Abstract
Na+/H+ exchanger-1 (NHE1) is a ubiquitous plasma membrane Na+/H+ exchanger typically associated with maintenance of intracellular volume and pH. In addition to the NHE1 role in electroneutral Na+/H+ transport, in renal tubular epithelial cells in vitro the polybasic, juxtamembrane NHE1 cytosolic tail domain acts as a scaffold, by binding with ezrin/radixin/moesin (ERM) proteins and phosphatidylinositol 4,5-bisphosphate, which initiates formation of a signaling complex that culminates in Akt activation and opposition to initial apoptotic stress. With robust apoptotic stimuli renal tubular epithelial cell NHE1 is a caspase substrate, and proteolytic cleavage may permit progression to apoptotic cell death. In vivo, genetic or pharmacological NHE1 loss of function causes renal tubule epithelial cell apoptosis and renal dysfunction following streptozotocin-induced diabetes, ureteral obstruction, and adriamycin-induced podocyte toxicity. Taken together, substantial in vivo and in vitro data demonstrate that NHE1 regulates tubular epithelial cell survival. In contrast to connotations of NHE1 as an unimportant “housekeeping” protein, this review highlights that NHE1 activity is critical for countering tubular atrophy and chronic renal disease progression.
Keywords: apoptosis, chronic kidney disease, ezrin/radixin/moesin, necrosis, tubular atrophy
although chronic kidney disease (CKD) is initiated by glomerular injury in most instances, careful morphometric studies have demonstrated that interstitial fibrosis and tubular atrophy correlate better than glomerular pathology with glomerular filtration rate (92, 97), suggesting that tubular atrophy is a superior predictor of CKD progression. A commonly cited hypothesis for the pathogenesis of tubular atrophy, which is merely a term for the absence of renal tubular epithelial cells, has been ischemia secondary to an imbalance between high oxygen demand of tubule cells (particularly proximal tubule) and relatively decreased blood flow and oxygen supply following peritubular capillary loss (14, 49). However, if ischemia were the only mechanism, one would expect tubulointerstitial pathology to parallel glomerular pathology in severity, which is often not observed (92, 97). Furthermore, chronic ischemia usually leads to a combination of apoptosis and necrosis, and the latter is a rare pathological feature of most types of CKD. Additional factors must therefore contribute to the pathogenesis of tubular atrophy.
Enhanced tubular epithelial cell apoptosis has been noted in both human renal disease and animal models of CKD (80). Because cell number is tightly regulated by a balance between apoptosis and survival factors (113), these data suggest that tubular atrophy may be partially governed by apoptosis pathways (49, 98, 99). The purpose of this review is to summarize data regarding the role of Na+/H+ exchanger-1 (NHE1) in cell survival, particularly in proximal tubule, in the context of CKD.
NHE1 Biology
Na+/H+ exchange activity was initially demonstrated in 1972 in the bacterium Streptococcus faecalis (38), and cDNA encoding the first mammalian NHE isoform (NHE1) was cloned in 1989 (96). Since then, NHEs have been described in virtually all prokaryotic and eukaryotic cells and nine mammalian isoforms have been identified (reviewed in Refs. 74, 79). Human NHE1 is an 815-amino acid glycoprotein with conserved sequence between mammalian isoforms. NHE1 structure includes a 14-amino acid NH2-terminal cytoplasmic tail, 12 α-helical transmembrane domains through which Na+ and H+ are exchanged, and a long (315 amino acids in human) cytoplasmic domain that serves a regulatory function. The NHE1 gene (SLC9A1) maps to chromosome 1p36.11 in humans and to chromosome 4D2.3 in mice.
NHE1 is ubiquitously expressed in a plasma membrane distribution. In polarized epithelial cells, newly synthesized NHE1 sorts to both apical and basolateral plasma membranes, but mature NHE1 is localized almost exclusively to the basolateral membrane (27). Detailed NHE1 targeting mechanisms have not been described, although NHE1 appears to specify its own membrane distribution and retention through organization of, and docking with, actin (30) as well as through interactions with other basolateral membrane proteins within lipid rafts (18). Additional studies have confirmed that NHE1 localizes to lipid rafts (21, 32), which are enriched for phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2], an inner leaflet phospholipid that binds and activates both NHE1 and ezrin/radixin/moesin (ERM) (see below). However, the significance of NHE1 within lipid rafts has been questioned because lipid raft disruption with β-methylcyclodextrin did not affect NHE1-dependent Na+/H+ exchange and NHE1 regulation by clustering within lipid domains may be cell type specific (21, 32). NHE1 anchorage to cytoskeleton and discrete membrane domains is consistent with minimal NHE1 regulation by endocytic cycling (102) and a long basolateral membrane half-life of 20–24 h (23, 27).
NHE1 is often referred to as a housekeeping protein because major functions include maintenance of intracellular pH and volume. H+-sensing and osmotic regulatory domains have been localized to specific transmembrane and cytosolic regions of the transporter, respectively (13, 119). NHE1 activation by either intracellular acidosis or cell shrinkage results in electroneutral (1:1 stoichiometry) Na+/H+ exchange. Under basal conditions, NHE1 activity is minimal. However, multiple extracellular cues, including growth factors, hormones, integrin engagement, and shear stress activate NHE1, which then cooperatively regulates many cell phenotypes, such as proliferation (12, 72, 83), differentiation (84, 121), adhesion (114), and migration (29, 100).
The NHE1 cytosolic tail regulates Na+/H+ exchange by binding to multiple partners [calmodulin, calcineurin homologous protein (CHP), carbonic anhydrase II, ERM, heat shock protein 70 (HSP70), PI(4,5)P2, and the 14-3-3 adaptor protein]. The COOH-terminal portion of the NHE1 cytoplasmic tail contains several Ser/Thr residues, which are constitutively phosphorylated in quiescent cells (95) but can then be further phosphorylated in response to the above-mentioned extracellular stimuli. A notable exception is NHE1 activation by hypertonicity, which does not require NHE1 phosphorylation (37, 69).
Multiple Ser/Thr kinases phosphorylate NHE1, including Ca2+/calmodulin-dependent kinase II (CaMKII), extracellular signal-regulated kinases (ERK1/2), Janus-activated kinase 2 (JAK2), NCK-interacting kinase (NIK), p38 MAP kinase, p90 ribosomal S6 kinase (p90rsk), and Rho kinase (p160ROCK) (reviewed in Refs. 8, 103). Protein kinase C and phosphatidylinositol 3-kinase (PI3K) have also been implicated in NHE1 regulation, although neither kinase directly phosphorylates NHE1 (reviewed in Ref. 103). Rho kinase is of particular interest because, in addition to directly phosphorylating NHE1 (114, 115, 118), it also contributes to activation of ERM by phosphorylating a conserved COOH-terminal Thr (5, 20, 66, 116), suggesting a pivotal role for Rho kinase in the assembly of an NHE1-based signaling complex that regulates cell survival (discussed below).
To summarize, NHE1 is ubiquitously expressed along the basolateral membranes of polarized epithelial cells. NHE1 is normally inactive, but in response to specific stimuli (including apoptotic stress), the exchanger maintains homeostatic cell volume and pH through Na+/H+ transport. In most circumstances, Na+/H+ antiporter activity is regulated by NHE1 cytosolic tail phosphorylation and interaction with adaptor proteins.
