Abstract
1-β-d-Arabinofuranosylcytosine (Ara-C) is a nucleoside analog commonly used in the treatment of leukemias. Ara-C inhibits DNA polymerases and can be incorporated into DNA. Its mechanism of cytotoxicity is not fully understood. Using oligonucleotides and purified human topoisomerase I (top1), we found a 4- to 6-fold enhancement of top1 cleavage complexes when ara-C was incorporated at the +1 position (immediately 3′) relative to a unique top1 cleavage site. This enhancement was primarily due to a reversible inhibition of top1-mediated DNA religation. Because ara-C incorporation is known to alter base stacking and sugar puckering at the misincorporation site and at the neighboring base pairs, the observed inhibition of religation at the ara-C site suggests the importance of the alignment of the 5′-hydroxyl end for religation with the phosphate group of the top1 phosphotyrosine bond. This study also demonstrates that ara-C treatment and DNA incorporation trap top1 cleavage complexes in human leukemia cells. Finally, we report that camptothecin-resistant mouse P388/CPT45 cells with no detectable top1 are crossresistant to ara-C, which suggests that top1 poisoning is a potential mechanism for ara-C cytotoxicity.
Keywords: camptothecin, DNA repair, DNA damage, nucleoside analog
DNA topoisomerases I (top1) are essential and ubiquitous enzymes (1, 2). They are critical for DNA replication and transcription by regulating the topological state of DNA by means of reversible transesterification reactions (3–5). Eukaryotic top1 reversibly cleaves one strand of the DNA by binding covalently to the 3′ end of the broken DNA (6). This intermediate is referred to as the top1 cleavage complex. Top1 also mediates religation of the DNA. Under normal conditions, the religation step of the equilibrium is favored and only a small fraction of the DNA is cleaved (4). Top1 inhibitors, such as camptothecin (CPT) and its derivatives, stabilize (trap) the cleavage complexes by inhibiting top1-mediated DNA religation (7, 8). Trapping of cleavage complexes by CPT converts the top1 enzyme into a cellular poison, and top1-mediated DNA damage probably results from replication or transcription complex collisions with CPT-arrested top1-DNA covalent complexes (7, 8).
DNA damages, such as base mismatches, abasic sites, UV photo-lesions, and ethenoadenine adducts, can also stabilize top1 cleavage complexes by inhibiting top1-mediated DNA religation (9–11). Oxidized bases, and benzo[a]pyrene adducts, on the other hand, enhance top1 cleavage complexes by enhancing the cleavage step (forward rate) of the nicking-closing reaction (12, 13).
1-β-d-Arabinofuranosylcytosine (Ara-C) is one of the most potent antitumor agents for the treatment of acute leukemias and other hematopoietic malignancies (14). Ara-C is a nucleoside analog that differs from cytosine by the presence of a hydroxyl group at the 2′ position of the sugar residue (Fig. 1A). Its active metabolite, ara-CTP is a competitive inhibitor of DNA polymerase α and, to a lesser extent, polymerase β (15–18). At relatively low concentrations, ara-CTP slows down but does not halt DNA synthesis, and is incorporated into DNA (19, 20). Interestingly, cell killing and DNA synthesis inhibition by ara-C can be observed under such conditions (16, 21), suggesting that other factors than chain termination are involved in ara-C-mediated cytotoxicity.
Figure 1.
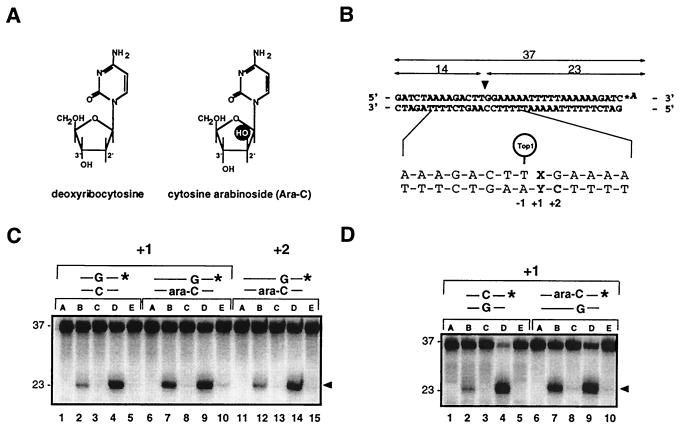
Enhancement of top1 cleavage complexes by ara-C incorporation at the +1 position of a top1 cleavage site. (A) Structures of deoxycytidine (C) and ara-C. (B) Modified Tetrahymena hexadecameric rDNA sequence with a strong top1 cleavage site (10, 31) indicated by the arrowhead was labeled with [32P]cordycepin (*A) at the 3′ terminus of the scissile (upper) strand. Top1 cleavage yields a 23-mer product. Oligonucleotides were synthesized with either C or ara-C at the indicated positions. (C) Enhancement of top1 cleavage by ara-C at the +1 but not at the +2 position of the nonscissile strand. Oligonucleotides with the indicated base pair at the +1 or +2 position relative to the top1 cleavage site are shown above lanes. (D) Enhancement of top1 cleavage by ara-C at the +1 position of the scissile strand. For each oligonucleotide used in C and D: Lanes A, DNA alone; lanes B and C, + top1; lanes D and E, + top1 + 10 μM CPT. Reactions were performed at 25°C for 15 min and stopped either immediately with 0.5% SDS (lanes B and D) or were first incubated with 0.5 M NaCl (final concentration) for an additional 30 min at 25°C before addition of 0.5% SDS (lanes C and E).
