Summary
The mechanisms for delivering components to nerve terminals are diverse and highly regulated. The diversity of kinesin motors alone is insufficient to account for the specificity of delivery. Additional specificity and control are contributed by adaptor proteins and associated regulatory molecules. The interaction of cargos with these complexes can confer distinct behaviors on the transport of synaptic organelles. The rich regulatory mechanisms of transport are only now emerging as the cargo-motor complexes are defined and subsequent local events that regulate their dynamic relationship are examined. Here we review recent studies of kinesin-related axonal transport of three crucial synaptic components, Piccolo-bassoon Transport Vesicles (PTVs), Synaptic Vesicle Precursors (SVPs), and mitochondria, and the mechanisms that modulate their transport.
Introduction
Do you have a lengthy commute to work? If so, you may sympathize with the many organelles in your neurons that require several days to travel to their synaptic place of work. Neurons evolved long axons to maximize circuitry, not to minimize transportation costs from the cell soma to the terminals. Given the challenging geometry of the neuron, the mechanisms that deliver synaptic materials to the developing synapse and subsequently sustain the synapse are of the utmost importance. Indeed, mutations in motor proteins and other defects in intracellular transport are increasingly linked to neuropathologies [1,2], and the failure to maintain synapses is apparent as an early hallmark of some degenerative diseases [3,4]. This review will focus on recent studies of the delivery mechanism for synaptic components, on the need for different regulatory rules to govern the delivery of different components, and on how that specificity can be achieved through the interplay of motors, adaptor proteins, and regulatory cascades. The return trip, mediated by dynein complex proteins has been reviewed elsewhere [5,6].
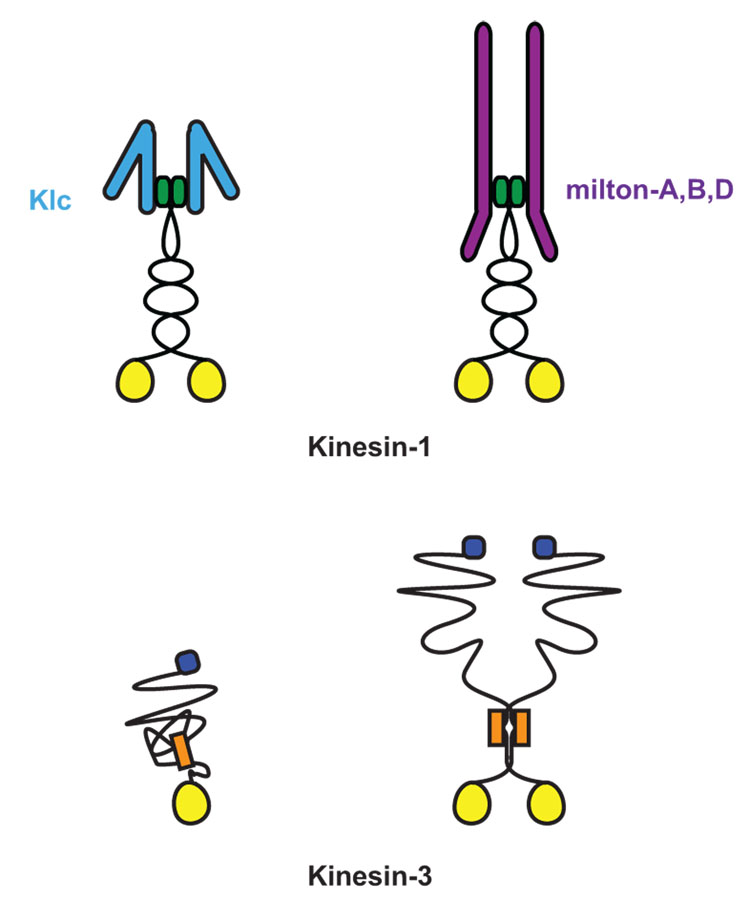
The fundamentals of axonal transport are clear: long distance movement is primarily dependent on microtubules, axonal microtubules are predominantly oriented with +-ends in the periphery, and +-end directed traffic is accomplished by kinesin motors. The kinesins involved in axonal transport of synaptic cargo are chiefly members of the conventional kinesins (Kinesin-1 family) and of the Kinesin-3 family [7,8] (Figure 1). Although the biophysics of kinesins is increasingly well understood, many cell biological questions remain mysterious. How is the motor matched with appropriate cargo? How does a motor share its cargo with other motors? How does the motor know when to stop and “unload”?
Figure 1. Meet the family of Kinesin-1 and Kinesin-3.
Kinesins are defined by their highly conserved ATP binding and microtubule binding motor domain (yellow circle) [8]. Both Kinesin-1 and Kinesin-3 family members have their motor domain at their N-termini and move toward the (+)-end microtubules. The Kinesin-1 family subfamily of KIF5, or kinesin heavy chain (KHC), is a homodimer that dimerizes via coiled-coil domains at its neck. KHC associates with two kinesin light chains (KLC) to link it to multiple cargo complexes [7]. Although initially believed to act as an obligate tetramer with KLC, KHC can also associate with cargos via a specialized adaptors independent of KLC, as is the case with the mitochondrial adaptor protein, Milton [•36]. In contrast, Kinesin-3 family members have been found as both monomers and dimers and are able to associate to vesicular cargo directly. Kinesin-3 family members share a conserved fork-head association domain (orange box) and multiple coiled-coil domains at the neck of the motor [7,8]. The defining motor of the Kinesin-3 family, Unc-104, has a pleckstrin homology domain (blue square) that is necessary for its association with synaptic vesicle precursors [24].
Consider a growing axon forming en passant synapses on nearby dendrites while its growth cone continues to advance: active zone proteins and synaptic vesicles arrest at the new synaptic locations but vesicles carrying new membrane and guidance molecules move past them into the growth cone. The mechanistic differences that must underly the behaviors of these cargos can be illustrated by the phenotype of immaculate connections (imac), a Drosophila Kinesin-3. In imac embryos, motoneuron axons extend properly and are guided to and arrest on the appropriate muscle fibers, but synapses cannot form. Active zone proteins are greatly reduced, synaptic vesicles are absent, and the nerve endings do not mature into rounded boutons [••9]. Thus the imac motor is selectively required in these neurons for many synaptogenic cargos but other kinesins must mediate membrane addition and growth cone guidance.
