Abstract
Purpose
To evaluate whether childhood cancer survivors receive regular medical care focused on the specific morbidities that can arise from their therapy.
Patients and Methods
We conducted a cross-sectional survey of health care use in 8,522 participants in the Childhood Cancer Survivor Study, a multi-institutional cohort of childhood cancer survivors. We assessed medical visits in the preceding 2 years, whether these visits were related to the prior cancer, whether survivors received advice about how to reduce their long-term risks, and whether screening tests were discussed or ordered. Completion of echocardiograms and mammograms were assessed in patients at high risk for cardiomyopathy or breast cancer. We examined the relationship between demographics, treatment, health status, chronic medical conditions, and health care use.
Results
Median age at cancer diagnosis was 6.8 years (range, 0 to 20.9 years) and at interview was 31.4 years (range, 17.5 to 54.1 years). Although 88.8% of survivors reported receiving some form of medical care, only 31.5% reported care that focused on their prior cancer (survivor-focused care), and 17.8% reported survivor-focused care that included advice about risk reduction or discussion or ordering of screening tests. Among survivors who received medical care, those who were black, older at interview, or uninsured were less likely to have received risk-based, survivor-focused care. Among patients at increased risk for cardiomyopathy or breast cancer, 511 (28.2%) of 1,810 and 169 (40.8%) of 414 had undergone a recommended echocardiogram or mammogram, respectively.
Conclusion
Despite a significant risk of late effects after cancer therapy, the majority of childhood cancer survivors do not receive recommended risk-based care.
INTRODUCTION
With contemporary therapy, 80% of children diagnosed with cancer will become long-term survivors.1 Consequently, more than 270,000 survivors of childhood cancer are alive in the United States,2 many of whom are at risk of long-term morbidity3,4 and premature mortality5,6 as a result of their therapy. We have estimated that by 30 years from their cancer diagnosis, 73% will develop at least one chronic physical health condition and 42% will develop a severe, life-threatening, or disabling condition or die from a chronic condition.4 Compared with their siblings, survivors are 10 times more likely to develop a serious chronic disease (eg, second cancer or heart disease), and the risk of morbidity and premature mortality does not plateau.4 Because the risk for many serious health problems can be reduced by prevention or early detection, the Institute of Medicine (IOM) has recommended lifelong risk-based health care for all cancer survivors.2 This requires a systematic plan for periodic screening, surveillance, and prevention that is adapted to the risks arising from the previous cancer, its therapy, genetic predispositions, lifestyle behaviors, and comorbid health conditions.2,7 The frequency and intensity of surveillance should be adapted to each survivor's risk, with those at low risk for sequelae requiring less contact and those at significantly increased risk of morbidity or premature mortality (eg, those treated with radiation therapy or stem-cell transplantation) requiring annual monitoring.
The purpose of this study was to characterize the patterns and predictors of health care use among a large cohort of geographically and socioeconomically diverse childhood cancer survivors. In particular, we were interested in determining the proportion of individuals who received medical care that was focused specifically on addressing and reducing the risks arising from their prior cancer therapy.
PATIENTS AND METHODS
Childhood Cancer Survivor Study
The Childhood Cancer Survivor Study (CCSS) methodology and a description of the participants have been published.8 Briefly, the cohort includes individuals diagnosed with cancer before age 21 years at one of 26 centers (25 centers in the United States and one in Canada) from 1970 to 1986 who were alive at least 5 years from their original diagnosis. The eligible cohort consisted of 20,720 patients; 17,703 were successfully contacted and 14,366 (81.2%) enrolled in the study. There were no significant differences between participants and nonparticipants by sex, age at diagnosis or at cohort assembly, cancer type, or treatment.8,9 Detailed medical information was abstracted from participants’ hospital records. Participants completed a comprehensive baseline questionnaire and several subsequent questionnaires. Eligibility for this analysis was limited to participants who completed a questionnaire in 2002 to 2003 that addressed health care use and for whom information regarding treatment for their original cancer was available. Questionnaires and data abstraction forms are available at www.stjude.org/ccss. The study was approved by the institutional review board at each participating institution, and informed consent was obtained from each participant or parent/guardian.
