Abstract
The 5-HT re-uptake inhibitor (SSRI) fluoxetine and the adrenal hormone dehydroepiandrosterone (DHEA) both increase the proliferation of progenitor cells in the adult hippocampus and also have antidepressant activity. This paper explores the combined ability of fluoxetine and DHEA to affect this process in the dentate gyrus of adult rats. We show that DHEA can render an otherwise ineffective dose of fluoxetine (2.5 mg/kg) able to increase progenitor cell proliferation to the same extent as doses four times higher (10 mg/kg). This synergistic action does not appear to be mediated by alterations in brain-derived neurotrophic factor (BDNF) gene expression; or by TrkB, mineralocorticoid, glucocorticoid, or 5-HT (5HT1A) receptor expression in the dentate gyrus; or by altered levels of plasma corticosterone. In a second experiment, the synergism between DHEA and fluoxetine was replicated. Furthermore, flattening the diurnal rhythm of plasma corticosterone by implanting additional corticosterone pellets s.c. prevented the effect of fluoxetine on progenitor cell division. This was not overcome by simultaneous treatment with DHEA, despite the latter’s reported anti-glucocorticoid actions. The cellular mechanism for the potentiating action of DHEA on the pro- proliferative effects of fluoxetine in the adult hippocampus remains to be revealed. Since altered neurogenesis has been linked to the onset or recovery from depression, one consequence of these results is to suggest DHEA as a useful adjunct therapy for depression.
Keywords: neurogenesis, fluoxetine, dehydroepiandrosterone, corticosterone, synergism, dentate gyrus
The generation of neurons in the adult hippocampus from progenitor cells is now well established in several mammalian species, though its functional significance is still debated (Christie and Cameron, 2006; Duman et al., 2001; Jagasia et al., 2006). One of its striking features is its lability. This is due to the sensitivity of neurogenesis to a wide range of regulatory factors, among which steroid hormones and central neural 5-HT are among the most potent. Glucocorticoids (GRs) have both a primary regulatory role and a permissive one for some other controls (Wong and Herbert, 2006). Raised levels—such as those observed after a severe stress—reduce the proliferation rate of the progenitor cells located in the innermost layer of the dentate gyrus, whereas reducing endogenous corticoids by adrenalectomy has the converse effect (Wong and Herbert, 2004). Flattening the marked diurnal rhythm characteristic of corticoids (corticosterone (CORT) in the rat) itself has little effect, but prevents the action of at least two other controlling agents: drugs that either act on 5-HT (such as the SSRI fluoxetine (FLX)) or those that inhibit nitric oxide synthase (NOS) that otherwise stimulate progenitor cell proliferation (Huang and Herbert, 2006; Pinnock et al., 2007). So corticoids are not only a major determinant of variations in hippocampal neurogenesis, they also interact with other control systems.
Dehydroepiandrosterone (DHEA), another steroid derived from the adrenal, can also regulate neurogenesis. The blood of rats contains hardly any DHEA, but in humans levels of this steroid (and its sulfated derivative) are higher than any other (Schlinger et al., 2008). Giving rats DHEA, at doses that mimic blood levels in young humans, stimulated progenitor cell division, and racts the suppressive action of excess CORT (Karishma and Herbert, 2002). Whether DHEA is also sensitive to an intact CORT rhythm is not known. Neither is there any information on whether DHEA interacts with the serotonergic system or pharmacological agents which target this system such as FLX. Plasma DHEA has been reported to be lower in adult depression (Michael et al., 2000).
FLX is a potent inhibitor of the 5-HT transporter (5-HTT, SERT; Ki ~ 1 nM) (Fazzino et al., 2008). However, stimulation of progenitor cell activity only appears at 14 days of treatment (Encinas et al., 2006; Huang and Herbert, 2006; Malberg et al., 2000). The mechanism for this latency is not understood, but has aroused considerable interest since it corresponds to a similar interval preceding clinical response to such anti-depressants in the treatment of major depression (Wong and Licinio, 2001). 5HT1A receptors seem to be implicated in the action of FLX on neurogenesis (Duman et al., 2001; Holick et al., 2007; Santarelli et al., 2003). It is absent in 5HT1A knockout mice, blocked by 5HT1A antagonists (such as WAY 100635), and replicated by 5HT1A agonists (such as 8-OH-DPAT) (Huang and Herbert, 2005).
Abolishing the diurnal CORT rhythm without changing daily mean levels attenuates 5HT1A autoreceptor function, though whether this underlies the permissive role of this rhythm on the stimulating action of FLX on neurogenesis is not known. There are some reports that additional CORT reduces hippocampal 5HT1A receptor mRNA, but these are not unanimous (Glatz et al., 2003). One attempt to reconcile these results has suggested posttranslational control of 5HT1A expression by GRs (Flugge et al., 1998). CORT may inhibit the expression of tryptophan hydroxylase, the rate limiting enzyme in 5HT synthesis (Azmitia and McEwen, 1969). However, antagonizing 5HT1A receptors does not prevent increased progenitor cell division following adrenalectomy, so not all the actions of GRs seem dependent on altered 5HT1A activity (Huang and Herbert, 2005). FLX itself has an inconsistent effect on blood CORT levels (Weber et al., 2006). Diurnal rhythms in cortisol are flattened during a substantial number of depressive episodes (Keller et al., 2006).
A number of growth factors have been shown to regulate hippocampal neurogenesis (Chen et al., 2007). BDNF has been implicated in neuronal survival, morphological and synaptic plasticity during development (Thomas and Davies, 2005). Not surprisingly, therefore it has also been implicated in adult neurogenesis. Intracerebral BDNF infusions increase proliferation of progenitor cells (Pinnock and Herbert, 2008; Scharfman et al., 2005). FLX increases BDNF mRNA, and its effects on neurogenesis are attenuated in BDNF+/- mice (Guiard et al., 2008). Various regimens of stress reduce BDNF mRNA expression, as they do neurogenesis (Ivy et al., 2003; Jacobsen and Mork, 2006). Whether or not DHEA alters BNDF, or synergizes with FLX on the expression of BDNF has not been studied. Since both FLX and DHEA have been shown to be effective antidepressants (Schmidt et al., 2005), and polymorphisms in the BDNF gene increase the risk of depression (Martinowich et al., 2007), this interaction has both clinical as well as experimental interest.
In this paper we explore the interaction between FLX and DHEA on the proliferation rate of progenitor cells in the dentate gyrus of the adult male rat. We show that DHEA can render an otherwise inactive dose of FLX highly effective on progenitor cell activity, and we explore whether this is related to concomitant alterations in BDNF, TrkB, mineralocorticoid (MR), GR and 5HT1A receptor mRNA expression in the dentate gyrus. In a second experiment, we confirm the synergism between DHEA and FLX, and show that DHEA does not overcome the need for an intact diurnal CORT rhythm if FLX is to stimulate progenitor cell division.
