Abstract
Parathyroid hormone (PTH) inhibits Na+-K+-ATPase activity by serine phosphorylation of the α1-subunit through ERK-dependent phosphorylation and translocation of protein kinase Cα (PKCα). On the basis of previous studies, we postulated that PTH regulates sodium pump activity through Src kinase, PLC, and calcium-dependent ERK phosphorylation. In the present work utilizing opossum kidney cells, a model of renal proximal tubule, PTH-stimulated ERK phosphorylation and membrane translocation of PKCα were prevented by inhibition of Src kinase, PLC, and calcium entry. Pharmacological inhibition of PLA2 did not prevent PTH-stimulated ERK phosphorylation but completely prevented PKCα translocation. Silencing the expression of cytosolic or calcium-independent PLA2 also prevented PTH-mediated phosphorylation of Na+-K+-ATPase α1-subunit and PKCα without blocking ERK phosphorylation. Inhibition of Na+-K+-ATPase activity by the PLA2 metabolites arachidonic acid and 20-hydroxyeicosatetraenoic acid was prevented by specific inhibition of PKCα but not by U0126, a MEK-1 inhibitor. Transient transfection of constitutively active MEK-1 cDNA induced phosphorylation of Na+-K+-ATPase α1-subunit and PKCα, which was prevented by PLA2 inhibition. We conclude that PTH stimulates Na+-K+-ATPase phosphorylation and decreases the activity of Na+-K+-ATPase by a sequential activation of a signaling pathway involving Src kinase, PLC, ERK, PLA2, and PKCα.
Keywords: Na+-K+-ATPase α1-subunit, parathyroid hormone, phospholipase C, extracellular signal-regulated kinase, phospholipase A2, protein kinase Cα
an energy-dependent enzyme, Na+-K+-ATPase is responsible for maintenance of intracellular sodium and potassium balance. In renal proximal tubules, the sodium pump is restricted to the basolateral membrane, where it provides the driving force for the vectorial transport of various solutes and ions. Regulation of proximal tubule sodium reabsorption is a major determinant of total body sodium homeostasis and blood pressure control (39). Abnormalities in regulation of Na+-K+-ATPase activity have been implicated in several forms of hypertension (14).
Na+-K+-ATPase is regulated in kidney proximal tubules by several hormones through the interplay of multiple signaling cascades. Our laboratory has demonstrated that the extracellular signal-regulated kinase (ERK) plays an essential role in the regulation of the sodium pump by parathyroid hormone (PTH) (16–18, 20). PTH stimulates phosphorylation of the α1-subunit of Na+-K+-ATPase at Ser11 through an ERK-dependent pathway leading to its endocytosis via clathrin-coated vesicles (16). This pathway involves ERK-dependent protein kinase Cα (PKCα) activation, translocation, and association with the Na+-K+-ATPase α1-subunit (17). The signaling mechanisms involved in PTH-stimulated ERK phosphorylation and PKCα activation are not well understood. We (21) have previously shown that PTH activates ERK in a biphasic fashion, initially through phosphatidylinositol 3-kinase (PI 3-kinase) and secondly through a PKC-dependent mechanism. PTH activates several other intracellular signals, including phospholipase A2 (PLA2) (4, 10, 31), phospholipase C (PLC) (7), Src kinase (8), and intracellular calcium (5, 24, 26), but the interaction of these other signals with ERK in PTH regulation of Na+-K+-ATPase has not been defined. In the present study, we tested whether PTH-stimulated ERK phosphorylation is dependent on one or more of these pathways.
Studies were conducted to examine the ability of PTH to phosphorylate ERK1/2 in the presence and absence of inhibitors of c-Src kinase, PLC, and PLA2 in opossum kidney (OK) cells. We also studied the effects of these inhibitors on PTH-mediated translocation of PKCα, Na+-K+-ATPase α1-subunit phosphorylation and surface expression, and Na+-K+-ATPase activity. Our data demonstrate that PTH increases ERK1/2 phosphorylation in a manner that is Src kinase and PLC dependent. PTH-mediated ERK1/2 phosphorylation was found to be PLA2 independent. However, ERK1/2 phosphorylation is a necessary step leading to PLA2 stimulation, and this affects the interaction between PKCα and Na+-K+-ATPase α1-subunit.
METHODS
Materials.
PTH (bovine 1–34) was purchased from Bachem Biosciences (King of Prussia, PA). Polyclonal antibodies against Na+-K+-ATPase α1-subunit (for immunoprecipitation), antibodies against phospho-PKCα, and MEK-1 cDNA were purchased from Upstate Biotechnology (Waltham, MA). Monoclonal antibodies against Na+-K+-ATPase α1-subunit (for Western blots) were purchased from Sigma-RBI (Natick, MA). Phosphoserine antibodies were purchased from Zymed (San Francisco, CA). PP2, edelfosine (ET), 20-hydroxyeicosatetraenoic acid (20-HETE), and arachidonic acid were purchased from EMD Biochemical (Gibbstown, NJ). Bromoenol lactone [BEL; a specific inhibitor of calcium-independent PLA2 (iPLA2)] and methyl arachidonyl fluorophosphonate [MAFP; an inhibitor of both cytosolic (cPLA2) and iPLA2] were purchased from Cayman Chemicals (Ann Arbor, MI). Opti-MEM, fura-2 AM, and Pluronic acid were purchased from Invitrogen (Carlsbad, CA). A biotinylation kit was purchased from Pierce (Rockford, IL). Phospho- and total ERK antibodies, horseradish peroxidase (HRP)-linked mouse secondary antibodies, and cPLA2 and iPLA2 small interfering (si)RNA were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Geneporter (cDNA) and Genesilencer (siRNA) transfection reagents were purchased from Gene Therapy Systems (San Diego, CA). All other chemicals were purchased from Sigma (St. Louis, MO) unless otherwise specified.
Cell culture.
OK cells used in this study are a continuous cell line derived from Virginia opossum and are a widely used model for mammalian renal proximal tubule (18). Cells were maintained at 37°C in a humidified atmosphere with 5% CO2 in minimum essential medium with Earl's salts (EMEM) supplemented with 10% (vol/vol) fetal calf serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. The cells were fed twice a week and split once a week at a 1:4 ratio. All experiments were carried out using cells at 90–95% confluence. Cells grown on six-well culture plates were washed with serum-free medium 24 h before use.
Treatment with PTH.
Unless otherwise stated, the cells were treated for 15 min with 100 nM PTH (1–34) at 37°C in 95% air-5% CO2.
86Rb uptake.
Ouabain-sensitive 86Rb uptake was measured as an index of Na+-K+-ATPase-mediated ion transport as described previously (17).
Measurement of intracellular calcium.
Intracellular calcium concentration was measured using fura-2 AM, a calcium-sensitive fluorescent dye, as described previously (19). Briefly, cultured OK cells attached to the glass coverslips were loaded with fura-2 AM for 30 min at 37°C. Fura-2 AM was dissolved in 20% Pluronic F127 and then added to Krebs solution (120 mM NaCl, 4.8 mM KCl, 5.5 mM dextrose, 3.12 mM NaH2PO4, and 12.48 mM Na2HPO4). The pH was adjusted to 7.4, after which 1.2 mM MgSO4 and 0.54 mM CaCl2 were added slowly to avoid precipitate formation at a final concentration of 5 μM fura-2 AM and 0.04% Pluronic F127. After being loaded, the cells were washed with Krebs solution. The glass coverslip was placed on the stage of a microscope (Carl Zeiss, Thornwood, NY) equipped with a digital fluorescence imaging system (Attofluor; Atto Instruments, Rockville, MD). The cells were continuously superfused with Krebs solution and maintained at 37°C using a heated microscope stage. The emission wavelength was 520 nm. Alternating excitation wavelengths of 334 and 380 nm were used, and fluorescence was continuously quantified. To calibrate the fluorescent signal, we permeabilized the cells at the end of each experiment by adding 10 μM ionomycin to the calcium-containing superfusate to establish the maximum signal. Next, 5 mM EGTA was added to the superfusate to obtain the minimum signal. In each experiment, data were averaged from 30–40 cells. The calcium concentration was computed according to the formula supplied by the fura-2 manufacturer (Invitrogen).