Health Care Use
A series of questions was constructed to characterize the medical care received by survivors and to determine whether this care focused on the previous cancer and the risk of future health problems arising from its therapy. Participants were asked whether they had visited a health care provider (physician or nurse) within the preceding 2 years, whether the visit was related to their previous cancer, and whether their health care provider had given them advice on how to reduce their risks or discussed or ordered screening tests for cancer-related sequelae. Responses to these questions were used to categorize health care into one of four mutually exclusive groups: (1) no health care, (2) general medical care (patient reported one or more medical visits, none of which was related to their previous cancer), (3) general survivor-focused care (patient reported at least one visit related to their previous cancer, but did not report receiving advice on how to reduce risks or that screening tests for cancer-related sequelae were discussed or ordered), and (4) risk-based, survivor-focused care (survivor-focused care that included advice about how to reduce risks or discussion or ordering of screening tests for cancer-related sequelae). The hierarchy was constructed to classify levels of medical care related specifically to the prior cancer and its risks and is not intended to imply a level of quality of care for health issues unrelated to the previous cancer. Each participant was classified according to the highest category of care received during the 2-year study period. The assigned level of care was independent of who delivered the care (cancer specialist or primary care clinician) or where the care was received (cancer center or community setting). Additionally, we classified a subset of the cohort as being at high risk for developing a cardiomyopathy (survivors who had received ≥ 300 mg/m2 of an anthracycline or any anthracycline dose plus chest radiation) or breast cancer (females who had received radiation to the chest and who were ≥ 27 years old), two late effects for which there is general consensus concerning the need for regular surveillance with an echocardiogram or mammogram, respectively.10-12
Predictors of Health Care Use
Demographic and treatment information.
Demographic data were obtained on the baseline questionnaire. Sociodemographic status (household income, health insurance, education, and employment status) was assessed in the 2002 to 2003 questionnaire. Disease and treatment variables were abstracted from medical records.
Chronic medical conditions and health status.
We evaluated the association between health status, chronic medical conditions, and health care use. The severity of chronic health conditions reported on the baseline questionnaire was classified as (1) mild, (2) moderate, (3) severe, or (4) life-threatening or disabling, using the National Cancer Institute's Common Terminology Criteria for Adverse Events (version 3), as published previously.4 Health status was measured on the 2002 to 2003 questionnaire using a previously defined set of domains (emotional health,13,14 physical functioning,15 cancer-related pain, and cancer-related anxiety and fears14).
Statistical Analysis
Descriptive statistics were calculated for each of the sociodemographic, treatment, and health status variables. The probability of reporting a particular level of care, adjusted for age at interview and sex, was calculated by diagnosis using a generalized logit model, treating levels of care as nominal response variables.16 The relative odds of receiving no medical care versus any medical care, of receiving general medical care versus risk-based and survivor-focused care, and of not having either a mammogram or an echocardiogram, if indicated, were calculated in separate multivariable logistic regression models. A model was generated that included sociodemographic, health status, and chronic disease variables as predictors of the health care outcome, and a separate model was generated that included therapeutic exposures as predictors. Models evaluating the impact of therapeutic exposure were adjusted for age at diagnosis, age at interview, and sex. The frequency and percentage of those receiving either a mammogram or echocardiogram (if indicated) were evaluated by level of care using a contingency table and compared with χ2 statistics.
