Abstract
Described is a bioorthogonal reaction that proceeds with unusually fast reaction rates without need for catalysis: the cycloaddition of s-tetrazine and trans-cyclooctene derivatives. The reactions tolerate a broad range of functionality, and proceed in high yield in organic solvents, water, cell media or in cell lysate. The rate of the ligation between trans-cyclooctene and 3,6-di-(2-pyridyl)-s-tetrazine is very rapid (k2 2000 M−1s−1). This fast reactivity enables protein modification at low concentration.
Described herein is a bioorthogonal reaction that proceeds with unusually fast reaction rates without need for catalysis: the cycloaddition of s-tetrazine and trans-cyclooctene derivatives. The reactions tolerate a broad range of functionality, and proceed in high yield in organic solvents, water, cell media or in cell lysate. The rate of the ligation between trans-cyclooctene and 3,6-di-(2-pyridyl)-s-tetrazine is very rapid (k2 2000 M−1s−1). This fast reactivity enables protein modification at low concentration.
Bioorthogonal reactions— unnatural transformations that are unaffected by biological functionality— are broadly useful tools with applications that span synthesis, chemical biology, and materials science.1 The utility of bioorthogonal reactivity has been augmented by recent developments in post-translational strategies for incorporating bioorthogonal functionality into proteins.2 Bioorthogonal reactions must be exceptionally fast in order to be useful at the low concentrations relevant to many biological applications. Recently, Bertozzi and coworkers described strain-driven click reactions that take advantage of the intrinsic reactivity of cyclooctyne toward organic azides,3,4 and work by Bertozzi4a–d and by Boons4e has shown that click reactions of cyclooctyne derivatives are fast (up to k2 2.3 M−1s−1).4 Importantly, this method avoids Cu-catalysts, which are cytotoxic, and the enhanced reactivity enables applications for dynamic in vivo imaging.4 Recently, Lin and coworkers elegantly described bioconjugation based on a photoinducible 1,3-dipolar cycloaddition reaction that proceeds with fast rates (k2 11 M−1s−1) with acrylamide.5 Faster ligation chemistry will allow for the assembly of complex biomaterials under dilute conditions. Ultimately, fast bioconjugation reactions should facilitate the intracellular assembly of molecular structures that are too large to cross cell membranes.
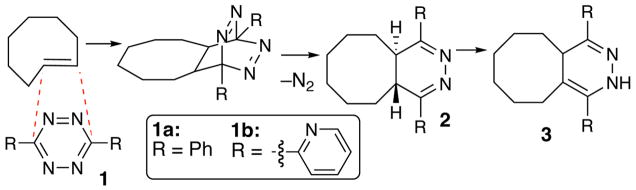
Unlike normal-electron-demand Diels-Alder chemistry,6 inverse-electron demand Diels-Alder reactions have not previously been applied to bioconjugation. Tetrazines are voracious dienes for inverse-electron-demand Diels-Alder reactions, and N2 is produced as the only byproduct upon subsequent retro-[4+2] cycloaddition.7 In 1990, Sauer described the kinetics of electron deficient tetrazines (Scheme 1, structure 1, where R=CO2Me or CF3) with a number of dienophiles, and quantitatively demonstrated that their reactions with strained alkenes are exceptionally fast.8 The most reactive dienophile is trans-cyclooctene, which is 7 orders of magnitude more reactive than cis-cyclooctene toward these tetrazines.8 In protic solvents, the 4,5-dihydropyridizine 2 rapidly rearranges to isomeric 3.
Scheme 1.