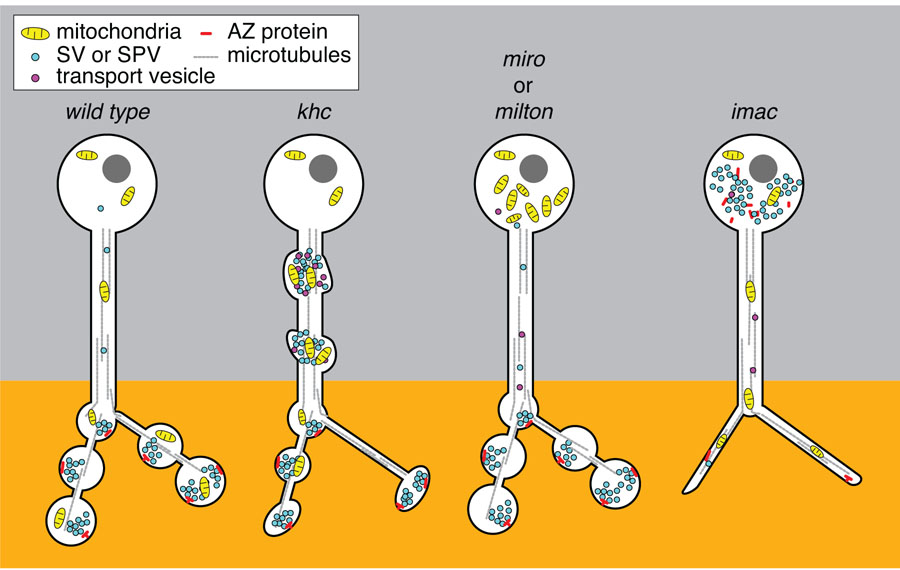
Once a synapse is established, new components must continue to arrive to replace proteins and organelles targeted for degradation; whether or not the arrival and departure of components are balanced may determine whether a synapse is strengthened, weakened, or in a steady state [•10]. Moreover, while some cargos need to find their way selectively to axon terminals, other cargos need to distribute themselves according to their own rules; mitochondria, for example, must be present in the axon but also concentrate at synapses and growth cones (Figure 2). Thus axonal transport is more than a monotonous crawl down the axon towards the end.
Figure 2. Distinct functions of Kinesin-1 and Kinesin-3 family motors for transport in the axon.
The presynaptic compartment of the neuron is normally enriched with mitochondria and SVs in close apposition to a density of active zone proteins. Loss of the lone Kinesin-1 motor in the fly, KHC, leads to accumulations of mitochondria, SPV as well as post-golgi transport vesicles in the axon [7]. This is striking when compared to what occurs in the absence of either milton or Miro, the adaptor complex that associates KHC to mitochondria. Mitochondria are stranded in the cell body of both milton and miro mutants and do not generate traffic jams in the axonal processes or disrupt the distribution of SVs or active zone proteins [•36,38]. One interpretation of this discrepancy between the phenotype of Milton/Miro and KHC is that the traffic jams in the khc mutants are due to the mislocalization of additional cargos of KHC. For example, through binding of the JIP1 signaling complex associated with KLC and transport vesicles, decreased KHC dependent transport could compromise both the integrity of the cytoskeletal network as well as the signal for the proper sorting of cargos. In contrast, loss of the Kinesin-3 family motors Unc-104 or its fly homologue, Imac, leads to a severe reduction of synaptic vesicles (SVs) at terminals and an increase of SVs stranded in the cell body [7]. Mitochondrial distribution and axon guidance and growth remains normal in unc-104 and imac mutants demonstrating their specialized role for SVP transport. In addition, in the absence of Imac, morphologically mature synaptic endings do not form and active zone proteins are stranded in the cell body along with a notable reduction of active zones (AZ) at the synapse [••9]. Although loss of KHC does disrupt the normal trafficking of SVs and demonstrates a reduction of synapses, the severity of the imac phenotype implies that it is likely the primary motor to initiate the transport of SVs and as yet unidentified proteins critical for morphological synapse development.
Three classes of transport organelle are crucial to the nerve terminal and yet distinct in their transport mechanisms: Piccolo-bassoon Transport Vesicles or PTVs, named after the active zone proteins piccolo and bassoon [11], Synaptic Vesicle Precursors (SVPs), and mitochondria. We will next review the evidence linking particular kinesins to these organelles and review the early indications of regulatory mechanisms for controlling their transport.
Regulating synapse formation through motors
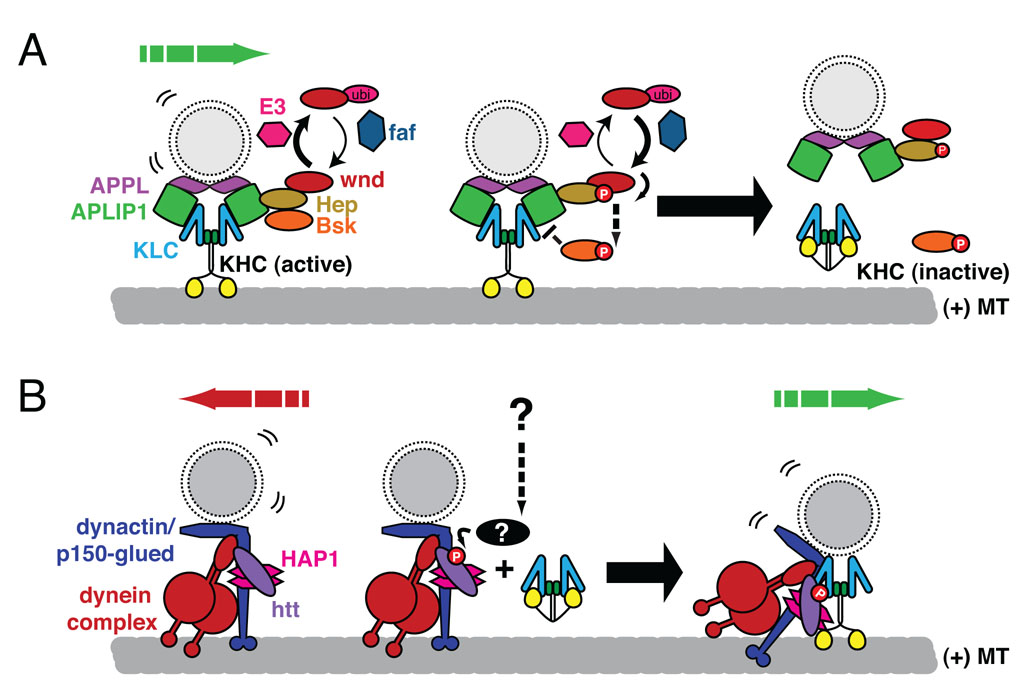
When a developing axon meets an appropriate target, a functioning synapse can form within minutes. This speed can only be achieved because axonal transport has already brought the key components of the synapse into the growing axon. The imac phenotype (see above) illustrates the absolute requirement of axonal transport for forming synapses. Additional Drosophila mutants have established a link between transport regulation and control of the size of a synapse. Mutations of a ubiquitin ligase (highwire), or overexpression of a ubiquitin hydrolase (fat facets) or MAPKKK (wallenda, DLK in mammals) causes dramatic overgrowth of synapses at the neuromuscular junction [••12] and homologs in C.elegans also regulate presynaptic development [13]. These signaling molecules have recently been shown to regulate a Kinesin-1 adaptor protein called JIP1 (APLIP1 in the fly) [••14] (Figure 3A). JIP1 binds MAPKK (hemipterous), which binds to the wallenda and the downstream JNK (basket) and links them to KLC and vesicular cargos [15]. Wallenda is enriched at the synapse and its kinase activity can decrease JIP1 binding to KLC, thereby releasing JIP1-associated cargo [••14] (Figure 3A). Analogous JNK-dependent phosphorylation events have been suggested to cause kinesin to dissociate from microtubules in other vertebrate and invertebrate systems [16,17] illustrating the potential conservation of this pathway for regulating Kinesin-1 transport. Potentially, by modulating kinesin activation and binding to cargo, the JNK pathway could regulate synapse growth by signaling when and where synaptic cargos are deposited. In this context, another recent finding is also noteworthy: Insulin growth factor (IGF) activation of Akt phosphorylates the vesicle-associated protein huntingtin and thereby regulates the ability of huntingtin to recruit kinesin to vesicles [••18] (Figure 3B). By recruiting kinesin, phospho-huntingtin promotes anterograde transport over retrograde. Huntingtin is likely to have multiple roles in axonal transport, and transport defects with consequent synaptic impairment are prominent in the pathology of Huntington’s Disease [19–21]. The regulation of kinesin/cargo association through adaptor proteins is thus a major control point for cargo-specific control of transport and can influence synaptic structure and function.