Pharmacological Inhibitors of NHE1
NHE1-dependent Na+/H+ exchange is inhibitable by both amiloride analogs [5-(N-ethyl-N-isopropyl)amiloride (EIPA), 5-(N,N-hexamethylene)amiloride (HMA)] as well as a newer class of NHE1-selective acylguanidine-derived compounds (51, 65), such as cariporide, eniporide, and sabiporide. Orlowski and Grinstein (78) previously showed enhanced NHE1 sensitivity to amiloride derivates compared with NHE3, the other major Na+/H+ antiporter in proximal tubule. Amiloride binds NHE1 at the extracellular face of transmembrane segment IV, as evidenced by a single mutation (Phe167Leu) in this region, which abrogates the amiloride effect on Na+/H+ exchange (78, 120).
Role of NHE1 as a Tubular Epithelial Cell Survival Factor
In studies involving ROP-Os/+ and p53 transgenic mouse models of glomerulosclerosis due to reduced nephron number (35, 39, 127), progressive renal disease was accompanied by significant tubular epithelial cell apoptosis within the proximal tubule (49, 98, 99). One plausible mechanism by which NHE1 might regulate proximal tubule cell survival is enhancing Na+ entry as a part of regulatory volume increase (RVI)-mediated defense against apoptotic volume decrease (77). When cells are induced to shrink, RVI is coordinated by activation of NHE1 and, in some cells, the AE2 Cl−/HCO3− exchanger and the Na+-K+-2Cl− symporter (57, 68, 77). The net effect of RVI activation is Na+, K+, Cl−, and H2O influx, which leads to cell volume reexpansion. However, neither AE2 nor the BSC1 or BSC2 isoforms of the Na+-K+-2Cl− symporter are expressed in proximal tubule (2, 34, 45), suggesting a prominent role for NHE1 in the opposition of proximal tubule cell volume decrease induced by apoptotic or hypertonic stress (58, 77, 82, 91, 124).
Neither mice with targeted NHE1 gene deletion nor a spontaneous point mutation that introduces a premature stop codon, resulting in truncation between the 11th and 12th NHE1 transmembrane domains and loss of NHE1 function (Swe mice), have an overt renal phenotype (11, 28). These mouse studies are consistent with redundancy of renal transporters or, alternatively, with the concept that NHE1 “housekeeping” activity is not required under normal circumstances but may be important in the context of cellular stress. The latter possibility is supported by studies that show that NHE1 activity is increased in cell lines derived from patients with diabetic nephropathy (76). The role of NHE1 in kidney disease was further tested in Swe mice treated with the podocyte toxin adriamycin, which developed significantly greater cortical tubular epithelial cell apoptosis compared with wild-type control animals (124). Tubules from heterozygous Swe/+ mice had intermediate levels of apoptosis compared with wild-type and homozygous Swe/Swe animals, suggesting that the NHE1 cytoprotective effect was gene dose dependent. Similar results were observed in studies with Swe mice that were rendered diabetic by repeated streptozotocin injection; mice with the Swe genotype had significant increases in albuminuria, azotemia, and tubular epithelial cell apoptosis compared with wild-type control animals (50). In both reports, renal injury was induced in C57BL/6 mice, which are generally resistant to nephrotoxic insults. However, the combination of NHE1 loss of function and glomerular injury from adriamycin or streptozotocin was sufficient to overcome the protective genetic background effects. More recently, Manucha et al. (63) demonstrated that tubular epithelial cell apoptosis was associated with decreased NHE1 expression in a neonatal rat model of ureteral obstruction. Administration of EIPA after ureteral ligation further diminished NHE1 expression and significantly increased several indexes of apoptosis. Taken together, the in vivo data from multiple renal disease models indicate that tubular epithelial cell NHE1 is cytoprotective.
In vitro, proximal tubule cell lines subjected to staurosporine-induced apoptotic stress demonstrate increased NHE1 Na+/H+ exchange activity, which peaks at 30–60 min and decreases precipitously thereafter (123). The explanation for subsequent loss of NHE1 function, which correlates with apoptotic features, has not been thoroughly investigated. However, preliminary studies indicate that the NHE1 cytosolic tail may be a caspase-3 substrate (124). Because the cleavage site(s) has not yet been identified, we can only speculate that decreased NHE1 function may be due, at least in part, to caspase-mediated degradation. A cytoprotective role of NHE1 is also supported by experiments in which NHE1-null proximal tubule cells demonstrated enhanced sensitivity to multiple apoptotic stimuli compared with wild-type cells (50, 123). PS120 fibroblasts, which do not express NHE1, are also more susceptible to apoptotic stress compared with wild-type fibroblasts (6, 124), a phenomenon that is partially reversed by NHE1 reconstitution (61, 124).
Collectively, the data indicate that NHE1 activation represents an initial defense against apoptotic stress. However, with overwhelming or sustained apoptotic stimuli, the prosurvival NHE1 effect can be surmounted, which allows cells to proceed toward apoptosis.
Regulation of Cell Survival by NHE1-Mediated Na+/H+ Exchange
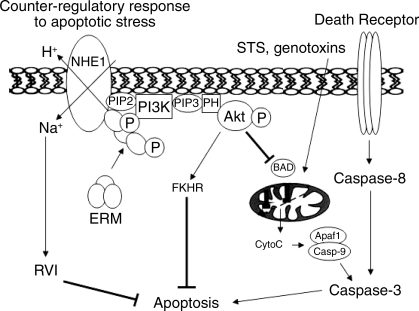
In studies with lymphocytes, Bortner and Cidlowski (16, 17) demonstrated that apoptosis induction is accompanied by initial, rapid exchange of intracellular K+ for Na+, although the net intracellular ion content is decreased, resulting in cell shrinkage (77). In contrast to lymphocytes and mesenchymal cells, epithelial cells are relatively resistant to shrinkage (15) because of robust expansion through activation of RVI pathways (77). As mentioned above, NHE1 is an important component of this pathway (68, 77, 107), with activation resulting in Na+ and H2O influx that effects cytoplasmic volume expansion. Consistent with this notion, NHE1 regulation of cell volume has been implicated as a mechanism of survival in multiple cell lines (Fig. 1, left; Refs. 58, 59, 61, 124).
Fig. 1.
Na+/H+ exchanger-1 (NHE1)-dependent mechanisms of cell survival. Apoptotic stress elicits 2 major, NHE1-regulated cell survival pathways. Decreased cytoplasmic volume leads to NHE1-dependent Na+/H+ exchange, which mediates regulatory volume increase (RVI) and cytosolic alkalinization. NHE1 activation also stimulates phosphorylation and recruitment of cytoskeleton ezrin/radixin/moesin (ERM) linkers to the NHE1 cytosolic tail. The NHE1-ERM interaction leads to the formation of a signaling complex that includes phosphatidylinositol 3-kinase (PI3K) and, ultimately, the prosurvival kinase Akt, which phosphorylates multiple substrates, some of which are depicted, resulting in apoptosis inhibition. Apaf1, apoptotic protease-activating factor-1; BAD, proapoptotic Bcl-2 family member; CytoC, cytochrome c; FKHR, forkhead transcription factor; PH, pleckstrin homology domain; PIP2, phosphatidylinositol 4,5-bisphosphate; PIP3, phosphatidylinositol 3,4,5-trisphosphate; STS, staurospsorine. Reprinted with permission from Wu et al. (123).
NHE1-dependent Na+ entry is also accompanied by H+ extrusion and intracellular alkalinization, which could defend against apoptosis by inhibiting caspase (67, 94, 101), endonuclease (3, 7) or acidic sphingomyelinase (86) catalysis, preventing conformational changes in Bax, a proapoptotic Bcl-2 family member (4, 108), or removing cytosolic H+ resulting from apoptosis-induced mitochondrial H+ release (67). Stimulation of mitochondrion-mediated apoptosis has been associated with brief intracellular alkalinization (10, 47) due to NHE1 activation (41, 48, 123), consistent with an NHE1 role in the early rescue response to apoptotic stress (Fig. 1).
