Abstract
Objective
Human immunodeficiency virus type 1 blood plasma viral load is correlated with the sexual transmission of HIV, although transmission from men involves virus from semen instead of blood. We quantified HIV-1 RNA in the blood and semen of men who did or did not transmit HIV to their sex partners. We compared the relationships of HIV-1 transmission risk with blood plasma viral load, seminal plasma viral load, herpes simplex virus 2 serostatus and other factors.
Design
A case–control study.
Methods
Participants were men evaluated for primary HIV infection and their recent male sex partners. They were interviewed, and clinical specimens were collected. Epidemiologic and phylogenetic linkages were determined by history and molecular techniques. Couples were grouped on the basis of transmission after exposure. Fisher’s exact test and Wilcoxon tests were used for comparisons between groups. Multivariable logistic regressions were fit to identify independent predictors of transmission.
Results
HIV-transmitting partners (n=15) had a higher median seminal plasma viral load (P<0.015) and median blood plasma viral load (P<0.001) than nontransmitting partners (n=32). Herpes simplex virus 2 serostatus was associated with transmission only when the HIV-infected source partner was herpes simplex virus 2 seropositive and the HIV-exposed partner was not (odds ratio 16, P<0.03). Adjusting for other factors, HIV transmission was significantly associated with blood plasma viral load (odds ratio 13.4, P<0.02) but not seminal plasma viral load (odds ratio 0.69, P=not significant).
Conclusion
Blood and seminal plasma viral load were both associated with human immunodeficiency virus type 1 transmission, but blood plasma viral load was the stronger predictor in this cohort. Herpes simplex virus 2 coinfection was associated with the risk of transmission but not acquisition of human immunodeficiency virus type 1.
Keywords: herpes simplex virus 2, HIV, men who have sex with men, seminal viral load, transmission
Introduction
The majority of new HIV infections originate from sexual exposures to virus transmitted from semen [1]; however, not every exposure results in new infection. Many virological, biological, and behavioral factors are associated with risk of transmission, like human immunodeficiency virus type 1 (HIV-1) subtype [2,3], mode of sexual exposure [4], condom use [5], penile circumcision [6,7], mucosal inflammation [8], use of drug therapy, stage of infection [9], hormonal factors [10] or host genetic background [11,12]. The HIV-seropositive source partner’s infectiousness may be the most important determinant. Stage of infection [9,13] and HIV-1 blood plasma viral load (BPVL) [14] correlate strongly with the risk of sexual transmission even though it involves virus from genital secretions rather than blood. Although the seminal plasma viral load (SPVL), reflecting the concentration of cell-free HIV-1 in semen, roughly correlates to BPVL [15,16], no empirical studies to date have directly examined the relationship between SPVL and HIV-1 transmission [17].
Materials and methods
Participants
Men who have sex with men (MSM) being evaluated for possible HIV-1 infection were recruited for participation in the San Diego HIV Transmission Study or the San Diego cohort of the Acute Infection and Early Disease Research Program (AIEDRP). Consent was obtained from eligible participants, observing all guidelines pertaining to research involving individuals as outlined by the US Department of Health and Human Services and the University of California, San Diego, Human Research Protection Program. Participants were interviewed and examined by research staff, who administered pretest and posttest HIV counseling and treatment for infection with Neisseria gonorrhea, Chlamydia trachomatis, or Treponema pallidum, as indicated.
Biological samples
Participants submitted urine specimens and then blood and semen samples at specified intervals. Samples of blood and semen were collected and processed as described previously [18] Reference laboratories measured Rapid Plasma Reagin titers, total CD4+ T-cell counts, and performed nucleic acid amplification tests (LabCorp, San Diego, California,USA) on urine to assess for gonorrhea or Chlamydia.HSV-2 serostatuswas determined as previously described [19].
Human immunodeficiency virus type 1 RNA
HIV-1 RNAwas extracted and quantified from 500µl of blood plasma (Amplicor HIV-1 Monitor Test; Roche Molecular Systems Inc., Alameda, California, USA) and from 500µl of seminal plasma (qc-HIV Assay; GenProbe Inc., San Diego, California, USA) according to the manufacturers’ protocols.
Transmission confirmation
Population-based pol sequencing of HIV-1RNAextracted from blood plasma was used to confirm transmission with maximum-likelihood phylogenetic analysis as previously described [18]. Direction of transmission was based upon clinical history and the estimated duration of infection of the partners, with the more recently infected partner termed as ‘recipient’ and the more chronically infected partner as ‘source.’