Figure 3. Phosphorylation dependent regulation of transport.
Two recent examples of how transport of vesicular cargos is modulated by adaptor complexes are illustrated above. (A) Recent work in the fly indicates that the interaction between the KHC/KLC kinesin complex and the JNK adaptor, JIP1 (APLIP1 in the fly) is modulated by the JNK signaling pathway [••14]. Synaptic material accumulates in axons when APLIP1 is overexpressed. These accumulations are alleviated by the overexpression of either the ubiquitin hydrolase, fat facets (faf) or the MAPKKK, wallenda (wnd). Transport defects are similarly apparent with the loss of wnd or the activity of its downstream signaling partners the MAPKK, hemipterous (Hep), or the JNK homologue, basket (Bsk), suggesting that JNK signaling is necessary for maintaining or initializing the transport of APLIP1 cargos, i.e. APPL- associated transport vesicles. The kinase activity of wnd promotes the activation of Hep, increased phospho-JNK, and decreased binding of APLIP1 with KLC. Thus the activity of the ubiquitin proteasome system may normally reduce wnd activity and thereby allow adaptor-cargo complexes to assemble. Increasing wnd activity, via modulation of the ubiquitination pathway, e.g via the E3 ubiquitin ligase Highwire, or direct activation of wnd would promote the dissociation of the motor and cargo and inactivation of the kinesin [64,65]. (B) A recent study helps to resolve how the Huntingtin protein (htt) may regulate the bidirectional movement of vesicles [18]. Htt associates with both the dynein/dynactin retrograde motor complex and kinesin-1, in part via huntingtin associated protein (HAP1) [•20,66]. Insulin growth factor, via the kinase Akt, phosphorylates htt at serine 421 and increased the association of KHC with vesicles, thereby promoting anterograde transport of BDNF-containing vesicles. In this model, phosphorylation of htt controls the balance between retrograde and anterograde forces on this cargo and others and places htt at the center of signaling pathways that may modulate transport normally and in disease states.
Transport of Synaptic Vesicle Precursors
In a developing neuron, the components needed for synaptogenesis travel in armadas composed of multiple vesicular organelles that contain at least two classes of transport vesicle. One vesicle class, the PTVs, are dense-core 80 nm vesicles with a coat of electron dense material that contains active zone proteins. The second component, SVPs, contains markers of synaptic vesicles [22]. There is some evidence that SVPs are heterogeneous; different components of the mature synaptic vesicle may be transported separately and subsequently united, possibly through a recycling endosome [23]. Though PTVs and SVPs cluster together while moving and are recruited together to new synaptic sites, they may in fact use distinct kinesins.
Genetic and biochemical evidence demonstrate that SVPs are dependent on a Kinesin-3 family member: UNC-104 in C. elegans, imac in Drosophila and KIF1A in vertebrates [7,••9,24]. These three motors possess a pleckstrin homology (PH) domain whose affinity for phosphatidylinositol-4,5-bisphosphate (PI(4,5)P(2)) on the vesicle surface is important for SVP binding, though protein-protein interactions are also likely to be needed [24]. When mutations disrupt these motors, the vast majority of SVPs are stranded in the cell body and do not enter the axon [7,••9,24] leaving little doubt that these motors are the primary motor for axonal transport of SVPs.
Nonetheless, some SVPs remain in the neuropil of unc-104 and kif1A animals. How did these SVPs travel? In some cases, the alleles examined may not have been null or a related redundant motor (i.e. KIF1B) may account for the residuum. Another possibility, however, is that additional unrelated motors can carry SVPs. Indeed, recent mass spectrometry of synaptic vesicles from adult rat brain found the Kinesin-1 family motors, KIF5A and KIF5B, but not the KIF1 motors [25]. Interestingly, Kinesin-1 mutants [7] or animals with mutations in proteins associated with Kinesin-1 motors including kinesin light chain (KLC), the JNK scaffolding adaptor (JIP3/UNC-16/SYD [15] or JIP1/APLIP1 [14,26] and its associated MAPKKK/MAPKK/JNK signaling complex, liprin-α [27] or Unc-76 [28] all share an SVP-transport phenotype. Unlike the mutations in Kinesin-3 motors, altering Kinesin-1 motor complexes led to accumulations of SVPs and other organelles in axonal traffic jams. Why are two classes of kinesin implicated in SVP transport? Three explanations can be suggested. 1) A Kinesin-3 may be required to move the vesicles from the cell body into the axon, but Kinesin-1 and the JIP complex may make long range movement within the axon more efficient. 2) Different cell types and different developmental stages may primarily use one motor or the other, with Kinesin-3 motors dominant during development. 3) The Kinesin-3 motor may be the predominant motor and the effects of Kinesin-1 mutations may be indirect. For example, mutations in Kinesin-1 and its associated JIP complex and regulatory molecules may disrupt transport of other post-Golgi vesicles or activate signaling cascades that alter microtubule structure or cargo binding. In the resulting traffic jams, SVPs and other organelles may get stuck despite possessing functional motors.
Very little is known at present about the regulation of SVP transport. One motor-associated protein, liprin-α (syd-2 in C. elegans), has been suggested to be either a regulator of motor activity for SVPs within the axon [27] or a molecule that signals the motor to unload its vesicles at the synapse [•29]. As discussed earlier, phosphorylation can also cause cargo unloading [••14,30], but the signals that tell SVPs when to stop their journey remain poorly understood.
