Abstract
Cancer progression and metastasis involves interactions between tumor cells and the tumor microenvironment (TME). We reported that mice deficient for cytosolic phospholipase A2 (cPLA2-KO) are protected against the development of lung tumors. The goal of this study was to examine the role of cPLA2 in the TME. Mouse lung cancer cells (CMT167 and Lewis lung carcinoma cells) injected directly into lungs of syngeneic mice formed a primary tumor, and then metastasized to other lobes of the lung and to the mediastinal lymph nodes. Identical cells injected into cPLA2-KO mice showed a dramatic decrease in the numbers of secondary metastatic tumors. This was associated with decreased macrophage staining surrounding the tumor. Wild-type mice transplanted with cPLA2-KO bone marrow had a marked survival advantage following inoculation with tumor cells compared to mice receiving WT bone marrow. In vitro, co-culturing CMT167 cells with bone marrow-derived macrophages from WT mice increased production of interleukin 6 (IL-6) by cancer cells. This increase was blocked in co-cultures using cPLA2-KO macrophages. Correspondingly, IL-6 staining was decreased in tumors grown in cPLA2-KO mice. These data suggest that stromal cPLA2 plays a critical role in tumor progression by altering tumor-macrophage interactions and cytokine production.
Introduction
Lung cancer is the leading cause of cancer deaths. At diagnosis, the majority of patients display metastases, and available treatments fail to prolong survival. During the past 25 years, researchers studying epithelial cancers have focused on genetic changes in tumor cells. However, it has become apparent that cancer progression and metastasis requires complex interactions between tumor cells and the surrounding stroma(1). We have studied the role of cPLA2 in the development of lung cancer, focusing on tumor cells themselves. Activation of cPLA2, resulting in arachidonic acid release, controls production of a family of eicosanoids, including prostaglandins. In non-small cell lung cancer (NSCLC) cells oncogenic K-Ras leads to elevated levels of cPLA2 and PGE2 production; blocking this pathway inhibits transformed growth in vitro and in xenograft models(2). However, little is known regarding the role of cPLA2 in the TME. While global deletion of cPLA2 in mice inhibits chemically induced lung tumorigenesis(3), these studies do not discriminate between the actions of cPLA2 in tumor cells versus the TME. In this study we employed a model using mouse lung tumor cells injected into the lungs of syngeneic, immune-competent mice. These cells form well-defined primary tumors, which metastasize to other lobes of the lungs and into the mediastinal lymph nodes. Comparing tumor progression in wild-type versus cPLA2-KO mice allowed us to specifically assess the role of this enzyme in the TME.
Materials and Methods
Cells
CMT167 cells obtained from Dr. Alvin Malkinson (School of Pharmacy, University of Colorado Denver) and Lewis lung carcinoma cells were transfected with pGL3-Control Vector (Promega) containing firefly luciferase constitutively driven by an SV40 promoter and a vector containing neomycin-resistance. Stable transfectants were selected using media containing G418, and individual clones were screened for luciferase activity. A clone with high luciferase activity (CMT167/luc or LLC/luc) was used for injection into animals. Bone marrow derived cells were isolated from femurs and tibias of wild type and cPLA2 knockout mice and cultured in the presence of M-CSF to promote macrophage maturation as previously described(4). After 3-7 days in culture, these cells have the morphology of macrophages, and were > 95% F4/80 positive.
Mice
cPLA2-KO mice(5) were backcrossed >10 generations to C57BL/6 mice in the Center for Laboratory Animal Care (CLAC) at the University of Colorado Denver. Wild-type littermates were used as controls in all studies. All procedures were performed under a protocol approved by the IACUC at the University of Colorado Denver. For bone marrow transplant, donor mice were sacrificed, femurs and tibias were aseptically removed, and bone marrow obtained by aspiration. Cells were suspended in sterile PBS + 1% FCS. Recipient wild type and cPLA2-KO mice were γ-irradiated (900-1200 RAD split doses) by Cesium source at 7-8 weeks of age. Twenty-four hours later, halothane anesthetized, irradiated recipients were injected with donor marrow via retro-orbital injection (5×106 BM MNC/mouse). UBI-EGFP mice were used as wild type bone marrow donors to track bone marrow by GFP expression; cPLA2-KO mice, without GFP-labeled bone marrow, were used for knockout bone marrow transplants.