Diels-Alder reactions of tetrazines with trans-cyclooctene
We surmised that such fast and selective reactivity could form the basis of a powerful bioorthogonal reaction. However, Sauer’s conditions could not be directly applied to bioconjugation, as the tetrazines that he studied immediately react with water.9 From a survey of substituted s-tetrazines, 3,6-diaryl-s-tetrazines were identified as suitable derivatives for bioorthogonal reactivity.10
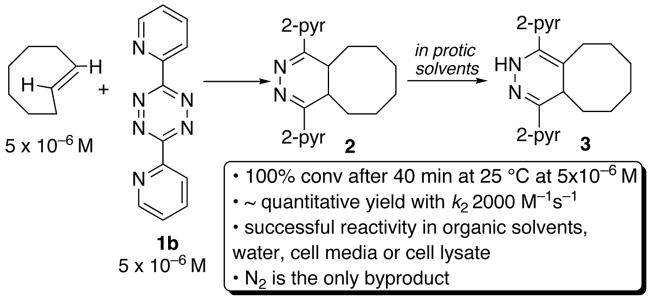
As a model study, it was shown that 1b and trans-cyclooctene combine to give 3 in quantitative yield after epimerization (Scheme 2). In separate experiments, EtSH (100 mM) and BuNH2 (100 mM) were introduced to trans-cyclooctene (120 mM) before combination with 1b (100 mM), but these had no effect on the efficiency of the Diels-Alder reaction. As more stringent tests of tolerance of biological functionality, it was shown that the reaction of 1b and trans-cyclooctene can be carried out in cell media (DMEM +5% FBS) or in an aqueous solution containing 10% untreated rabbit reticulocyte lysate. The reactions were carried out at rt for 1h with 50 μM 1b and 500 μM trans-cyclooctene, and monitored by ESI-MS. The yields in cell lysate and media were estimated to be >80% (vs internal MS standards).
Scheme 2.
Fast reactivity at low micromolar concentrations
The second order rate constant for 1b + trans-cyclooctene at 25 °C is k2 2000 (+/− 400) M−1s−1 in 9:1 methanol: water. As anticipated,10 there is a hydrophobic effect for the Diels-Alder reaction: slower rates were observed for reactions in pure methanol [k2 1140 (+/− 40) M−1s−1] and in THF [k2 400 (+/− 20) M−1s−1]. The reaction to form 2 is complete within 40 min at 25 °C at 5 μM in THF without using an excess of either 1b or trans-cyclooctene. The half-life is 7 s when 1b (20 μM) is reacted with excess trans-cyclooctene (200 μM) in 9:1 methanol:water at 25 °C. The reaction between 1b and trans-cyclooctene is much faster than background reactivity toward water or exogenous nucleophiles.9,11 Further, 1a also displays fast reactivity toward trans-cyclooctene [k2 3.1 (+/− 0.1) M−1s−1 in THF] and does not display background reactivity toward BuSH or BuNH2 after 8 h at rt.11,12
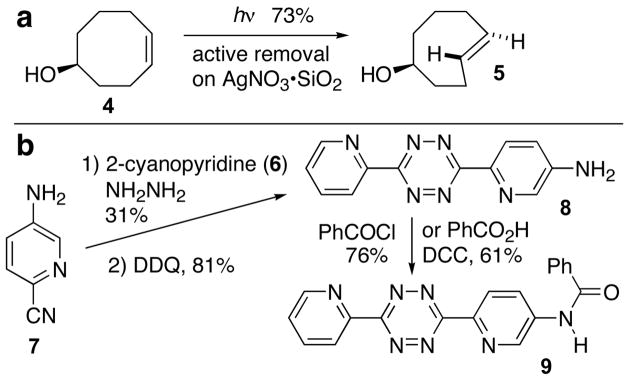
The practicality of the tetrazine ligation is augmented by facile access to starting materials. trans-Cyclooctene derivative 5 is readily accessible from cis-cyclooctene 4 by a photochemical protocol that we described recently (Scheme 3a).13 Functionalized analogs of 1a are known.14 Unsymmetrical tetrazine 8 can be prepared in gram quantities from the reaction of hydrazine with commercially available 5-amino-2-cyanopyridine (7) and 2-cyanopyridine (6) (Scheme 3b). The amino group of tetrazine 8 provides a handle for functionalization via acyl transfer (e.g. 9).15
Scheme 3.