RESULTS
Characteristics of the Study Cohort
Of the 14,366 survivors who were enrolled in the CCSS, 1,773 did not have medical records available, 1,017 were dead at the time of the baseline assessment and had their questionnaire completed by a proxy respondent, and 462 died before the mailing out of the 2002 to 2003 questionnaire. Thus 11,114 survivors were eligible for this study of health care use. Of these, 1,919 (17.3%) were nonrespondents and 673 (6.1%) were lost to follow-up, resulting in 8,522 (76.7%) survivors being available for this analysis. The 8,522 participants did not differ from the 2,592 eligible nonparticipants by diagnosis, therapy, or current age, but were more likely to be female (79.8% of eligible females v 73.9% of eligible males; P < .001) and older when diagnosed with their original cancer (mean age, 8.2 v 7.5 years; P = .006). Demographic, treatment, and health status characteristics of the participants are presented in Table 1.
Table 1.
Characteristics of the Study Cohort and Their Self-Reported Medical Care Within the Last 2 Years
| Characteristic | Full Study Cohort (N = 8,522)
|
% of Patients
|
||||
|---|---|---|---|---|---|---|
| No. | % | No Medical Care (n = 953)* | General Medical Care (n = 4,882) | General Survivor-Focused Care (n = 1,166) | Risk-Based Survivor-Focused Care (n = 1,521) | |
| Sex | ||||||
| Male | 4,273 | 50.1 | 15.6 | 55.8 | 12.7 | 15.9 |
| Female | 4,249 | 49.9 | 6.7 | 58.8 | 14.7 | 19.8 |
| Race/ethnicity | ||||||
| White, non-Hispanic | 7,368 | 86.5 | 10.6 | 57.6 | 14.1 | 17.8 |
| Hispanic | 135 | 1.6 | 11.1 | 57.0 | 9.6 | 22.2 |
| Black | 231 | 2.7 | 18.6 | 61.5 | 9.1 | 10.8 |
| Other | 788 | 9.2 | 14.5 | 53.7 | 12.2 | 19.7 |
| Age, years | ||||||
| At diagnosis | ||||||
| Mean | 7.5 | 8.1 | 8.3 | 8.8 | ||
| SD | 5.5 | 5.8 | 5.9 | 6.1 | ||
| At interview | ||||||
| Mean | 30.9 | 31.8 | 31.4 | 32.2 | ||
| SD | 7.1 | 7.5 | 7.8 | 8.1 | ||
| Time since diagnosis, years | ||||||
| Mean | 23.4 | 23.7 | 23.1 | 23.4 | ||
| SD | 4.5 | 4.5 | 4.5 | 4.7 | ||
| Annual household income | ||||||
| < $40,000 | 2,643 | 31.0 | 14.5 | 55.7 | 13.9 | 15.9 |
| $40,000-$79,000 | 2,721 | 31.9 | 8.6 | 59.4 | 13.5 | 18.5 |
| ≥ $80,000 | 1,957 | 23.0 | 7.8 | 59.4 | 12.5 | 20.2 |
| Unknown | 1,201 | 14.1 | 15.3 | 52.5 | 15.4 | 16.7 |
| Educational attainment | ||||||
| < High school | 376 | 4.4 | 12.2 | 51.9 | 16.8 | 19.2 |
| High school graduate | 4,387 | 51.5 | 14.0 | 55.5 | 14.0 | 16.5 |
| College graduate | 3,666 | 43.0 | 7.8 | 60.0 | 12.9 | 19.3 |
| Employment status | ||||||
| Unemployed | 1,023 | 12.0 | 11.3 | 44.8 | 21.1 | 22.8 |