EXPERIMENTAL PROCEDURES
Animals
All procedures were carried out in accordance with the regulations set by the UK home office Animal Scientific Procedures Act (1986). This corresponds to international guidelines, and ensures minimal animal use and suffering. Adult male Sprague-Dawley rats, aged c. 8 weeks and weighing between 250 g to 300 g, were used. They were kept in groups of five/cage under 12-h light/dark reversed lighting (lights off 10:00 h). Food and water were available ad libitum.
Experimental design
Experiment 1
There were eight groups (n=5 per group). Half the groups were implanted with a 100 mg pellet of DHEA s.c., the other half with cholesterol (control). One group from each half was given 0, 2.5, 5 or 10 mg/kg/day FLX for 14 days, delivered from an osmotic minipump (Alzet, Cupertino, CA) implanted s.c. under isoflurane/NO anesthesia.
Experiment 2
There were two groups (n=10 per group). The first group was implanted with cholesterol pellets, the second group 100 mg CORT pellets. After 14 days half of each group was killed at 10:00 h and the other half at 16:00 h (CT12 and CT18); blood samples were taken and plasma CORT levels were assayed.
Experiment 3
There were four groups (n=5 per group). All were implanted with an osmotic minipump under isoflurane/NO anesthesia delivering 2.5 mg/kg/day FLX for 14 days. Half were also implanted s.c. with a 100 mg pellet of CORT (Innovative Research of America, Florida, USA) to flatten the diurnal CORT rhythm, the rest with a cholesterol pellet (control). One group from each half received a DHEA pellet as above, the others a second control pellet.
All animals were terminally anesthetized with i.p. pentobarbitone after 14 days, a heparinized sample of blood was taken from the heart within 4 min of the injection, and the brains were removed and kept frozen at -70 °C. The blood was centrifuged and the plasma stored at -20 °C until assay.
Brain samples
Brains were sectioned in the coronal plane at 20 μm in a standard manner. The brain from each rat was sampled at level -2.80 behind the bregma to -4.52 .A standardized counting area which contains 20 μm thick coronal sections in a one-in-six serious of sections representing the dorsal hippocampus was used. For each rat 12 sections were analyzed 720 μm apart. All positive Ki67 cells within the SGZ of the dentate gyrus were quantified. The number of immunopositive cells was determined from the values obtained from each DG in the 12 brain sections. The mean number of immunopositive cells in the 12 sections of each rat was taken as n=1. The number of immunopositive cells is expressed as the mean±S.E. for each treatments (number of rats/group=5). Sections were mounted onto mRNA-free polylysine-coated glass slides (BDH, Leicestershire, UK), left in a fume hood to dry overnight and stored at -70 °C. Before staining they were immersed in 4% paraformaldehyde for 5 min, followed by 2×5 min washes in KPBS.
Ki67 immunohistochemistry
Sections were incubated in 0.01 M citric acid for 40 min at 98 °C in a waterbath. They were cooled to room temperature for 15 min before 2×5 min washes in KPBS. Endogenous peroxidase activity was quenched with 3% H2O2 solution for 10 min followed by 2×5 min KPBS washes. Five hundred microliters of primary antibody mixture containing 1:100 mouse monoclonal IgG anti-human Ki67 (Novocastra, Newcastle upon Tyne, UK) was applied to each slide and left overnight in a humidified chamber at room temperature in darkness, followed by 2×5 min KPBS wash. Then, 500 μl of secondary antibody mixture containing 1:200 biotinylated mouse IgG (Vector Laboratories Ltd., Peterborough, UK) was applied to each slide and incubated in humidified chamber at room temperature for 1 h, followed by 2×5 min KPBS wash. Five hundred microliters of avidin-biotin-peroxidase reagent (Vector Laboratories Ltd.) was applied to each slide and incubated for 1 h in a humidified chamber at room temperature, followed by 2×5 min KPBS wash. With lights off, 3,3-diaminobenzidine (DAB) solution was applied and incubated for 5 min. Excess DAB was removed by dipping in double-distilled water and washing in KPBS for 30 min. Slides were counterstained by being immersed in 10% Cresyl Violet solution followed by step dehydration through double-distilled water, 70%, 95%, 100% ethanol and Histoclear solution. Finally, they were coverslipped with DPX for light microscopy at ×40 magnification.
In situ hybridization
Sections were allowed to air dry at room temperature and were then fixed with 4% paraformaldehyde (Sigma, Dorset, UK) for 5 min, washed in PBS and then dehydrated in 70% ethanol and 95% ethanol for 5 min before finally storing in fresh 95% ethanol. In situ hybridization was carried out under RNAase-free conditions. The accuracy of synthetic antisense oligonucleotide probes was confirmed by BLAST searches on NCBI. 3′ Synthetic antisense oligonucleotide probes were used for the following genes: BDNF (5′ AGT TCC AGT GCC TTT TGT CAT GCC CCT GCA GCT TCC TTC GTG TAA CCC 3′), TrkB (5′ GAG AGG GCT GGC AGA GTC ATC GTC GTT GCT GAT GAC GGA AGC TGG 3′) (Al-Majed et al., 2000), MR (5′ TTC GGA ATA GCA CCG GAA ACG CAG CTG ACG TTG ACA ATC T 3′) (van Riel et al., 2003), GR (5′ AGG AGA ATC CTC TGC TGC TTG GAA TCT GCC TGA 3′)(McQuade et al., 2004), 5HT1A (5′ GGT TAG CGT GGG AGG AAG GGA GAC TAG CTG TCT GAG CGA CAT ACA AG 3′), and 5HTT (5′ ACT GCA GAG TAC CCA TTG GAT ATT TGG CTA GGC TCT GCC CTG TCC GCT GT 3′) (van Riel et al., 2003). All probes were end-labeled with 35S-ATP as follows: 2 μl of purified oligonucleotide (5 ng/μl) was added to 1.25 μl buffer and 1.25 μl cobalt chloride (New England Biosystem, UK). DEPC-treated water (6.5 μl) was added, followed by 1 μl terminal 35S deoxyadenosine 5′ (α-thio) triphosphate (10 mCi/ml) (Amersham, Buckinghamshire, UK) and 0.5 μl (15-20 U) terminal deoxynucleotide transferase enzyme (New England Biosystem). Probes were incubated at 37 °C for 1 h before 40 μl of DEPC was added to terminate the reaction. Purification of labeled probe from unincorporated nucleotides was accomplished by centrifugation (3000 rpm for 2 min) through a G-50 sephadex micro-column (Amersham). Probes were evaluated for incorporation of radiolabel by scintillation counting. All hybridizations were carried out at 2500-5000 cpm/μl in hybridization buffer (50% deionized formamide, 4× saline sodium citrate (SSC), 5× Denhardt’s, 100 μg/ml polyadenylic (potassium salt) acid, 200 μg/ml salmon sperm DNA, 120 μg/ml heparin (BDH), 25 mM sodium phosphate pH 7.0,1 mM sodium pyrophosphate, 10% (w/v) dextran sulfate in DEPC-treated water) (all Sigma). Sections were covered with parafilm and hybridized overnight at 44 °C in a humid atmosphere. Excess unbound probe was removed using the following washes: slides were rinsed in 1× SSC (Sigma) at room temperature, washed twice for 30 min at 55 °C with 1× SSC and then rinsed at room temperature for 2 min, each in 1× SSC, 0.1× SSC, 18 Ω water, 50%, 70%, and 95% ethanol (BDH). Sections were thoroughly air-dried at room temperature before exposure to autoradiographic X-ray film (Amersham) for 14 days for BDNF, and 6 days for 5HT1A. Sense probes for each of the mRNAs were run as negative controls.