Most studies have demonstrated that apoptosis is ultimately accompanied by cytosol acidification (reviewed in Ref. 55). NHE1 inhibition has been implicated as an explanation for diminished intracellular pH (pHi) (40, 64), with possible mechanisms of inactivation including ATP depletion, NHE1 dephosphorylation, or cleavage (55, 124). Defining the exact role of NHE1-regulated H+ transport in apoptosis is complicated, though, because in vitro experiments often do not employ HCO3−-containing buffer, in which pHi can be controlled by multiple ion exchangers, resulting in minimal change in pHi (61). In addition, it has recently been recognized that NHE1-dependent H+ extrusion and acidification of the extracellular microenvironment regulates membrane protein and cell behavior (9, 18, 106).
In light of these findings, we postulate that apoptotic stress triggers early NHE1-dependent defense against cell volume reduction and intracellular acidification. Later stages of apoptosis are accompanied by diminished NHE1 activity, which facilitates apoptotic cell death by allowing cell shrinkage and intracellular acidification, which is favorable for the activity of many proapoptotic proteins.
Regulation of Cell Survival by NHE1 Na+/H+ Transport-Independent Mechanisms
In renal tubular epithelial cells exposed to apoptotic stress (123) or migrating fibroblasts (29, 31), a region of polybasic amino acids within the juxtamembrane domain of the NHE1 cytosolic tail physically associates with the NH2 terminus of ERM proteins, which tether transmembrane proteins, such as NHE1, to cytoskeleton (31). ERM are predominantly expressed in an apical distribution of polarized epithelial cells and, in proximal tubule cells, indirectly interact with NHE3 through Na+/H+ exchanger regulatory factor (NHERF) (56, 73). Because NHE1 localizes to the basolateral membrane, it is important to note that in epithelial cells expressing endogenous NHE1 and ERM ezrin transiently shuttled to the lateral membrane in response to NHE1 stimulation (50), where it could then potentially interact with NHE1.
The NHE1-ERM interaction is at least partly independent of Na+/H+ exchange (31, 123), indicating that the NHE1 cytoplasmic tail appears to serve as a scaffold to direct signal transduction pathways and regulate ion transport-independent cell functions (Fig. 1, right; Ref. 70). On exposure to apoptotic stress or growth factor stimulation, NHE1 and ERM are both rapidly activated and bind to one another (25, 123). ERM activation requires both phosphorylation of a conserved COOH-terminal Thr and localization with the membrane phospholipid PI(4,5)P2 (20). Interestingly, PI(4,5)P2 binding is also required for NHE1 activation (1); we speculate that PI(4,5)P2 may therefore target NHE1 and ERM to the same plasma membrane domain and dock these molecules for juxtaposition to downstream effectors. Phosphorylation of activated ERM at Tyr353 recruits the prosurvival PI3K (33), which phosphorylates PI(4,5)P2 and subsequently leads to Akt-dependent regulation of multiple survival pathways (Fig. 1), including in renal tubular epithelial cells (110, 123). This specific cell survival pathway has also been demonstrated in the DU-145 prostate carcinoma cell line, which revealed that amiloride enhanced TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis by preventing PI3K and Akt phosphorylation (52).
In migrating cells NHE1, ERM, and Akt colocalize at the leading edge of fibroblasts (29), and PI3K is activated in conjunction with NHE1 in breast cancer cells (88), suggesting confluence of cell migration and survival signaling pathways. This concept is consistent with the hypothesis that integrins, which mediate cell migration and are cooperatively activated with NHE1 (42, 100, 106, 114), represent a type of dependence receptor that regulates cell survival (19, 104). According to this hypothesis, migrating cells survive through perpetual probing of the extracellular environment by integrins for the appropriate matrix ligand. Adherent cells expressing unligated integrins undergo apoptosis by a mechanism, which is distinct from detachment and anoikis, that involves recruitment of caspase-8 (104, 105). This phenomenon of integrin-mediated cell death suggests that migration may be an in vitro surrogate for cell survival and further substantiates a role for NHE1/ERM/PI3K/Akt complex in this process.
The preponderance of evidence indicates that NHE1 and ERM localization and activation are concomitantly upregulated (29, 31, 43, 123) and culminate in inhibition of apoptosis (43, 123). In addition to a role in apoptosis, the NHE1-ERM interaction has also been implicated in the pathophysiology of necrosis. In a recent study by Jung et al. (44), the death domain-associated protein Daxx was shown to interact with NHE1 at the ERM binding domain. In in vitro models of ischemia, Daxx displaced ezrin binding to NHE1, which was associated with NHE1 activation and release of lactate dehydrogenase, a marker of necrotic cell death. This observation is consistent with NHE1 mediation of Na+ influx, leading to cell swelling and necrosis (77). The precise role and ordering of NHE1-ERM interaction will require further investigation, however, inasmuch as ERM have also recently been described to be upstream and inhibitory to NHE1 in some systems (62, 85).
As mentioned above, the best-described function of ERM is to provide structural support by linking transmembrane proteins with actin cytoskeleton (20). Although ERM function in this capacity has not been specifically addressed in the setting of NHE1-regulated cell survival, multiple studies have shown that ERM-directed cytoarchitecture is critical for normal and apoptotic cell morphology. In the initial stages of CD95 (Fas)-stimulated T-cell apoptosis, Fas is clustered in a polarized fashion, and both the Fas expression pattern and apoptosis execution require ERM colocalization and actin polymerization (81). We speculate that one ramification of ERM translocation to these polarized death domains could be depletion from prosurvival sites, such as the NHE1-regulated survival signaling complex. In later stages of apoptosis, multiple cytoskeletal and structural proteins are degraded by caspases (26), which leads to the apoptotic cell volume decrease. We therefore propose that by binding the NHE1 cytosolic tail ERM promote cell survival signaling as well as structural integrity in cells under apoptotic siege.
Role of NHE1 in Survival of Nonrenal Cells
Because NHE1 is ubiquitously expressed, it is beyond the scope of this review to summarize the effects of NHE1 on survival in all tissues. Some of the studies mentioned above regarding NHE1 in cell migration were conducted in cancer cell lines (88, 106), and the role of NHE1 in tumor invasiveness and metastasis has been extensively reviewed (22). Resistance to apoptosis is a cardinal feature of oncogenesis, although few studies have examined a direct role for NHE1 in this process. Several studies have demonstrated that pharmacological inhibition of NHE1 with amiloride analogs caused apoptosis in leukemia cells (24, 54, 82, 91). In a recent report with the HT29 colon carcinoma cell line, sensitivity to the chemotherapeutic agent cisplatin was associated with cytosol acidification, which may be due to NHE1 inhibition (86). Similar results were observed in breast cancer cells, because paclitaxel-induced apoptosis was accompanied by abrogation of NHE1 activity and administration of paclitaxel plus the NHE1 inhibitor cariporide caused synergistic induction of apoptosis (90). An interesting possible mechanism of tumor cell survival is related to NHE1-regulated intracellular alkalinization and stimulation of aerobic glycolysis (Warburg effect) (89), which has been linked to Akt-mediated apoptosis suppression (53, 93). Taken together, the data support that the idea that NHE1 activation promotes cancer cell survival, proliferation, invasiveness, and metastasis, which has led to proposals that NHE1 might be an exploitable therapeutic target, particularly for metastasis blockade (22, 25, 91).