Tumor cell injections
Mice were directly injected with the indicated tumor cells (105/40 μl), suspended in 10% Growth Factor Reduced Matrigel™ Matrix (BD Biosciences) PBS, through the rib cage into the left lobe of the lung using 30 g needles. For bioluminescence imaging, mice were injected i.p. with 300mg/kg body weight luciferin prior to sedation and imaged using The IVIS Imaging System 50 Series (Caliper Life Sciences/Xenogen Corp.). At time of sacrifice, some lungs were inflated with India ink to better visualize and quantify additional large (>0.4 mm) and micro (<0.3 mm) secondary tumors. Tumor size was quantitated using digital calipers.
Immunohistochemistry/fluorescence staining
For immunohistochemistry, formalin-fixed, paraffin-embedded tissues were deparaffinized, rehydrated and underwent antigen retrieval by heating for 20 min at 115°C in a decloaking chamber (Biocare). Sections were then exposed to specific antibodies overnight at 4°C. Antigen:antibody complexes were visualized using kits from Vector Laboratories and sections lightly counterstained with hematoxylin. Negative controls included the use of mouse or rabbit IgG. Sections were visualized using an Olympus light microscope equipped with SPOT software. For double immunofluorescence labeling, tissue sections were treated as above. Following incubations with primary antibodies, antigen:antibody complexes were visualized using rhodamine (Alexa Fluor-568)-coupled or fluorescein isothiocyanate (Alexa Fluor-488)-coupled secondary antibodies (Molecular Probes); sections were sequentially incubated with specific primary and secondary antibodies. Coverslips were mounted with VectaShield medium containing DAPI to detect all cell nuclei (Vector Laboratories), and sections were visualized using a Nikon inverted fluorescence microscope equipped with Metamorph software. Antibodies used include polyclonal anti-F4/80 (1:100; Caltag), FITC-conjugated anti-GFP (1:100; Abcam), and polyclonal anti-IL-6 (1:2000; Abcam).
Co-Culture
Bone marrow-derived macrophages were grown on the bottom of Transwell inserts. CMT/167 cells were grown on Transwell filters. After 24 hours in co-culture, cells were separated and each cell type was placed in fresh media. Condition media was collected after 24 hours and assayed for IL-6 production by specific ELISA.
Results and Discussion
The CMT167 cell line derived from a spontaneous alveolar lung carcinoma of a C57BL/6 female mouse(6) was stably transfected with firefly luciferase (CMT167/luc) to allow bioluminescent imaging to be performed. CMT167/luc cells suspended in Matrigel were injected through the rib cage into the left lobe of the lung of C57/BL6 mice as previously described(7, 8), and tumor formation analyzed as a function of time by bioluminescent imaging (Figure 1A). At 3 weeks, mice consistently developed large primary tumors at the sight of injection (Figure 1 A,B). Lungs were inflated with India ink to visualize and quantify additional large (>0.4 mm) and micro (<0.3 mm) secondary tumors. Wild-type mice exhibited multiple secondary tumors throughout the left and right lung lobes (Figure 1 C,Da-c). By four weeks post-injection, all mice showed clinical features of mediastinal lymph node metastasis (Figure 1Dd).
Figure 1. Immune-competent lung cancer model.