Because ara-C incorporation alters DNA structure by changing the backbone torsion angles and base stacking (22) and because the top1 nicking-closing activity is very sensitive to DNA structure alterations (9–12, 23, 24), we investigated the effects of ara-C incorporation on the activity of purified human top1. In this report, we demonstrate that top1 cleavage complexes can be trapped by ara-C incorporation immediately 3′ from a top1 cleavage site in oligonucleotides. We also found that top1 cleavage complexes can be induced in ara-C-treated human cells and that leukemia cells with no detectable top1 are crossresistant to ara-C.
Materials and Methods
Chemicals and Enzymes.
CPT was provided by M. E. Wall (Research Triangle Institute, Research Triangle Park, NC). Ara-C and etoposide (VP-16) were purchased from Sigma. Ten or 100 mM aliquots of drugs in DMSO were stored at −20°C, thawed, and diluted just before use. [14C]thymidine, [methyl-3H]thymidine, and [α-32P]cordycepin 5′-triphosphate were purchased from New England Nuclear. Polyacrylamide was purchased from Bio-Rad. Terminal deoxynucleotidyl transferase and T4 polynucleotide kinase were purchased from GIBCO/BRL. Human recombinant top1 was purified from Sf9 cells by using a baculovirus construct for the N terminus-truncated human top1 cDNA (12, 25, 26). The purified recombinant human Y727F top1 mutant was a kind gift from Lance Stewart (Emerald Biostructures, Bainbridge Island, WA).
Cell Lines and Cytotoxicity Assays.
P388 and P388/CPT45 mouse leukemia cells were a kind gift from Michael R. Mattern and Randal K. Johnson (SmithKline Beecham). P388/CPT45-resistant cells were obtained by exposing CPT-5 cell lines (27, 28) to stepwise increasing concentrations of CPT until they grew in the presence of 45 μM of CPT. Human leukemia CEM and P388 cells were cultured in RPMI 1640 medium (Life Technologies, Gaithersburg, MD) containing 10% heat-inactivated FCS and 2 mM glutamine in a 5% CO2 incubator at 37°C. P388/CPT45 cells have a reduced doubling-time (40 h as compared with 16 h for P388 cells). Cytotoxicity in P388 cell lines was measured by using standard MTT assays after continuous treatment with the drug for 2.5 doubling time (3 days for P388 and 5 days for P388/45 cells). IC50s are the mean of two independent experiments.
Oligonucleotide Labeling and Annealing Procedures.
HPLC-purified oligonucleotides were purchased from Midland Certified Reagent (Midland, TX). Fig. 1B shows the full 36-bp duplex sequence used in standard experiments. Suicide substrates shown in Fig. 2C were generated by annealing a 3′ end-labeled 19-mer upper strand [5′-GATCTAAAAGACTTGGAA(A)-3′, where (A) corresponds to the α-32P-labeled cordycepin] with the 36-bp lower strand shown in Fig. 1B. 3′ Labeling and 5′ phosphorylation of single-stranded oligonucleotides and annealing were performed as described (10).
Figure 2.
