH, Nagelkerken L. Osteopontin levels and increased disease activity in relapsing-remitting multiple sclerosis patients. Journal of neuroimmunology. 2004;155:155–160. doi: 10.1016/j.jneuroim.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Hur EM, Youssef S, Haws ME, Zhang SY, Sobel RA, Steinman L. Osteopontin-induced relapse and progression of autoimmune brain disease through enhanced survival of activated T cells. Nature immunology. 2007;8:74–83. doi: 10.1038/ni1415. [DOI] [PubMed] [Google Scholar]
- 12.Stromnes IM, Goverman JM. Osteopontin-induced survival of T cells. Nature immunology. 2007;8:19–20. doi: 10.1038/ni0107-19. [DOI] [PubMed] [Google Scholar]
- 13.Ashkar S, Weber GF, Panoutsakopoulou V, Sanchirico ME, Jansson M, Zawaideh S, Rittling SR, Denhardt DT, Glimcher MJ, Cantor H. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science (New York, NY. 2000;287:860–864. doi: 10.1126/science.287.5454.860. [DOI] [PubMed] [Google Scholar]
- 14.Sospedra M, Martin R. Immunology of multiple sclerosis. Annual review of immunology. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 15.Ferber IA, Brocke S, Taylor-Edwards C, Ridgway W, Dinisco C, Steinman L, Dalton D, Fathman CG. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) J Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- 16.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nature immunology. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 17.Vaknin-Dembinsky A, Balashov K, Weiner HL. IL-23 is increased in dendritic cells in multiple sclerosis and down-regulation of IL-23 by antisense oligos increases dendritic cell IL-10 production. J Immunol. 2006;176:7768–7774. doi: 10.4049/jimmunol.176.12.7768. [DOI] [PubMed] [Google Scholar]
- 18.Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 19.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nature immunology. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stromnes IM, Cerretti LM, Liggitt D, Harris RA, Goverman JM. Differential regulation of central nervous system autoimmunity by T(H)1 and T(H)17 cells. Nature medicine. 2008;14:337–342. doi: 10.1038/nm1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manuel SL, Rahman S, Wigdahl B, Khan ZK, Jain P. Dendritic cells in autoimmune diseases and neuroinflammatory disorders. Front Biosci. 2007;12:4315–4335. doi: 10.2741/2390. [DOI] [PubMed] [Google Scholar]
- 22.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nature immunology. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 23.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 24.Shinohara ML, Jansson M, Hwang ES, Werneck MB, Glimcher LH, Cantor H. T-bet-dependent expression of osteopontin contributes to T cell polarization. Proc Natl Acad Sci U S A. 2005;102:17101–17106. doi: 10.1073/pnas.0508666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nature immunology. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y, Langrish CL, McKenzie B, Joyce-Shaikh B, Stumhofer JS, McClanahan T, Blumenschein W, Churakovsa T, Low J, Presta L, Hunter CA, Kastelein RA, Cua DJ. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. The Journal of clinical investigation. 2006;116:1317–1326. doi: 10.1172/JCI25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Wakkach A, Fournier N, Brun V, Breittmayer JP, Cottrez F, Groux H. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity. 2003;18:605–617. doi: 10.1016/s1074-7613(03)00113-4. [DOI] [PubMed] [Google Scholar]
- 30.Li Q, Lifson JD, Duan L, Schacker TW, Reilly C, Carlis J, Estes JD, Haase AT. Potential roles of follicular dendritic cell-associated osteopontin in lymphoid follicle pathology and repair and in B cell regulation in HIV-1 and SIV infection. The Journal of infectious diseases. 2005;192:1269–1276. doi: 10.1086/444430. [DOI] [PubMed] [Google Scholar]
- 31.Renkl AC, Wussler J, Ahrens T, Thoma K, Kon S, Uede T, Martin SF, Simon JC, Weiss JM. Osteopontin functionally activates dendritic cells and induces their differentiation toward a Th1-polarizing phenotype. Blood. 2005;106:946–955. doi: 10.1182/blood-2004-08-3228. [DOI] [PubMed] [Google Scholar]
- 32.Miller SD, McMahon EJ, Schreiner B, Bailey SL. Antigen presentation in the CNS by myeloid dendritic cells drives progression of relapsing experimental autoimmune encephalomyelitis. Annals of the New York Academy of Sciences. 2007;1103:179–191. doi: 10.1196/annals.1394.023. [DOI] [PubMed] [Google Scholar]
- 33.Sobel RA, Hinojoza JR, Maeda A, Chen M. Endothelial cell integrin laminin receptor expression in multiple sclerosis lesions. The American journal of pathology. 1998;153:405–415. doi: 10.1016/S0002-9440(10)65584-8. [DOI] [PMC free article] [PubMed] [Google Scholar]