In contrast to studies in renal tubular epithelial or cancer cell lines, NHE1 activation has convincingly been found to promote cell death in studies involving cardiac myocytes (46). NHE1 is the predominant Na+/H+ exchanger isoform in sarcolemma and a major source of H+ extrusion in cardiac myocytes (117). NHE1 activation is deleterious in a number of cardiac conditions, including left ventricular hypertrophy, congestive heart failure, and myocardial infarction (36, 75, 126). Importantly, isolated perfused hearts from NHE1-null mice, or hearts pretreated with an NHE1 inhibitor, are protected from ischemia-reperfusion injury (122). These studies are consistent with limited in vitro experiments that demonstrate that NHE1 activation regulates cardiac myocyte apoptosis (60, 111).
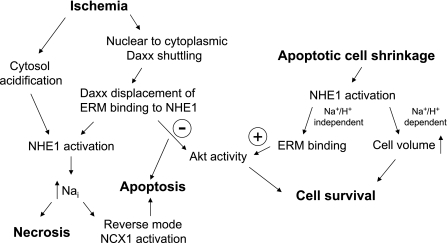
The apparent opposite effects of NHE1 on cell survival in cardiac myocytes and proximal tubule cells are largely due to different stimuli for NHE1 activation and/or cell type specificity (see Fig. 2). In CKD we speculate that apoptotic proximal tubule cell volume shrinkage is the proximate trigger for NHE1 stimulation, whereas intracellular acidosis from anaerobic metabolism stimulates NHE1 in ischemic myocardial cells, which can lead to necrosis or apoptosis, both of which have been described in the pathophysiology of myocardial infarction (36, 109, 126). After apoptotic stress the resulting increase in intracellular Na+ (Nai+) would lead to desirable restoration of cell volume, whereas with ischemia elevation in Nai+ would cause pathological cell swelling (77), a central feature of necrosis.
Fig. 2.
Working model to address different NHE1 roles in common pathophysiological processes. NCX1, Na+/Ca2+ exchanger-1; Nai, intracellular Na+.
Compensatory mechanisms to extrude Nai+ include reverse-mode activation of the NCX1 Na+/Ca2+ exchanger, which may lead to increased Cai2+ and apoptosis by the mitochondrial pathway. NCX1 is abundant in sarcolemma of cardiac myocytes, but in kidney NCX1 expression is scant and restricted to connecting tubules (87). A recent study demonstrates that ischemic kidney damage was abrogated by NHE1 inhibition (125), indicating that heart and kidney respond similarly to NHE1 blockade therapy for acute ischemic insults. Because proximal tubular epithelial cells do not express NCX1, and would therefore have limited capacity to generate compensatory Na+/Ca2+ exchange, we postulate that NHE1 primarily mediates cell swelling and necrosis in renal ischemia.
The NHE1 data in heart models are so compelling that large clinical trials have been undertaken to test the efficacy of the NHE1 inhibitor cariporide as adjunctive therapy for myocardial infarction. The GUARDIAN trial showed no mortality benefit compared with placebo (112). EXPEDITION showed decreased myocardial infarction incidence with cariporide therapy but overall increase in mortality, primarily from excess stroke (71). Interestingly, unspecified renal side effects were significantly more common in the cariporide group (http://www.medscape.com/viewarticle/464672), which raises the issue that NHE1 may theoretically be beneficial to the heart in the peri-infarct period, but could be deleterious to the kidney.
Summary
NHE1 is often referred to as a housekeeping protein, implying that it directs pedestrian functions. On the contrary, we propose that tubular epithelial cell NHE1 is a critical survival factor, which may regulate key aspects of CKD progression. In addition to facilitating Na+/H+ exchange, in vitro studies have shown that NHE1 functions as a molecular scaffold, by binding ERM and PI(4,5)P2 and directing the formation of a signaling complex, which leads to opposition of renal tubular epithelial cell apoptosis. Data from multiple in vivo models of kidney disease demonstrate that NHE1 promotes renal tubule epithelial cell survival, suggesting that strategies to preserve NHE1 activity may be therapeutically beneficial for CKD.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-067528.
Acknowledgments
We appreciate the critique of the manuscript by John Sedor.
REFERENCES
- 1.Aharonovitz O, Zaun HC, Balla T, York JD, Orlowski J, Grinstein S. Intracellular pH regulation by Na+/H+ exchange requires phosphatidylinositol 4,5-bisphosphate. J Cell Biol 150: 213–224, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alper SL, Stuart-Tilley AK, Biemesderfer D, Shmukler BE, Brown D. Immunolocalization of AE2 anion exchanger in rat kidney. Am J Physiol Renal Physiol 273: F601–F614, 1997. [DOI] [PubMed] [Google Scholar]
- 3.Altairac S, Zeggai S, Perani P, Courtois Y, Torriglia A. Apoptosis induced by Na+/H+ antiport inhibition activates the LEI/L-DNase II pathway. Cell Death Differ 10: 548–557, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Antonsson B, Conti F, Ciavatta A, Montessuit S, Lewis S, Martinou I, Bernasconi L, Bernard A, Mermod JJ, Mazzei G, Maundrell K, Gambale F, Sadoul R, Martinou JC. Inhibition of Bax channel-forming activity by Bcl-2. Science 277: 370–372, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Barret C, Roy C, Montcourrier P, Mangeat P, Niggli V. Mutagenesis of the phosphatidylinositol 4,5-bisphosphate (PIP2) binding site in the NH2-terminal domain of ezrin correlates with its altered cellular distribution. J Cell Biol 151: 1067–1079, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrière H, Poujeol C, Tauc M, Blasi JM, Counillon L, Poujeol P. CFTR modulates programmed cell death by decreasing intracellular pH in Chinese hamster lung fibroblasts. Am J Physiol Cell Physiol 281: C810–C824, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Barry MA, Eastman A. Endonuclease activation during apoptosis: the role of cytosolic Ca2+ and pH. Biochem Biophys Res Commun 186: 782–789, 1992. [DOI] [PubMed] [Google Scholar]
- 8.Baumgartner M, Patel H, Barber DL. Na+/H+ exchanger NHE1 as plasma membrane scaffold in the assembly of signaling complexes. Am J Physiol Cell Physiol 287: C844–C850, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Beg AA, Ernstrom GG, Nix P, Davis MW, Jorgensen EM. Protons act as a transmitter for muscle contraction in C. elegans. Cell 132: 149–160, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belaud-Rotureau MA, Leducq N, Macouillard Poulletier de Gannes F, Diolez P, Lacoste L, Lacombe F, Bernard P, Belloc F. Early transitory rise in intracellular pH leads to Bax conformation change during ceramide-induced apoptosis. Apoptosis 5: 551–560, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Bell SM, Schreiner CM, Schultheis PJ, Miller ML, Evans RL, Vorhees CV, Shull GE, Scott WJ. Targeted disruption of the murine Nhe1 locus induces ataxia, growth retardation, and seizures. Am J Physiol Cell Physiol 276: C788–C795, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Besson P, Fernandez-Rachubinski F, Yang W, Fliegel L. Regulation of Na+/H+ exchanger gene expression: mitogenic stimulation increases NHE1 promoter activity. Am J Physiol Cell Physiol 274: C831–C839, 1998. [DOI] [PubMed] [Google Scholar]