Estimations of timing
The stage of infectionwas defined as chronic (>12months) or recent, the latter incorporating both acute and early infections, using the previously described AIEDRP algorithm for estimating the date of infection (EDI) [20,21]. The number of days after HIV-1 exposure until collection of HIV-infected source partners’ samples was estimated for each pair. For the group in which viral transmission occurred, the date of the sexual exposure was taken to be the same as the EDI. When viral transmission between putative partners was not confirmed, there was no EDI. For HIV-seronegative partners in the nontransmitting group, the date of sexual exposure was taken as 14 days before screening, on the basis of participants having been instructed to recruit only people with whom they had had sex within 2 weeks of screening. Some men identified sex partners who were already participants in the study and had biological samples in storage. Samples collected closest to the estimated date of exposure were used in the analysis. Thus, sometimes a HIV-infected source partner’s samples were collected before the HIV-exposed seronegative partners’ enrollment. In such cases, the number of days ‘after’ enrollment until collection was assigned a negative value.
Statistical analysis
All viral load data were log-transformed. Bivariate comparisons for both groups were tested using Fisher’s exact test and Wilcoxon tests (Table 1). We examined bivariate relationships between HIV-infected source partner characteristics and HIV transmission in contingency table analysis, and multivariable logistic regressions were then fit. Vectors of HSV-2 serostatus and presence of sexually transmitted infection (STI) were created to ascertain influence of partner discordance. STAT version 9.1 (SAS Institute Inc., Cary, North Carolina, USA) was used.
Table 1.
Comparisons between groups.
| MSM | Transmitting | Nontransmitting |
|---|---|---|
| Number of pairs | 15 | 32 |
| Age of source, years (mean, range) | 33 (22–41) | 30 (20–51) |
| Age of recipient, years (mean, range) | 34 (20–47) | 35 (19–57) |
| Source taking ART | 1/15 | 5/31 |
| HSV-2(+) source taking (val)acyclovir | 1/8 | 1/14 |
| HSV-2(+) recipient taking (val)acyclovir | 1/5 | 1/13 |
| HSV-2 serostatus, source (+) recipient (+) | 3/15 | 10/29 |
| HSV-2 Serostatus, source (+) recipient (−)a | 5/15 | 1/29 |
| HSV-2 Serostatus, source (−) recipient (−) | 5/15 | 16/29 |
| HSV-2 Serostatus, source (−) recipient (+) | 2/15 | 2/29 |
| Bacterial STI, infected source | 1/14 | 4/30 |
| Bacterial STI, infected recipienta | 7/14 | 2/29 |
| Estimated number of days from sexual exposure | 103 days (20–146 days after) | 24 days (107 days before–42 days after) |
| that source partners’ genital secretions | ||
| were collected, (median, range)a | ||
| Recently HIV-infected sourcea | 4/15 | 19/32 |
| CD4, cells/µl (median, range) | 469 (147–737) | 548 (229–927) |
| Log10 BPVL, copies/ml (median, range)a | 4.74 (4.12–6.66) | 4.07 (1.70–5.83) |
| Log10 SPVL, copies/ml (median, range) | 3.63 (2.04–4.84) | 2.58 (1.68–5.05) |
ART, antiretroviral therapy; BPVL, blood plasma viral load; HSV-2, herpes simplex virus 2; SPVL, seminal plasma viral load; STI, sexually transmitted infection.
Differences between transmitting and nontransmitting groups were not statistically significant (P>0.05) except for these. Bivariate analysis determined an odds ratio (OR) of 16 [confidence interval (CI) 1.49–171, P<0.03] for HIV transmission with the source (+) recipient (−) pattern of HSV-2 serotypes. Bacterial STI, when the HIV-exposed recipient partner tested positive, was associated with HIV transmission (P<0.003) using Fisher’s Exact test. The estimated number of days between sexual exposure and collection of genital secretions was longer for the transmitting group (P<0.001) by the Wilcoxon Test. Multivariate analysis determined that more recent HIV infection (OR=0.09, CI 0.01–0.93, P<0.05) and higher BPVL (OR=13.4, CI 1.52–118, P<0.02) were each independently associated with an increased risk of HIV transmission.