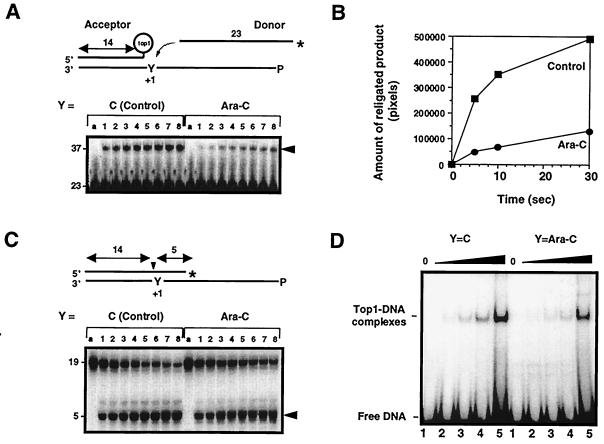
Ara-C incorporation at the +1 position of a top1 cleavage site inhibits the religation of top1 cleavage complexes. (A) Ara-C inhibits the religation of top1 cleavage complexes. Acceptors were generated from substrates shown in C (see text for details). The unlabeled acceptors were then incubated with 10-fold excess of 3′-labeled, 23-mer donor strand for 5 s, 10 s, 0.5 min, 1 min, 3 min, 5 min, 10 min, or 30 min (lanes 1–8, respectively), and reactions were stopped with 0.5% SDS. (B) Quantitation of the gel shown in A. (C) Kinetics of top1-induced DNA cleavage. Oligonucleotides containing either C or ara-C at the +1 position were incubated with top1 for 5 s, 10 s, 0.5 min, 1 min, 3 min, 5 min, 10 min, or 30 min (lanes 1–8, respectively), and reactions were stopped with 0.5% SDS. (D) Effects of Ara-C incorporation on noncovalent top1 binding to DNA. Oligonucleotides with C or ara-C at the +1 position of the nonscissile strand (see Fig. 1B) were incubated with 0, 25, 50, 100, or 250 ng of purified human top1Y727Fp for 5 min at 25°C (lanes 1–5, respectively), and electromobility shift assay was performed. (A and C) Lanes a represent the DNA alone.
Top1 Reactions.
DNA substrates (approximately 50 fmol/reaction) were incubated with 5 ng of top1 with or without CPT for the indicated times at 25°C in reaction buffer [10 mM Tris⋅HCl (pH 7.5)/50 mM KCl/5 mM MgCl2/0.1 mM EDTA/15 μg/ml BSA]. Reactions were stopped by adding SDS (final concentration 0.5%). For the reversal experiments, the SDS stop was preceded by the addition of 0.5 M NaCl. For electromobility shift assay, approximately 25 fmol of 3′-end-labeled duplex DNA (Fig. 1B) were incubated with the human Y727F mutant top1 in 50 mM Tris⋅HCl (pH 7.5) in the presence of 50 ng of double-stranded poly dI-dC DNA for 5 min at 25°C. Reaction products were loaded on 6% nondenaturing polyacrylamide gels as described (12).
Gel Electrophoresis and Analysis of Cleavage Products.
Before loading samples to gels, 3.3 vol of Maxam Gilbert loading buffer (98% formamide/0.01 M EDTA/10 mM NaOH/1 mg/ml xylene cyanol/1 mg/ml bromophenol blue) were added to reaction mixtures. Sixteen or 20 percent denaturing polyacrylamide gels (7 M urea) were run at 40 V/cm at 50°C for 1–2 h. After drying the gels on 3MM Whatman paper sheets, imaging and quantitations were performed by using a PhosphorImager (Molecular Dynamics).
Detection of Covalent Top1-DNA Complexes in CEM Cells.
Top1-DNA adducts were isolated by using the in vivo complex of enzyme (ICE) bioassay (29, 30). Briefly, 106 treated or untreated cells were pelleted and immediately lysed with 1 ml of 1% sarkosyl. After homogenization with a Dounce, cell lysates were gently layered on step gradients containing four different CsCl solutions (2 ml of each) of the following densities: 1.82, 1.72, 1.50, and 1.45 (30). Tubes were centrifuged at 165,000 × g in a Beckman SW40 rotor for 24 h at 20°C. Half-milliliter fractions were collected from the bottom of the tubes. Aliquots of each fraction (100 μl) were diluted with an equal volume of 25 mM sodium phosphate buffer (pH 6.5) and applied to Immobilon-P membranes (Millipore) by using a slot-blot vacuum manifold. Detection of topoisomerase-DNA adducts was performed by Western blotting by using the top1 mAb C21 obtained from Yung-Chi Cheng (Yale University, New Haven, CT) or a top2 mAb from TopoGEN (Columbus, OH) according to standard procedures.
Results
Ara-C Incorporation Enhances Reversible Top1 Cleavage Complexes.