Isolation of endosomes and plasma membrane.
Early and late endosomes and plasma membranes were isolated by sucrose density gradient as described previously (16). Briefly, cells were incubated with PTH (100 nM) or vehicle for 15 min at 37°C. The response to PTH was stopped by washing the cells with ice-cold PBS and adding ice-cold homogenization buffer containing 250 mM sucrose and 3 mM imidazole, pH 7.4. The cells were gently homogenized using a Dounce homogenizer and centrifuged at 4°C, 3,000 g for 5 min. The supernatant was adjusted to 40.6% sucrose and was loaded (1.5 ml) at the bottom of a 5-ml centrifuge tube. To this, a 16% sucrose solution (1.5 ml) in 3 mM imidazole, 0.5 mM EDTA, and 10% sucrose (1 ml) and 1 ml of homogenization buffer was added sequentially, and then the mixture was centrifuged for 1 h at 110,000 g in a Beckman SW 50.1 rotor. Early endosomes were collected at the homogenization buffer-10% sucrose interface, late endosomes were collected from the 10–16% sucrose interface, and the plasma membrane was collected from the 16–40.6% sucrose interface. Western blots for early endosome antigen 1 (EEA1) and mannose-6-phosphate receptor were carried out to identify the early and late endosomes, respectively.
Membrane preparation.
Cells were treated with 100 nM PTH (bovine 1–34) or vehicle, washed twice with PBS, and homogenized in 50 mM mannitol and 5 mM Tris (pH 7.4), and the crude membranes were prepared as described previously (18).
Biotinylation of surface proteins.
Cell surface proteins were biotinylated using sulfo-NHS-LC biotin (Pierce) following treatment for 15 min with 100 nM PTH in the presence or absence of PP2, BEL, or ET according to the manufacturer's protocol with slight modifications. Briefly, after PTH treatment, cells were washed four times with phosphate-buffered saline (PBS; pH 8.0) containing 0.5 mM CaCl2 and 1 mM MgSO4. Cells were then incubated for 2 h at 4°C with 1 mg/ml sulfo-NHS-LC biotin in PBS. Cells were washed four times with ice-cold PBS. Unbound biotin was then quenched with 100 mM glycine in PBS for exactly 15 min. Cells were washed four times with ice-cold PBS. Cells were scraped in 50 mM mannitol, 5 mM Tris-HEPES (pH 7.4) containing 10 μl/ml protease inhibitor cocktail (Sigma) and homogenized by being passed through a 27.5-gauge needle. Crude membranes were prepared as described above and solubilized in RIPA buffer [150 mM NaCl, 50 mM Tris·HCl (pH 7.4), 5 mM EDTA, 1% (vol/vol) Triton X-100, 1% (wt/vol) sodium deoxycholate, 0.1% (wt/vol) SDS, and 10 μl/ml protease inhibitor cocktail]. Crude membranes were incubated overnight at 4°C with an equal amount of prewashed immobilized streptavidin beads (Pierce). The samples were centrifuged in a microfuge for 5 min at top speed to collect streptavidin beads. The beads were washed four times with RIPA buffer and boiled for 5 min in an equal amount of 2× Laemmli buffer. Streptavidin-bound proteins were separated by 10% SDS-PAGE, transferred to nitrocellulose membrane, and analyzed by Western blotting using anti-Na+-K+-ATPase α1-subunit antibodies.
Western blot analysis.
OK cells were treated with 100 nM PTH for 15 min in the continued presence or absence of inhibitors of Src kinase, PLC, or PLA2. Cells were lysed in lysis buffer containing 20 mM Tris (pH 7.4), 150 mM NaCl, 20 mM NaF, 1 mM EGTA, 1 mM EDTA, 10 μl/ml protease inhibitor cocktail 1, 10 μl/ml phosphatase inhibitor cocktail 1, 0.5% Nonidet P-40 (NP-40), and 1% Triton X-100. The cell lysate was homogenized by being passed a 27.5-gauge needle and centrifuged at 20,000 g at 4°C. The supernatant proteins were separated by 10% SDS-PAGE and transferred to nitrocellulose membrane. The nitrocellulose membrane was incubated in 5% milk in 20 mM Tris, 150 mM NaCl, and 0.05% Tween 20 (TTBS) at room temperature for 1 h to inhibit nonspecific binding, followed by overnight incubation at 4°C with anti-phospho-ERK or anti-PLA2 antibodies in 5% milk or BSA in TTBS. Location of specific antibodies was detected by incubation with peroxidase-labeled secondary antibodies at 1:2,000 dilution in 5% milk in TTBS, followed by development with enhanced chemiluminescence (New England Biolabs). The blots were stripped and reprobed with total ERK or PKCα antibodies to confirm equal loading of proteins. The bands imaged by chemiluminescence were scanned using a Personal Densitometer SI (Molecular Dynamics) and analyzed using ImageJ software (NIH).
Immunoprecipitation.
The crude membranes were solubilized in immunoprecipitation buffer containing 20 mM Tris·HCl (pH 7.4), 150 mM NaCl, 20 mM NaF, 1 mM EDTA, 1 mM EGTA, 10 μl/ml protease inhibitor cocktail 1, 10 μl/ml phosphatase inhibitor cocktail 1, 1% Triton X-100, 0.5% NP-40, and 0.5% SDS and centrifuged at 70,000 g for 1 h in a Beckman ultracentrifuge. Na+-K+-ATPase α1-subunit was immunoprecipitated from 100 μg of supernatant protein overnight at 4°C as described previously (16, 17). Western blotting was performed as described above.
Confocal imaging.
Confocal microscopy was performed as described previously (20). Briefly, multichambered cover glass wells (Nunc) were seeded with OK cells. Cells were washed with serum-free medium 24 h before treatment with PTH. After the treatments, cells were rinsed three times with PBS containing 0.5 mM CaCl2 and 1 mM MgCl2, incubated in 4% paraformaldehyde in PBS for 10 min, rinsed five times with PBS, solubilized with 0.025% saponin in PBS for 15 min, incubated at 20°C with an appropriate dilution of primary antibody (1:250 in PBS-saponin for both phospho-PKCα and Na+-K+-ATPase α1-subunit antibodies), rinsed five times with PBS-saponin, and incubated at 20°C with the appropriate Alexa Fluor secondary antibody (1:1,000) conjugated to different fluorescent tags. Alexa Fluor 488 was used for identification of phospho-PKCα (green), and Alexa Fluor 555 was used for identification of the Na+-K+-ATPase α1-subunit (red). The cells were rinsed five times with PBS-saponin, incubated with 300 nM 4,6-diamidino-2-phenylindole for 5 min, rinsed three times with PBS, and mounted with 300 μl/well PBS. Images were acquired using a Zeiss confocal microscope and analyzed using LSM510 software. The images for PKCα and the Na+-K+-ATPase α1-subunit were merged in a single image to compare the cellular distribution of the two proteins.
MEK-1 transfection.
Transient transfection of MEK-1 was performed as described previously (15).