| Employed, student, caring for home | 7,313 | 85.8 | 11.1 | 59.1 | 12.5 | 17.3 |
| Health insurance status | ||||||
| No, United States | 971 | 11.4 | 28.5 | 51.3 | 10.4 | 9.8 |
| Yes, United States | 6,918 | 81.2 | 8.8 | 58.5 | 13.8 | 18.8 |
| Canadian resident | 559 | 6.6 | 9.3 | 53.0 | 17.5 | 20.2 |
| Care at cancer center in last 2 years | ||||||
| No | 7,276 | 85.4 | NA | 63.8 | 11.0 | 12.3 |
| Yes | 1,246 | 14.6 | NA | 19.5 | 29.5 | 50.4 |
| Cancer diagnosis | ||||||
| Leukemia | 2,910 | 34.1 | 13.0 | 61.1 | 11.8 | 14.1 |
| CNS tumor | 1,076 | 12.6 | 11.2 | 44.5 | 22.4 | 21.9 |
| Hodgkin's disease | 1,086 | 12.7 | 7.2 | 48.3 | 15.6 | 29.0 |
| Non-Hodgkin's lymphoma | 628 | 7.4 | 11.2 | 62.9 | 8.6 | 14.7 |
| Wilms’ tumor | 794 | 9.3 | 11.1 | 65.5 | 8.6 | 14.7 |
| Neuroblastoma | 576 | 6.8 | 12.7 | 62.8 | 9.6 | 14.9 |
| Soft tissue sarcoma | 750 | 8.8 | 10.4 | 55.3 | 16.5 | 17.7 |
| Osteosarcoma | 481 | 5.6 | 10.6 | 60.1 | 13.3 | 16.0 |
| Ewing's sarcoma | 221 | 2.6 | 7.7 | 53.4 | 19.5 | 19.5 |
| RT | ||||||
| Brain | 2,604 | 30.6 | 13.1 | 52.7 | 16.1 | 18.1 |
| Chest | 1,562 | 18.3 | 7.7 | 50.0 | 14.5 | 27.7 |
| Other | 1,213 | 14.2 | 9.7 | 57.4 | 14.6 | 18.4 |
| None | 2,873 | 33.7 | 11.9 | 65.6 | 10.4 | 12.1 |
| Unknown | 270 | 3.2 | 11.1 | 54.8 | 17.0 | 17.0 |
| Cardiotoxic therapies | ||||||
| Anthracyclines, no chest RT | 2,408 | 28.3 | 12.2 | 58.4 | 12.5 | 16.9 |
| Chest RT, no anthracyclines | 1,035 | 12.1 | 8.1 | 48.6 | 15.6 | 27.4 |
| Anthracyclines + chest RT | 589 | 6.9 | 7.6 | 51.5 | 12.4 | 28.9 |
| No anthracyclines, no chest RT | 4,490 | 52.7 | 11.8 | 59.5 | 14.0 | 14.7 |
| Alkylating agent dose† | ||||||
| None | 1,708 | 20.0 | 12.2 | 65.8 | 10.8 | 11.2 |
| First tertile | 733 | 8.6 | 12.1 | 65.9 | 9.7 | 12.3 |
| Second tertile | 270 | 3.2 | 10.7 | 64.8 | 10.0 | 14.4 |
| Third tertile | 162 | 1.9 | 9.9 | 62.9 | 9.9 | 17.3 |
| Poor emotional health | ||||||
| No | 7,829 | 91.9 | 11.4 | 58.0 | 13.2 | 17.4 |
| Yes | 693 | 8.1 | 9.0 | 49.0 | 19.1 | 22.9 |
| Cancer-related anxiety | ||||||
| None, a small amount | 7,756 | 91.0 | 11.5 | 58.5 | 13.1 | 17.0 |
| Moderate, a lot, extreme | 766 | 9.0 | 7.6 | 45.6 | 20.1 | 26.8 |
| Cancer-related pain | ||||||
| None, a small amount | 7,821 | 91.8 | 11.6 | 58.8 | 12.7 | 16.9 |
| Moderate, a lot, extreme | 701 | 8.2 | 6.6 | 39.9 | 24.5 | 29.0 |
| Poor physical health | ||||||
| No | 6,461 | 75.8 | 11.3 | 59.7 | 12.3 | 16.7 |
| Yes | 2,061 | 24.2 | 10.9 | 49.6 | 18.0 | 21.5 |
| Chronic disease status, grade‡ | ||||||
| 0, 1, 2 | 6,415 | 75.3 | 12.0 | 60.3 | 12.1 | 15.6 |
| 3, 4 | 2,106 | 24.7 | 8.6 | 48.2 | 18.4 | 24.8 |
Abbreviations: SD, standard deviation; RT, radiation therapy.