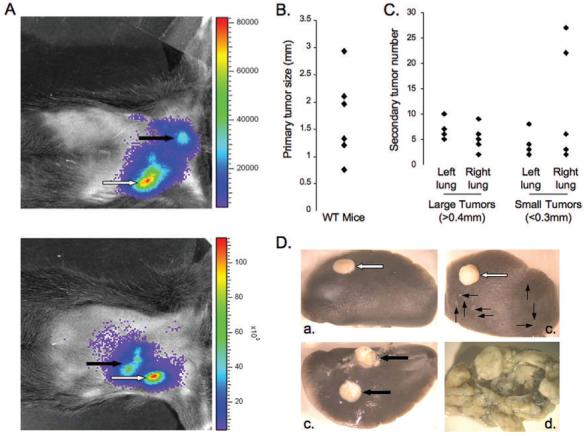
(A). CMT/167 cells (105/40μl of 10% Matrigel) were injected into the left lung lobe of WT mice. Four weeks after injection, mice were injected with luciferin and imaged for luciferase expression. White arrows = primary tumor; black arrows = secondary tumor metastases; 2 separate mice shown. (B&C). Lungs from injected mice were harvested and inflated with India ink to identify tumors. Primary tumor diameter was measured by digital calipers. (B). Secondary tumors were identified, measured, and recorded as large (>0.4mm) or micro (<0.3mm)(C). Individual points represent single animals. (D). Macroscopic images of injected lungs (a-c) and mediastinal lymph involvement (d). White arrows = primary tumors; thick black arrows = large secondary tumors; thin black arrows = micro secondary tumors
To assess the role of cPLA2 in the TME, equal numbers of CMT167/luc cells were injected into WT or cPLA2-KO mice, and lungs harvested after four weeks. Quantification of tumor metastases was limited to large secondary tumors visible under a dissecting microscope. Growth of the primary tumor was not significantly different between the two groups of mice (Figure 2A). CMT167 cells injected into WT mice metastasized to other lung lobes and into mediastinal lymph nodes. However, CMT167 cells growing in cPLA2-KO mice showed fewer metastases and little lymph involvement; 5/5 WT mice, but only 1/5 KO mice, exhibited lung metastases and lymph involvement by macroscopic morphological examination (Figure 2B). To extend the generality of these findings we performed similar experiments with Lewis lung carcinoma cells, another lung cancer cell line derived from C57BL/6 mice. These cells are more aggressive as reflected by more rapid proliferation of the primary tumor and increased numbers of secondary tumors. However, as with CMT167 cells, we observed no statistical difference in the size of primary tumors (not shown), but a marked decrease in secondary tumor number (Figure 2C) in cPLA2-KO mice compared to WT. CMT167 cells were used for all subsequent studies.
Figure 2. cPLA2 in the tumor microenvironment is essential for lung cancer progression.
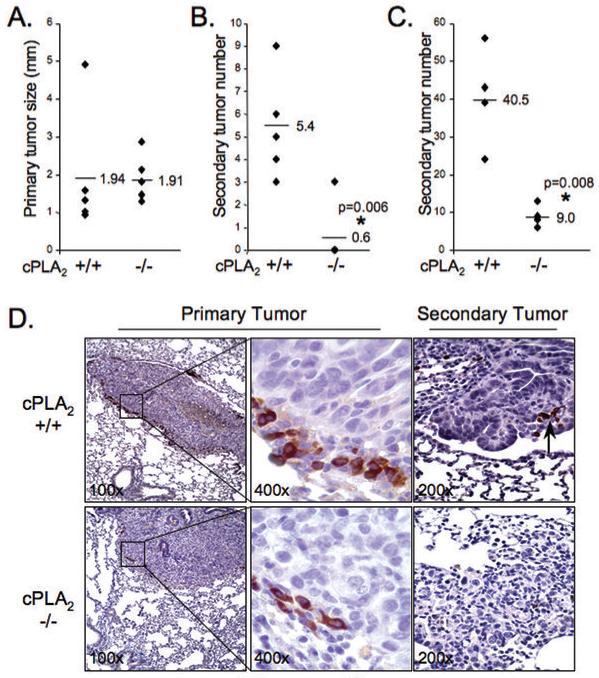
105 CMT167 cells were injected into 5 WT or 5 cPLA2-KO mice as above. Lungs were harvested 4 wks after injection. (A). Primary tumor size (diameter) was measured by caliper. Each point represents a single animal. (B). Numbers of large secondary tumors on left and right lungs were determined. Each point represents a single animal. Four cPLA2 KO mice had no secondary tumors. (C) 105 LLC cells were injected into 4 WT or 4 cPLA2-KO mice. Lungs were harvested as described above, and primary and secondary tumors evaluated. (D). Lung sections from WT (top panels) and KO (bottom panels) mice injected with CMT/167 cells were immunohistochemically stained for F4/80, a macrophage marker (brown reaction color). Arrow = F4/80(+) cells in secondary tumors growing in WT mice. Insets in left panels shown at higher magnification in middle panels.