Silencer RNA transfection.
cPLA2 and iPLA2 siRNA were transfected as described previously using Genesilencer siRNA transfection reagent (20).
Protein concentration.
Protein concentration was measured with the bicinchoninic acid method (Sigma) using BSA as standard.
Statistics.
Data are means ± SE. All experiments were repeated at least three times unless otherwise stated. P values were calculated by ANOVA, followed by Bonferroni's analysis using Prizm software (GraphPad). A P value <0.05 was considered statistically significant.
RESULTS
Role of Src kinase, PLC, PLA2, and calcium in PTH-mediated Na+-K+-ATPase regulation.
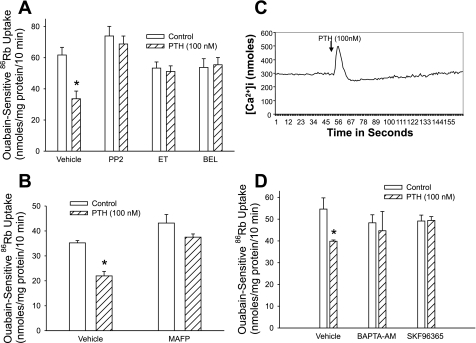
Several laboratories have demonstrated that PTH signals through activation of Src kinase, PLC, and PLA2 and by increasing intracellular calcium transients. To determine whether these signaling pathways are involved in the regulation of Na+-K+-ATPase, we treated OK cells for 15 min with 100 nM PTH in the presence or absence of inhibitors of Src kinase (PP2, 100 nM), PLC (ET, 30 μM), PLA2 [BEL (iPLA2 inhibitor) or MAFP (cPLA2 and iPLA2 inhibitor), 1 μM], and calcium [BAPTA-AM, 20 μM; EGTA, 1 mM; or SKF-96365, 25 μM (an inhibitor of store-operated calcium channels)] and measured Na+-K+-ATPase-mediated 86Rb uptake. As shown in Fig. 1, inhibition of Src kinase (A), PLC (A), and PLA2 (A and B) prevented PTH-mediated inhibition of 86Rb uptake. PTH increased intracellular calcium concentration (Fig. 1C). Chelation of intracellular calcium (BAPTA) or inhibition of store-operated calcium channels (SKF-96365) also prevented PTH-mediated inhibition of 86Rb uptake (Fig. 1D).
Fig. 1.
Effect of Src kinase, PLC, and PLA2 on parathyroid hormone (PTH) regulation of Na+-K+-ATPase-mediated 86Rb uptake. Opossum kidney (OK) cells were treated for 15 min with 100 nM PTH in the presence or absence of the Src kinase inhibitor PP2 (100 nM; A), PLC inhibitor edelfosine (ET; 30 μM; A), the calcium-independent PLA2 (iPLA2) inhibitor bromoenol lactone (BEL; 1 μM; A), the cytosolic (cPLA2) and iPLA2 inhibitor methyl arachidonyl fluorophosphonate (MAFP; 1 μM; B), or the calcium inhibitors BAPTA-AM (20 μM) or SKF-96365 (25 μM) (D). Ouabain-sensitive 86Rb uptake (A, B, and D) or intracellular calcium (C) was measured as described in methods. Bars represent ouabain-sensitive 86Rb uptake as means ± SE from 6 individual experiments run in triplicate (n = 6). *P < 0.05 by ANOVA followed by Bonferroni analysis.
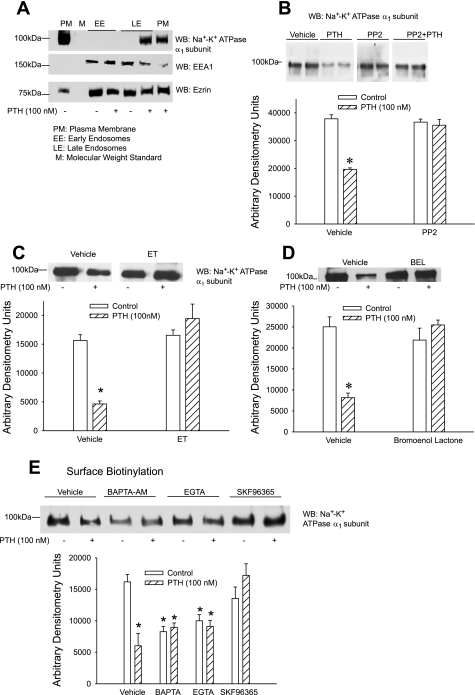
We (16) had previously demonstrated that inhibition of Na+-K+-ATPase-mediated 86Rb uptake is associated with endocytosis and/or phosphorylation of Na+-K+-ATPase α1-subunit. As previously demonstrated, treatment of OK cells for 15 min with 100 nM PTH increased Na+-K+-ATPase α1-subunit endocytosis (Fig. 2A). To determine whether Src kinase, PLC, PLA2, and calcium are required for PTH-stimulated endocytosis of the Na+-K+-ATPase α1-subunit, we examined the effect of the signaling pathway inhibitors on surface expression of the Na+-K+-ATPase α1-subunit by using surface biotinylation. As shown in Fig. 2, inhibition of Src kinase (B), PLC (C), PLA2 (D), and calcium (E) prevented Na+-K+-ATPase α1-subunit endocytosis by PTH. Of note, chelating intracellular (BAPTA) or extracellular (EGTA) calcium decreased surface biotinylation of Na+-K+-ATPase α1-subunit in the absence of PTH. Addition of PTH to either BAPTA or EGTA did not result in a further decrease in surface biotinylation. Treatment of cells with SKF-96365, the inhibitor of store-operated calcium channels, had no effect on surface biotinylation of Na+-K+-ATPase α1-subunit but prevented PTH-mediated decrease in surface biotinylation (Fig. 2E). Interestingly, treatment of cells with BAPTA had no effect on basal Na+-K+-ATPase-mediated 86Rb uptake (Fig. 1C).
Fig. 2.
Effect of Src kinase, PLC, and PLA2 on Na+-K+-ATPase α1-subunit surface expression. A: OK cells were treated for 15 min with 100 nM PTH, and plasma membrane (PM) and early (EE) and late endosomes (LE) were prepared as described in methods. Proteins were separated by 10% SDS-PAGE and analyzed by Western blotting (WB) for expression of Na+-K+-ATPase α1-subunit, early endosome antigen 1 (EEA1), and ezrin. B–E: OK cells were treated for 15 min with 100 nM PTH in the presence or absence of the Src kinase inhibitor PP2 (100 nM; B), the PLC inhibitor ET (30 μM; C), the PLA2 inhibitor BEL (1 μM; D), or the calcium inhibitors BAPTA-AM (20 μM) or SKF-96365 (25 μM) (D). Cell surface proteins were biotinylated, crude membranes were prepared, and biotinylated proteins were precipitated using streptavidin beads as described in methods. Biotinylated proteins were subjected to 10% SDS-PAGE, transferred, and probed with anti-Na+-K+-ATPase α1-subunit antibodies. A representative Western blot is shown. Bar diagrams show arbitrary densitometry units of Na+-K+-ATPase α1-subunit as means ± SE (n = 3). *P < 0.05 by ANOVA followed by Bonferroni analysis.