Results
Sexual partners were characterized and sorted into groups of transmitting and nontransmitting pairs. For the transmitting group (n=15 pairs) and the non-transmitting group (n=32 pairs) the respective mean ages of HIV-infected source (33 and 30 years) and HIV-exposed (34 and 35 years) partners, median CD4 cell counts (469 and 548 cells/µl), and rates of antiviral (HIV-1 and HSV-2) treatment were similar (Table 1).
Bacterial STI were more common in the group of transmitting pairs, particularly when the HIV-exposed partner tested positive (P<0.003). HSV-2 serostatus was associated with HIV-1 transmission only when the HIV-infected source partner was HSV-2 seropositive and the HIV-exposed partner was not (P<0.014). No factor was associated with transmission in multivariate analysis.
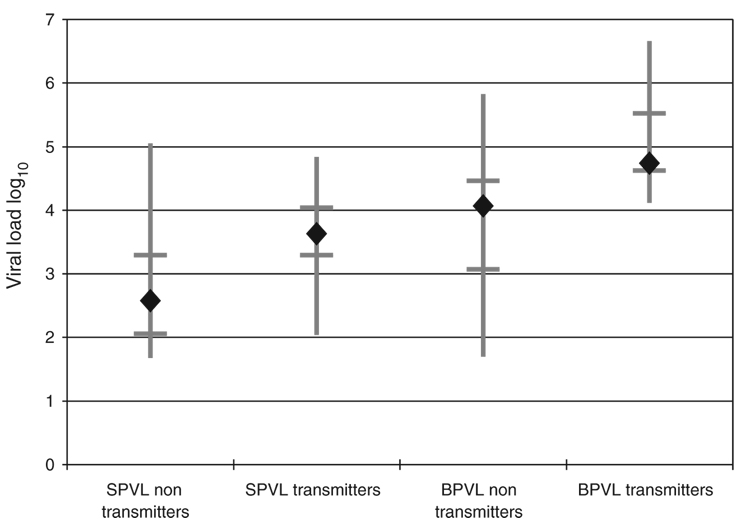
The median BPVL of the transmitting group was higher than that of the nontransmitting group (log10 4.74 vs. log10 4.07, P<0.001), as was the median SPVL (log10 3.63 vs. log10 2.58, P<0.015) (Fig. 1). The SPVL range, however, for the transmitting group (log10 2.04–4.84) was entirely within the range of the nontransmitting group (log10 1.68–5.05). This was not the case with BPVL, wherein the transmitting group demonstrated a narrower range of values with a higher maximum (log10 4.12–6.66) than the nontransmitting group (log101.70–5.83). SPVL correlated with BPVL in both the nontransmitting and transmitting groups of HIV-infected source partners (R2 correlations of 0.49 and 0.22, respectively). These correlations were similar to previous report [17]. Multivariate logistic regression analysis adjusted for race, ethnicity, age, BPVL, SPVL, stage of infection with HIV, and source-positive and recipient-negative HSV-2 serostatus with an area under the ROC curve (AUROC) of 0.92. Using multivariate analysis to identify independent risk associations, higher BPVL was associated with transmission [odds ratio (OR) 13.4, confidence interval (CI) 1.52–118, P<0.02] and SPVL was not (OR 0.99, CI 0.16–2.93, P>0.05) (Table 1).
Fig. 1. Comparisons of viral loads of HIV-1.
Vertical bars represent the ranges of seminal plasma viral loads (SPVL) and blood plasma viral loads (BPVL) for the two groups. Horizontal bars denote the first and third quartiles. Diamonds indicate the median viral load for the group.
The estimated time between exposures and collecting HIV-infected source partners’ semen differed (P<0.001, Table 1) between the two groups. For the HIV-transmitting partners, samples were collected after a median of 103 days (range 20–146 days). For the nontransmitting partners, samples were collected after a median of just 24 days (range 107 days before to 42 days after the exposure).