Percentages calculated by row.
Limited to those who did not receive radiation.
Grade 0, 1, 2: either no chronic condition (grade 0) or at least one grade 1 (mild) or grade 2 (moderate) chronic condition; grade 3, 4: at least one grade 3 (severe) or grade 4 (life-threatening or disabling) chronic condition.
Health Care Use
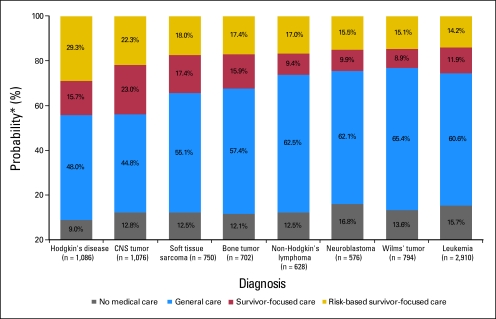
During the 2-year study period, 953 survivors (11.2%) reported receiving no medical care, 4,882 survivors (57.3%) reported receiving general medical care, 1,166 survivors (13.7%) reported receiving general survivor-focused care, and 1,521 survivors (17.8%) reported receiving risk-based, survivor-focused care. Table 1 lists the percentage of survivors who reported each level of care by demographic, treatment, and health status variables. Only 1,246 patients (14.6%) received care at a cancer center. The probability of receiving a particular level of care according to cancer diagnosis is shown in Figure 1. General survivor-focused or risk-based survivor-focused care was most likely among survivors of CNS tumors. Survivors of neuroblastoma were most likely to have received no medical care.
Fig 1.
Probability of reporting a particular level of medical care according to initial cancer diagnosis. (*) Probabilities adjusted for sex, age at diagnosis, and age at time of interview using a generalized logit model.
The relationship between demographic and health status variables and the odds of having received no medical care is summarized in Table 2. Male survivors, uninsured survivors, and those with a household income less than $40,000/year were more likely to report no medical visits. In contrast, survivors who reported moderate to extreme cancer-related pain were more likely to have received care, as were those with a severe, life-threatening, or disabling chronic health condition. Age at diagnosis and at interview were not associated with having received medical care.
Table 2.
Relative Odds of Receiving No Medical Care Versus Any Care and of Receiving General Care Versus Risk-Based, Survivor-Focused Care by Demographic and Outcome Variables
| Variable | No Medical Care (n = 953) v Any Care (n = 7,569)*
|
General Care (n = 4,882) v Risk-Based, Survivor-Focused Care (n = 1,521)*
|
||
|---|---|---|---|---|
| OR† | 95% CI | OR† | 95% CI | |
| Sex | ||||
| Male | 2.5 | 2.2 to 2.9‡ | 1.1 | 1.0 to 1.3 |
| Female (referent) | 1.0 | 1.0 | ||
| Race/ethnicity | ||||
| White, non-Hispanic (referent) | 1.0 | 1.0 | ||
| Hispanic | 1.0 | 0.6 to 1.8 | 0.8 | 0.5 to 1.3 |
| Black | 1.4 | 0.9 to 2.0 | 2.1 | 1.3 to 3.3‡ |
| Other | 1.2 | 1.0 to 1.5‡ | 0.8 | 0.7 to 1.0‡ |
| Age, years | ||||
| At diagnosis | 0.99 | 0.97 to 1.01 | 0.97 | 0.95 to 0.98‡ |
| At interview | 1.01 | 0.99 to 1.03 | 1.03 | 1.02 to 1.04‡ |
| Annual household income | ||||
| < $40,000 | 1.4 | 1.2 to 1.8‡ | 1.1 | 1.0 to 1.4 |
| $40,000-$79,000 | 1.0 | 0.8 to 1.2 | 1.1 | 0.9 to 1.2 |
| ≥ $80,000 (referent) | 1.0 | 1.0 | ||
| Unknown | 1.6 | 1.2 to 2.1‡ | 1.0 | 0.8 to 1.3 |