- 13.Bianchini L, Kapus A, Lukacs G, Wasan S, Wakabayashi S, Pouyssegur J, Yu FH, Orlowski J, Grinstein S. Responsiveness of mutants of NHE1 isoform of Na+/H+ antiport to osmotic stress. Am J Physiol Cell Physiol 269: C998–C1007, 1995. [DOI] [PubMed] [Google Scholar]
- 14.Bohle A, Mackensen-Haen S, von Gise H. Significance of tubulointerstitial changes in the renal cortex for the excretory function and concentration ability of the kidney: a morphometric contribution. Am J Nephrol 7: 421–433, 1988. [DOI] [PubMed] [Google Scholar]
- 15.Bortner CD, Cidlowski JA. Absence of volume regulatory mechanisms contributes to the rapid activation of apoptosis in thymocytes. Am J Physiol Cell Physiol 271: C950–C961, 1996. [DOI] [PubMed] [Google Scholar]
- 16.Bortner CD, Cidlowski JA. Uncoupling cell shrinkage from apoptosis reveals that Na+ influx is required for volume loss during programmed cell death. J Biol Chem 278: 39176–39184, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Bortner CD, Sifre MI, Cidlowski JA. Cationic gradient reversal and cytoskeletal independent volume regulatory pathways define an early stage of apoptosis. J Biol Chem 283: 7219–7229, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bourguignon LYW, Singleton PA, Diedrich F, Stern R, Gilad E. CD44 interaction with Na+-H+ exchanger (NHE1) creates acidic microenvironments leading to hyaluronidase-2 and cathepsin B activation and breast tumor cell invasion. J Biol Chem 279: 26991–27007, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Bredesen DE, Mehlen P, Rabizadeh S. Receptors that mediate cellular dependence. Cell Death Differ 12: 1031–1043, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol 3: 586–599, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Bullis BL, Li XJ, Rieder CV, Singh DN, Berthiaume LG, Fliegel L. Properties of the Na+/H+ exchanger protein—detergent-resistant aggregation and membrane microdistribution. Eur J Biochem 269: 4887–4895, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Cardone RA, Casavola V, Reshkin SJ. The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat Rev Cancer 5: 786–795, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Cavet ME, Akhter S, Murtazina R, de Medina FS, Tse CM, Donowitz M. Half-lives of plasma membrane Na+/H+ exchangers NHE1-3: plasma membrane NHE2 has a rapid rate of degradation. Am J Physiol Cell Physiol 281: C2039–C2048, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Chen Q, Benson RS, Whetton AD, Brant SR, Donowitz M, Montrose MH, Dive C, Watson AJ. Role of acid/base homeostasis in the suppression of apoptosis in haemopoietic cells by v-Abl protein tyrosine kinase. J Cell Sci 110: 379–387, 1997. [DOI] [PubMed] [Google Scholar]
- 25.Chiang Y, Chou CY, Hsu KF, Huang YF, Shen MR. EGF upregulates Na+/H+ exchanger NHE1 by post-translational regulation that is important for cervical cancer cell invasiveness. J Cell Physiol 214: 810–819, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Coleman ML, Olson MF. Rho GTPase signalling pathways in the morphological changes associated with apoptosis. Cell Death Differ 9: 493–504, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Coupaye-Gerard B, Bookstein C, Duncan P, Chen XY, Smith PR, Musch M, Ernst SA, Chang EB, Kleyman TR. Biosynthesis and cell surface delivery of the NHE1 isoform of Na+/H+ exchanger in A6 cells. Am J Physiol Cell Physiol 271: C1639–C1645, 1996. [DOI] [PubMed] [Google Scholar]
- 28.Cox GA, Lutz CM, Yang CL, Biemesderfer D, Bronson RT, Fu A, Aronson PS, Noebels JL, Frankel WN. Sodium/hydrogen exchanger gene defect in slow-wave epilepsy mutant mice. Cell 91: 139–148, 1997. [DOI] [PubMed] [Google Scholar]
- 29.Denker SP, Barber DL. Cell migration requires both ion translocation and cytoskeletal anchoring by the Na-H exchanger NHE1. J Cell Biol 159: 1087–1096, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Denker SP, Barber DL. Ion transport proteins anchor and regulate the cytoskeleton. Curr Opin Cell Biol 14: 214–220, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Denker SP, Huang DC, Orlowski J, Furthmayr H, Barber DL. Direct binding of the Na-H exchanger NHE1 to ERM proteins regulates the cortical cytoskeleton and cell shape independently of H+ translocation. Mol Cell 6: 1425–1436, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Fuster D, Moe OW, Hilgemann DW. Lipid- and mechanosensitivities of sodium/hydrogen exchangers analyzed by electrical methods. Proc Natl Acad Sci USA 101: 10482–10487, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gautreau A, Poullet P, Louvard D, Arpin M. Ezrin, a plasma membrane-microfilament linker, signals cell survival through the phosphatidylinositol 3-kinase/Akt pathway. Proc Natl Acad Sci USA 96: 7300–7305, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ginns SM, Knepper MA, Ecelbarger CA, Terris J, He X, Coleman RA, Wade JB. Immunolocalization of the secretory isoform of Na-K-Cl cotransporter in rat renal intercalated cells. J Am Soc Nephrol 7: 2533–2542, 1996. [DOI] [PubMed] [Google Scholar]
- 35.Godley LA, Kopp JB, Eckhaus M, Paglino JJ, Owens J, Varmus HE. Wild-type p53 transgenic mice exhibit altered differentiation of the ureteric bud and possess small kidneys. Genes Dev 10: 836–850, 1996. [DOI] [PubMed] [Google Scholar]
- 36.Gottlieb RA, Burleson KO, Kloner RA, Babior BM, Engler RL. Reperfusion injury induces apoptosis in rabbit cardiomyocytes. J Clin Invest 94: 1621–1628, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grinstein S, Woodside M, Sardet C, Pouyssegur J, Rotin D. Activation of the Na+/H+ antiporter during cell volume regulation. Evidence for a phosphorylation-independent mechanism. J Biol Chem 267: 23823–23828, 1992. [PubMed] [Google Scholar]
- 38.Harold FM, Papineau D. Cation transport and electrogenesis by Streptococcus faecalis. II. Proton and sodium extrusion. J Membr Biol 8: 45–62, 1972. [DOI] [PubMed] [Google Scholar]