We first investigated the effects of ara-C on recombinant purified human top1 in vitro. We used oligonucleotides where ara-C was introduced either in the scissile or the nonscissile strand of a well-characterized top1 cleavage site (12, 31) (Fig. 1). As shown in Fig. 1C, incorporation of ara-C on the nonscissile strand at the +1 position relative to the top1 site resulted in a 4- to 6-fold increase of cleavage complexes (Fig. 1C, compare lanes 2 and 7). A comparable increase was observed when ara-C was incorporated at the same position on the scissile strand (Fig. 1D, compare lanes 2 and 7). However, ara-C incorporation at the +2 position of the nonscissile strand did not affect top1-mediated DNA cleavage (Fig. 1C, compare lanes 2 and 12). We then tested whether CPT activity would be affected by ara-C incorporation at the +1 position, since two recent studies hypothesized that CPT contacts top1-DNA complex at the +1 base immediately flanking the cleavage site (32, 33). Incorporation of ara-C at the +1 position did not inhibit CPT activity in the different conditions of enzyme-DNA ratios used (Fig. 1, and data not shown).
The equilibrium between top1-mediated DNA cleavage and religation can be shifted toward religation upon addition of salt, which presumably prevents enzyme-mediated DNA cleavage (binding) once religation has taken place (4, 34). Top1 trapping by ara-C incorporation at the +1 positions was also reversed by addition of 0.5 M NaCl (Fig. 1 C and D, lanes 8). Thus, the top1 cleavage complexes induced by ara-C incorporation are reversible.
Ara-C Incorporation Slows Down the Religation of Top1 Cleavage Complexes.
To investigate the mechanism by which ara-C incorporation at the +1 position enhances top1 cleavage complexes, we used different substrates to assay the different steps of the top1-mediated DNA cleavage/religation equilibrium (Fig. 2). We first investigated the effect of ara-C incorporation on top1-mediated religation. To detect a difference in religation rates, we performed kinetic experiments using a “donor-acceptor” system (Fig. 2 A and B). In these experiments, an unlabeled top1-linked suicide product referred to as “acceptor” (see Fig. 2A) was generated from the substrate shown in Fig. 2C after reaction with purified top1 for 1 h at 37°C. Such conditions generated more than 95% of acceptor oligonucleotide (data not shown). The control (C)- or ara-C-containing acceptors were then incubated with 10-fold excess of 3′-end-labeled complementary single-stranded 23-mer oligonucleotides referred to as “donor,” and appearance of the religated product (37-mer) was measured as a function of time (Fig. 2A, arrowhead). Quantitation of early time points of the gel shown in Fig. 2A showed a marked difference in the initial slopes of the religation kinetics (Fig. 2B). We estimate an approximate 2- to 3-fold decrease in the religation rate in the case of the ara-C-containing substrate by assimilating the religation step as a first-order reaction (data not shown). Such estimation was complicated by the fact that religated products could in turn become cleavage substrates for top1, leading to an equilibrium reaction (Fig. 2A, lanes 6–8). These results demonstrate that ara-C incorporation inhibits the religation of top1 cleavage complexes.
To determine whether the enhancement of top1 cleavage complexes by ara-C incorporation might also be related to an induction of the top1 cleavage complexes, suicide substrates containing either C or ara-C at the +1 position of the nonscissile strand (Y in Fig. 2C and Fig. 1B for sequence) were used. Under such conditions, top1 generates a 5-mer product that dissociates from the top1 cleavage complex and cannot be religated by the enzyme (23, 35, 36). The lower strand was 5′-end phosphorylated to prevent intramolecular religation (12). Even though top1 may have a different binding affinity for these substrates as compared with full duplex oligonucleotides, kinetics of DNA cleavage could be directly measured. Quantitation of the kinetics shown in Fig. 2C showed a comparable rate of cleavage for both substrates.
We next investigated top1 binding to an ara-C-containing DNA using a human truncated top1 mutant where the catalytic tyrosine 723 was mutated to phenylalanine (37) (Fig. 2D). Noncovalent top1-DNA binding can be measured because this enzyme cannot perform the incision step but retains normal DNA binding (37). Electrophoretic mobility shift assay did not show a significant difference in the noncovalent binding of top1 when ara-C was incorporated at the +1 position (Fig. 2D).
Together, results of Fig. 2 demonstrate that trapping of top1 cleavage complexes by ara-C incorporation at the +1 position of a top1 cleavage site is primarily due to an inhibition of the religation step of the top1 reaction. Although ara-C is incorporated irreversibly into the DNA, the top1 cleavage complexes trapped by ara-C remained salt-reversible.
Ara-C Induces Top1 Cleavage Complexes in Human Cells.