| Educational attainment | ||||
| < High school | 1.0 | 0.7 to 1.5 | 1.0 | 0.8 to 1.4 |
| High school graduate | 1.5 | 1.3 to 1.8‡ | 1.1 | 1.0 to 1.2 |
| College graduate (referent) | 1.0 | 1.0 | ||
| Employment status | ||||
| Unemployed | 0.9 | 0.7 to 1.1 | 0.7 | 0.6 to 0.8‡ |
| Employed§ (referent) | 1.0 | 1.0 | ||
| Health insurance status | ||||
| No, United States | 3.4 | 2.8 to 4.0‡ | 1.7 | 1.3 to 2.2‡ |
| Yes, United States (referent) | 1.0 | 1.0 | ||
| Canadian resident | 1.1 | 0.8 to 1.4 | 0.8 | 0.6 to 1.0‡ |
| Poor emotional health | ||||
| No (referent) | 1.0 | 1.0 | ||
| Yes | 0.8 | 0.6 to 1.1 | 1.0 | 0.8 to 1.2 |
| Cancer-related anxiety | ||||
| None, a small amount (referent) | 1.0 | 1.0 | ||
| Moderate, a lot, extreme | 0.8 | 0.6 to 1.1 | 0.6 | 0.5 to 0.8‡ |
| Cancer-related pain | ||||
| None, a small amount (referent) | 1.0 | 1.0 | ||
| Moderate, a lot, extreme | 0.6 | 0.4 to 0.8‡ | 0.5 | 0.4 to 0.6‡ |
| Poor physical health | ||||
| No (referent) | 1.0 | 1.0 | ||
| Yes | 0.9 | 0.7 to 1.1 | 0.7 | 0.6 to 0.8‡ |
| Chronic disease status, grade | ||||
| 0, 1, 2 (referent) | 1.0 | 1.0 | ||
| 3, 4 | 0.8 | 0.7 to 1.0‡ | 0.6 | 0.5 to 0.7‡ |
Abbreviation: OR, odds ratio.
Adjusted for all other variables in the model.
OR > 1 represents increased odds of receiving lower level of medical care.
Significant at P < .05.
Employed, student, or caring for home.
Additionally, Table 2 presents predictors of reporting general medical care rather than risk-based, survivor-focused care among survivors who reported some form of care (n = 7,569). Survivors who were older, male, black, or uninsured were more likely to report general care rather than risk-based, survivor-focused care. In contrast, survivors who reported moderate to extreme cancer-related pain or anxiety, poor physical health, or more serious morbidity were more likely to report risk-based, survivor-focused care. Among survivors who received some form of survivor-focused care, only the location of care (cancer center v other location; odds ratio = 1.52, 95% CI, 1.29 to 1.80) and “other” race (v white race; odds ratio = 1.38, 95% CI, 1.05 to 1.84) influenced whether this care was risk-based.
The relationship between therapeutic exposures associated with an increased risk of late effects and the level of care is summarized in Table 3. Patients treated with an alkylating agent or anthracycline (without chest radiation), two therapies that are strongly associated with long-term morbidity, were no more likely to report receiving any health care rather than no health care. Similarly, survivors who received an anthracycline (without chest radiation) were no more likely to receive risk-based, survivor-focused care than general care, although risk-based, survivor-focused care was more likely in survivors who received the highest tertile of alkylating agent dosing. In contrast, all survivors treated with radiation therapy were more likely to report risk-based, survivor-focused care.
Table 3.