Steroid assays
Plasma CORT concentrations and DHEA were measured by radioimmunoassay according to a validated procedure described previously (Chen and Herbert, 1995). For experiment 1, the intra-assay coefficient of variation (CV) for CORT was 3.34% and, for DHEA 4.72%; the sensitivity of the assay was 0.98 ng/mL and 0.1 ng/mL, respectively. For experiment 2, the CV for CORT was 5.5%. For experiment 2, the CV was 4.63% for CORT and 3.84% for DHEA.
Quantification and statistical analysis
Proliferating cells
All slides were randomized and coded prior to quantitative analysis. Sections were examined using a 40× objective. Ki67-labeled stained cells were counted under experimentally ‘blind’ conditions. Only cells on the internal border of the subgranular zone were included. The data shown are the mean count per section obtained from 12 sections per animal.
For quantification of mRNA, the sections and C14-labeled standards of known radioactivity (Amersham) were placed in X-ray cassettes and then exposed to autoradiographic film. The optical density (OD) of the autoradiographic images was measured using a computerized PC-based image analysis system (NIH Image). ODs from the dentate gyrus from three consecutive sections per rat (between Bregma -3.14 mm and -3.30 mm) were obtained and averaged together. The mean value for each rat was entered into the equation derived from the C14 standards and the final value was used to calculate group means. Sections from all groups were hybridized at the same time to avoid intrinsic variations between different in situ hybridizations.
Between-group one/two-way analysis of variance (ANOVA) and Bonferroni’s post hoc test were used when applicable. Log transformation was used to ensure homogeneity of variance before ANOVA when appropriate. Results were considered statistically significant if P<0.05.
RESULTS
Experiment 1: The effect of DHEA on the response of progenitor cells in the dentate gyrus to graded doses of FLX
Plasma steroids
Treatment with DHEA raised plasma levels from less than 1 ng/mL to levels within the range found in young humans (Table 1). There was no difference between the four DHEA-treated groups (F(3,16)=1.6, P=0.232). Neither FLX nor DHEA had any effect on plasma CORT (F(3,28)=1.8 and F(1,28)=0.03, both ns; Table 1).
Table 1.
Plasma CORT and DHEA concentrations for experiment 1
| Hormone | Treatment | FLX (mg/kg/day) |
|||
|---|---|---|---|---|---|
| 0 | 2.5 | 5 | 10 | ||
| CORT | No DHEA | 209.2 (138.2) | 226.0 (53.2) | 145.8 (80.1) | 149.2 (29.9) |
| DHEA | 169.8 (54.8) | 214.8 (77.9) | 228.2 (63.8) | 134.6 (67.4) | |
| DHEA | No DHEA | 0.4 (0.1) | 0.4 (0.1) | 0.7 (0.6) | 0.3 (0.1) |
| DHEA | 31.1 (8.6) | 24.0 (1.6) | 22.5 (6.6) | 21.8 (6.4) | |
There were no significant main or interaction effects of DHEA and FLX on CORT. Plasma DHEA levels were much higher in rats treated with s.c. DHEA, and the levels were similar across FLX groups. Values are mean ng/ml (S.D.).
Ki67
Labeled cells are found in the innermost layer of the dentate gyrus (Fig. 1). In the absence of DHEA, FLX increased the number of Ki67 labeled cells to 280% of control values only at 10 mg/kg/day. Lower doses of FLX (2.5 and 5.0 mg/kg/day) had no effect (Fig. 2). Adding DHEA markedly shifted the dose response to FLX to the left; 2.5 mg/kg/day now increased Ki67 staining to the extent seen in control rats given 10 mg/kg/day (Fig. 2). There was no further increase in rats treated with both DHEA and higher doses of FLX. DHEA by itself had no effect (pairwise comparison: Bonferroni P>0.99). These results were confirmed by a two-way ANOVA: there was a significant FLX×DHEA interaction (F(3,32)=98.5, P<0.001) and main effects of both FLX (F(3,32)=242.5, P<0.001) and DHEA (F(3,32) 348.5, P<0.001). Pairwise comparisons within the groups not treated with DHEA showed no difference between those receiving 0, 2.5 or 5 mg/kg/day FLX, but a highly significant increase following 10 mg/kg/day (Bonferroni: P<0.001). In those treated with DHEA, there was a highly significant difference between the groups receiving 0 and 2.5 mg/kg/day FLX, (P<0.001) but no difference thereafter at higher doses.
Fig. 1.
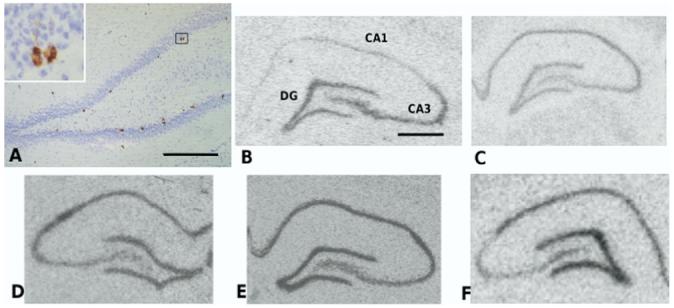
(A) Ki67 labeling in the inner layer of the dentate gyrus of the adult male rat (inset: high-power view of a characteristic group of labeled cells) (B) BDNF (C) trkB (D) GR receptor (E) MR receptor and (F) 5HT1A receptor mRNAs in the hippocampus (in situ hybridization). Scale bar=0.5 mm (A); 1 mm (B).
Fig. 2.