While numerous cell types comprise the TME, attention has focused on tumor-associated macrophages (TAMs) as mediators of tumor progression and metastasis(9). Macrophages express high levels of cPLA2, and produce pro-angiogenic cytokines. We demonstrated that macrophage recruitment to the lung is impaired in cPLA2-KO mice following an inflammatory stimulus(10). TAMs surrounding tumors were assessed by F4/80 staining. Tumors grown in WT mice showed abundant accumulation of TAMs surrounding developing tumors. In contrast, fewer TAMs were detected in tumors of KO mice (Figure 2D).
Recruitment of bone marrow-derived circulating monocytes/macrophages is associated with more aggressive, malignant tumors. To assess the role of cPLA2 in bone marrow-derived cells on tumor progression, wild-type mice received bone marrow transplants from either WT or cPLA2-KO mice. Animals were allowed to recover for 5 weeks, and then injected with CMT167/luc cells. Mice transplanted with cPLA2-KO bone marrow had a marked survival advantage over mice transplanted with WT bone marrow (Figure 3A). In separate studies, bone marrow derived from either WT or cPLA2-KO mice was transplanted into a limited number of either WT or cPLA2-KO recipients to examine recruitment of bone marrow-derived macrophages to the tumor. These studies used UBI-EGFP/B6 transgenic mice, which express EGFP in all cells, as WT bone marrow donors. Lungs were harvested 4 weeks after CMT167/luc injection and analyzed histologically. Mice receiving cPLA2-KO bone marrow, independent of genotype, exhibited less inflammatory infiltrate surrounding the primary tumor compared to mice receiving WT bone marrow (Figure 3B). Mice receiving wild-type bone marrow were examined for EGFP and stained for F4/80. Both groups of mice receiving WT bone marrow showed large numbers of EGFP+;F4/80+ cells surrounding the tumor (Figure 3C) indicating recruitment of bone marrow-derived macrophages. In both groups of mice receiving cPLA2-KO bone marrow, fewer F4/80 positive cells were detected, consistent with fewer macrophages. These data suggest that the stromal protective effects of cPLA2 depletion are mediated in large part through bone marrow-derived cells, and support a model in which cPLA2 expression in bone marrow-derived macrophages is critical for recruitment of these cells to the site of the tumor.
Figure 3. Increased survival and alterations in TAMs surrounding tumors grown in WT and cPLA2-KO mice transplanted with cPLA2 KO bone marrow.
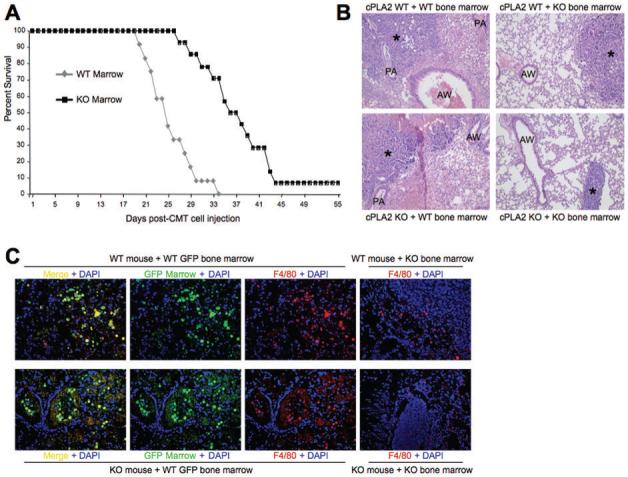
(A). Wild type mice (N=60) were lethally irradiated and transplanted with bone marrow from either UBI-EGFP/B6 WT or cPLA2-KO mice. Five weeks after transplant, mice were inoculated with CMT167 cells. Kaplan-Meier survival curve of WT mice of the indicated bone marrow genotype is shown. (B). H&E stain for tumors and surrounding stroma 4 weeks after injection with CMT167 cells in either WT or cPLA2-KO mice transplanted with the indicated bone marrow. * = primary tumor; AW = airway; PA = pulmonary artery. (C). Immunofluorescence for GFP and F4/80 in bone marrow-transplanted mice. Lung sections from the indicated mice transplanted with either WT or cPLA2-KO bone marrow were examined for accumulation of macrophages (F4/80+) and bone marrow-derived cells (GFP+). Mice receiving UBI-EGFP/B6-derived WT bone marrow were examined for GFP (green) and stained for F4/80 (red). Double positive cells were detected surrounding the primary tumor (Left-most panels, top and bottom). Mice receiving cPLA2-KO bone marrow were stained for F4/80 to detect macrophages (Right-most panels, top and bottom).