Transport of active zone components
Whereas Kinesin-3 family members predominate in transport of SVPs, the transport of PTVs is more complex. In hippocampal neurons, a protein called syntabulin is implicated in the transport of PTVs [•10,31]. This protein acts as an adaptor for PTV transport via its binding to the Kinesin-1 motor KIF5b. Knockdown of syntabulin or interference with its binding to KIF5b impaired the transport of several PTV components without disrupting the dynamics of SVPs [•10,31]. Reduced syntabulin also decreased activity dependent formation of new synapses, demonstrating that PTV transport is required for this form of plasticity. There are, however, no invertebrate homologues for syntabulin, bassoon, or piccolo and the vesicles that deliver active zone proteins in C. elegans and Drosophila remain uncharacterized. However, some evidence implicates Kinesin-3 motors in the establishment of active zones. The neuromuscular junctions of imac mutant flies, in addition to lacking synaptic vesicles, had greatly reduced levels of the active zone protein, Brp/ELKS/CAST/ERC, [••9]. Indeed, the absence of synaptic boutons in imac indicates that imac is responsible for transporting as yet unidentified synaptogenic molecules that are necessary for the morphological transformation of the axonal endings into rounded boutons. Unc-104 mutant C. elegans also have fewer synapses, but several active zone components localize to synapses independently of Unc-104 [•29]. Passive diffusion of active zone proteins may suffice in these relatively small neurons. Alternatively, additional kinesin motors or trafficking mechanisms may carry the presynaptic machinery. Much remains to be learned about the transport of active zone proteins and, perhaps more importantly, how it is regulated by synaptogenic cues.
Mitochondrial Movement and its Regulation
In contrast to the cargos discussed above, neuronal mitochondria do not have a final destination. They remain in flux and their dynamics are influenced by moment-to-moment changes in energy demands of the cell. Neurons are particularly vulnerable to defects in mitochondrial movement [1] because energetic needs are high at the synaptic extremities of the cell [32], and because efficient ATP supply and Ca2+-buffering must be satisfied locally rather than by slow diffusion. As the activity of the nervous system changes, so do the energy demands on its neurons. Consequently, many neuronal mitochondria are in motion, traveling in both anterograde and retrograde directions through the interplay of kinesin and dynein motors [33]. Their transport would seem to have nothing in common with that of PTVs and SPVs. Those vesicles efficiently leave the soma and exclusively travel down the axon, but neurons could not function without a pool of mitochondria remaining in the cell body and the distinction between axon and dendrite may be irrelevant to mitochondria. SPVs and PTVs only accumulate in axons in pathological conditions but a large pool of mitochondria are stationary in axons and accumulate at Nodes of Ranvier where they are essential for maintaining ion gradients. Nonetheless, the primary motor for their anterograde movement seems to be the conventional Kinesin-1 already discussed above. The distinguishing features of mitochondrial transport are therefore likely to reside not in the motors per se but in the adaptor proteins and regulatory proteins that are specific to mitochondrial transport.
Kinesins and Adaptors for Mitochondrial Movement
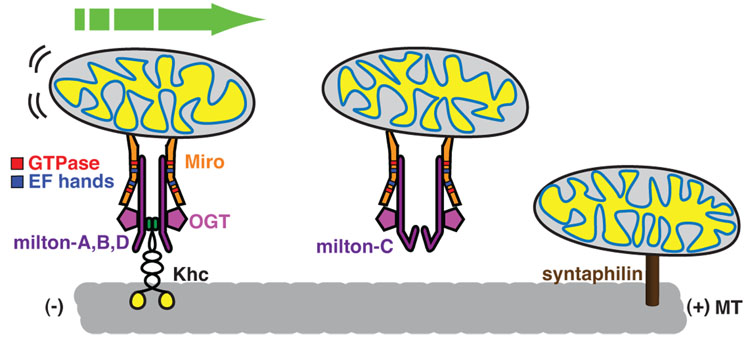
Genetic studies in Drosophila, in tandem with biochemical analysis, has revealed a kinesin-adaptor complex that is essential for the transport of mitochondria into axons and dendrites [34–38]. The complex, best characterized in Drosophila but also present in mammalian cells, is cartooned in Figure 4 and consists of 3 essential proteins: Miro, a protein in the outer mitochondrial membrane [39], Kinesin Heavy Chain (KHC) which is the essential motor component of the conventional Kinesin-1 in Drosophila [7] and milton, an adaptor protein that links KHC to Miro and thereby recruits the motor to the mitochondrial surface [35,•36]. In flies lacking either milton or Miro, mitochondria are retained in the cell body and do not enter axons [35,38]. The phenotype of khc mutants is too severe to permit an analysis of a neuron completely lacking the protein, but alleles that strongly reduce it cause mitochondria to accumulate in traffic jams [7,34]. One exceptional feature of mitochondrial transport is that, whereas Kinesin-1 is normally a tetramer of two KHCs and two Kinesin Light Chains (KLC), milton appears to replace the light chain in the complex and mitochondrial transport does not require the light chain [•36].
Figure 4. Mitochondria on the move.
The KHC/milton/Miro complex is one of the best-defined motor/adaptor/cargo complexes. The association of milton splice isoforms that allow for binding to KHC (milton-A, B or D) links the motor with the mitochondrial membrane protein, Miro. In the presence of milton-C, miro and mitochondria do not associate with KHC, perhaps allowing for a competitive pathway to stop mitochondria when they reach a destination of high milton-C expression. Miro has GTPase domains as well as EF hands that may allow it to differentially bind to the Milton/KHC complex to link mitochondria to a microtubule transport pathway. Milton binds to and is glycosylated by O-GlcNAc transferase (OGT) binds. Glycosylation of milton has an unknown function but may serve as an additional signal links the metabolism of the cell with regulation of mitochondrial transport. Recent identification of a synaptic protein, synaptophilin, critical for the docking of mitochondria by linking it directly to microtubules adds a new layer of possibilities to how the motility of mitochondria are controlled in neurons.
Although in Drosophila the evidence is strong that this mechanism is essential, Kinesin-1 may not be the only motor that moves mitochondria. In mammalian cells, both KIF5B, a Kinesin-1 motor, and KIF1B, a Kinesin-3 motor, are reported to be present on mitochondria [7]. The phenotype of kif5B mutant murine cells, however, indicates that the Kinesin-1 motor, as in Drosophila, has a major role [7]. Additional adaptor proteins for mitochondrial association with KIF5 and KIF1B, including syntabulin, RanBP2, and KBP have also been reported [26,40–42].
Mitochondrial anchors may be as significant for controlling mitochondrial distribution as the motors themselves. Recently, the protein syntaphilin has been shown to be selectively present on those axonal mitochondria that are stationary. Syntaphilin, by simultaneously binding to the mitochondrial surface and microtubules, may provide such an anchor. Consistent with this model, genetic disruption of syntaphilin increases the percentage of mitochondria in motion in the axon [••43].