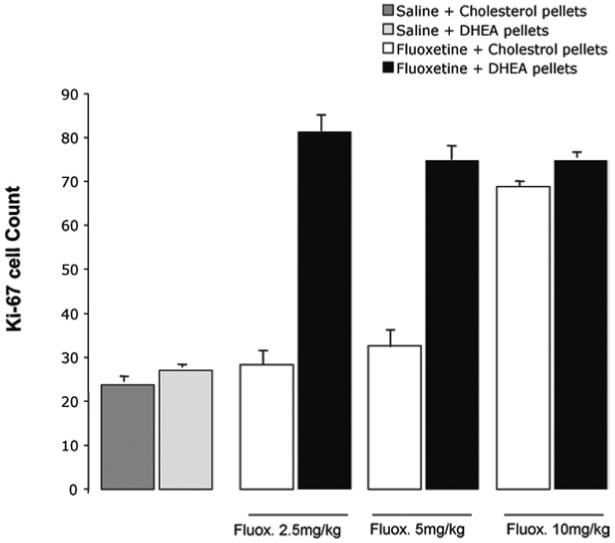
Synergistic effect of DHEA and FLX on proliferation of hippocampal neural progenitor cells. In the absence of DHEA, only 10 mg/kg of FLX increased the number of Ki67 cells in the subgranular zone of the dentate gyrus. In the presence of DHEA however, 2.5 mg/kg of FLX was sufficient to cause a similar increase in the number of Ki67 cells (DHEA×FLX interaction, P<0.001).
Gene expression
Neither FLX nor DHEA had any effect on 5HT1A receptor mRNA, nor was there any interaction between the two (Table 2). There were also no effects of either FLX or DHEA on the expression of either MR or GR mRNA in the dentate gyrus (Table 2). In addition, there were no significant effects of FLX or DHEA on BDNF or TrkB mRNA expression in the dentate gyrus (Table 2). It is noteworthy that BDNF mRNA is particularly prominent in the dentate gyrus and CA3 field of the hippocampal pyramidal cell layer, sites of active neurogenesis and synaptogenesis respectively (Fig. 1).
Table 2.
Gene expression in the dentate gyrus
| Gene | Treatment | FLX (mg/kg/day) |
|||
|---|---|---|---|---|---|
| 0 | 2.5 | 5 | 10 | ||
| 5HT1A | No DHEA | 51.8 (9.7) | 65.0 (12.5) | 53.9 (6.2) | 56.3 (7.4) |
| DHEA | 51.9 (9.30) | 53.3 (8.9) | 60.0 (11.7) | 63.2 (8.1) | |
| MR | No DHEA | 106.9 (13.8) | 101.1 (8.5) | 88.4 (7.9) | 112.4 (9.5) |
| DHEA | 106.6 (13.9) | 96.8 (8.5) | 97.1 (22.3) | 116.3 (7.2) | |
| GR | No DHEA | 28.2 (1.0) | 29.5 (1.4) | 29.2 (2.9) | 28.6 (2.9) |
| DHEA | 29.5 (1.4) | 25.6 (2.8) | 29.2 (2.1) | 29.7 (2.0) | |
| TrkB | No DHEA | 32.6 (1.1) | 33.0 (2.2) | 32.3 (2.6) | 32.7 (1.5) |
| DHEA | 33.5 (1.4) | 31.7 (1.7) | 32.0 (2.0) | 32.6 (2.0) | |
| BDNF | No DHEA | 24.0 (1.7) | 24.2 (2.8) | 22.3 (2.7) | 25.3 (2.1) |
| DHEA | 22.5 (1.3) | 22.2 (1.1) | 24.1 (1.6) | 24.4 (2.7) | |
There were no significant main or interaction effects of DHEA or FLX. Values are mean pixels (S.D.).
Experiment 2: The effect of 100 mg CORT pellets on the daily CORT rhythm in adrenal-intact rats
Fig. 3 shows a clear diurnal rhythm in plasma CORT in intact rats, even when sampled at a relatively short interval (6 h). This was abolished by the s.c. CORT implant.
Fig. 3.
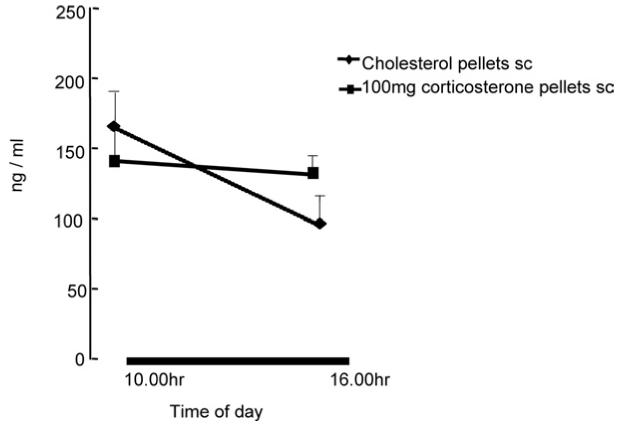
Diunal variation in plasma CORT in intact rats sampled at either 10:00 or 16:00 h (lights off: 10:00 h: L:D 12:12) or in those implanted with a 100 mg CORT pellet.
Experiment 3: Moderation by DHEA of the effect of ‘clamping’ CORT on sensitivity to FLX
Plasma steroids
There was a significant interaction between CORT and DHEA treatments on plasma CORT at the single time point studied in this experiment (F(1,16)=4.55, P=0.04). DHEA overall had no effect (Table 3).
Table 3.
Plasma CORT for experiment 3
| Treatment | No DHEA | DHEA |
|---|---|---|
| No CORT | 96.0 (38.6) | 148.3 (23.2) |
| CORT | 123.0 (28.1) | 133.4 (39.8) |
There were no significant main or interaction effects of DHEA and CORT. Values are mean ng/ml (S.D.).
Ki67
There was a highly significant interaction between CORT treatment (s.c. pellet) and DHEA (F(1,16)=35.8, P<0.001), as well as significant main effects (CORT: F(1,16)=172.2, P<0.001, DHEA: F(1,16)=100.7, P<0.001. Post hoc tests showed that, as in experiment 1, adding DHEA to a daily dose of 2.5 mg/kg FLX significantly increased the number of Ki67-labeled cells in the dentate gyrus (Bonferroni: P<0.001; Fig. 4). However, this was not observed in rats treated with s.c. CORT (previously shown to flatten the diurnal rhythm; P=0.674). Thus CORT not only decreased progenitor cell activity, it also prevented the synergistic actions of DHEA on FLX (Fig. 5).
Fig. 4.
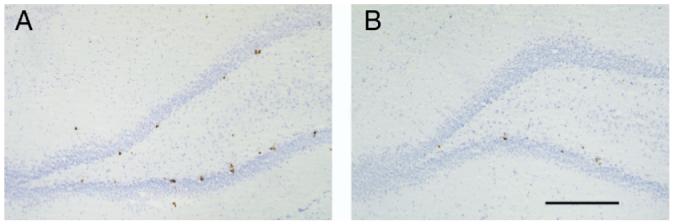
Photomicrograph on Ki67-labeled cells in the dentate gyrus of a rat (A) treated with FLX 2.5 mg/kg/day and a DHEA s.c. pellet or (B) the same FLX and DHEA treatment but in the presence of a flattened diurnal CORT rhythm (s.c. CORT pellet). Scale bar=0.5 mm.
Fig. 5.