Discussion
We describe two groups of MSM in which a recently HIV-exposed individual identified an HIV-infected sex partner: one group in which HIV transmission occurred and one group in which it did not. As participants in this case–control study were identified recently after exposure, we could characterize and compare the relationships between SPVL, BPVL, and other biologic factors with the risk of HIV-1 transmission. Here, HSV-2 infection was associated with HIV-1 transmission, but only in HSV-2 serodiscordant couples whose HIV-1-infected source partner was HSV-2 seropositive and HIV-exposed partner was not. HIV-uninfected participants who were HSV-2 seropositive were not more likely to acquire HIV-1 from their sex partners than the HSV-2-seronegative participants, consistent with recent reports in which treatment of HSV-2-seropositive participants with acyclovir did not reduce their rate of HIV-1 acquisition [22,23]. These results suggest that asymptomatic HSV-2 infection may have an impact on the risk of transmission rather than acquisition of HIV-1.
Symptomatic HSV-2 episodes, with genital ulceration and viral shedding, are associated with a greater increase in the risk of HIV-1 acquisition [24] than the risk during asymptomatic reactivations of chronic HSV-2 [25]. The relative risk of transmitting HIV-1 during an asymptomatic reactivation of chronic HSV-2 is unknown. An increase in HIV-1 RNA levels has been reported in the genital secretions of persons with untreated HSV-2 infection [26,27], but what effect treatment of asymptomatic HSV-2 may have on SPVL, or whether a threshold SPVL exists below which transmission does not occur, has not been determined. Future studies will need to evaluate shedding of HSV-2 in genital secretions among transmitting and nontransmitting HIV-infected source partners and correlate HSV-2 shedding with SPVL. A role for suppressive therapy of asymptomatic HSV-2 infection may exist, but it may be indicated for HIV-infected people to reduce their risk of transmitting HIV-1 rather than for HIV-uninfected people to reduce their risk of acquiring HIV-1, as has been investigated to date. This must be determined empirically.
Although sexual transmissions of HIV-1 are mediated by exposure to virus in genital secretions and not the blood, BPVL statistically correlated better than SPVL with the risk of HIV-1 transmission in this study. This may well be artifactual owing to the lag between sexual exposures and specimen collections. Longitudinal quantification of HIV-1 from paired blood and semen samples collected from HIV-infected men on antiretroviral therapy (ART) have shown that SPVL vary over time and over a broader range than matched BPVL [28]. Because SPVL may fluctuate so much over time, it is crucial to report the time intervals between sexual exposure, sample collection, and the estimated date of infection of the source partner. Current methods for EDI are not precise, but reporting the EDI is important nonetheless. In this report, we utilized the AIEDRP algorithm, which is well suited for our cohort, although other methods [29,30] may be more appropriate in other contexts. Consistent reporting of these time intervals will be necessary to enable meaningful comparisons between studies when considering the relationships between BPVL, SPVL, and transmission risk. More study participants with collections timed more closely to the time of transmission are needed to better delineate these relationships.
With our report, the concentration of cell-free HIV-1 in the semen of HIV-infected source partners has been correlated with the risk of HIV-1 transmission between MSM. The circumstances under which HIV-1 transmission occurs from seminal cell-associated virus and those under which it is mediated by seminal plasma virus, however, have yet to be defined. This study has only correlated SPVL with transmission; should transmitted HIV-1 prove to have its origin among the cell-associated virus instead, then the amount of cell-free virus (SPVL) would be less relevant and may explain why BPVL was a better predictor of HIV-1 transmission in this cohort. This has obvious implications for efforts directed at preventing transmission of HIV-1 by reducing SPVL through pharmacotherapy or by targeting cell-associated virus with the use of a cytotoxic T lymphocyte (CTL)-based vaccine. Other limitations of our study includes the relatively small size of the cohort, the lack of information about sexual positioning, frequency of intercourse in the partner pairs, HSV-2 shedding, and the human leukocyte antigen (HLA) similarity within partner pairs. A better understanding of these determinants of HIV-1 infection should provide crucial insights for the prevention of sexual transmissions from the male genital tract and for reducing the worldwide burden of disease caused by HIV/AIDS.
Acknowledgements
The present work was supported by: National Institutes of Health grants AI07384, 5K23AI055276, AI69432, AI38858, AI043638, AI29164, AI27670, AI57167, and MH62512; CDC contract 200-2002-00656; the UCSD Center for AIDS Research (AI36214, AI29164, and AI047745); and the San Diego Veterans Affairs Healthcare System.
S.J.L. and D.M.S. coordinated recruitment of participants who provided the information and biological samples used for analysis. G.K.H. and D.S.M. established the phylogenetic linkages. E.R.C. determined HSV-2 serostatus patterns and performed the statistical analyses. C.T.N. was instrumental in quantifying SPVLs. D.M.B. analyzed data and wrote the paper; all authors contributed to the final manuscript. D.D.R., S.J.L., and D.M.S. supervised the entire project from its inception.