Synthesis of trans-cyclooctene and tetrazine derivatives
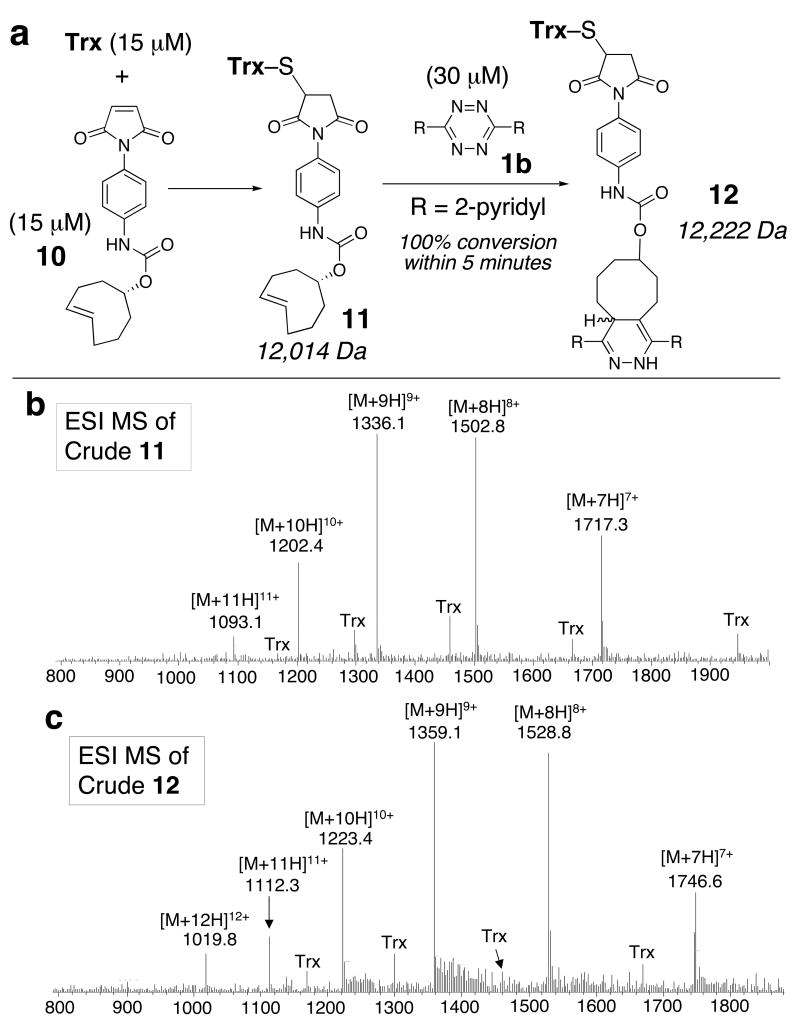
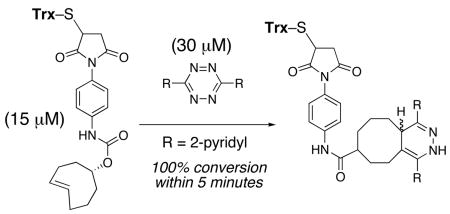
To illustrate compatibility of the tetrazine ligation with proteins, we functionalized thioredoxin (Trx) with trans-cyclooctene derivative 10. Trx is a 11.7 kDa protein that contains a single disulfide. Upon reduction, the solvent exposed cysteine can be selectively functionalized by maleimides.16 Thus, Trx (15 μM) was reduced with tri(3-hydroxypropyl)phosphine (THP, 1 mM), and combined with 10 (15 μM) in acetate buffer (pH 6). ESI mass spectral analysis (Fig 1b) indicated that most of the Trx had been consumed and that the conjugate 11 had formed. Subsequent combination of 11 with 1b (30 μM) indicated that the formation of 12 was complete within 5 min. A control experiment with the cis-cyclooctene analog of 10 gave the analog of 11, but reaction with 1b did not give the analog of 12 even after 24 h. HPLC was also used to monitor the bioconjugation reactions that gave 11 and 12, and to demonstrate that the tetrazine ligation to form 12 was fast and high yielding (see Supporting Information).
Figure 1.