Production of IL-6 by both tumor and stromal cells contributes to tumor progression as well as angiogenesis(11). To determine if cPLA2 plays a role in IL-6 production in vivo, sections from tumors grown in WT or cPLA2-KO mice were stained for IL-6. Tumors grown in cPLA2-KO mice had markedly lower levels of IL-6 both within and surrounding the tumor, compared to tumors grown in WT mice (Figure 4A). To examine IL-6 production in vitro, bone marrow-derived cells isolated from WT or cPLA2-KO mice were cultured in the presence of M-CSF to promote macrophage maturation as previously described(4). These cells have the morphology of macrophages, and are >95% F4/80 positive. Macrophages derived from WT mice produced twice the levels of IL-6 compared to macrophages derived from cPLA2-KO mice (Figure 4B). Since interactions between cancer cells and macrophages impact cytokine production(12), IL-6 production was also assessed in co-cultures of bone marrow-derived macrophages with CMT167 cells using Transwells, which allows diffusible mediators to act on each cell type. Co-culture of macrophages with CMT167 cells increased IL-6 production in both WT and cPLA2-KO macrophages, with WT macrophages continuing to produce twice the levels compared to cPLA2-KO macrophages (Figure 4B). CMT cells grown alone failed to produce detectable levels of IL-6. However, co-culture with macrophages resulted in significant IL-6 production. Importantly, IL-6 production by CMT167 co-cultured with cPLA2-KO macrophages was only 40% of the levels seen with WT macrophage-CMT cell co-culture (Figure 4C).
Figure 4. cPLA2 deficiency attenuates production of IL-6 in co-cultures of CMT cells and bone marrow-derived macrophages.
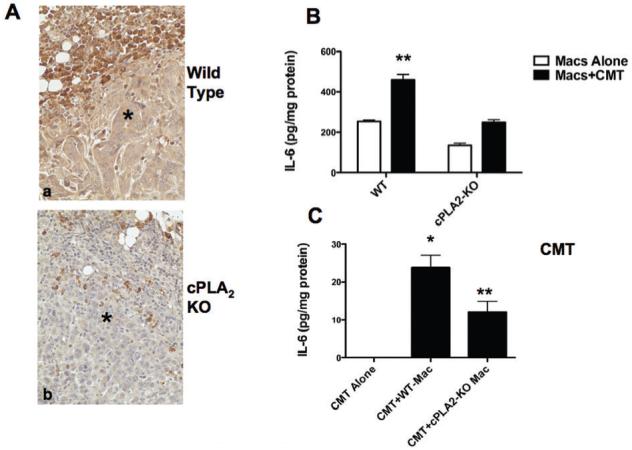
(A). Tumor-bearing lung sections from WT or cPLA2-KO mice were immunohistochemically stained for IL-6 (brown reaction color). Representative images of tumors in WT and cPLA2-KO mice are shown. * = tumor. (B,C). Bone marrow-derived macrophages were prepared as described in the “Materials and Methods” from either WT or cPLA2-KO mice. CMT/167 cells were placed in co-culture with macrophages using Transwell filters. Control cultures contained either CMT/167 cells or macrophages with medium alone. After 24 hours, cells in co-culture were separated and all cells received fresh media for an additional 24 hours. Conditioned media was then collected and assayed for IL-6 production; levels were normalized to cell protein. (B). IL-6 production by macrophages; (C). IL-6 production by CMT/167 cells. *P<0.05 vs. each cell type grown alone; **P<0.05 vs. co-culture with WT macrophages. Note: production of IL-6 by CMT cells alone was undetectable.