- 39.He CJ, Esposito C, Phillips C, Zalups RK, Henderson DA, Striker GE, Striker LJ. Dissociation of glomerular hypertrophy, cell proliferation, and glomerulosclerosis in mouse strains heterozygous for a mutation (Os) which induces a 50% reduction in nephron number. J Clin Invest 97: 1242–1249, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hirpara JL, Clément MV, Pervaiz S. Intracellular acidification triggered by mitochondrial-derived hydrogen peroxide is an effector mechanism for drug-induced apoptosis in tumor cells. J Biol Chem 276: 514–521, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Huc L, Sparfel L, Rissel M, Dimanche-Boitrel MT, Guillouzo A, Fardel O, Lagadic-Gossmann D. Identification of Na+/H+ exchange as a new target for toxic polycyclic aromatic hydrocarbons in liver cells. FASEB J 17: NIL451–NIL476, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Ingber DE, Prusty D, Frangioni JV, Cragoe EJ Jr, Lechene C, Schwartz MA. Control of intracellular pH and growth by fibronectin in capillary endothelial cells. J Cell Biol 110: 1803–1811, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Janket ML, Dericco JS, Borowski L, Ayyavoo V. Human immunodeficiency virus (HIV-1) Vpr induced downregulation of NHE1 induces alteration in intracellular pH and loss of ERM complex in target cells. Virus Res 126: 76–85, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jung YS, Kim HY, Kim J, Lee MG, Pouyssegur J, Kim E. Physical interactions and functional coupling between Daxx and sodium hydrogen exchanger 1 in ischemic cell death. J Biol Chem 283: 1018–1025, 2008. [DOI] [PubMed] [Google Scholar]
- 45.Kaplan MR, Plotkin MD, Brown D, Hebert SC, Delpire E. Expression of the mouse Na-K-2Cl cotransporter, mBSC2, in the terminal inner medullary collecting duct, the glomerular and extraglomerular mesangium, and the glomerular afferent arteriole. J Clin Invest 98: 723–730, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karmazyn M, Gan XT, Humphreys RA, Yoshida H, Kusumoto K. The myocardial Na+-H+ exchange: structure, regulation, and its role in heart disease. Circ Res 85: 777–786, 1999. [DOI] [PubMed] [Google Scholar]
- 47.Khaled AR, Kim K, Hofmeister R, Muegge K, Durum SK. Withdrawal of IL-7 induces Bax translocation from cytosol to mitochondria through a rise in intracellular pH. Proc Natl Acad Sci USA 96: 14476–14481, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khaled AR, Moor AN, Li AQ, Ferris DK, Muegge K, Fisher RJ, Fliegel L, Durum SK. Trophic factor withdrawal: p38 mitogen-activated protein kinase activates NHE1, which induces intracellular alkalinization. Mol Cell Biol 21: 7545–7557, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khan S, Cleveland RP, Koch CJ, Schelling JR. Hypoxia induces renal tubular epithelial cell apoptosis in chronic renal disease. Lab Invest 79: 1089–1099, 1999. [PubMed] [Google Scholar]
- 50.Khan S, Wu KL, Sedor JR, Abu Jawdeh BG, Schelling JR. The NHE1 Na+/H+ exchanger regulates cell survival by activating and targeting ezrin to specific plasma membrane domains. Cell Mol Biol (Noisy-le-grand) 52: 115–121, 2006. [PubMed] [Google Scholar]
- 51.Kim J, Jung YS, Han W, Kim MY, Namkung W, Lee BH, Yi KY, Yoo SE, Lee MG, Kim KH. Pharmacodynamic characteristics and cardioprotective effects of new NHE1 inhibitors. Eur J Pharmacol 567: 131–138, 2007. [DOI] [PubMed] [Google Scholar]
- 52.Kim KM, Lee YJ. Amiloride augments TRAIL-induced apoptotic death by inhibiting phosphorylation of kinases and phosphatases associated with the PI3K-Akt pathway. Oncogene 24: 355–366, 2005. [DOI] [PubMed] [Google Scholar]
- 53.Kroemer G Mitochondria in cancer. Oncogene 25: 4630–4632, 2006. [DOI] [PubMed] [Google Scholar]
- 54.Lachapelle G, Radicioni SM, Stankiewicz AR, Mosser DD. Acute acidification or amiloride treatment suppresses the ability of Hsp70 to inhibit heat-induced apoptosis. Apoptosis 12: 1479–1488, 2007. [DOI] [PubMed] [Google Scholar]
- 55.Lagadic-Gossmann D, Huc L, Lecureur V. Alterations of intracellular pH homeostasis in apoptosis: origins and roles. Cell Death Differ 11: 953–961, 2004. [DOI] [PubMed] [Google Scholar]
- 56.Lamprecht G, Weinman EJ, Yun CH. The role of NHERF and E3KARP in the cAMP-mediated inhibition of NHE3. J Biol Chem 273: 29972–29978, 1998. [DOI] [PubMed] [Google Scholar]
- 57.Lang F, Busch GL, Ritter M, Volkl H, Waldegger S, Gulbins E, Haussinger D. Functional significance of cell volume regulatory mechanisms. Physiol Rev 78: 247–306, 1998. [DOI] [PubMed] [Google Scholar]
- 58.Lang F, Madlung J, Bock J, Lükewille U, Kaltenbach S, Lang KS, Belka C, Wagner CA, Lang HJ, Gulbins E, Lepple-Wienhues A. Inhibition of Jurkat-T-lymphocyte Na+/H+-exchanger by CD95(Fas/Apo-1)-receptor stimulation. Pflügers Arch 440: 902–907, 2000. [DOI] [PubMed] [Google Scholar]
- 59.Li J, Eastman A. Apoptosis in an interleukin-2-dependent cytotoxic T lymphocyte cell line is associated with intracellular acidification. Role of the Na+/H+-antiport. J Biol Chem 270: 3203–3211, 1995. [DOI] [PubMed] [Google Scholar]
- 60.Maekawa N, Abe J, Shishido T, Itoh S, Ding B, Sharma VK, Sheu SS, Blaxall BC, Berk BC. Inhibiting p90 ribosomal S6 kinase prevents Na+-H+ exchanger-mediated cardiac ischemia-reperfusion injury. Circulation 113: 2516–2523, 2006. [DOI] [PubMed] [Google Scholar]
- 61.Maeno E, Takahashi N, Okada Y. Dysfunction of regulatory volume increase is a key component of apoptosis. FEBS Lett 580: 6513–6517, 2006. [DOI] [PubMed] [Google Scholar]
- 62.Magro F, Fraga S, Soares-da-Silva P. Interferon-γ-induced STAT1-mediated membrane retention of NHE1 and associated proteins ezrin, radixin and moesin in HT-29 cells. Biochem Pharmacol 70: 1312–1319, 2005. [DOI] [PubMed] [Google Scholar]
- 63.Manucha W, Carrizo L, Ruete C, Valles PG. Apoptosis induction is associated with decreased NHE1 expression in neonatal unilateral ureteric obstruction. BJU Int 100: 191–198, 2007. [DOI] [PubMed] [Google Scholar]
- 64.Marches R, Vitetta ES, Uhr JW. A role for intracellular pH in membrane IgM-mediated cell death of human B lymphomas. Proc Natl Acad Sci USA 98: 3434–3439, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Masereel B, Pochet L, Laeckmann D. An overview of inhibitors of Na+/H+ exchanger. Eur J Med Chem 38: 547–554, 2003. [DOI] [PubMed] [Google Scholar]
- 66.Matsui T, Maeda M, Doi Y, Yonemura S, Amano M, Kaibuchi K, Tsukita S, Tsukita S. Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association. J Cell Biol 140: 647–657, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matsuyama S, Llopis J, Deveraux QL, Tsien RY, Reed JC. Changes in intramitochondrial and cytosolic pH: early events that modulate caspase activation during apoptosis. Nat Cell Biol 2: 318–325, 2000. [DOI] [PubMed] [Google Scholar]
- 68.McManus ML, Churchwell KB, Strange K. Regulation of cell volume in health and disease. N Engl J Med 333: 1260–1266, 1995. [DOI] [PubMed] [Google Scholar]
- 69.McSwine RL, Li J, Villereal ML. Examination of the role for Ca2+ in regulation and phosphorylation of the Na+/H+ antiporter NHE1 via mitogen and hypertonic stimulation. J Cell Physiol 168: 8–17, 1996. [DOI] [PubMed] [Google Scholar]