(a) Rapid reactivity to form 12 was monitored by ESI-MS and HPLC. (b,c) Crude ESI-MS data for 11 and 12 in experiments that began with 15 μM Trx.
In summary, a new method for bioconjugation based on inverse-electron demand Diels-Alder chemistry has been described. The reaction proceeds with very fast rates and tolerates a broad range of biological functionality.
Supplementary Material
Experiments in which HPLC was used to monitor bioconjugation reactions are described. Full experimental details and 1H, 13C NMR spectra are provided. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
This work was supported by NIH grant GM068640-01. We thank Colin Thorpe for donating Trx, and Danny Ramadan for HPLC assistance. We thank Ryan Mehl for insight into the aqueous stability of 1b. We thank Colin Thorpe and John Koh for insightful discussions.
References
- 1.(a) Kolb HC, Finn MG, Sharpless KB. Angew Chem Int Ed. 2001;40:2004. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]; (b) Kohn M, Breinbauer R. Angew Chem Int Ed. 2004;43:3106. doi: 10.1002/anie.200401744. [DOI] [PubMed] [Google Scholar]; (c) Chen I, Ting AY. Curr Opin Biotech. 2005;16:35. doi: 10.1016/j.copbio.2004.12.003. [DOI] [PubMed] [Google Scholar]; (d) Antos JM, Francis MB. Curr Opin Chem Biol. 2006;10:253. doi: 10.1016/j.cbpa.2006.04.009. [DOI] [PubMed] [Google Scholar]; (e) Hahn ME, Muir TW. Trends Biochem Sci. 2005;30:26. doi: 10.1016/j.tibs.2004.10.010. [DOI] [PubMed] [Google Scholar]; (f) Soellner MB, Nilsson BL, Raines RT. J Am Chem Soc. 2006;128:8820. doi: 10.1021/ja060484k. [DOI] [PubMed] [Google Scholar]; (g) Lin FL, Hoyt HM, van Halbeek H, Bergman RG, Bertozzi CR. J Am Chem Soc. 2005;127:2686. doi: 10.1021/ja044461m. [DOI] [PubMed] [Google Scholar]; (h) Kolakowski RV, Shangguan N, Sauers RR, Williams LJ. J Am Chem Soc. 2006;128:5695. doi: 10.1021/ja057533y. [DOI] [PubMed] [Google Scholar]
- 2.Recent examples: Duckworth BP, Xu JH, Taton TA, Guo A, Distefano MD. Bioconj Chem. 2006;17:967. doi: 10.1021/bc060125e.Gauchet C, Labadie GR, Poulter CD. J Am Chem Soc. 2006;128:9274. doi: 10.1021/ja061131o.Esser-Kahn AP, Francis MB. Angew Chem Int Ed. 2008;47:3751. doi: 10.1002/anie.200705564.
- 3.Wittig G, Krebs A. Chem Ber. 1961;94:3260. [Google Scholar]
- 4.(a) Agard NJ, Prescher JA, Bertozzi CR. J Am Chem Soc. 2004;126:15046. doi: 10.1021/ja044996f. [DOI] [PubMed] [Google Scholar]; (b) Baskin JM, Prescher JA, Laughlin ST, Agard NJ, Chang PV, Miller IA, Lo A, Codelli JA, Bertozzi CR. Proc Natl Acad Sci USA. 2007;104:16793. doi: 10.1073/pnas.0707090104. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Codelli JA, Baskin JM, Agard NJ, Bertozzi CR. J Am Chem Soc. 2008;130:11486. doi: 10.1021/ja803086r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Sletten EM, Bertozzi CR. Org Lett. 2008;10:3097. doi: 10.1021/ol801141k. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Ning XH, Guo J, Wolfert MA, Boons GJ. Angew Chem, Int Ed. 2008;47:2253. doi: 10.1002/anie.200705456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song W, Wang Y, Qu J, Madden MM, Lin Q. Angew Chem Int Ed. 2008;47:2832. doi: 10.1002/anie.200705805. [DOI] [PubMed] [Google Scholar]; (b) Song W, Wang Y, Qu J, Lin Q. J Am Chem Soc. 2008;130:9654. doi: 10.1021/ja803598e. [DOI] [PubMed] [Google Scholar]