While cPLA2 expression by NSCLC has been demonstrated to be important for transformed growth(2), this is to our knowledge the first report indicating the importance of this enzyme in the TME. Our data indicate that two independent mouse lung cancer cell lines show a marked impairment in formation of secondary tumors when grown in cPLA2-deficient mice. While the TME comprises many types of cells, we have focused on the role of bone marrow-derived cells, specifically macrophages. Specific deletion of cPLA2 in bone marrow-derived cells inhibits tumor progression and metastasis and promotes cancer survival. This protection is associated with decreased numbers of TAMs surrounding the tumors in cPLA2-KO mice. In human lung cancer, increased numbers of macrophages surrounding the tumor has been associated with an unfavorable prognosis(13). Our findings are consistent with these observations.
Macrophages play a complex role in cancer progression. While initially mediating cytotoxic effects on tumors, TAMs have been implicated in promoting tumor progression and metastasis. Production by TAMs of pro-angiogenic cytokines in cooperation with tumor cells stimulates tumor angiogenesis(14). TAMs secrete factors with immunomodulatory activity, inhibiting T-cell function and other immune anti-tumorigenic effects. IL-6, a critical cytokine for tumor progression, promotes cancer progression through effects on tumor cells or stroma(15). Our data indicate that tumors grown in cPLA2-KO mice are exposed to lower levels of IL-6, associated with reduced tumor progression and metastasis. Consistent with this, in vitro data indicate that cPLA2 contributes to IL-6 production by macrophages, and is critical for the synergistic induction of IL-6 seen in co-cultures of cancer cells and macrophages.
The mechanism whereby cPLA2 regulates IL-6 production remains to be established. cPLA2 is critical for PGE2 production, the major prostaglandin produced by macrophages and NSCLC cells. PGE2 can induce IL-6 production in cholangiocarcinoma cells(16), and modulates cytokine production by macrophages leading to promotion of M2-type macrophages (17). PGE2 can act as an immune suppressor by inhibiting proliferation of T cells, effecting immunoglobulin production by B cells, and regulating dendritic cell maturation(18). Further studies will be required to define the role of PGE2 in this model. Since cPLA2 is critical for production of other eicosanoids, including leukotrienes, other mediators may contribute to the effects of stromal cPLA2. In addition, our studies also do not rule out a role for cPLA2 in other stromal cells, including fibroblasts and vascular cells. Recent studies have implicated production of PGE2 by myeloid-derived suppressor cells as a mediator of breast cancer progression(19). Finally it should be noted that numerous studies have examined NSAIDs as therapeutic treatments for lung cancer. It appears that these agents may not only impact tumor growth, but may also directly impact metastasis through targeting prostaglandin production in macrophages and other stromal cells(20).
Acknowledgments
This work was supported by grants from the NIH (CA103618, CA108610, and CA58187) as well as a University of Colorado Cancer Center Seed Grant to Dr. Weiser-Evans.