- 70.Meima ME, Mackley JR, Barber DL. Beyond ion translocation: structural functions of the sodium-hydrogen exchanger isoform-1. Curr Opin Nephrol Hypertens 16: 365–372, 2007. [DOI] [PubMed] [Google Scholar]
- 71.Mentzer RM, Bartels C, Bolli R, Boyce S, Buckberg GD, Chaitman B, Haverich A, Knight J, Menasche P, Myers ML, Nicolau J, Simoons M, Thulin L, Weisel RD. Sodium-hydrogen exchange inhibition by cariporide to reduce the risk of ischemic cardiac events in patients undergoing coronary artery bypass grafting: results of the EXPEDITION study. Ann Thorac Surg 85: 1261–1270, 2008. [DOI] [PubMed] [Google Scholar]
- 72.Moolenaar WH, Tsien RY, van der Saag PT, de Laat SW. Na+/H+ exchange and cytoplasmic pH in the action of growth factors in human fibroblasts. Nature 304: 645–648, 1983. [DOI] [PubMed] [Google Scholar]
- 73.Murthy A, Gonzalez-Agosti C, Cordero E, Pinney D, Candia C, Solomon F, Gusella J, Ramesh V. NHE-RF, a regulatory cofactor for Na+-H+ exchange, is a common interactor for merlin and ERM (MERM) proteins. J Biol Chem 273: 1273–1276, 1998. [DOI] [PubMed] [Google Scholar]
- 74.Nakamura N, Tanaka S, Teko Y, Mitsui K, Kanazawa H. Four Na+/H+ exchanger isoforms are distributed to Golgi and post-Golgi compartments and are involved in organelle pH regulation. J Biol Chem 280: 1561–1572, 2005. [DOI] [PubMed] [Google Scholar]
- 75.Narula J, Haider N, Virmani R, DiSalvo TG, Kolodgie FD, Hajjar RJ, Schmidt U, Semigran MJ, Dec GW, Khaw BA. Apoptosis in myocytes in end-stage heart failure. N Engl J Med 335: 1182–1189, 1996. [DOI] [PubMed] [Google Scholar]
- 76.Ng LL, Davies JE, Siczkowski M, Sweeney FP, Quinn PA, Krolewski B, Krolewski AS. Abnormal Na+/H+ antiporter phenotype and turnover of immortalized lymphoblasts from type 1 diabetic patients with nephropathy. J Clin Invest 93: 2750–2757, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Okada Y, Maeno E, Shimizu T, Dezaki K, Wang J, Morishima S. Receptor-mediated control of regulatory volume decrease (RVD) and apoptotic volume decrease (AVD). J Physiol 532: 3–16, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Orlowski J, Grinstein S. Na+/H+ exchangers of mammalian cells. J Biol Chem 272: 22373–22376, 1997. [DOI] [PubMed] [Google Scholar]
- 79.Orlowski J, Grinstein S. Diversity of the mammalian sodium/proton exchanger SLC9 gene family. Pflügers Arch 447: 549–565, 2004. [DOI] [PubMed] [Google Scholar]
- 80.Ortiz A Renal cell loss through cell suicide. Kidney Int 58: 2235–2236, 2000. [DOI] [PubMed] [Google Scholar]
- 81.Parlato S, Giammarioli AM, Logozzi M, Lozupone F, Matarrese P, Luciani F, Falchi M, Malorni W, Fais S. CD95 (APO-1/Fas) linkage to the actin cytoskeleton through ezrin in human T lymphocytes: a novel regulatory mechanism of the CD95 apoptotic pathway. EMBO J 19: 5123–5134, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Perez-Sala D, Collado-Escobar D, Mollinedo F. Intracellular alkalinization suppresses lovastatin-induced apoptosis in HL-60 cells through the inactivation of a pH-dependent endonuclease. J Biol Chem 270: 6235–6242, 1995. [DOI] [PubMed] [Google Scholar]
- 83.Putney LK, Barber DL. Na-H exchange-dependent increase in intracellular pH times G2/M entry and transition. J Biol Chem 278: 44645–44649, 2003. [DOI] [PubMed] [Google Scholar]
- 84.Rao GN, Sardet C, Pouyssegur J, Berk BC. Na+/H+ antiporter gene expression increases during retinoic acid-induced granulocytic differentiation of HL60 cells. J Cell Physiol 151: 361–366, 1992. [DOI] [PubMed] [Google Scholar]
- 85.Rasmussen M, Alexander RT, Darborg BV, Mobjerg N, Hoffmann EK, Kapus A, Pedersen SF. Osmotic cell shrinkage activates ezrin/radixin/moesin (ERM) proteins: activation mechanisms and physiological implications. Am J Physiol Cell Physiol 294: C197–C212, 2008. [DOI] [PubMed] [Google Scholar]
- 86.Rebillard A, Tekpli X, Meurette O, Sergent O, LeMoigne-Muller G, Vernhet L, Gorria M, Chevanne M, Christmann M, Kaina B, Counillon L, Gulbins E, Lagadic-Gossmann D, Dimanche-Boitrel MT. Cisplatin-induced apoptosis involves membrane fluidification via inhibition of NHE1 in human colon cancer cells. Cancer Res 67: 7865–7874, 2007. [DOI] [PubMed] [Google Scholar]
- 87.Reilly RF, Shugrue CA, Lattanzi D, Biemesderfer D. Immunolocalization of the Na+/Ca2+ exchanger in rabbit kidney. Am J Physiol Renal Fluid Electrolyte Physiol 265: F327–F332, 1993. [DOI] [PubMed] [Google Scholar]
- 88.Reshkin SJ, Bellizzi A, Albarani V, Guerra L, Tommasino M, Paradiso A, Casavola V. Phosphoinositide 3-kinase is involved in the tumor-specific activation of human breast cancer cell Na+/H+ exchange, motility, and invasion induced by serum deprivation. J Biol Chem 275: 5361–5369, 2000. [DOI] [PubMed] [Google Scholar]
- 89.Reshkin SJ, Bellizzi A, Caldeira S, Albarani V, Malanchi I, Poignee M, Alunni-Fabbroni M, Casavola V, Tommasino M. Na+/H+ exchanger-dependent intracellular alkalinization is an early event in malignant transformation and plays an essential role in the development of subsequent transformation-associated phenotypes. FASEB J 14: 2185–2197, 2000. [DOI] [PubMed] [Google Scholar]
- 90.Reshkin SJ, Bellizzi A, Cardone RA, Tommasino M, Casavola V, Paradiso A. Paclitaxel induces apoptosis via protein kinase A- and p38 mitogen-activated protein-dependent inhibition of the Na+/H+ exchanger (NHE) isoform 1 in human breast cancer cells. Clin Cancer Res 9: 2366–2373, 2003. [PubMed] [Google Scholar]
- 91.Rich IN, Worthington-White D, Garden OA, Musk P. Apoptosis of leukemic cells accompanies reduction in intracellular pH after targeted inhibition of the Na+/H+ exchanger. Blood 95: 1427–1434, 2000. [PubMed] [Google Scholar]
- 92.Risdon RA, Sloper JC, De Wardener HE. Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis. Lancet 2: 363–366, 1968. [DOI] [PubMed] [Google Scholar]
- 93.Robey RB, Hay N. Mitochondrial hexokinases, novel mediators of the antiapoptotic effects of growth factors and Akt. Oncogene 25: 4683–4696, 2006. [DOI] [PubMed] [Google Scholar]
- 94.Roy S, Bayly CI, Gareau Y, Houtzager VM, Kargman S, Keen SL, Rowland K, Seiden IM, Thornberry NA, Nicholson DW. Maintenance of caspase-3 proenzyme dormancy by an intrinsic “safety catch” regulatory tripeptide. Proc Natl Acad Sci USA 98: 6132–6137, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sardet C, Fafournoux P, Pouyssegur J. α-Thrombin, epidermal growth factor, and okadaic acid activate the Na+/H+ exchanger, NHE-1, by phosphorylating a set of common sites. J Biol Chem 266: 19166–19171, 1991. [PubMed] [Google Scholar]
- 96.Sardet C, Franchi A, Pouyssegur J. Molecular cloning, primary structure, and expression of the human growth factor-activatable Na+/H+ antiporter. Cell 56: 271–280, 1989. [DOI] [PubMed] [Google Scholar]
- 97.Schainuck LI, Striker GE, Cutler RE, Benditt EP. Structural-functional correlations in renal disease. Hum Pathol 1: 631–641, 1970. [DOI] [PubMed] [Google Scholar]