- 6.(a) Dantas de Araujo A, Palomo JM, Cramer J, Seitz O, Alexandrov K, Waldmann H. Angew Chem, Int Ed. 2006;45:296. doi: 10.1002/anie.200502266. [DOI] [PubMed] [Google Scholar]; (b) Latham-Timmons HA, Wolter A, Roach JS. Nucleosides, Nucleotides Nucleic Acids. 2003;22:1495. doi: 10.1081/NCN-120023019. [DOI] [PubMed] [Google Scholar]; (c) Yousaf MN, Houseman BT, Mrksich M. Proc Natl Acad Sci USA. 2001;98:5992. doi: 10.1073/pnas.101112898. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Yousaf MN, Mrksich M. J Am Chem Soc. 1999;121:4286. [Google Scholar]; (e) Seelig B, Jaschke A. Tetrahedron Lett. 1997;38:7729. [Google Scholar]
- 7.Boger DL. Chem Rev. 1986;86:781. [Google Scholar]
- 8.Thalhammer F, Wallfahrer U, Sauer J. Tetrahedron Lett. 1990;31:6851. [Google Scholar]
- 9.Hunter D, Nielson DG, Tweakley T., Jr J Chem Soc Perkin Trans I. 1985:2709. and references therein. [Google Scholar]
- 10.For Diels-Alder reactions of styrene and 1b in water: Wijnen JW, Zavarise S, Engberts JBFN, Charton M. J Org Chem. 1996;61:2001.
- 11.No reaction was observed by 1H NMR after 8 h when 1a (2×10−2 M) was combined with BuNH2 (2×10−2 M) or EtSH (2×10−2 M) in CD3OD. When a CD3OD solution of 1b (2×10−2 M) was combined with BuNH2 (2×10−2 M), 50% of 1b had reacted after 4 h. In a similar experiment with EtSH (2×10−2 M), 50% of 1b had reacted after 10 min. When 1b (2×10−2 M) was allowed to stir in pure water, 20% decomposition of 1b was noted after 2 h.
- 12.The product from trans-cyclooctene and 1a aromatizes upon workup. see Padwa A, Rodriguez A, Tohidi M, Fukunaga T. J Am Chem Soc. 1983;105:933.
- 13.Royzen M, Yap GPA, Fox JM. J Am Chem Soc. 2008;130:3760. doi: 10.1021/ja8001919. [DOI] [PubMed] [Google Scholar]
- 14.Soloducho J, Doskocz J, Cabaj J, Roszak S. Tetrahedron. 2003;59:4761. [Google Scholar]
- 15.Acyl transfer to 8 was most effective when aromatic acylating agents were employed. When aliphatic acylating agents were used, undesired reactivity (presumably via ketene formation) competes with the rate of acylation. It is likely that the rate of acyl transfer to 8 is slow because it is a very electron-deficient aniline. Sugars and peptides can be appended to aromatic acylating agents via terephthaloyl linkers, e.g.: Angelastro MR, Baugh LE, Bey P, Burkhart JP, Chen TM, Durham SL, Hare CM, Huber EW, Janusz MJ, Koehl JR, Marquart AL, Mehdi S, Peet NP. J Med Chem. 1994;37:4538. doi: 10.1021/jm00052a013.Kanie O, Grotenbreg G, Wong C-H. Angew Chem Int Ed. 2000;39:4545.Czifrák K, Hadady Z, Docsa T, Gergely P, Schmidt J, Wessjohann L, Somsák L. Carbohydr Res. 2006;341:947. doi: 10.1016/j.carres.2006.03.002.
- 16.Kallis G-B, Holmgren A. J Biol Chem. 1980;255:10261. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experiments in which HPLC was used to monitor bioconjugation reactions are described. Full experimental details and 1H, 13C NMR spectra are provided. This material is available free of charge via the Internet at http://pubs.acs.org.