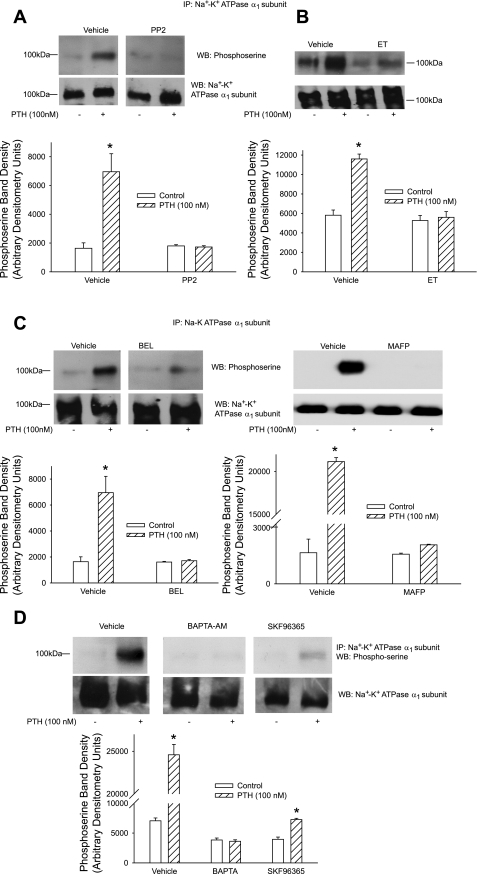
We (16) and others (29) had previously demonstrated that inhibition Na+-K+-ATPase and endocytosis of Na+-K+- ATPase α1-subunit also involve phosphorylation of the Na+-K+-ATPase α1-subunit. To determine whether the above-described signaling pathways are involved in PTH-mediated phosphorylation of Na+-K+-ATPase α1-subunit, cells were treated for 15 min with 100 nM PTH in the presence or absence of inhibitors of Src kinase, PLC, PLA2, or calcium, and Na+-K+-ATPase α1-subunit was immunoprecipitated from crude membranes followed by Western blotting with phosphoserine antibodies. Figure 3 demonstrates that PTH stimulates Na+-K+-ATPase α1-subunit phosphorylation. As shown in Fig. 3, PTH-mediated Na+-K+-ATPase α1-subunit phosphorylation was prevented by inhibition of Src kinase (A), PLC (B), PLA2 (C), and calcium (BAPTA and SKF-96365; D).
Fig. 3.
Effect of Src kinase, PLC, and PLA2 on PTH-stimulated Na+-K+-ATPase α1-subunit phosphorylation. OK cells were treated for 15 min with 100 nM PTH in the presence or absence of the Src kinase inhibitor PP2 (100 nM; A), the PLC inhibitor ET (30 μM; B), the PLA2 inhibitors BEL (1 μM) or MAFP (1 μM) (C), or the calcium inhibitors BAPTA-AM (20 μM) or SKF-96365 (25 μM) (D). Cells were lysed, and crude membranes were prepared as described in methods. Na+-K+-ATPase α1-subunit was immunoprecipitated (IP) using anti-Na+-K+-ATPase α1-subunit antibodies as described in methods. Immunoprecipitated proteins were subjected to 10% SDS-PAGE, transferred, and probed with anti-phosphoserine antibodies. Nitrocellulose membranes were stripped and reprobed with anti-Na+-K+-ATPase α1-subunit antibodies (data not shown). A representative Western blot is shown. Bar diagrams show arbitrary densitometry units of phosphorylated Na+-K+-ATPase α1-subunit as means ± SE (n = 3). *P < 0.05 by ANOVA followed by Bonferroni analysis.
Role of Src kinase, PLC, and PLA2 in PTH-mediated ERK phosphorylation.
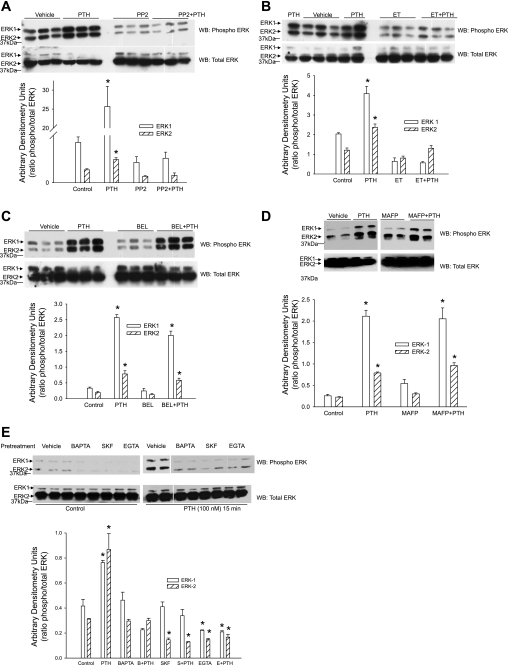
We (17) had previously demonstrated that ERK phosphorylation plays a central role in PTH-mediated regulation of Na+-K+-ATPase. The above-mentioned data suggest that PTH-mediated regulation of Na+-K+-ATPase requires Src kinase, PLC, PLA2, and calcium. To determine whether these signaling events are involved in PTH-stimulated ERK phosphorylation, OK cells were treated for 15 min with 100 nM PTH in the presence or absence of inhibitors of Src kinase, PLC, PLA2, or calcium entry. Cells were lysed and subjected to Western blot analysis for phospho-ERK1/2. As shown in Fig. 4, PTH-stimulation of ERK phosphorylation was prevented by inhibition of Src kinase (A) and PLC (B) and by manipulating calcium with SKF-96365, EGTA, and BAPTA (E) but not by PLA2 inhibition (C and D).
Fig. 4.
Effect of Src kinase, PLC, and PLA2 on PTH-stimulated ERK phosphorylation. OK proximal tubule cells were treated for 15 min with 100 nM PTH in the presence or absence of the Src kinase inhibitor PP2 (100 nM; A), the PLC inhibitor ET (30 μM; B), the iPLA2 inhibitor BEL (1 μM; D), the cPLA2 and iPLA2 inhibitor MAFP (1 μM; D), or the calcium inhibitors BAPTA-AM (20 μM), SKF-96365 (25 μM), or EGTA (1 mM) (E). Cells were lysed, and the lysate proteins were separated by 10% SDS-PAGE, transferred, and immunoblotted for phospho-ERK1/2. Nitrocellulose membranes were stripped and reprobed for total ERK using ERK2 antibodies. A representative blot from 3 different experiments is shown. Bar diagrams show arbitrary densitometry units as the ratio of phosphorylated to total ERK as means ± SE (n = 3). *P < 0.05 by ANOVA followed by Bonferroni analysis.
Role of Src kinase, PLC, and PLA2 in PTH-mediated PKCα translocation.
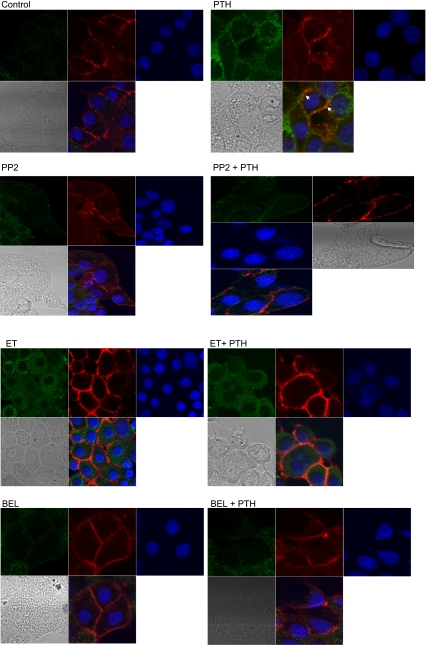
We (17) had previously demonstrated that PTH-mediated phosphorylation of Na+-K+-ATPase α1-subunit requires ERK-dependent PKCα activation and translocation. To determine whether Src kinase, PLC, and/or PLA2 play a role in PTH-mediated translocation of PKCα to the membrane, cells were treated for 15 min with 100 nM PTH in the presence or absence of inhibitors of Src kinase, PLC, or PLA2. Cells were then fixed with 4% paraformaldehyde and stained with antibodies to phospho-PKCα (green) or Na+-K+-ATPase α1-subunit (red). Confocal microscopy of the PTH-treated cells showed increased green fluorescent staining consistent with an increase in PKCα phosphorylation. There also was an increase in colocalization of green with the red staining, suggesting an increased association between Na+-K+-ATPase α1-subunit and PKCα. There was no visible difference between the intensity of Na+-K+-ATPase α1-subunit staining in control and PTH-treated cells. Inhibition of Src kinase, PLC, or PLA2 suppressed the PTH-mediated increase in phospho-PKCα as well as the increase of PKCα colocalization (arrows) with Na+-K+-ATPase α1-subunit (Fig. 5).