Regulation of Mitochondrial Movement
The complexity of mitochondrial distribution and its responsiveness to the changing energy demands of neurons implies an elaborate mechanism for controlling the mitochondrial motors. Several intracellular signals have been found that influence mitochondrial movement and distribution [33,••43,44,45]. Potential points of regulation in the motor mechanisms have also been identified and it is likely that we will soon be able to link signaling pathways to specific mechanisms for altering mitochondrial behavior.
Elevated cytosolic Ca++, the most studied mitochondrial regulator, halts microtubule-based mitochondrial movement in many cell types [46–50]. This phenomenon may explain aspects of mitochondrial behavior: in neurons, Ca++ influx occurs at pre- post- synaptic membranes, where much energy is required to maintain the ion gradients and mitochondria are abundant. Moreover, when local ATP levels are poor, the pumping of Ca++ across the plasma membrane is compromised and sustained elevation of cytosolic Ca++ results. Thus, locally elevated Ca++, by arresting passing mitochondria, can hold them at a site where the energy requirement is high and supply is low. Conversely, where ATP is high and mitochondria are in surplus, Ca++ will be low and mitochondria will be free to move away [46–50]. How does Ca++ regulate mitochondrial movement? A pair of Ca++ binding EF-hands in Miro are strong candidates for mediating this regulation (Figure 4).
The mitochondrial motor-adaptor complex has many additional sites that are likely to provide regulation, though their significance is not yet known. In addition to the Ca++ -binding EF hands, Miro has two domains homologous to small GTPases. Mutation of these domains alters mitochondrial distribution in transfected cells [37,51]. In addition, milton undergoes alternative splicing in both Drosophila and mammals [•36,52,53]. Although the significance of the resulting variants is largely unknown, one amino-terminus variant of Drosophila milton, milton-C, inhibits KHC binding to milton and thereby prevents KHC recruitment to mitochondria (Figure 4) [•36].
Another intriguing type of regulation may be mediated by O-GlcNAcylation, a posttranslational modification of serine and threonine residues [54]. This reaction, quite distinct from the addition of sugar residues in the ER and Golgi, involves the addition of a single sugar residue. Like the better studied phosphorylations, GlcNAcylations occur in the cytoplasm and are reversible. The enzyme that mediates this modification, O-GlcNAc transferase (OGT), binds and glycosylates milton in both mammals [55] and Drosophila [•36]. Notably, OGT activity has been reported to be an index of glucose concentration in the cell [56]. Thus, whereas cytosolic Ca++ may signal to mitochondria the local demand for mitochondria, OGT may convey information about the supply of available substrate in subcellular regions. OGT signaling may be important in the etiology of diabetes [57], suggesting the possibility that glycosylation-related modification of milton may be relevant to diabetic neuropathy.
Stimulation of Nerve Growth Factor (NGF) receptors also induces a local capture of mitochondria, a phenomenon that likely contributes to the accumulation of mitochondria in active growth cones. NGF acts, at least in part, through the phosphatidylinositol (3,4,5) – trisphosphate (PtdIns(3,4,5)P3) and MAPK signaling pathways [44,45]. The targets of these kinases are not certain, but as discussed earlier, phosphorylation of Kinesin-1 can control its association with cargos and microtubules [••14,16,58,59] and may act in a similar manner to regulate milton or an additional mitochondrial adaptor/cargo complex.
Conclusions
Diverse cargos are needed at the synapse, but despite their common destiny they have distinct regulatory needs that are reflected in a diversity of kinesins, adaptors, and regulator proteins. The extreme length of neuronal processes makes them vulnerable to transport defects. These may be specific for an individual cargo, such as the disruption of mitochondrial traffic in forms of Charcot-Marie-Tooth disease where mitochondrial fussion and fission are perturbed. Alternatively, they may involve multiple cargos, as in Alzheimer’s disease, Hungtinton’s disease, frontotemporal dementia with Parkinsonism -17, and amyotrophic lateral sclerosis where trafficking defects arise from mutations in APP, Huntingtin, tau, and SOD1 [1,2]. For these reasons, understanding axonal cell biology has an added urgency. With the identification of some of the key elements of the mechanism, it is now possible to take the next step toward understanding how intracellular traffic is managed: how the right amount of a cargo moves in the proper direction; how it is received at the proper location; and how that mechanism is thwarted in pathological states.
Table 1.
Critical motors for transport of presynaptic organelles
| Kinesin family |
Axonal motor | Phenotypes of motor loss/disruption |
Associated neuronal proteins |
|---|---|---|---|
| Severe reduction of mitochondrial flux and number in axons [34] |
Milton [35,•36] | ||
| KLC [7] | |||
|
C. elegans UNC-116 |
|||
| Kinesin-1 |
D. melanogastor KHC |
Mislocalized accumulations of SVs and mitochondria in axons [7] |
Syntabulin [31] |
| Liprin-α [27] | |||
|
Mammalian KIF5A KIF5B KIF5C |
Reduced synapse formation and function [7] |
RanBP2 [41] | |
| SNAP-25, SNAP-23 [60] | |||
| Calsyntenin [61] | |||
| Reduced neurite outgrowth [7] |
β-dystrobrevin [62] | ||
|
C. elegans UNC-104 |
SVs stranded in cell body and severely reduced at synapse [7] |
||
| D. melanogastor Imac | Decrease in synapse number [7] |
Liprin-α[63] | |
| Kinesin-3 | |||
|
Mammalian KIF1A KIF1Bα KIF1Bβ KIF1C |
Failed development of morphological synapse [••9] |
KBP [42] | |
| Decrease in mitochondria motility [7] |
|||
Acknowledgements
This work was supported by NIH RO1 grants MH075058 and GM069808 to TLS. AYNG was supported by a postdoctoral NRSA award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethics in Publishing
All opinions are of the authors and all figures and the table are original artwork generated for this review.
Conflicts of interest
The authors have no conflicts of interest with the publication of this article.
Contributor Information
Ann Y. N. Goldstein, Email: Ann.Goldstein@childrens.harvard.edu.
Xinnan Wang, Email: Xinnan.Wang@childrens.harvard.edu.
Thomas L Schwarz, Email: Thomas.Schwarz@childrens.harvard.edu.