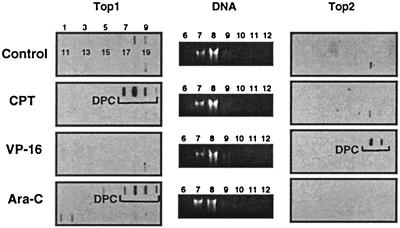
Ara-C-mediated top1 trapping in CEM treated cells was measured by using the ICE assay (for Immuno Complex of Ezyme assay). This technique allows the detection of top1 covalently linked to genomic DNA after cesium chloride gradient centrifugation. This technique has been used for the detection of top1-DNA complexes in tissue culture or tumor samples (25, 29, 30). Fractionation and immunoblotting of the cesium chloride gradients showed top1 signals in the DNA-containing fractions for the ara-C-treated cells. Induction of top1-DNA complexes by ara-C was dose-dependent (see Fig. 4A). As expected, top1-DNA complexes were formed in CPT-treated cells (Fig. 3). Low levels of top1-DNA complexes were also detected in untreated cells, which probably reflects the “physiological” levels of top1 covalently bound to genomic DNA. Immunoblotting with top2 antibody revealed that only the VP-16-treated cells showed a signal in the DNA fractions. The ara-C-treated cells showed no detectable signal for top2 (Fig. 3). These results demonstrate that ara-C induces top1 cleavage complexes in CEM cells.
Figure 4.
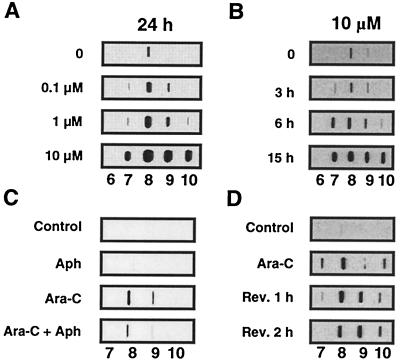
Ara-C incorporation produces top1 cleavage complexes in human leukemia CEM cells. Detection of top1 cleavage complexes was performed by using the ICE assay as described in Fig. 3, and top1 immunoblotting of the DNA-containing fractions (numbers at the bottom of each panel) are presented. Dose-response and kinetics of top1 trapping by ara-C in CEM cells are shown in A and B, respectively. (C) Aphidicolin cotreatment inhibits the production of top1-DNA complexes by ara-C. Cells were treated with 1 μM aphidicolin alone (Aph), 10 μM ara-C alone (Ara-C) for 7 h, or with a combination of ara-C and aphidicolin (Ara-C + Aph) (1 h pretreatment with aphidicolin followed by aphidicolin and ara-C for 6 h) at 37°C. (D) Persistence of ara-C-induced top1-DNA complexes. Cells were treated with Ara-C (1 μM, 15 h), and top1-DNA complexes were measured either immediately (Ara-C) or after removal of the drug and incubation in drug-free medium for 1 or 2 h (Rev. 1 h and Rev. 2 h, respectively).
Figure 3.
Top1 cleavage complexes in human leukemia CEM cells treated with ara-C. Approximately 106 cells were treated with 10 μM ara-C for 15 h or with 1 μM CPT or 100 μM VP-16 for 1 h at 37°C. Cells were then lysed with 1% sarkosyl, and subjected to the ICE bioassay (see text for details). Cesium chloride fractions are indicated by numbers 1–20. (A) Top1 immunoblotting. (B) DNA staining of fractions 6–12 with ethidium bromide after electrophoresis. (C) Top2 immunoblotting. Presence of covalent topoisomerase cleavage complexes is indicated by the brackets.
To test whether induction of top1 cleavage complexes depended on ara-C incorporation into DNA during replication, we studied the effect of aphidicolin on the formation of top1-DNA complexes in ara-C-treated cells. Fig. 4C shows that aphidicolin treatment decreased the ara-C-induced top1-DNA complexes. We also performed kinetic studies and found that ara-C-induced top1-DNA complexes were time-dependent (Fig. 4B), which is consistent with the possibility that the top1-DNA complexes were formed in response to ara-C incorporation. Finally, as shown in Fig. 4D, ara-C-induced top1-DNA complexes did not reverse after drug removal after a 2-h incubation in drug-free medium, as opposed to CPT treatment where there is a near complete reversion of top1-DNA complexes within the first 30 min after drug removal (data not shown, and ref. 38).
Resistance of Top1-Deficient Cells to Ara-C.