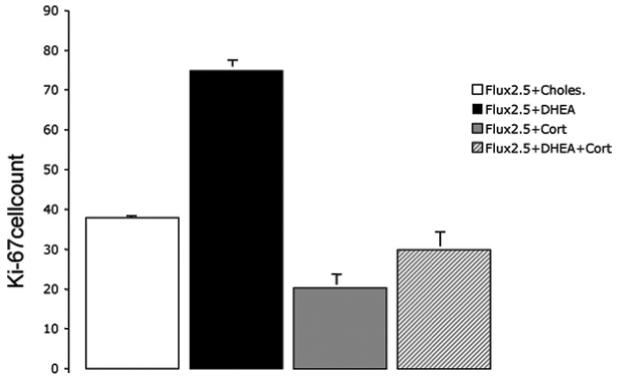
A flattened CORT rhythm inhibits the effect of DHEA and FLX. All rats in this experiment were given 2.5 mg/kg of FLX. Similar to the first experiment, the addition of DHEA (in the absence of CORT) increased the number of Ki67 cells in the subgranular zone compared with the no DHEA control group (Bonferroni: P<0.001). This effect was completely abolished in rats with a flattened CORT rhythm (DHEA×CORT interaction, P<0.001).
DISCUSSION
The primary finding in this paper is that DHEA, in a dose which itself had no discernible effect on the proliferation rate of progenitor cells in the dentate gyrus, markedly sensitizes them to FLX. There are no previous reports of this steroid accentuating a 5-HT-acting drug on proliferation of progenitor cells. Doses of FLX which, in controls, had no effect on proliferation, markedly stimulated it in the presence of DHEA. It should be noted that although the blood levels of DHEA we achieved in treated rats resemble those in young adult humans, this is nevertheless a pharmacological state, since rats ordinarily have hardly any DHEA in their blood (see our control values).
In the first set of experiments described here, DHEA itself failed to induce proliferation, contrasting with the results from (Karishma and Herbert, 2002). This may be due to the higher dose of DHEA used in our previous experiment (200-250 mg pellet) compared with the one used here (100 mg). However the most interesting result in this study is the presence of a synergistic effect on proliferation when both DHEA and FLX were administered together; 2.5 mg/kg and 5 mg/kg FLX on their own failed to stimulate proliferation, but when administered with DHEA, there was a marked stimulation to the extent seen for 10 mg/kg FLX alone. The absence of a further effect of DHEA on the response to 10 mg/kg FLX suggests that the proliferation rate had reached a maximum at this dose (‘ceiling effect’).
Previous reports have shown that FLX treatment (10 mg/kg/day) increased levels of BDNF mRNA (De Foubert et al., 2004; Koponen et al., 2005; Sairanen et al., 2005), though the change we observed in our experiments was not significant. However, it seems unlikely that the synergistic action of DHEA depends on this pathway, since there was no increase in BDNF at lower doses of FLX in the presence of DHEA in the present experiment, despite increased progenitor proliferation. This also suggests the existence of a DHEA-sensitive pathway alternative to BDNF for regulating proliferation. We wondered whether this might involve either the corticoid receptors, or TrkB, the principal BDNF receptor.
The anti-GR actions of DHEA do not seem to depend on interactions with either GR or MR. DHEA does not bind to either receptor and does not interfere with the binding of GRs, though it may slow down the translocation of GR to the nucleus (Saponaro et al., 2007). We found that the combination of DHEA and FLX (at any dose) had no significant effect on the levels of MR or GR mRNA, and we thus cannot account for its synergistic action on FLX through altered corticoid receptor activity. DHEA can bind to the pregnane-X receptor (Ripp et al., 2002) but whether this is implicated in the effects we describe in this paper awaits further investigation. We also found no change in trkB receptor expression, so we cannot ascribe our results to increased sensitivity to BDNF, though this is only one measure of responsiveness. Thus we have no evidence that DHEA sensitizes the progenitor cells to FLX though altering the expression of any of these factors. Since FLX is thought to act on neurogenesis via 5HT1A receptors (Santarelli et al., 2003), DHEA might interact with this pathway to bring about the synergistic effect. But we were unable to find alterations in the expression of 5HT1A receptor mRNA, though our result do not exclude other actions—for example, post-translational changes in this or other receptors.
In humans, DHEA together with its sulfated derivative DHEAS represents one of the most abundant steroid products of the adrenal cortex. A major metabolic pathway involves 7-hydroxylation (Khalil et al., 1993; Morfin and Starka, 2001; Muller et al., 2006; Schmitt et al., 2001) 7α-OH DHEA stimulates progenitor cell activity whereas 7β-OH DHEA does not (Bandpey and Herbert, unpublished observations). It remains uncertain whether or not the synergistic action we report between DHEA and FLX depends on this conversion, or whether it might be altered by FLX. The molecular mechanisms underlying the endocrine actions of DHEA or its derivatives are still unclear. DHEA has been shown to exert multiple effects in the rodent CNS (Baulieu and Robel, 1996) through a variety of mechanisms that include negative modulation of GABAA receptors and a positive effect on N-methyl d-aspartate (NMDA) receptors (Kaasik et al., 2001). It can activate the serine-threonine protein kinase Akt in cultures of embryonic forebrain (Zhang et al., 2002). In addition, DHEA has been reported to increase the plasma concentration of the insulin-like growth factor IGF-1 in rats and in humans (Aberg et al., 2000; Ribeiro and Garcia-Segura, 2002).
The action of DHEA on sigma 1 (σ1) receptor may be particularly relevant. DHEA can act as an agonist at the σ1 receptor (Ueda et al., 2001) and σ1 antagonists blocked the increase in proliferation mediated by DHEA in neural stem cell cultures (Nakata et al., 2003). σ1 Agonists act as antidepressants in animal studies (Matsuno et al., 1996) and σ1 antagonists blocked the antidepressant actions of DHEAS, a sulfated derivative of DHEA (Dhir and Kulkarni, 2008). The action of σ1 agonists bears some resemblance to the interaction between DHEA and FLX. They often have little or no effect on their own but may act as amplifiers of intracellular signaling cascades (Su and Hayashi, 2003). DHEA might bind to σ1 receptors to amplify 5HT1A-dependent signaling to bring about synergism in proliferation. Consistent with this idea, the antidepressant effects of 5HT1A agonist were potentiated by co-administration of a σ1 agonist (Yamada et al., 2000). σ1 Agonists desensitize the 5HT1A autoreceptor in the DRN after only 2 days of treatment (Bermack et al., 2002), whereas antidepressants such as FLX typically do this only after chronic treatment. DHEA may bind to an unidentified plasma membrane receptor to mediate proliferation of endothelial cells via a Gi-mediated MAPK pathway (Liu et al., 2008). If this happens in the hippocampus, it could be related to ERK activation and downregulated PKA activity (Cormaci et al., 2007). 5HT1A activation may therefore increase σ1 receptor expression by both activating the MAPK cascade and inhibiting the cAMP-PKA signaling. This would enhance DHEA-σ1 signaling, which may in turn amplify 5HT1A. These ideas need experimental testing.