Fig. 5.
Effect of Src kinase, PLC, and PLA2 on PTH-stimulated PKCα translocation. OK cells were plated on 8-well confocal tissue culture plates. Cells were treated for 15 min with 100 nM PTH in the presence or absence of the Src kinase inhibitor PP2 (100 nM), the PLC inhibitor ET (30 μM), or the PLA2 inhibitor BEL (1 μM). Cells were fixed, permeabilized, stained with anti-Na+-K+-ATPase α1-subunit (red) and anti-phospho-PKCα (green), and visualized using a Zeiss confocal microscope. Representative confocal pictures are shown (n = 3). Arrows show colocalization of Na+-K+-ATPase α1-subunit with phosphorylated PKCα.
Role of PLA2 in PTH-mediated Na+-K+-ATPase regulation.
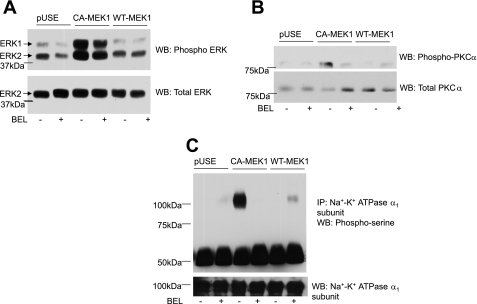
The above-described data suggest that PTH-stimulated ERK phosphorylation is independent of PLA2, whereas PTH-stimulated phosphorylation and translocation of PKCα and inhibition of Na+-K+-ATPase function are dependent on PLA2. To confirm that PLA2 is required in ERK-dependent regulation of Na+-K+ ATPase by PTH, OK cells were transiently transfected with vector (pUSE), constitutively active (CA-MEK1) or wild-type MEK-1, the upstream activator of ERK1/2 in the presence or absence of the PLA2 inhibitor BEL. Cells were lysed 24 h after transfection, and samples were subjected to either 10% SDS-PAGE or immunoprecipitation with Na+-K+-ATPase α1-subunit antibodies. Figure 6A shows that transfection of the CA-MEK1 resulted in the predicted increase in ERK phosphorylation. Transient transfection with CA-MEK1 also increased phosphorylation of PKCα (Fig. 6B) and Na+-K+-ATPase α1-subunit (Fig. 6C). CA-MEK1-mediated phosphorylation of PKCα and Na+-K+-ATPase α1-subunit, but not ERK phosphorylation, was prevented by inhibition of PLA2.
Fig. 6.
Effect of MEK-1 transfection and PLA2 on PKCα and Na+-K+-ATPase α1-subunit phosphorylation. OK cells were transiently transfected with empty vector (pUSE) or constitutively active (CA-MEK1) or wild-type MEK-1 (WT-MEK1) as described in methods. Cells were lysed 24 h after transfection and subjected to 10% SDS-PAGE (A–C) for Western blot analysis for phospho-ERK1/2 (A) and phospho-PKCα (B). Samples from the same lysates were analyzed for Na+-K+-ATPase α1-subunit phosphorylation by immunoprecipitation as described above (C). A representative blot from 2 independent experiments is shown. Western blots are shown for phospho-ERK1/2, phospho-PKCα, and phosphoserine. Nitrocellulose membranes were stripped and reprobed for total ERK, PKCα, and Na+-K+-ATPase α1-subunit (n = 2).
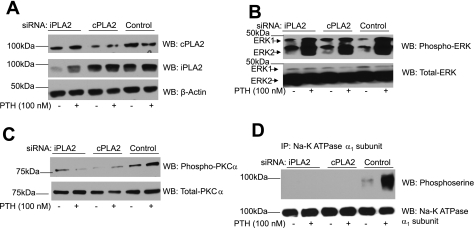
Two different isoforms of phospholipase have been described in many cells: one is intracellular calcium-dependent cPLA2, and the other is membrane-associated calcium-independent iPLA2. To determine which isoform of PLA2 is involved in regulation of Na+-K+-ATPase by PTH, cells were transfected with hairpin loop siRNA for cPLA2 or iPLA2 for 24 h to inhibit their expression. The cells were then treated with 100 nM PTH for 15 min, after which cells were lysed and ERK, PKCα, and Na+-K+-ATPase α1-subunit phosphorylation were determined as described above. As shown in Fig. 7A, transfection of cPLA2 and iPLA2 blocked the expression of the respective proteins without affecting the expression of the other PLA2 isoform. Inhibition of cPLA2 or iPLA2 expression had no effect on PTH-induced ERK phosphorylation (Fig. 7B). However, inhibition of cPLA2 and iPLA2 expression prevented PTH-mediated phosphorylation of PKCα (Fig. 7C) and Na+-K+-ATPase α1-subunit (Fig. 7D).
Fig. 7.
Effect of cPLA2 and iPLA2 small interfering (si)RNA on PTH-mediated regulation of Na+-K+-ATPase α1-subunit. OK cells were transiently transfected with control, cPLA2, or iPLA2 siRNA as described in methods. Cells were treated for 15 min with 100 nM PTH for 24 h after transfection. Cells were lysed and subjected to 10% SDS-PAGE (A and B) for Western blot analysis for phospho-ERK1/2 (A) and phospho-PKCα (B). Samples from the same lysates were analyzed for Na+-K+-ATPase α1-subunit phosphorylation by immunoprecipitation as described above (C). A representative blot from 2 independent experiments is shown. Western blots are shown for phosphorylated ERK1/2, phospho-PKCα, and phosphoserine. Nitrocellulose membranes were stripped and reprobed for total ERK, PKCα, and Na+-K+-ATPase α1-subunit (n = 2).