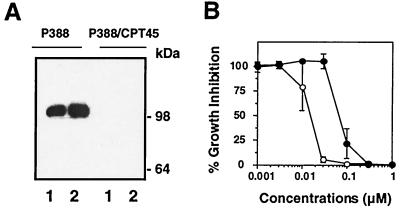
To test whether top1 trapping might contribute to the cytotoxicity of ara-C, we performed cell survival assays using the murine leukemia P388 cell line and its CPT-resistant derivative P388/CPT45. P388/CPT45 cells are highly resistant to CPT (≥1000-fold) (27, 28) due to the lack of detectable top1 as analyzed by Western blotting (Fig. 5A). Growth inhibition was measured after continuous treatment for approximately 2.5 doubling-times for each cell line. Fig. 5B shows that P388/CPT45 cells were 7- to 10-fold more resistant to ara-C than P388 cells. P388/CPT45 cells did not show crossresistance to the alkylating agent 1-methyl-3-nitro-1-nitrosoguanidine (MNNG) (IC50 of 2.3 μg/ml for P388 vs. 1.5 μg/ml for P388/CPT45), the topoisomerase II inhibitor etoposide (IC50 of 85 nM for P388 vs. 14 nM for P388/CPT45), or the tubulin poison vincristine (IC50 of 1.2 nM for P388 vs. 0.7 nM for P388/CPT45). Together, these results suggest that top1 poisoning is a cytotoxic mechanism for ara-C incorporation into genomic DNA.
Figure 5.
Top1-deficient P388/CPT45 cells are resistant to ara-C. (A) Top1 content of P388 and P388/CPT45 cells were analyzed by Western blotting using the C21 human top1 mAb. Lanes 1, 105 cells; lanes 2, 2 × 105 cells. Numbers on the right indicate the migration position of molecular mass markers. (B) Growth inhibition in P388 (○) and P388/CPT45 cells (●) was measured by MTT assay after continuous treatment with ara-C for 3 and 5 days, respectively.
Discussion
This study suggests the importance of DNA structure for top1-mediated DNA religation. Comparative crystal and NMR studies show that the presence of a single ara-C does not produce a large modification in the overall structure of the DNA helix (22, 39). Changes appear to be localized to the ara-C site and to the neighboring 3′ and 5′ base pairs. They include alterations in sugar puckering, backbone torsion angles, and base stacking (22). The 2′-hydroxy group of the ara-C residue in the major groove of the DNA has been reported to possibly form an intramolecular hydrogen bond with the 3′ phosphate group of the DNA backbone reducing the mobility of this moiety (22). We found that the enhancement of top1 cleavage by ara-C incorporation immediately 3′ from the top1 cleavage site was the result of an inhibition of the DNA religation step of the top1 cleavage-religation reaction. DNA modifications that alter base pairing of the DNA immediately 3′ from a top1 cleavage site also inhibit top1-mediated religation (9, 10). Optimum religation probably requires alignment of the DNA 5′-hydroxyl with the enzyme-DNA tyrosyl phosphodiester bond for nucleophilic attack (SN2 reaction). Biochemical studies indicated that the enzyme-DNA contacts were weak for the 4 nt 3′ from the cleavage site from the top1-mediated cleavage site (40). More recently, the crystal structure of the top1-DNA complex also indicated that the major contacts were with the phosphate residues of the DNA backbone upstream from the top1 cleavage site (for the nonscissile strand with the 5′ phosphates of the nucleotide from positions −5 to +1, and for scissile strand with the 3′ phosphates of the nucleotides from positions −5 to +2) (41). Thus, it appears that alterations of either base stacking (in the case of ara-C) or base pairing (in the case of mismatches) affect top1-mediated DNA religation, probably by misaligning the 5′-hydroxyl end of the DNA for the religation reaction. Hence, both base pairing and base stacking appear critical for optimum religation of the top1 cleavage complexes.
In this study, we also demonstrate that incorporation of ara-C in cellular DNA can induce top1-covalent complexes in human leukemia cells. The following observations indicate that ara-C incorporation into DNA is needed for the formation of the top1 cleavage complexes. First, free ara-C had no effect on purified top1 (data not shown). Second, ara-C-induced top1-DNA complexes were found to depend on DNA synthesis and incubation time and to persist after drug removal. The levels of top1 cleavage complexes induced by ara-C were less than for camptothecin. Because of its chain termination property, it is possible that high concentrations of ara-C prevent further ara-C incorporation into DNA and thus, may limit further trapping of top1 cleavage complexes. Although ara-C has multiple effects on DNA synthesis, the total amount of incorporated ara-C is correlated with the cytotoxicity of the drug (21). To determine whether ara-C incorporation and top1 trapping could play a role in the cytotoxicity of ara-C, we first attempted to compare the cytotoxicity of ara-CMP in top1-deficient and normal yeast (42). However, these experiments were inconclusive because of a lack of cell killing by ara-CMP in either yeast strain (data not shown). We next used highly CPT-resistant murine leukemia cells with no detectable top1 and found that the top1-deficient cells were resistant to ara-C. The degree of resistance to ara-C was less than for CPT, probably because the cytotoxic mechanisms of ara-C also involve its inhibitory effects on DNA polymerization (21). Our study suggests that top1 poisoning is a mechanism for the antitumor activity of ara-C.