Clamping the CORT level and hence abolishing the diurnal corticoid rhythm has been shown to prevent the effect of FLX on the rate of progenitor cell proliferation (Huang and Herbert, 2006). We wondered whether the DHEA-dependent increased sensitivity to FLX might also extend to restoring the effect of this SSRI in CORT-clamped rats. However, we found that DHEA does not overcome the need for an intact diurnal CORT rhythm. There may be interactions between DHEA and corticoid metabolism (Apostolova et al., 2005), but the regulation of neurogenesis exerted by the diurnal corticoid rhythm seems to be independent of DHEA. Successful antidepressant treatment, including that with SSRIs is associated with resolution of impairment in HPA axis negative feedback and normalizing the HPA axis in depressed patients (Heuser et al., 1996; Linkowski et al., 1987; Nickel et al., 2003). Recent reports also suggest limited efficacy of SSRI in patients with abnormal HPA axis function (Young et al., 2004). If the proposed link between neurogenesis in the hippocampus and clinical depression (Jacobs et al., 2000; Kempermann et al., 2008) is sustained by future work, then the results reported here suggest that DHEA might be a useful adjunct to SSRI therapy, and that this might well be modulated by the diurnal corticoid rhythm.
Acknowledgments
Supported by a grant from the Wellcome Trust. We thank Helen Shiers and Sarah Cleary for the corticosterone and DHEA assays.
Abbreviations
- ANOVA
analysis of variance
- CORT
corticosterone
- CV
coefficient of variation
- DAB
3,3-diaminobenzidine
- DHEA
dehydroepiandrosterone
- FLX
fluoxetine
- GR
glucocorticoid
- MR
mineralocorticoid
- OD
optical density
- SSC
saline sodium citrate
REFERENCES
- Aberg MA, Aberg ND, Hedbacker H, Oscarsson J, Eriksson PS. Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. J Neurosci. 2000;20:2896–2903. doi: 10.1523/JNEUROSCI.20-08-02896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Majed AA, Brushart TM, Gordon T. Electrical stimulation accelerates and increases expression of BDNF and trkB mRNA in regenerating rat femoral motoneurons. Eur J Neurosci. 2000;12:4381–4390. [PubMed] [Google Scholar]
- Apostolova G, Schweizer RA, Balazs Z, Kostadinova RM, Odermatt A. Dehydroepiandrosterone inhibits the amplification of glucocorticoid action in adipose tissue. Am J Physiol Endocrinol Metab. 2005;288:E957–E964. doi: 10.1152/ajpendo.00442.2004. [DOI] [PubMed] [Google Scholar]
- Azmitia EC, Jr, McEwen BS. Corticosterone regulation of tryptophan hydroxylase in midbrain of the rat. Science. 1969;166:1274–1276. doi: 10.1126/science.166.3910.1274. [DOI] [PubMed] [Google Scholar]
- Baulieu EE, Robel P. Dehydroepiandrosterone and dehydroepiandrosterone sulfate as neuroactive neurosteroids. J Endocrinol. 1996;150(Suppl):S221–S239. [PubMed] [Google Scholar]
- Bermack J, Lavoie N, Dryver E, Debonnel G. Effects of sigma ligands on NMDA receptor function in the bulbectomy model of depression: a behavioural study in the rat. Int J Neuropsychopharmacol. 2002;5:53–62. doi: 10.1017/S1461145701002760. [DOI] [PubMed] [Google Scholar]
- Chen K, Henry RA, Hughes SM, Connor B. Creating a neurogenic environment: the role of BDNF and FGF2. Mol Cell Neurosci. 2007;36:108–120. doi: 10.1016/j.mcn.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Chen X, Herbert J. Regional changes in c-fos expression in the basal forebrain and brainstem during adaptation to repeated stress: correlations with cardiovascular, hypothermic and endocrine responses. Neuroscience. 1995;64:675–685. doi: 10.1016/0306-4522(94)00532-a. [DOI] [PubMed] [Google Scholar]
- Christie BR, Cameron HA. Neurogenesis in the adult hippocampus. Hippocampus. 2006;16:199–207. doi: 10.1002/hipo.20151. [DOI] [PubMed] [Google Scholar]
- Cormaci G, Mori T, Hayashi T, Su TP. Protein kinase A activation down-regulates, whereas extracellular signal-regulated kinase activation up-regulates sigma-1 receptors in B-104 cells: Implication for neuroplasticity. J Pharmacol Exp Ther. 2007;320:202–210. doi: 10.1124/jpet.106.108415. [DOI] [PubMed] [Google Scholar]
- De Foubert G, Carney SL, Robinson CS, Destexhe EJ, Tomlinson R, Hicks CA, et al. Fluoxetine-induced change in rat brain expression of brain-derived neurotrophic factor varies depending on length of treatment. Neuroscience. 2004;128:597–604. doi: 10.1016/j.neuroscience.2004.06.054. [DOI] [PubMed] [Google Scholar]
- Dhir A, Kulkarni SK. Involvement of sigma ({sigma}1) receptors in modulating the anti-depressant effect of neurosteroids (dehydroepiandrosterone or pregnenolone) in mouse tail-suspension test. J Psychopharmacol. 2008;22:691–696. doi: 10.1177/0269881107082771. [DOI] [PubMed] [Google Scholar]
- Duman RS, Malberg J, Nakagawa S. Regulation of adult neurogenesis by psychotropic drugs and stress. J Pharmacol Exp Ther. 2001;299:401–407. [PubMed] [Google Scholar]
- Encinas JM, Vaahtokari A, Enikolopov G. Fluoxetine targets early progenitor cells in the adult brain. Proc Natl Acad Sci U S A. 2006;103:8233–8238. doi: 10.1073/pnas.0601992103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazzino F, Montes C, Urbina M, Carreira I, Lima L. Serotonin transporter is differentially localized in subpopulations of lymphocytes of major depression patients. Effect of fluoxetine on proliferation. J Neuroimmunol. 2008;196:173–180. doi: 10.1016/j.jneuroim.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Flugge G, Kramer M, Rensing S, Fuchs E. 5HT1A-receptors and behaviour under chronic stress: selective counteraction by testosterone. Eur J Neurosci. 1998;10:2685–2693. [PubMed] [Google Scholar]
- Glatz K, Mossner R, Heils A, Lesch KP. Glucocorticoid-regulated human serotonin transporter (5-HTT) expression is modulated by the 5-HTT gene-promotor-linked polymorphic region. J Neurochem. 2003;86:1072–1078. doi: 10.1046/j.1471-4159.2003.01944.x. [DOI] [PubMed] [Google Scholar]