References
- 1.UNAIDS. 2006 Report on the global AIDS epidemic. Geneva: UNAIDS; 2006. [Google Scholar]
- 2.Kunanusont C, Foy HM, Kreiss JK, Rerks-Ngarm S, Phanuphak P, Raktham S, et al. HIV-1 subtypes and male-to-female transmission in Thailand. Lancet. 1995;345:1078–1083. doi: 10.1016/s0140-6736(95)90818-8. [DOI] [PubMed] [Google Scholar]
- 3.Hudgens MG, Longini IM, Jr, Vanichseni S, Hu DJ, Kitayaporn D, Mock PA, et al. Subtype-specific transmission probabilities for human immunodeficiency virus type 1 among injecting drug users in Bangkok, Thailand. Am J Epidemiol. 2002;155:159–168. doi: 10.1093/aje/155.2.159. [DOI] [PubMed] [Google Scholar]
- 4.Royce RA, Sena A, Cates W, Cohen MS. Sexual transmission of HIV. N Engl J Med. 1997;336:1072–1078. doi: 10.1056/NEJM199704103361507. [DOI] [PubMed] [Google Scholar]
- 5.Pinkerton SD, Abramson PR. Effectiveness of condoms in preventing HIV transmission. Soc Sci Med. 1997;44:1303–1312. doi: 10.1016/s0277-9536(96)00258-4. [DOI] [PubMed] [Google Scholar]
- 6.Cameron DW, D’Costa L, Maitha G, Cheang M, Piot P, Simonsen JN, et al. Female to male transmission of human immunodeficiency virus type 1: risk factors for seroconversion in men. Lancet. 1989;334:403–407. doi: 10.1016/s0140-6736(89)90589-8. [DOI] [PubMed] [Google Scholar]
- 7.Sullivan PS, Kilmarx PH, Peterman TA, Taylor AW, Nakashima AK, Kamb ML, et al. Male circumcision for prevention of HIV transmission: what the new data mean for HIV prevention in the United States. PLoS Med. 2007;4:e223. doi: 10.1371/journal.pmed.0040223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen MS. Sexually transmitted diseases enhance HIV transmission: no longer a hypothesis. Lancet. 1998;351 Suppl 3:5–7. doi: 10.1016/s0140-6736(98)90002-2. [DOI] [PubMed] [Google Scholar]
- 9.Wawer MJ, Gray RH, Sewankambo NK, Serwadda D, Li X, Laeyendecker O. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191:1403–1409. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 10.Coombs RW, Reichelderfer PS, Landay AL. Recent observations on HIV type-1 infection in the genital tract of men and women. AIDS. 2003;17:455–480. doi: 10.1097/00002030-200303070-00001. [DOI] [PubMed] [Google Scholar]
- 11.Paxton WA, Kang S, Koup RA. The HIV type 1 coreceptor CCR5 and its role in viral transmission and disease progression. AIDS Res Hum Retroviruses. 1998;14 Suppl 1:S89–S92. [PubMed] [Google Scholar]
- 12.Carrington M, Nelson GW, Martin MP, Kissner T, Vlahov D, Goedert JJ, et al. HLA and HIV-1: heterozygote advantage and B*35-Cw*04 disadvantage. Science. 1999;283:1748–1752. doi: 10.1126/science.283.5408.1748. [DOI] [PubMed] [Google Scholar]
- 13.Pinkerton SD. Probability of HIV transmission during acute infection in Rakai, Uganda. AIDS Behav. 2007 doi: 10.1007/s10461-007-9329-1. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 15.Vernazza PL, Gilliam BL, Dyer J, Fiscus SA, Eron JJ, Frank AC, Cohen MS. Quantification of HIV in semen: correlation with antiviral treatment and immune status. AIDS. 1997;11:987–993. [PubMed] [Google Scholar]
- 16.Pinto-Neto LF, Vieira NF, Soprani M, Cunha CB, Dietze R, Ribeiro-Rodrigues R. Lack of correlation between seminal and plasma HIV-1 viral loads is associated with CD4 T cell depletion in therapy-naive HIV-1Rpatients. Mem Inst Oswaldo Cruz. 2002;97:563–567. doi: 10.1590/s0074-02762002000400021. [DOI] [PubMed] [Google Scholar]