References and recommended readings
- 1.De Vos KJ, Grierson AJ, Ackerley S, Miller CC. Role of Axonal Transport in Neurodegenerative Diseases. Annu Rev Neurosci. 2008;31:151–173. doi: 10.1146/annurev.neuro.31.061307.090711. [DOI] [PubMed] [Google Scholar]
- 2.Stokin GB, Goldstein LS. Axonal transport and Alzheimer's disease. Annu Rev Biochem. 2006;75:607–627. doi: 10.1146/annurev.biochem.75.103004.142637. [DOI] [PubMed] [Google Scholar]
- 3.Saxena S, Caroni P. Mechanisms of axon degeneration: from development to disease. Prog Neurobiol. 2007;83:174–191. doi: 10.1016/j.pneurobio.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Schaefer AM, Sanes JR, Lichtman JW. A compensatory subpopulation of motor neurons in a mouse model of amyotrophic lateral sclerosis. J Comp Neurol. 2005;490:209–219. doi: 10.1002/cne.20620. [DOI] [PubMed] [Google Scholar]
- 5.Ibanez CF. Message in a bottle: long-range retrograde signaling in the nervous system. Trends Cell Biol. 2007;17:519–528. doi: 10.1016/j.tcb.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Levy JR, Holzbaur EL. Cytoplasmic dynein/dynactin function and dysfunction in motor neurons. Int J Dev Neurosci. 2006;24:103–111. doi: 10.1016/j.ijdevneu.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Hirokawa N, Noda Y. Intracellular Transport and Kinesin Superfamily Proteins, KIFs: Structure, Function, and Dynamics. Physiol Rev. 2008;88:1089–1118. doi: 10.1152/physrev.00023.2007. [DOI] [PubMed] [Google Scholar]
- 8.Vale RD. The molecular motor toolbox for intracellular transport. Cell. 2003;112:467–480. doi: 10.1016/s0092-8674(03)00111-9. [DOI] [PubMed] [Google Scholar]
- 9. Pack-Chung E, Kurshanx PT, Dickman DK, Schwarz TL. A Drosophila kinesin required for synaptic bouton formation and synaptic vesicle transport. Nat Neurosci. 2007;10:980–989. doi: 10.1038/nn1936.. Mutations in a Kinesin-3 prevent synapse formation at the Drosophila neuromuscular junction although axon outgrowth and guidance are normal. Not only are synaptic vesicles stranded in the cell soma, but few active zones form and growth cones do not undergo the correct remodeling into boutons. The phenotype illustrates the selectivity of this Kinesin-3 motor for synaptogenesis.
- 10. Cai Q, Pan PY, Sheng ZH. Syntabulin-kinesin-1 family member 5B-mediated axonal transport contributes to activity-dependent presynaptic assembly. J Neurosci. 2007;27:7284–7296. doi: 10.1523/JNEUROSCI.0731-07.2007.. An intriguing case is made for syntabulin as an adaptor protein between kif5B and vesicles carrying active zone components. Tagged syntabulin and bassoon colocalize on a subset of PTVs that move anterogradely. RNAi against syntabulin reduced axonal and increased somatic bassoon indicating that syntabulin is not just another active zone protein but is engaged in transport.
- 11.Zhai RG, Vardinon-Friedman H, Cases-Langhoff C, Becker B, Gundelfinger ED, Ziv NE, Garner CC. Assembling the presynaptic active zone: a characterization of an active one precursor vesicle. Neuron. 2001;29:131–143. doi: 10.1016/s0896-6273(01)00185-4. [DOI] [PubMed] [Google Scholar]
- 12. Collins CA, Wairkar YP, Johnson SL, DiAntonio A. Highwire restrains synaptic growth by attenuating a MAP kinase signal. Neuron. 2006;51:57–69. doi: 10.1016/j.neuron.2006.05.026.. The ubiquitinylation of a MAPKKK can profoundly modify the anatomy of the Drosophila neuromuscular junction and the number of synapses formed. Lovely genetics establishes a pathway leading to the MAPkinase JNK. This study lays the foundation for Horiuchi et al below.
- 13.Nakata K, Abrams B, Grill B, Goncharov A, Huang X, Chisholm AD, Jin Y. Regulation of a DLK-1 and p38 MAP kinase pathway by the ubiquitin ligase RPM-1 is required for presynaptic development. Cell. 2005;120:407–420. doi: 10.1016/j.cell.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 14. Horiuchi D, Collins CA, Bhat P, Barkus RV, Diantonio A, Saxton WM. Control of a kinesin-cargo linkage mechanism by JNK pathway kinases. Curr Biol. 2007;17:1313–1317. doi: 10.1016/j.cub.2007.06.062.. JNK, a MAP kinase, is known to bind JIP1, a kinesin adaptor protein, and is transported down the axon. This paper shows that the ubiquitinylation, MAPKKK, and JNK pathway that controls synapse number at the fly NMJ also controls the association of kinesin light chain with JIP1. Kinesin-associated JNK therefore is a regulator of kinesin-cargo interactions - a possible explanation for the synaptic phenotypes of the signaling pathway.
- 15.Koushika SP. "JIP"ing along the axon: the complex roles of JIPs in axonal transport. Bioessays. 2008;30:10–14. doi: 10.1002/bies.20695. [DOI] [PubMed] [Google Scholar]
- 16.Stagi M, Gorlovoy P, Larionov S, Takahashi K, Neumann H. Unloading kinesin transported cargoes from the tubulin track via the inflammatory c-Jun N-terminal kinase pathway. Faseb J. 2006;20:2573–2575. doi: 10.1096/fj.06-6679fje. [DOI] [PubMed] [Google Scholar]
- 17.Morfini G, Pigino G, Szebenyi G, You Y, Pollema S, Brady ST. JNK mediates pathogenic effects of polyglutamine-expanded androgen receptor on fast axonal transport. Nat Neurosci. 2006;9:907–916. doi: 10.1038/nn1717. [DOI] [PubMed] [Google Scholar]
- 18. Colin E, Zala D, Liot G, Rangone H, Borrell-Pages M, Li XJ, Saudou F, Humbert S. Huntingtin phosphorylation acts as a molecular switch for anterograde/retrograde transport in neurons. EMBO J. 2008;27:2124–2134. doi: 10.1038/emboj.2008.133.. The authors show that the direction of trafficking of a vesicle can be controlled by whether or not kinesin is recruited to the vesicle. Recruitment is promoted by the phosphorylation of Huntingtin by Akt, placing Huntingtin in at a key control point for axonal transport.
- 19.Gauthier LR, Charrin BC, Borrell-Pages M, Dompierre JP, Rangone H, Cordelieres FP, De Mey J, MacDonald ME, Lessmann V, Humbert S, et al. Huntingtin controls neurotrophic support and survival of neurons by enhancing BDNF vesicular transport along microtubules. Cell. 2004;118:127–138. doi: 10.1016/j.cell.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 20. Caviston JP, Ross JL, Antony SM, Tokito M, Holzbaur EL. Huntingtin facilitates dynein/dynactin-mediated vesicle transport. Proc Natl Acad Sci U S A. 2007;104:10045–10050. doi: 10.1073/pnas.0610628104.. The authors offer a mechanism by which Huntingtin (Htt) participates in retrograde transport. Htt binds directly to dynein intermediate chain and serves as a scaffold for motor interactions with cargos. Antibodies to Htt compromised vesicle movements on microtubules in both directions, consistent with Htt interactions with kinesin as well.