- Guiard BP, David DJ, Deltheil T, Chenu F, Le Maitre E, Renoir T, et al. Brain-derived neurotrophic factor-deficient mice exhibit a hippocampal hyperserotonergic phenotype. Int J Neuropsychopharmacol. 2008;11:79–92. doi: 10.1017/S1461145707007857. [DOI] [PubMed] [Google Scholar]
- Heuser IJ, Schweiger U, Gotthardt U, Schmider J, Lammers CH, Dettling M, et al. Pituitary-adrenal-system regulation and psychopathology during amitriptyline treatment in elderly depressed patients and normal comparison subjects. Am J Psychiatry. 1996;153:93–99. doi: 10.1176/ajp.153.1.93. [DOI] [PubMed] [Google Scholar]
- Holick KA, Lee DC, Hen R, Dulawa SC. Behavioral effects of chronic fluoxetine in BALB/cJ mice do not require adult hippocampal neurogenesis or the serotonin 1A receptor. Neuropsychopharmacology. 2007;33:406–417. doi: 10.1038/sj.npp.1301399. [DOI] [PubMed] [Google Scholar]
- Huang GJ, Herbert J. The role of 5-HT1A receptors in the proliferation and survival of progenitor cells in the dentate gyrus of the adult hippocampus and their regulation by corticoids. Neuroscience. 2005;135:803–813. doi: 10.1016/j.neuroscience.2005.05.056. [DOI] [PubMed] [Google Scholar]
- Huang GJ, Herbert J. Stimulation of neurogenesis in the hippocampus of the adult rat by fluoxetine requires rhythmic change in corticosterone. Biol Psychiatry. 2006;59:619–624. doi: 10.1016/j.biopsych.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Ivy AS, Rodriguez FG, Garcia C, Chen MJ, Russo-Neustadt AA. Noradrenergic and serotonergic blockade inhibits BDNF mRNA activation following exercise and antidepressant. Pharmacol Biochem Behav. 2003;75:81–88. doi: 10.1016/s0091-3057(03)00044-3. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Praag H, Gage FH. Adult brain neurogenesis and psychiatry: a novel theory of depression. Mol Psychiatry. 2000;5:262–269. doi: 10.1038/sj.mp.4000712. [DOI] [PubMed] [Google Scholar]
- Jacobsen JP, Mork A. Chronic corticosterone decreases brain-derived neurotrophic factor (BDNF) mRNA and protein in the hippocampus, but not in the frontal cortex, of the rat. Brain Res. 2006;1110:221–225. doi: 10.1016/j.brainres.2006.06.077. [DOI] [PubMed] [Google Scholar]
- Jagasia R, Song H, Gage FH, Lie DC. New regulators in adult neurogenesis and their potential role for repair. Trends Mol Med. 2006;12:400–405. doi: 10.1016/j.molmed.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Kaasik A, Kalda A, Jaako K, Zharkovsky A. Dehydroepiandrosterone sulphate prevents oxygen-glucose deprivation-induced injury in cerebellar granule cell culture. Neuroscience. 2001;102:427–432. doi: 10.1016/s0306-4522(00)00489-9. [DOI] [PubMed] [Google Scholar]
- Karishma KK, Herbert J. Dehydroepiandrosterone (DHEA) stimulates neurogenesis in the hippocampus of the rat, promotes survival of newly formed neurons and prevents corticosterone-induced suppression. Eur J Neurosci. 2002;16:445–453. doi: 10.1046/j.1460-9568.2002.02099.x. [DOI] [PubMed] [Google Scholar]
- Keller J, Flores B, Gomez RG, Solvason HB, Kenna H, Williams GH, Schatzberg AF. Cortisol circadian rhythm alterations in psychotic major depression. Biol Psychiatry. 2006;60:275–281. doi: 10.1016/j.biopsych.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Krebs J, Fabel K. The contribution of failing adult hippocampal neurogenesis to psychiatric disorders. Curr Opin Psychiatry. 2008;21:290–295. doi: 10.1097/YCO.0b013e3282fad375. [DOI] [PubMed] [Google Scholar]
- Khalil MW, Strutt B, Vachon D, Killinger DW. Metabolism of dehydroepiandrosterone by cultured human adipose stromal cells: identification of 7 alpha-hydroxydehydroepiandrosterone as a major metabolite using high performance liquid chromatography and mass spectrometry. J Steroid Biochem Mol Biol. 1993;46:585–595. doi: 10.1016/0960-0760(93)90186-z. [DOI] [PubMed] [Google Scholar]
- Koponen E, Rantamaki T, Voikar V, Saarelainen T, MacDonald E, Castren E. Enhanced BDNF signaling is associated with an antidepressant-like behavioral response and changes in brain monoamines. Cell Mol Neurobiol. 2005;25:973–980. doi: 10.1007/s10571-005-8468-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkowski P, Mendlewicz J, Kerkhofs M, Leclercq R, Golstein J, Brasseur M, et al. 24-Hour profiles of adrenocorticotropin, cortisol, and growth hormone in major depressive illness: effect of antidepressant treatment. J Clin Endocrinol Metab. 1987;65:141–152. doi: 10.1210/jcem-65-1-141. [DOI] [PubMed] [Google Scholar]
- Liu D, Iruthayanathan M, Homan LL, Wang Y, Yang L, Wang Y, Dillon JS. Dehydroepiandrosterone stimulates endothelial proliferation and angiogenesis through extracellular signal-regulated kinase 1/2-mediated mechanisms. Endocrinology. 2008;149:889–898. doi: 10.1210/en.2007-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nat Neurosci. 2007;10:1089–1093. doi: 10.1038/nn1971. [DOI] [PubMed] [Google Scholar]
- Matsuno K, Kobayashi T, Tanaka MK, Mita S. Sigma 1 receptor subtype is involved in the relief of behavioral despair in the mouse forced swimming test. Eur J Pharmacol. 1996;312:267–271. doi: 10.1016/0014-2999(96)00497-9. [DOI] [PubMed] [Google Scholar]
- McQuade R, Leitch MM, Gartside SE, Young AH. Effect of chronic lithium treatment on glucocorticoid and 5-HT1A receptor messenger RNA in hippocampal and dorsal raphe nucleus regions of the rat brain. J Psychopharmacol. 2004;18:496–501. doi: 10.1177/026988110401800406. [DOI] [PubMed] [Google Scholar]
- Michael A, Jenaway A, Paykel ES, Herbert J. Altered salivary dehydroepiandrosterone levels in major depression in adults. Biol Psychiatry. 2000;48:989–995. doi: 10.1016/s0006-3223(00)00955-0. [DOI] [PubMed] [Google Scholar]
- Morfin R, Starka L. Neurosteroid 7-hydroxylation products in the brain. Int Rev Neurobiol. 2001;46:79–95. doi: 10.1016/s0074-7742(01)46059-4. [DOI] [PubMed] [Google Scholar]