Acknowledgments
We thank Drs. Kurt W. Kohn (Laboratory of Molecular Pharmacology) and Lance Stewart (Emerald Biostructures) for suggestions and comments during the course of this work. We also thank Dr. Michael R. Mattern (SmithKline Beecham) for providing the P388 cell lines and Dr. Yung-Chi Cheng (Yale University) for the kind gift of the top1 mAb.
Abbreviations
- ara-C
1-β-d-arabinofuranosylcytosine
- C
cytosine
- CPT
camptothecin
- G
guanine
- ICE assay
in vivo complex of enzyme assay
- top1
DNA topoisomerase I
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Morham S G, Kluckman K D, Voulomanos N, Smithies O. Mol Cell Biol. 1996;16:6804–6809. doi: 10.1128/mcb.16.12.6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsieh T, Lee M P, Brown S D. Adv Pharmacol. 1994;29:191–200. doi: 10.1016/s1054-3589(08)60546-3. [DOI] [PubMed] [Google Scholar]
- 3.Wang J C. Annu Rev Biochem. 1996;65:635–692. doi: 10.1146/annurev.bi.65.070196.003223. [DOI] [PubMed] [Google Scholar]
- 4.Champoux J. In: Mechanistic Aspects of Type-I Topoisomerases. Wang J C, Cozarelli N R, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1990. pp. 217–242. [Google Scholar]
- 5.Gupta M, Fujimori A, Pommier Y. Biochim Biophys Acta. 1995;1262:1–14. doi: 10.1016/0167-4781(95)00029-g. [DOI] [PubMed] [Google Scholar]
- 6.Champoux J J. J Biol Chem. 1981;256:4805–4809. [PubMed] [Google Scholar]
- 7.Chen A Y, Liu L F. Annu Rev Pharmacol Toxicol. 1994;94:194–218. doi: 10.1146/annurev.pa.34.040194.001203. [DOI] [PubMed] [Google Scholar]
- 8.Pommier Y, Pourquier P, Fan Y, Strumberg D. Biochim Biophys Acta. 1998;1400:83–105. doi: 10.1016/s0167-4781(98)00129-8. [DOI] [PubMed] [Google Scholar]
- 9.Pourquier P, Bjornsti M-A, Pommier Y. J Biol Chem. 1998;273:27245–27249. doi: 10.1074/jbc.273.42.27245. [DOI] [PubMed] [Google Scholar]
- 10.Pourquier P, Ueng L-M, Kohlhagen G, Mazumder A, Gupta M, Kohn K W, Pommier Y. J Biol Chem. 1997;272:7792–7796. doi: 10.1074/jbc.272.12.7792. [DOI] [PubMed] [Google Scholar]
- 11.Lanza A, Tornaletti S, Rodolfo C, Scanavini M C, Pedrini A M. J Biol Chem. 1996;271:6978–6986. doi: 10.1074/jbc.271.12.6978. [DOI] [PubMed] [Google Scholar]
- 12.Pourquier P, Ueng L M, Fertala J, Wang D, Park H J, Essigmann J M, Bjornsti M A, Pommier Y. J Biol Chem. 1999;274:8516–8523. doi: 10.1074/jbc.274.13.8516. [DOI] [PubMed] [Google Scholar]
- 13.Pommier, Y., Kohlhagen, G., Pourquier, P., Sayer, J. M., Kroth, H. & Jerina, D. (2000) Proc. Natl. Acad. Sci. USA, in press. [DOI] [PMC free article] [PubMed]
- 14.Grant S. Adv Cancer Res. 1998;72:197–233. doi: 10.1016/s0065-230x(08)60703-4. [DOI] [PubMed] [Google Scholar]
- 15.Furth J J, Cohen S S. Cancer Res. 1968;28:2061–2067. [PubMed] [Google Scholar]
- 16.Graham F L, Whitmore G F. Cancer Res. 1970;30:2627–2635. [PubMed] [Google Scholar]
- 17.Yoshida S, Yamada M, Masaki S. Biochim Biophys Acta. 1977;477:144–150. doi: 10.1016/0005-2787(77)90230-1. [DOI] [PubMed] [Google Scholar]
- 18.Dunn W C, Regan J D. Mol Pharmacol. 1979;15:367–374. [PubMed] [Google Scholar]
- 19.Cozzarelli N R. Annu Rev Biochem. 1977;46:641–668. doi: 10.1146/annurev.bi.46.070177.003233. [DOI] [PubMed] [Google Scholar]