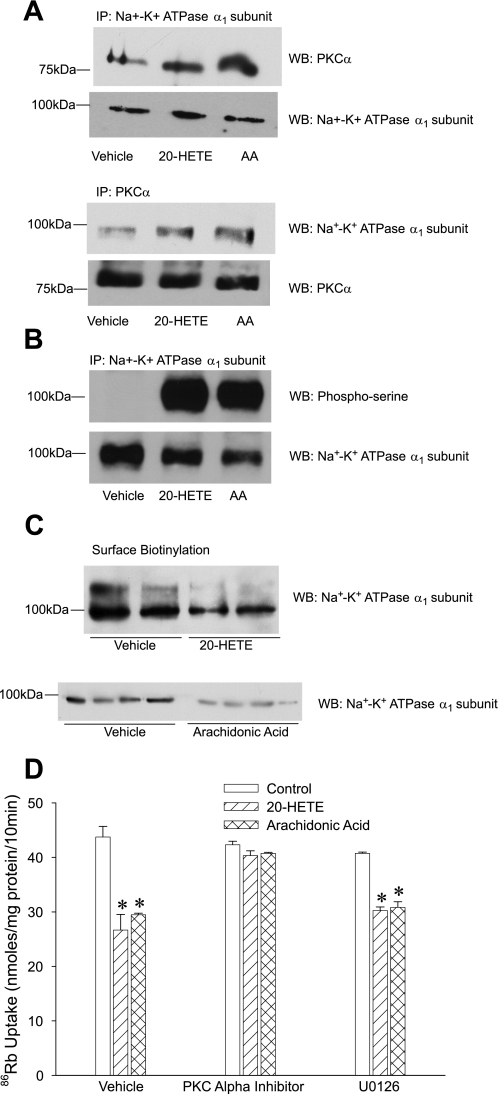
20-HETE and arachidonic acid (products of PLA2) inhibit Na+-K+-ATPase activity in OK cells through pathways that are independent of ERK activation but dependent on PKC (18). To determine whether 20-HETE and arachidonic acid mimic the effect of PTH on PKCα phosphorylation and translocation and Na+-K+-ATPase regulation, cells were exposed to 10 μM 20-HETE or 5 μM arachidonic acid. Immunoprecipitation with anti-Na+-K+-ATPase α1-subunit or anti-PKCα antibodies demonstrated that 15-min treatment with 20-HETE and arachidonic acid increased PKCα association with Na+-K+-ATPase α1-subunit (Fig. 8A). Treatment with 20-HETE and arachidonic acid also increased Na+-K+-ATPase α1-subunit phosphorylation (Fig. 8B) and decreased the availability of Na+-K+-ATPase α1-subunit to surface biotinylation (Fig. 8C). Importantly, Na+-K+-ATPase-mediated 86Rb uptake was significantly decreased in cells treated with 20-HETE or arachidonic acid (Fig. 8D). The effect of 20-HETE and arachidonic acid on 86Rb uptake was prevented by a PKCα-specific peptide inhibitor but not by U0126 (MEK-1 inhibitor).
Fig. 8.
Effect of 20-hydroxyeicosatetraenoic acid (20-HETE) and arachidonic acid on Na+-K+-ATPase regulation. OK cells were treated for 15 min with 10 μM 20-HETE or 5 μM arachidonic acid. A: cells were lysed, and Na+-K+-ATPase α1-subunit (top) or PKCα (bottom) was immunoprecipitated from crude membranes. Immunoprecipitated proteins were subjected to 10% SDS-PAGE, transferred to nitrocellulose membrane, and analyzed by Western blotting using anti-PKCα or anti-Na+-K+-ATPase α1-subunit antibodies. The blots were stripped and reprobed for total Na+-K+-ATPase α1-subunit or PKCα immunoprecipitated. A representative blot is shown (n = 3). B: a sample from the Na+-K+-ATPase α1-subunit immunoprecipitate was used to analyze for Na+-K+-ATPase α1-subunit phosphorylation using anti-phosphoserine antibodies. The blots were stripped and reprobed for total Na+-K+-ATPase α1-subunit immunoprecipitated. A representative blot is shown (n = 3). C: OK cells were treated for 15 min with 10 μM 20-HETE or 5 μM arachidonic acid. Cell surface proteins were biotinylated as described in methods. Biotinylated proteins were precipitated using streptavidin beads, separated by 10% SDS-PAGE, transferred to nitrocellulose membranes, and analyzed by Western blotting using anti-Na+-K+-ATPase α1-subunit antibodies. A representative blot from 3 independent experiments is shown (n = 3). D: OK cells were treated for 15 min with 10 μM 20-HETE or 5 μM arachidonic acid in the presence or absence of 100 nM PKCα inhibitory peptide or 10 μM U0126. 86Rb uptake was measured as described in methods. Bars represent ouabain-sensitive 86Rb uptake as means ± SE from 6 individual experiments run in triplicate (n = 6). *P < 0.05 by ANOVA followed by Bonferroni analysis.
DISCUSSION
The present findings provide new information on the signaling mechanisms through which PTH mediates ERK phosphorylation leading to changes in Na+-K+-ATPase function. Although ERK is recognized as one of the essential signaling elements through which PTH regulates Na+-K+-ATPase activity, the mechanism of PTH-mediated ERK phosphorylation in OK cells is not well understood. In different tissues, PTH can activate ERK through a variety of signaling cascades. For example, in enterocytes, PTH activates ERK1/2 through a pathway involving sequential activation of c-Src and PLCγ (7–10). In kidney distal tubules, PTH-mediated ERK activation is dependent on expression of β-arrestin (34), calcium (35), matrix metalloproteinase (36), and EGF receptor transactivation (36). In the present study, we have demonstrated that inhibition of Src kinase, PLC, and chelation of extracellular or intracellular calcium prevented PTH-mediated phosphorylation of ERK in OK cells. In contrast, inhibition of PLA2 had no effect on PTH-mediated ERK phosphorylation. We had previously demonstrated that ERK phosphorylation stimulates translocation of PKCα to the membrane and association with Na+-K+-ATPase α1-subunit (17). In the present study, we have shown that PTH-mediated PKCα translocation, Na+-K+-ATPase α1-subunit phosphorylation, and Na+-K+-ATPase α1-subunit endocytosis also are dependent on Src kinase and PLC.
We (17) and others (13) recently demonstrated that ERK might directly phosphorylate PKCα. PKCα can be directly phosphorylated by ERK1/2 on Thr638 (http://scansite.mit.edu/) (1, 38). However, complete PKCα activation also requires phosphorylation at Thr497 and Ser657 (3, 12), which are not classic ERK phosphorylation sites since these amino acid residues lack the required subsequent proline residue. In the present work, we tested whether transfection with CA-MEK1 into native OK cells could phosphorylate PKCα and Na+-K+-ATPase α1-subunit. The results show that phosphorylation occurred in these cells but was completely blocked by an inhibitor of PLA2. Furthermore, the data suggest that inhibition of MEK-1 by U0126 blocked PTH-mediated phosphorylation of PKCα, Na+-K+-ATPase α1-subunit, and inhibition of Na+-K+-ATPase activity. Although we cannot exclude the possibility that PTH-stimulated ERK directly phosphorylated PKCα and/or Na+-K+-ATPase α1-subunit, the data presented suggest that ERK stimulates activation and translocation of PKCα through a PLA2-dependent pathway. Arachidonic acid and 20-HETE, products of PLA2, are known to activate and translocate PKCα to the membrane in a variety of cells. For example, Lopez-Nicolas et al. (23) demonstrated that arachidonic acid directly stimulates PKCα activation and translocation in MCF-7 cells, a human breast adenocarcinoma cell line. They showed that the C2 domain of PKCα is important for the activation and translocation of PKCα. An arachidonic acid-mediated increase of intracellular calcium resulted in binding of arachidonic acid to the calcium binding region of PKCα, followed by the interaction of arachidonic acid with the C1A domain of PKCα, resulting in full activation and translocation of PKCα to the membrane (23). Similar results were shown by Russell and colleagues (32, 33), who demonstrated that angiotensin II- and insulin-like growth factor-I-mediated activation and translocation of PKCα are dependent on arachidonic acid release in C2C12 murine myoblasts. 20-HETE also has been shown to activate and translocate PKCα. 20-HETE increases phosphorylation and translocation of PKCα in a manner similar to stretching in canine basilar arteries, and the effects of 20-HETE can be prevented by damping the rise of intracellular calcium using nicardipine or gadolinium, an inhibitor of stretch-activated cation channels (29). Similar results were observed by Randriamboavonjy (30) in porcine coronary arteries. A direct relationship between 20-HETE mediated-PKC activation and Na+-K+-ATPase inhibition was demonstrated by Nowicki et al. (27) in microdissected single kidney proximal tubules.