- 98.Schelling JR, Cleveland RP. Involvement of Fas-dependent apoptosis in renal tubular epithelial cell deletion in chronic renal failure. Kidney Int 56: 1313–1316, 1999. [DOI] [PubMed] [Google Scholar]
- 99.Schelling JR, Nkemere N, Kopp JB, Cleveland RP. Fas-dependent fratricidal apoptosis is a mechanism of tubular epithelial cell deletion in chronic renal failure. Lab Invest 78: 813–824, 1998. [PubMed] [Google Scholar]
- 100.Schwartz MA, Lechene C, Ingber DE. Insoluble fibronectin activates the Na/H antiporter by clustering and immobilizing integrin α5β1, independent of cell shape. Proc Natl Acad Sci USA 88: 7849–7853, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Segal MS, Beem E. Effect of pH, ionic charge, and osmolality on cytochrome c-mediated caspase-3 activity. Am J Physiol Cell Physiol 281: C1196–C1204, 2001. [DOI] [PubMed] [Google Scholar]
- 102.Shrode LD, Gan BS, D'Souza SJ, Orlowski J, Grinstein S. Topological analysis of NHE1, the ubiquitous Na+/H+ exchanger using chymotryptic cleavage. Am J Physiol Cell Physiol 275: C431–C439, 1998. [DOI] [PubMed] [Google Scholar]
- 103.Slepkov ER, Rainey JK, Sykes BD, Fliegel L. Structural and functional analysis of the Na+/H+ exchanger. Biochem J 401: 623–633, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stupack DG Integrins as a distinct subtype of dependence receptors. Cell Death Differ 12: 1021–1030, 2005. [DOI] [PubMed] [Google Scholar]
- 105.Stupack DG, Puente XS, Boutsaboualoy S, Storgard CM, Cheresh DA. Apoptosis of adherent cells by recruitment of caspase-8 to unligated integrins. J Cell Biol 155: 459–470, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stuwe L, Muller M, Fabian A, Waning J, Mally S, Noel J, Schwab A, Stock C. pH dependence of melanoma cell migration: protons extruded by NHE1 dominate protons of the bulk solution. J Physiol 585: 351–360, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Su X, Pang TX, Wakabayashi S, Shigekawa M. Evidence for involvement of the putative first extracellular loop in differential volume sensitivity of the Na+/H+ exchangers NHE1 and NHE2. Biochemistry 42: 1086–1094, 2003. [DOI] [PubMed] [Google Scholar]
- 108.Tafani M, Cohn JA, Karpinich NO, Rothman RJ, Russo MA, Farber JL. Regulation of intracellular pH mediates Bax activation in HeLa cells treated with staurosporine or tumor necrosis factor-α. J Biol Chem 277: 49569–49576, 2002. [DOI] [PubMed] [Google Scholar]
- 109.Takemura G, Fujiwara H. Morphological aspects of apoptosis in heart diseases. J Cell Mol Med 10: 56–75, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Terada Y, Inoshita S, Hanada S, Shimamura H, Kuwahara M, Ogawa W, Kasuga M, Sasaki S, Marumo F. Hyperosmolality activates Akt and regulates apoptosis in renal tubular cells. Kidney Int 60: 553–567, 2001. [DOI] [PubMed] [Google Scholar]
- 111.Teshima Y, Akao M, Jones SP, Marbán E. Cariporide (HOE642), a selective Na+-H+ exchange inhibitor, inhibits the mitochondrial death pathway. Circulation 108: 2275–2281, 2003. [DOI] [PubMed] [Google Scholar]
- 112.Theroux P, Chaitman BR, Danchin N, Erhardt L, Meinertz T, Schroeder JS, Tognoni G, White HD, Willerson JT, Jessel A. Inhibition of the sodium-hydrogen exchanger with cariporide to prevent myocardial infarction in high-risk ischemic situations. Main results of the GUARDIAN trial. Guard during ischemia against necrosis (GUARDIAN) Investigators. Circulation 102: 3032–3038, 2000. [DOI] [PubMed] [Google Scholar]
- 113.Thompson CB Apoptosis in the pathogenesis and treatment of disease. Science 267: 1456–1462, 1995. [DOI] [PubMed] [Google Scholar]
- 114.Tominaga T, Barber DL. Na-H exchange acts downstream of RhoA to regulate integrin-induced cell adhesion and spreading. Mol Biol Cell 9: 2287–2303, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tominaga T, Ishizaki T, Narumiya S, Barber DL. p160ROCK mediates RhoA activation of Na-H exchange. EMBO J 17: 4712–4722, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tsukita S, Yonemura S. Cortical actin organization: lessons from ERM (ezrin/radixin/moesin) proteins. J Biol Chem 274: 34507–34510, 1999. [DOI] [PubMed] [Google Scholar]
- 117.Vaughan-Jones RD, Villafuerte FC, Swietach P, Yamamoto T, Rossini A, Spitzer KW. pH-regulated Na+ influx into the mammalian ventricular myocyte: the relative role of Na+-H+ exchange and Na+-HCO3− co-transport. J Cardiovasc Electrophysiol 17, Suppl 1: S134–S140, 2006. [DOI] [PubMed] [Google Scholar]
- 118.Vexler ZS, Symons M, Barber DL. Activation of Na+-H+ exchange is necessary for RhoA-induced stress fiber formation. J Biol Chem 271: 22281–22284, 1996. [DOI] [PubMed] [Google Scholar]
- 119.Wakabayashi S, Fafournoux P, Sardet C, Pouyssegur J. The Na+/H+ antiporter cytoplasmic domain mediates growth factor signals and controls “H+-sensing”. Proc Natl Acad Sci USA 89: 2424–2428, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wakabayashi S, Shigekawa M, Pouyssegur J. Molecular physiology of vertebrate Na+/H+ exchangers. Physiol Rev 77: 51–74, 1997. [DOI] [PubMed] [Google Scholar]
- 121.Wang H, Singh D, Fliegel L. The Na+/H+ antiporter potentiates growth and retinoic acid-induced differentiation of P19 embryonal carcinoma cells. J Biol Chem 272: 26545–26549, 1997. [DOI] [PubMed] [Google Scholar]
- 122.Wang YG, Meyer JW, Ashraf M, Shull GE. Mice with a null mutation in the NHE1 Na+-H+ exchanger are resistant to cardiac ischemia-reperfusion injury. Circ Res 93: 776–782, 2003. [DOI] [PubMed] [Google Scholar]
- 123.Wu KL, Khan S, Lakhe-Reddy S, Jarad G, Mukherjee A, Obejero-Paz CA, Konieczkowski M, Sedor JR, Schelling JR. The NHE1 Na+/H+ exchanger recruits ezrin/radixin/moesin proteins to regulate Akt-dependent cell survival. J Biol Chem 279: 26280–26286, 2004. [DOI] [PubMed] [Google Scholar]
- 124.Wu KL, Khan S, Lakhe-Reddy S, Wang LM, Jarad G, Miller RT, Konieczkowski M, Brown AM, Sedor JR, Schelling JR. Renal tubular epithelial cell apoptosis is associated with caspase cleavage of the NHE1 Na+/H+ exchanger. Am J Physiol Renal Physiol 284: F829–F839, 2003. [DOI] [PubMed] [Google Scholar]
- 125.Yamashita J, Ohkita M, Takaoka M, Kaneshiro Y, Matsuo T, Kaneko K, Matsumura Y. Role of Na+/H+ exchanger in the pathogenesis of ischemic acute renal failure in mice. J Cardiovasc Pharmacol 49: 154–160, 2007. [DOI] [PubMed] [Google Scholar]
- 126.Yaoita H, Ogawa K, Maehara K, Maruyama Y. Apoptosis in relevant clinical situations: contribution of apoptosis in myocardial infarction. Cardiovasc Res 45: 630–641, 2000. [DOI] [PubMed] [Google Scholar]
- 127.Zalups RK The Os/+ mouse: a genetic animal model of reduced renal mass. Am J Physiol Renal Fluid Electrolyte Physiol 264: F53–F60, 1993. [DOI] [PubMed] [Google Scholar]