- 21.Gunawardena S, Her LS, Brusch RG, Laymon RA, Niesman IR, Gordesky-Gold B, Sintasath L, Bonini NM, Goldstein LS. Disruption of axonal transport by loss of huntingtin or expression of pathogenic polyQ proteins in Drosophila. Neuron. 2003;40:25–40. doi: 10.1016/s0896-6273(03)00594-4. [DOI] [PubMed] [Google Scholar]
- 22.Jin Y, Garner CC. Molecular Mechanisms of Presynaptic Differentiation. Annu Rev Cell Dev Biol. 2008 doi: 10.1146/annurev.cellbio.23.090506.123417. [DOI] [PubMed] [Google Scholar]
- 23.Bonanomi D, Benfenati F, Valtorta F. Protein sorting in the synaptic vesicle life cycle. Prog Neurobiol. 2006;80:177–217. doi: 10.1016/j.pneurobio.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Klopfenstein DR, Vale RD. The lipid binding pleckstrin homology domain in UNC-104 kinesin is necessary for synaptic vesicle transport in Caenorhabditis elegans. Mol Biol Cell. 2004;15:3729–3739. doi: 10.1091/mbc.E04-04-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takamori S, Holt M, Stenius K, Lemke EA, Gronborg M, Riedel D, Urlaub H, Schenck S, Brugger B, Ringler P, et al. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 26.Horiuchi D, Barkus RV, Pilling AD, Gassman A, Saxton WM. APLIP1, a kinesin binding JIP-1/JNK scaffold protein, influences the axonal transport of both vesicles and mitochondria in Drosophila. Curr Biol. 2005;15:2137–2141. doi: 10.1016/j.cub.2005.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller KE, DeProto J, Kaufmann N, Patel BN, Duckworth A, Van Vactor D. Direct observation demonstrates that Liprin-alpha is required for trafficking of synaptic vesicles. Curr Biol. 2005;15:684–689. doi: 10.1016/j.cub.2005.02.061. [DOI] [PubMed] [Google Scholar]
- 28.Gindhart JG, Chen J, Faulkner M, Gandhi R, Doerner K, Wisniewski T, Nandlestadt A. The kinesin-associated protein UNC-76 is required for axonal transport in the Drosophila nervous system. Mol Biol Cell. 2003;14:3356–3365. doi: 10.1091/mbc.E02-12-0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Patel MR, Lehrman EK, Poon VY, Crump JG, Zhen M, Bargmann CI, Shen K. Hierarchical assembly of presynaptic components in defined C. elegans synapses. Nat Neurosci. 2006;9:1488–1498. doi: 10.1038/nn1806.. Examining a set of en passant synapses made by a selected C. elegans neuron, the authors focus on two active zone proteins, syd-1 and the kinesin-associated protein syd-2/liprinα, to determine which active zone and synaptic vesicle components depend on them for incorporation to the synapse. In the process, they also sort out which synaptic molecules and organelles depend on the Kinesin-3 motor unc-104.
- 30.Guillaud L, Wong R, Hirokawa N. Disruption of KIF17-Mint1 interaction by CaMKII-dependent phosphorylation: a molecular model of kinesin-cargo release. Nat Cell Biol. 2008;10:19–29. doi: 10.1038/ncb1665. [DOI] [PubMed] [Google Scholar]
- 31.Su Q, Cai Q, Gerwin C, Smith CL, Sheng ZH. Syntabulin is a microtubule-associated protein implicated in syntaxin transport in neurons. Nat Cell Biol. 2004;6:941–953. doi: 10.1038/ncb1169. [DOI] [PubMed] [Google Scholar]
- 32.Laughlin SB, de Ruyter van Steveninck RR, Anderson JC. The metabolic cost of neural information. Nat Neurosci. 1998;1:36–41. doi: 10.1038/236. [DOI] [PubMed] [Google Scholar]
- 33.Boldogh IR, Pon LA. Mitochondria on the move. Trends Cell Biol. 2007;17:502–510. doi: 10.1016/j.tcb.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 34.Pilling AD, Horiuchi D, Lively CM, Saxton WM. Kinesin-1 and Dynein are the primary motors for fast transport of mitochondria in Drosophila motor axons. Mol Biol Cell. 2006;17:2057–2068. doi: 10.1091/mbc.E05-06-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stowers RS, Megeath LJ, Gorska-Andrzejak J, Meinertzhagen IA, Schwarz TL. Axonal transport of mitochondria to synapses depends on milton, a novel Drosophila protein. Neuron. 2002;36:1063–1077. doi: 10.1016/s0896-6273(02)01094-2. [DOI] [PubMed] [Google Scholar]
- 36. Glater EE, Megeath LJ, Stowers RS, Schwarz TL. Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. J Cell Biol. 2006;173:545–557. doi: 10.1083/jcb.200601067.. Biochemical studies establish a model for mitochondrial motility in which Miro recruits Milton which binds to kinesin heavy chain. This paper also reports alternatively spliced forms of milton that differ in whether or not they recruit Khc to mitochondria.
- 37.Fransson S, Ruusala A, Aspenstrom P. The atypical Rho GTPases Miro-1 and Miro-2 have essential roles in mitochondrial trafficking. Biochem Biophys Res Commun. 2006;344:500–510. doi: 10.1016/j.bbrc.2006.03.163. [DOI] [PubMed] [Google Scholar]
- 38.Guo X, Macleod GT, Wellington A, Hu F, Panchumarthi S, Schoenfield M, Marin L, Charlton MP, Atwood HL, Zinsmaier KE. The GTPase dMiro is required for axonal transport of mitochondria to Drosophila synapses. Neuron. 2005;47:379–393. doi: 10.1016/j.neuron.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 39.Frederick RL, McCaffery JM, Cunningham KW, Okamoto K, Shaw JM. Yeast Miro GTPase, Gem1p, regulates mitochondrial morphology via a novel pathway. J Cell Biol. 2004;167:87–98. doi: 10.1083/jcb.200405100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cai Q, Gerwin C, Sheng ZH. Syntabulin-mediated anterograde transport of mitochondria along neuronal processes. J Cell Biol. 2005;170:959–969. doi: 10.1083/jcb.200506042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cho KI, Cai Y, Yi H, Yeh A, Aslanukov A, Ferreira PA. Association of the kinesin-binding domain of RanBP2 to KIF5B and KIF5C determines mitochondria localization and function. Traffic. 2007;8:1722–1735. doi: 10.1111/j.1600-0854.2007.00647.x. [DOI] [PubMed] [Google Scholar]
- 42.Wozniak MJ, Melzer M, Dorner C, Haring HU, Lammers R. The novel protein KBP regulates mitochondria localization by interaction with a kinesin-like protein. BMC Cell Biol. 2005;6:35. doi: 10.1186/1471-2121-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kang JS, Tian JH, Pan PY, Zald P, Li C, Deng C, Sheng ZH. Docking of axonal mitochondria by syntaphilin controls their mobility and affects short-term facilitation. Cell. 2008;132:137–148. doi: 10.1016/j.cell.2007.11.024.. By video microscopy, the authors show that syntaphilin is selectively on immobile mitochondria within axons and a knock-out for this gene increases the percentage of motile mitochondria. Syntaphilin, they conclude, can anchor mitochondria in place by binding them to microtubules.