A role for PLA2 in regulation of Na+-K+-ATPase is consistent with previous studies. Mandel and colleagues (4, 31), using the PLA2 inhibitor BEL, demonstrated that PTH-mediated inhibition of Na+-K+-ATPase activity requires PLA2 in rat renal proximal tubules. They also demonstrated that treating tubules with 20-HETE, a product of PLA2 metabolism, produces an effect similar to PTH. Previous studies from our laboratory (18) confirmed the involvement of PLA2 in PTH-mediated inhibition of Na+-K+-ATPase activity in OK cells. Arachidonic acid and 20-HETE inhibited Na+-K+-ATPase activity similarly to PTH. 20-HETE-mediated inhibition was prevented by a general PKC inhibitor calphostin-C but not by MEK inhibitor PD-98059, suggesting that PKC activation is PLA2 dependent but not ERK dependent (18). The present data suggest that a sequential signaling mechanism is activated by PTH to regulate Na+-K+-ATPase in OK cells in which ERK1/2 is activated through a Src kinase-, PLC-, and calcium-dependent mechanism. Activation of ERK1/2 stimulates PLA2 activation, leading to the release of 20-HETE and/or arachidonic acid. The substrates of PLA2 then activate PKCα, causing its translocation and association with Na+-K+-ATPase α1-subunit, resulting in its phosphorylation, endocytosis, and inhibition. Our data also demonstrate that both the PLA2 isoforms are involved in the PTH-mediated activation of PKCα and regulation of Na+-K+-ATPase. However, it remains to be determined whether ERK activates cPLA2 and/or iPLA2 directly or indirectly. Lin et al. (22), in a classic study, showed that cPLA2 is a substrate for MAP kinases. Phosphorylation of cPLA2 by a MAP kinase-dependent pathway stimulated the activity of cPLA2 in Chinese hamster ovary cells. Steer et al. (37) and Meyer et al. (25) demonstrated that iPLA2 is regulated through PKC-dependent phosphorylation in ventricular myocytes and in human coronary artery endothelial cells. Beckett et al. (2) demonstrated that treatment of ventricular myocytes with thrombin activates iPLA2 in an ERK-independent manner. From the present data, we cannot determine whether ERK is required for activation of cPLA2 and/or iPLA2. Further studies are required to identify a link between ERK and cPLA2/iPLA2 activation in PTH-mediated regulation of Na+-K+-ATPase. Whether ERK phosphorylation by PTH requires cross talk among Src kinase, PLC, and the intracellular calcium transients or whether each signal can individually stimulate ERK independently of the others remains to be elucidated. Data from other laboratories suggest that Src kinase activates PLC, which then signals an increase in intracellular calcium (5, 7, 8).
Previously published work (6, 11, 24, 26) demonstrates that PTH increases intracellular calcium. The present study confirms those studies and demonstrates that PTH-mediated ERK phosphorylation is partially calcium dependent. Chelation of extracellular calcium by EGTA, inhibition of store-operated calcium channel by SKF-96365, and chelation of intracellular calcium by BAPTA-AM partially suppressed ERK phosphorylation. Consistent with the requirement for ERK phosphorylation described above, EGTA, BAPTA-AM, and SKF-96365 also prevented PTH-mediated increase in Na+-K+-ATPase α1-subunit phosphorylation. Interestingly, chelation of intracellular (BAPTA) and extracellular (EGTA) calcium by themselves decreased surface biotinylation of Na+-K+-ATPase α1-subunit without further decreasing PTH-mediated decrease in Na+-K+-ATPase α1-subunit surface expression. This may be due to global decreased biotinylation of proteins or increased endocytosis of Na+-K+-ATPase α1-subunit in the absence of calcium. Treatment with BAPTA alone did not significantly change the basal Na+-K+-ATPase-mediated 86Rb uptake, suggesting that the decrease in biotinylation may be due to the requirement of calcium for protein biotinylation. However, the reason for the decreased biotinylation remains to be studied in detail.
In conclusion, the results of this study illustrate a sequential signal transduction pathway leading from PTH receptor ligation to inhibition of Na+-K+-ATPase activity in a model of proximal tubular cells, the opossum kidney cell line. The results demonstrate that PTH stimulates ERK by a mechanism that is Src kinase, PLC, and calcium dependent but PLA2 independent. ERK activation stimulates phosphorylation and, thus, activation of PLA2. The lipid by-products of PLA2 stimulate activation and translocation of PKCα, leading to enhanced association with Na+-K+-ATPase α1-subunit. This association facilitates subsequent phosphorylation of Na+-K+-ATPase α1-subunit, reduction in the amount of Na+-K+-ATPase α1-subunit at the cell surface, and thus reduction in sodium pump function.
GRANTS
This work was supported by a Veteran Affairs Merit Review grant (to E. D. Lederer), American Heart Association Scientist Development Grant 0435153N and American Heart Association, Ohio Valley Affiliate Postdoctoral Fellowship Grant 0120318B (to S. J. Khundmiri), National Eye Institute Grant EY040414 (to N. A. Delamere), and a Project Grant from the J. Graham Brown Cancer Center, University of Louisville (to N. A. Delamere).
Acknowledgments
We are grateful to Dr. D. Mochly-Rosen, Stanford University School of Medicine, Stanford, CA, for the gift of PKCα isoform-specific inhibitory peptides. We thank Nina Lesousky and Sudarshana Sengupta for expert technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Adayev T, Ray I, Sondhi R, Sobocki T, Banerjee P. The G protein-coupled 5-HT1A receptor causes suppression of caspase-3 through MAPK and protein kinase Calpha. Biochim Biophys Acta 1640: 85–96, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Beckett CS, Pennington K, McHowat J. Activation of MAPKs in thrombin-stimulated ventricular myocytes is dependent on Ca2+-independent PLA2. Am J Physiol Cell Physiol 290: C1350–C1354, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Bornancin F, Parker PJ. Phosphorylation of protein kinase C-α on serine 657 controls the accumulation of active enzyme and contributes to its phosphatase-resistant state. J Biol Chem 272: 3544–3549, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Derrickson BH, Mandel LJ. Parathyroid hormone inhibits Na+-K+-ATPase through Gq/G11 and the calcium-independent phospholipase A2. Am J Physiol Renal Physiol 272: F781–F788, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Friedman PA, Coutermarsh BA, Kennedy SM, Gesek FA. Parathyroid hormone stimulation of calcium transport is mediated by dual signaling mechanisms involving protein kinase A and protein kinase C. Endocrinology 137: 13–20, 1996. [DOI] [PubMed] [Google Scholar]
- 6.Fujimori A, Miyauchi A, Hruska KA, Martin KJ, Avioli LV, Civitelli R. Desensitization of calcium messenger system in parathyroid hormone-stimulated opossum kidney cells. Am J Physiol Endocrinol Metab 264: E918–E924, 1993. [DOI] [PubMed] [Google Scholar]