- 17.Kalichman S, Di Berto G, Eaton L. Human immunodeficiency virus viral load in blood plasma and semen: review and implications of empirical findings. Sex Transm Dis. 2008;35:55–60. doi: 10.1097/olq.0b013e318141fe9b. [DOI] [PubMed] [Google Scholar]
- 18.Smith DM, Wong JK, Shao H, Hightower GK, Mai SHT, Moreno JM, et al. Long-term persistence of transmitted HIV drug resistance in male genital tract secretions: implications for secondary transmission. J Infect Dis. 2007;196:356–360. doi: 10.1086/519164. [DOI] [PubMed] [Google Scholar]
- 19.Cachay ER, Frost SD, Richman DD, Smith DM, Little SJ. Herpes simplex virus type 2 infection does not influence viral dynamics during early HIV-1 infection. J Infect Dis. 2007;195:1270–1277. doi: 10.1086/513568. [DOI] [PubMed] [Google Scholar]
- 20.Smith DM, Strain MC, Frost SD, Pillai SK, Wong JK, Wrin T, et al. Lack of neutralizing antibody response to HIV-1 predisposes to superinfection. Virology. 2006;355:1–5. doi: 10.1016/j.virol.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 21.Kothe D, Byers RH, Caudill SP, Satten GA, Janssen RS, Hannon WH, Mei JV. Performance characteristics of a new less sensitive HIV-1 enzyme immunoassay for use in estimating HIV seroincidence. J Acquir Immune Defic Syndr. 2003;33:625–634. doi: 10.1097/00126334-200308150-00012. [DOI] [PubMed] [Google Scholar]
- 22.Celum C, Wald A, Hughes J, Sanchez J, Reid S, Delaney-Moretlwe S, et al. 15th Conference on Retroviruses and Opportunistic Infections. Boston, MA.: 2008. HSV-2 suppressive therapy for prevention of HIV acquisition: results of HPTN 039. [Google Scholar]
- 23.Watson-Jones D, Weiss HA, Rusizoka M, Changalucha J, Baisley K, Mugeye K, et al. Effect of herpes simplex suppression on incidence of HIV among women in Tanzania. N Engl J Med. 2008;358:1560–1571. doi: 10.1056/NEJMoa0800260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wald A, Link K. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. J Infect Dis. 2002;185:45–52. doi: 10.1086/338231. [DOI] [PubMed] [Google Scholar]
- 25.Corey L, Wald A, Celum CL, Quinn TC. The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: a review of two overlapping epidemics. J Acquir Immune Defic Syndr. 2004;35:435–445. doi: 10.1097/00126334-200404150-00001. [DOI] [PubMed] [Google Scholar]
- 26.Mbopi-Keou FX, Gresenguet G, Mayaud P, Weiss HA, Gopal R, Matta M, et al. Interactions between herpes simplex virus type 2 and human immunodeficiency virus type 1 infection in African women: opportunities for intervention. J Infect Dis. 2000;182:1090–1096. doi: 10.1086/315836. [DOI] [PubMed] [Google Scholar]
- 27.Nagot N, Ouedraogo A, Foulongne V, Konate I, Weiss HA, Vergne L, et al. Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus. N Engl J Med. 2007;356:790–799. doi: 10.1056/NEJMoa062607. [DOI] [PubMed] [Google Scholar]
- 28.Pinto Neto LFdS, Vieira NFR, Soprani M, Cunha CB, Cabral VP, Dietze R, Ribeiro-Rodrigues R. Longitudinal comparison between plasma and seminal HIV-1 viral loads during antiretroviral treatment. Rev Soc Bras Med Trop. 2003;36:689–694. doi: 10.1590/s0037-86822003000600008. [DOI] [PubMed] [Google Scholar]
- 29.Schupbach J, Gebhardt MD, Tomasik Z, Niederhauser C, Yerly S, Bürgisser P, et al. Assessment of recent HIV-1 infection by a line immunoassay for HIV-1/2 confirmation. PLoS Med. 2007;4:e343. doi: 10.1371/journal.pmed.0040343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parekh BS, McDougal JS. Application of laboratory methods for estimation of HIV-1 incidence. Indian J Med Res. 2005;121:510–518. [PubMed] [Google Scholar]