- 44.Chada SR, Hollenbeck PJ. Mitochondrial movement and positioning in axons: the role of growth factor signaling. J Exp Biol. 2003;206:1985–1992. doi: 10.1242/jeb.00263. [DOI] [PubMed] [Google Scholar]
- 45.Chada SR, Hollenbeck PJ. Nerve growth factor signaling regulates motility and docking of axonal mitochondria. Curr Biol. 2004;14:1272–1276. doi: 10.1016/j.cub.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 46.Chang DT, Honick AS, Reynolds IJ. Mitochondrial trafficking to synapses in cultured primary cortical neurons. J Neurosci. 2006;26:7035–7045. doi: 10.1523/JNEUROSCI.1012-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hollenbeck PJ, Saxton WM. The axonal transport of mitochondria. J Cell Sci. 2005;118:5411–5419. doi: 10.1242/jcs.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rintoul GL, Filiano AJ, Brocard JB, Kress GJ, Reynolds IJ. Glutamate decreases mitochondrial size and movement in primary forebrain neurons. J Neurosci. 2003;23:7881–7888. doi: 10.1523/JNEUROSCI.23-21-07881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Szabadkai G, Simoni AM, Bianchi K, De Stefani D, Leo S, Wieckowski MR, Rizzuto R. Mitochondrial dynamics and Ca2+ signaling. Biochim Biophys Acta. 2006;1763:442–449. doi: 10.1016/j.bbamcr.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 50.Yi M, Weaver D, Hajnoczky G. Control of mitochondrial motility and distribution by the calcium signal: a homeostatic circuit. J Cell Biol. 2004;167:661–672. doi: 10.1083/jcb.200406038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fransson A, Ruusala A, Aspenstrom P. Atypical Rho GTPases have roles in mitochondrial homeostasis and apoptosis. J Biol Chem. 2003;278:6495–6502. doi: 10.1074/jbc.M208609200. [DOI] [PubMed] [Google Scholar]
- 52.Cox RT, Spradling AC. Milton controls the early acquisition of mitochondria by Drosophila oocytes. Development. 2006;133:3371–3377. doi: 10.1242/dev.02514. [DOI] [PubMed] [Google Scholar]
- 53.Smith MJ, Pozo K, Brickley K, Stephenson FA. Mapping the GRIF-1 binding domain of the kinesin, KIF5C, substantiates a role for GRIF-1 as an adaptor protein in the anterograde trafficking of cargoes. J Biol Chem. 2006;281:27216–27228. doi: 10.1074/jbc.M600522200. [DOI] [PubMed] [Google Scholar]
- 54.Hart GW, Housley MP, Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446:1017–1022. doi: 10.1038/nature05815. [DOI] [PubMed] [Google Scholar]
- 55.Iyer SP, Hart GW. Roles of the tetratricopeptide repeat domain in O-GlcNAc transferase targeting and protein substrate specificity. J Biol Chem. 2003;278:24608–24616. doi: 10.1074/jbc.M300036200. [DOI] [PubMed] [Google Scholar]
- 56.Murrey HE, Hsieh-Wilson LC. The chemical neurobiology of carbohydrates. Chem Rev. 2008;108:1708–1731. doi: 10.1021/cr078215f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang X, Ongusaha PP, Miles PD, Havstad JC, Zhang F, So WV, Kudlow JE, Michell RH, Olefsky JM, Field SJ, et al. Phosphoinositide signaling links O-GlcNAc transferase to insulin resistance. Nature. 2008;451:964–969. doi: 10.1038/nature06668. [DOI] [PubMed] [Google Scholar]
- 58.Morfini G, Szebenyi G, Brown H, Pant HC, Pigino G, DeBoer S, Beffert U, Brady ST. A novel CDK5-dependent pathway for regulating GSK3 activity and kinesin-driven motility in neurons. Embo J. 2004;23:2235–2245. doi: 10.1038/sj.emboj.7600237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morfini G, Szebenyi G, Elluru R, Ratner N, Brady ST. Glycogen synthase kinase 3 phosphorylates kinesin light chains and negatively regulates kinesin-based motility. Embo J. 2002;21:281–293. doi: 10.1093/emboj/21.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Diefenbach RJ, Diefenbach E, Douglas MW, Cunningham AL. The heavy chain of conventional kinesin interacts with the SNARE proteins SNAP25 and SNAP23. Biochemistry. 2002;41:14906–14915. doi: 10.1021/bi026417u. [DOI] [PubMed] [Google Scholar]
- 61.Konecna A, Frischknecht R, Kinter J, Ludwig A, Steuble M, Meskenaite V, Indermuhle M, Engel M, Cen C, Mateos JM, et al. Calsyntenin-1 docks vesicular cargo to kinesin-1. Mol Biol Cell. 2006;17:3651–3663. doi: 10.1091/mbc.E06-02-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Macioce P, Gambara G, Bernassola M, Gaddini L, Torreri P, Macchia G, Ramoni C, Ceccarini M, Petrucci TC. Beta-dystrobrevin interacts directly with kinesin heavy chain in brain. J Cell Sci. 2003;116:4847–4856. doi: 10.1242/jcs.00805. [DOI] [PubMed] [Google Scholar]
- 63.Shin H, Wyszynski M, Huh KH, Valtschanoff JG, Lee JR, Ko J, Streuli M, Weinberg RJ, Sheng M, Kim E. Association of the kinesin motor KIF1A with the multimodular protein liprin-alpha. J Biol Chem. 2003;278:11393–11401. doi: 10.1074/jbc.M211874200. [DOI] [PubMed] [Google Scholar]
- 64.Cai D, Hoppe AD, Swanson JA, Verhey KJ. Kinesin-1 structural organization and conformational changes revealed by FRET stoichiometry in live cells. J Cell Biol. 2007;176:51–63. doi: 10.1083/jcb.200605097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blasius TL, Cai D, Jih GT, Toret CP, Verhey KJ. Two binding partners cooperate to activate the molecular motor Kinesin-1. J Cell Biol. 2007;176:11–17. doi: 10.1083/jcb.200605099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rong J, Li SH, Li XJ. Regulation of intracellular HAP1 trafficking. J Neurosci Res. 2007;85:3025–3029. doi: 10.1002/jnr.21326. [DOI] [PubMed] [Google Scholar]