- 7.Gentili C, Boland R, de Boland AR. PTH stimulates PLCβ and PLCγ isoenzymes in rat enterocytes: influence of ageing. Cell Signal 13: 131–138, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Gentili C, Boland R, de Boland AR. Implication of Gβγ proteins and c-Src tyrosine kinase in parathyroid hormone-induced signal transduction in rat enterocytes. J Endocrinol 188: 69–78, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Gentili C, Morelli S, Boland R, de Boland AR. Parathyroid hormone activation of MAP kinase in rat duodenal cells is mediated by 3′,5′-cyclic AMP and Ca2+. Biochim Biophys Acta 1540: 201–212, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Gentili C, Morelli S, de Boland AR. PTH and phospholipase A2 in the aging process of intestinal cells. J Cell Biochem 93: 312–326, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Goligorsky MS, Loftus DJ, Hruska KA. Cytoplasmic calcium in individual proximal cells in culture. Am J Physiol Renal Fluid Electrolyte Physiol 251: F938–F944, 1986. [DOI] [PubMed] [Google Scholar]
- 12.Gysin S, Imber R. Phorbol-ester-activated protein kinase C-α lacking phosphorylation at Ser657 is down-regulated by a mechanism involving dephosphorylation. Eur J Biochem 249: 156–160, 1997. [DOI] [PubMed] [Google Scholar]
- 13.Hansara G, Garcia-Paramio P, Prevostel C, Whelan RDH, Bornancin F, Parker P. Multisite dephosphorylation and desensitization of conventional protein kinase C isotypes. Biochem J 342: 337–344, 1999. [PMC free article] [PubMed] [Google Scholar]
- 14.Hussain T, Lokhandwala MF. Renal dopamine receptors and hypertension. Exp Biol Med (Maywood) 228: 134–142, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Khundmiri SJ, Amin V, Henson J, Lewis J, Ameen M, Rane MJ, Delamere NA. Ouabain stimulates protein kinase B (Akt) phosphorylation in opossum kidney proximal tubule cells through an ERK-dependent pathway. Am J Physiol Cell Physiol 293: C1171–C1180, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khundmiri SJ, Bertorello AM, Delamere NA, Lederer ED. Clathrin-mediated endocytosis of Na+,K+-ATPase in response to parathyroid hormone requires ERK-dependent phosphorylation of Ser-11 within the alpha1-subunit. J Biol Chem 279: 17418–17427, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Khundmiri SJ, Dean WL, McLeish KR, Lederer ED. Parathyroid hormone-mediated regulation of Na+-K+-ATPase requires ERK-dependent translocation of protein kinase C alpha. J Biol Chem 280: 8705–8713, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Khundmiri SJ, Lederer ED. PTH and DA regulate Na-K ATPase through divergent pathways. Am J Physiol Renal Physiol 282: F512–F522, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Khundmiri SJ, Metzler M, Ameen M, Amin V, Rane MJ, Delamere NA. Ouabain induces cell proliferation through calcium-dependent activation of PKB (Akt) in opossum kidney proximal tubule cells. Am J Physiol Cell Physiol 291: C1247–C1257, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Khundmiri SJ, Weinman EJ, Steplock D, Cole J, Ahmad A, Baumann PD, Barati M, Rane MJ, Lederer ED. Parathyroid hormone regulation of Na+, K+-ATPase requires the PDZ 1 domain of sodium hydrogen exchanger regulatory factor-1 in opossum kidney cells. J Am Soc Nephrol 16: 2598–2607, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Lederer ED, Sohi SS, McLeish KR. Parathyroid hormone stimulates extracellular signal-regulated kinase (ERK) activity through two independent signal transduction pathways: role of ERK in sodium-phosphate cotransport. J Am Soc Nephrol 9: 975–985, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Lin L, Wartmann M, Lin AY, Knopf JL, Seth A, Davis RJ. cPLA2 is phosphorylated and activated by MAP kinase. Cell 72: 269–278, 1993. [DOI] [PubMed] [Google Scholar]
- 23.Lopez-Nicolas R, Lopez-Andreo MJ, Marin-Vicente C, Gomez- Fernandez JC, Corbalan-Garcia S. Molecular mechanisms of PKCα localization and activation by arachidonic acid. The C2 domain also plays a role. J Mol Biol 357: 1105–1120, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Mahon MJ, Segre GV. Stimulation by parathyroid hormone of a NHERF-1-assembled complex consisting of the parathyroid hormone I receptor, phospholipase Cbeta, and actin increases intracellular calcium in opossum kidney cells. J Biol Chem 279: 23550–23558, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Meyer MC, Kell PJ, Creer MH, McHowat J. Calcium-independent phospholipase A2 is regulated by a novel protein kinase C in human coronary artery endothelial cells. Am J Physiol Cell Physiol 288: C475–C482, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Miyauchi A, Dobre V, Rickmeyer M, Cole J, Forte L, Hruska KA. Stimulation of transient elevations in cytosolic Ca2+ is related to inhibition of Pi transport in OK cells. Am J Physiol Renal Fluid Electrolyte Physiol 259: F485–F493, 1990. [DOI] [PubMed] [Google Scholar]
- 27.Nowicki S, Chen SL, Aizman O, Cheng XJ, Li D, Nowicki C, Nairn A, Greengard P, Aperia A. 20-Hydroxyeicosa-tetraenoic acid (20-HETE) activates protein kinase C. Role in regulation of Na+,K+ ATPase. J Clin Invest 99: 1224–1230, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Obara K, Koide M, Nakayama K. 20-Hydroxyeicosatetraenoic acid potentiates stretch-induced contraction of canine basilar artery via PKCα-mediated inhibition of KCa channel. Br J Pharmacol 137: 1362–1370, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pedemonte CH, Efendiev R, Bertorello AM. Inhibition of Na,K-ATPase by dopamine in proximal tubule epithelial cells. Semin Nephrol 25: 322–327, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Randriamboavonjy V, Kiss L, Falck JR, Busse R, Fleming I. The synthesis of 20-HETE in small porcine coronary arteries antagonizes EDHF-mediated relaxation. Cardiovasc Res 65: 487–494, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Ribeiro CM, Dubay GR, Falck JR, Mandel LJ. Parathyroid hormone inhibits Na+-K+-ATPase through a cytochrome P-450 pathway. Am J Physiol Renal Fluid Electrolyte Physiol 266: F497–F505, 1994. [DOI] [PubMed] [Google Scholar]
- 32.Russell ST, Eley H, Tisdale MJ. Mechanism of attenuation of angiotensin-II-induced protein degradation by insulin-like growth factor-I (IGF-I). Cell Signal 19: 1583–1595, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Russell ST, Wyke SM, Tisdale MJ. Mechanism of induction of muscle protein degradation by angiotensin II. Cell Signal 18: 1087–1096, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Sneddon WB, Friedman PA. β-Arrestin-dependent parathyroid hormone-stimulated extracellular signal-regulated kinase activation and parathyroid hormone type I receptor internalization. Endocrinology 148: 4073–4079, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Sneddon WB, Liu F, Gesek FA, Friedman PA. Obligate mitogen-activated protein kinase activation in parathyroid hormone stimulation of calcium transport but not calcium signaling. Endocrinology 141: 4185–4193, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Sneddon WB, Yang Y, Ba J, Harinstein LM, Friedman PA. Extracellular signal-regulated kinase activation by parathyroid hormone in distal tubule cells. Am J Physiol Renal Physiol 292: F1028–F1034, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Steer SA, Wirsig KC, Creer MH, Ford DA, McHowat J. Regulation of membrane-associated iPLA2 activity by a novel PKC isoform in ventricular myocytes. Am J Physiol Cell Physiol 283: C1621–C1626, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Yaffe MB, Leparc GG, Lai J, Obata T, Volinia S, Cantley LC. A motif-based profile scanning approach for genome-wide prediction of signaling pathways. Nat Biotechnol 19: 348–353, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Norian JM, Magyar CE, Holstein-Rathlou NH, Mircheff AK, McDonough AA. In vivo PTH provokes apical NHE3 and NaPi2 redistribution and Na-K-ATPase inhibition. Am J Physiol Renal Physiol 276: F711–F719, 1999. [DOI] [PubMed] [Google Scholar]