References
- 1.Kenny PA, Lee GY, Bissell MJ. Targeting the tumor microenvironment. Front Biosci. 2007;12:3468–74. doi: 10.2741/2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heasley LE, Thaler S, Nicks M, Price B, Skorecki K, Nemenoff RA. Induction of cytosolic phospholipase A2 by oncogenic Ras in human non-small cell lung cancer. J Biol Chem. 1997;272:14501–4. doi: 10.1074/jbc.272.23.14501. [DOI] [PubMed] [Google Scholar]
- 3.Meyer AM, Dwyer-Nield LD, Hurteau GJ, et al. Decreased lung tumorigenesis in mice genetically deficient in cytosolic phospholipase A2. Carcinogenesis. 2004;25:1517–24. doi: 10.1093/carcin/bgh150. [DOI] [PubMed] [Google Scholar]
- 4.Riches DW, Henson PM, Remigio LK, Catterall JF, Strunk RC. Differential regulation of gene expression during macrophage activation with a polyribonucleotide. The role of endogenously derived IFN. J Immunol. 1988;141:180–8. [PubMed] [Google Scholar]
- 5.Bonventre JV, Huang Z, Taheri MR, et al. Reduced fertility and postischaemic brain injury in mice deficient in cytosolic phospholipase A2. Nature. 1997;390:622–5. doi: 10.1038/37635. [DOI] [PubMed] [Google Scholar]
- 6.Layton MG, Franks LM. Heterogeneity in a spontaneous mouse lung carcinoma: selection and characterisation of stable metastatic variants. Br J Cancer. 1984;49:415–21. doi: 10.1038/bjc.1984.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen X, Su Y, Fingleton B, et al. An orthotopic model of lung cancer to analyze primary and metastatic NSCLC growth in integrin alpha1-null mice. Clin Exp Metastasis. 2005;22:185–93. doi: 10.1007/s10585-005-7453-8. [DOI] [PubMed] [Google Scholar]
- 8.Onn A, Isobe T, Itasaka S, et al. Development of an orthotopic model to study the biology and therapy of primary human lung cancer in nude mice. Clin Cancer Res. 2003;9:5532–9. [PubMed] [Google Scholar]
- 9.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–6. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Meyer AM, Dwyer-Nield LD, Hurteau G, et al. Attenuation of the pulmonary inflammatory response following butylated hydroxytoluene treatment of cytosolic phospholipase A2 null mice. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1260–6. doi: 10.1152/ajplung.00182.2005. [DOI] [PubMed] [Google Scholar]
- 11.Naugler WE, Karin M. The wolf in sheep’s clothing: the role of interleukin-6 in immunity, inflammation and cancer. Trends Mol Med. 2008;14:109–19. doi: 10.1016/j.molmed.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Hagemann T, Wilson J, Burke F, et al. Ovarian cancer cells polarize macrophages toward a tumor-associated phenotype. J Immunol. 2006;176:5023–32. doi: 10.4049/jimmunol.176.8.5023. [DOI] [PubMed] [Google Scholar]
- 13.Welsh TJ, Green RH, Richardson D, Waller DA, O’Byrne KJ, Bradding P. Macrophage and Mast-Cell Invasion of Tumor Cell Islets Confers a Marked Survival Advantage in Non-Small-Cell Lung Cancer. J Clin Oncol. 2005;23:8959–67. doi: 10.1200/JCO.2005.01.4910. [DOI] [PubMed] [Google Scholar]
- 14.Lewis CE, Pollard JW. Distinct Role of Macrophages in Different Tumor Microenvironments. Cancer Res. 2006;66:605–12. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 15.Ancrile B, Lim K-H, Counter CM. Oncogenic Ras-induced secretion of IL6 is required for tumorigenesis. Genes Dev. 2007;21:1714–9. doi: 10.1101/gad.1549407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han C, Demetris AJ, Stolz DB, Xu L, Lim K, Wu T. Modulation of Stat3 activation by the cytosolic phospholipase A2alpha and cyclooxygenase-2-controlled prostaglandin E2 signaling pathway. J Biol Chem. 2006;281:24831–46. doi: 10.1074/jbc.M602201200. [DOI] [PubMed] [Google Scholar]
- 17.Harizi H, Gualde N. Pivotal role of PGE2 and IL-10 in the cross-regulation of dendritic cell-derived inflammatory mediators. Cell Mol Immunol. 2006;3:271–7. [PubMed] [Google Scholar]
- 18.Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23:144–50. doi: 10.1016/s1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- 19.Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179:977–83. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]
- 20.Riedl K, Krysan K, Pold M, et al. Multifaceted roles of cyclooxygenase-2 in lung cancer. Drug Resist Updat. 2004;7:169–84. doi: 10.1016/j.drup.2004.04.003. [DOI] [PubMed] [Google Scholar]