- 20.Kufe D, Spriggs D, Egan E M, Munroe D. Blood. 1984;64:54–58. [PubMed] [Google Scholar]
- 21.Kufe D W, Major P P, Egan E M, Beardsley G P. J Biol Chem. 1980;255:8997–8900. [PubMed] [Google Scholar]
- 22.Schweitzer B I, Mikita T, Kellogg G W, Gardner K H, Beardsley G P. Biochemistry. 1994;33:11460–11475. [PubMed] [Google Scholar]
- 23.Pourquier P, Pilon A A, Kohlhagen G, Mazumder A, Sharma A, Pommier Y. J Biol Chem. 1997;272:26441–26447. doi: 10.1074/jbc.272.42.26441. [DOI] [PubMed] [Google Scholar]
- 24.Yeh Y-C, Liu H-F, Ellis C A, Lu A-L. J Biol Chem. 1994;269:15498–15504. [PubMed] [Google Scholar]
- 25.Takebayashi Y, Pourquier P, Yoshida A, Kohlhagen G, Pommier Y. Proc Natl Acad Sci USA. 1999;96:7196–7201. doi: 10.1073/pnas.96.13.7196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhelkovsky A M, Moore C L. Protein Expression Purif. 1994;5:364–370. doi: 10.1006/prep.1994.1053. [DOI] [PubMed] [Google Scholar]
- 27.Mattern M R, Hofmann G A, Polsky R M, Funk L R, McCabe F L, Johnson R K. Oncol Res. 1993;5:467–474. [PubMed] [Google Scholar]
- 28.Mattern M R, Hofmann G A, McCabe F L, Johnson R K. Cancer Res. 1991;51:5813–5816. [PubMed] [Google Scholar]
- 29.Subramanian D, Kraut E, Staubus A, Young D C, Muller M T. Cancer Res. 1995;55:2097–2103. [PubMed] [Google Scholar]
- 30.Shaw J L, Blanco J, Mueller G C A. Anal Biochem. 1975;65:125–131. doi: 10.1016/0003-2697(75)90498-4. [DOI] [PubMed] [Google Scholar]
- 31.Bonven B J, Gocke E, Westergaard O. Cell. 1985;41:541–551. doi: 10.1016/s0092-8674(85)80027-1. [DOI] [PubMed] [Google Scholar]
- 32.Fan Y, Weinstein J N, Kohn K W, Shi L M, Pommier Y. J Med Chem. 1998;41:2216–2226. doi: 10.1021/jm9605445. [DOI] [PubMed] [Google Scholar]
- 33.Redinbo M R, Stewart L, Kuhn P, Champoux J J, Hol W G. Science. 1998;279:1504–1513. doi: 10.1126/science.279.5356.1504. [DOI] [PubMed] [Google Scholar]
- 34.Tanizawa A, Kohn K W, Kohlhagen G, Leteurtre F, Pommier Y. Biochemistry. 1995;43:7200–7206. doi: 10.1021/bi00021a035. [DOI] [PubMed] [Google Scholar]
- 35.Shuman S. J Biol Chem. 1992;267:16755–16758. [PubMed] [Google Scholar]
- 36.Svejstrup J Q, Christiansen K, Gromova I I, Andersen A H, Westergaard O. J Mol Biol. 1991;222:669–678. doi: 10.1016/0022-2836(91)90503-x. [DOI] [PubMed] [Google Scholar]
- 37.Stewart L, Ireton G C, Parker L H, Madden K R, Champoux J J. J Biol Chem. 1996;271:7593–7601. doi: 10.1074/jbc.271.13.7593. [DOI] [PubMed] [Google Scholar]
- 38.Pommier Y, Tanizawa A, Kohn K W. Adv Pharmacol. 1994;29:73–92. doi: 10.1016/s1054-3589(08)61132-1. [DOI] [PubMed] [Google Scholar]
- 39.Gao Y G, van der Marel G A, van Boom J H, Wang A H. Biochemistry. 1991;30:9922–9931. doi: 10.1021/bi00105a016. [DOI] [PubMed] [Google Scholar]
- 40.Christiansen K, Svejstrup A B, Andersen A H, Westergaard O. J Biol Chem. 1993;268:9690–9701. [PubMed] [Google Scholar]
- 41.Stewart L, Redinbo M R, Qiu X, Hol W G, Champoux J J. Science. 1998;279:1534–1541. doi: 10.1126/science.279.5356.1534. [DOI] [PubMed] [Google Scholar]
- 42.Nitiss J L, Pourquier P, Pommier Y. Cancer Res. 1997;57:4564–4569. [PubMed] [Google Scholar]