- Muller C, Pompon D, Urban P, Morfin R. Inter-conversion of 7alpha- and 7beta-hydroxy-dehydroepiandrosterone by the human 11beta-hydroxysteroid dehydrogenase type 1. J Steroid Biochem Mol Biol. 2006;99:215–222. doi: 10.1016/j.jsbmb.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Nakata T, Takashima S, Shiotsu Y, Murakata C, Ishida H, Akinaga S, et al. Role of steroid sulfatase in local formation of estrogen in post-menopausal breast cancer patients. J Steroid Biochem Mol Biol. 2003;86:455–460. doi: 10.1016/s0960-0760(03)00357-1. [DOI] [PubMed] [Google Scholar]
- Nickel T, Sonntag A, Schill J, Zobel AW, Ackl N, Brunnauer A, et al. Clinical and neurobiological effects of tianeptine and paroxetine in major depression. J Clin Psychopharmacol. 2003;23:155–168. doi: 10.1097/00004714-200304000-00008. [DOI] [PubMed] [Google Scholar]
- Pinnock SB, Balendra R, Chan M, Hunt LT, Turner-Stokes T, Herbert J. Interactions between nitric oxide and corticosterone in the regulation of progenitor cell proliferation in the dentate gyrus of the adult rat. Neuropsychopharmacology. 2007;32:493–504. doi: 10.1038/sj.npp.1301245. [DOI] [PubMed] [Google Scholar]
- Pinnock SB, Herbert J. Brain-derived neurotropic factor and neurogenesis in the adult rat dentate gyrus: interactions with corticosterone. Eur J Neurosci. 2008;27:2493–2500. doi: 10.1111/j.1460-9568.2008.06250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro MF, Garcia-Segura LM. Dehydroepiandrosterone regulates insulin-like growth factor-1 system in adult rat hypothalamus. Endocrine. 2002;17:129–134. doi: 10.1385/ENDO:17:2:129. [DOI] [PubMed] [Google Scholar]
- Ripp SL, Fitzpatrick JL, Peters JM, Prough RA. Induction of CYP3A expression by dehydroepiandrosterone: involvement of the pregnane X receptor. Drug Metab Dispos. 2002;30:570–575. doi: 10.1124/dmd.30.5.570. [DOI] [PubMed] [Google Scholar]
- Sairanen M, Lucas G, Ernfors P, Castren M, Castren E. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci. 2005;25:1089–1094. doi: 10.1523/JNEUROSCI.3741-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Saponaro S, Guarnieri V, Pescarmona GP, Silvagno F. Long-term exposure to dehydroepiandrosterone affects the transcriptional activity of the glucocorticoid receptor. J Steroid Biochem Mol Biol. 2007;103:129–136. doi: 10.1016/j.jsbmb.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol. 2005;192:348–356. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Schlinger BA, Pradhan DS, Soma KK. 3beta-HSD activates DHEA in the songbird brain. Neurochem Int. 2008;52:611–620. doi: 10.1016/j.neuint.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt PJ, Daly RC, Bloch M, Smith MJ, Danaceau MA, St Clair LS, et al. Dehydroepiandrosterone monotherapy in midlife-onset major and minor depression. Arch Gen Psychiatry. 2005;62:154–162. doi: 10.1001/archpsyc.62.2.154. [DOI] [PubMed] [Google Scholar]
- Schmitt M, Klinga K, Schnarr B, Morfin R, Mayer D. Dehydroepiandrosterone stimulates proliferation and gene expression in MCF-7 cells after conversion to estradiol. Mol Cell Endocrinol. 2001;173:1–13. doi: 10.1016/s0303-7207(00)00442-1. [DOI] [PubMed] [Google Scholar]
- Su TP, Hayashi T. Understanding the molecular mechanism of sigma-1 receptors: towards a hypothesis that sigma-1 receptors are intracellular amplifiers for signal transduction. Curr Med Chem. 2003;10:2073–2080. doi: 10.2174/0929867033456783. [DOI] [PubMed] [Google Scholar]
- Thomas K, Davies A. Neurotrophins: a ticket to ride for BDNF. Curr Biol. 2005;15:R262–R264. doi: 10.1016/j.cub.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Ueda H, Yoshida A, Tokuyama S, Mizuno K, Maruo J, Matsuno K, Mita S. Neurosteroids stimulate G protein-coupled sigma receptors in mouse brain synaptic membrane. Neurosci Res. 2001;41:33–40. doi: 10.1016/s0168-0102(01)00258-9. [DOI] [PubMed] [Google Scholar]
- van Riel EMO, Steenbergen PJ, Joels M. Chronic unpredictable stress causes attenuation of serotonin responses in cornu ammonis 1 pyramidal neurons. Neuroscience. 2003;120:649–658. doi: 10.1016/s0306-4522(03)00355-5. [DOI] [PubMed] [Google Scholar]
- Weber CC, Eckert GP, Muller WE. Effects of antidepressants on the brain/plasma distribution of corticosterone. Neuropsychopharmacology. 2006;31:2443–2448. doi: 10.1038/sj.npp.1301076. [DOI] [PubMed] [Google Scholar]
- Wong EY, Herbert J. The corticoid environment: a determining factor for neural progenitors’ survival in the adult hippocampus. Eur J Neurosci. 2004;20:2491–2498. doi: 10.1111/j.1460-9568.2004.03717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EY, Herbert J. Raised circulating corticosterone inhibits neuronal differentiation of progenitor cells in the adult hippocampus. Neuroscience. 2006;137:83–92. doi: 10.1016/j.neuroscience.2005.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong ML, Licinio J. Research and treatment approaches to depression. Nat Rev Neurosci. 2001;2:343–351. doi: 10.1038/35072566. [DOI] [PubMed] [Google Scholar]
- Yamada S, Yamauchi K, Hisatomi S, Annoh N, Tanaka M. Effects of sigma(1) receptor ligand, MS-377 on apomorphine- or phencyclidine-induced disruption of prepulse inhibition of acoustic startle in rats. Eur J Pharmacol. 2000;402:251–254. doi: 10.1016/s0014-2999(00)00498-2. [DOI] [PubMed] [Google Scholar]
- Young EA, Altemus M, Lopez JF, Kocsis JH, Schatzberg AF, DeBattista C, Zubieta JK. HPA axis activation in major depression and response to fluoxetine: a pilot study. Psychoneuroendocrinology. 2004;29:1198–1204. doi: 10.1016/j.psyneuen.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Zhang L, Li B, Ma W, Barker JL, Chang YH, Zhao W, Rubinow DR. Dehydroepiandrosterone (DHEA) and its sulfated derivative (DHEAS) regulate apoptosis during neurogenesis by triggering the AKT signaling pathway in opposing ways. Brain Res Mol Brain Res. 2002;98:58–66. doi: 10.1016/s0169-328x(01)00315-1. [DOI] [PubMed] [Google Scholar]