Comparison of Medical Care According to Chemotherapy or Radiation Exposure During Cancer Treatment
| Variable | No Medical Care (n = 953) v Any Care (n = 7,569)*
|
General Care (n = 4,882) v Risk-Based, Survivor-Focused Care (n = 1,521)*
|
||
|---|---|---|---|---|
| OR† | 95% CI | OR† | 95% CI | |
| RT | ||||
| Brain | 1.1 | 0.9 to 1.3 | 0.5 | 0.4 to 0.6‡ |
| Chest | 0.7 | 0.5 to 0.8‡ | 0.3 | 0.2 to 0.4‡ |
| Other | 0.8 | 0.6 to 1.0 | 0.5 | 0.4 to 0.6‡ |
| None | 1.0 | 1.0 | ||
| Unknown | 0.9 | 0.6 to 1.3 | 0.5 | 0.4 to 0.8‡ |
| Cardiotoxic therapies | ||||
| Anthracyclines, no chest RT | 1.0 | 0.9 to 1.2 | 0.9 | 0.8 to 1.1 |
| Chest RT, no anthracyclines | 0.8 | 0.6 to 0.9‡ | 0.4 | 0.3 to 0.6‡ |
| Anthracyclines + chest RT | 0.6 | 0.5 to 0.9‡ | 0.5 | 0.4 to 0.6‡ |
| No anthracyclines, no chest RT (referent) | 1.0 | 1.0 | ||
| Alkylating agent therapy dose§ | ||||
| None (referent) | 1.0 | 1.0 | ||
| First tertile | 0.9 | 0.7 to 1.3 | 1.0 | 0.7 to 1.3 |
| Second tertile | 0.9 | 0.6 to 1.3 | 0.8 | 0.5 to 1.1 |
| Third tertile | 0.7 | 0.4 to 1.3 | 0.6 | 0.4 to 0.9‡ |
Abbreviations: OR, odds ratio; RT, radiation therapy.
Adjusted for sex, age at diagnosis, and age at time of interview.
OR > 1 represents increased odds of receiving lower level of care.
Significant at P < .05.
Limited to those who did not receive radiation.
An echocardiogram was indicated in 1,810 survivors and a mammogram was indicated in 414 women based on their high risk for developing a cardiomyopathy or breast cancer, respectively. However, only 511 (28.2%) of 1,810 survivors at risk for cardiomyopathy reported an echocardiogram. For women treated with chest radiation who were 27 years or older at the time of the 2002 to 2003 questionnaire and were younger than the age at which routine screening mammography is recommended for the general population (United States, age 40 years; Canada, age 50 years), 169 (40.8%) of 414 survivors reported receiving a mammogram. Survivors who were uninsured, Canadian, or not seen at a cancer center were more likely to not report an indicated echocardiogram, whereas those who had moderate to extreme anxiety after their cancer were more likely to have received an echocardiogram (Table 4). Only care outside of a cancer center was associated with not having received an indicated mammogram. Survivors who received care in a cancer center were more likely than those who received their care elsewhere to report an indicated echocardiogram (53.2% v 22.3%) or mammogram (62.4% v 34.6%).
Table 4.
Relative Odds of Not Receiving Indicated Echocardiogram or Mammogram by Demographic and Outcome Variables
| Variable | Did Not Receive Indicated Echocardiogram* (n = 1,299 of 1,810)
|
Did Not Receive Indicated Mammogram* (n = 245 of 414)
|
||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Care category | ||||
| No medical care | NA | NA | 7.8 | 1.4 to 43.0† |
| General medical care | 3.9 | 3.0 to 5.0† | 2.5 | 1.5 to 4.2† |
| General survivor-focused care | 2.1 | 1.5 to 3.0† | 1.6 | 0.8 to 3.0 |
| Risk-based survivor-focused care (referent) | 1.0 | 1.0 | ||
| Sex | ||||
| Male | 1.0 | 0.8 to 1.2 | NA | NA |
| Female (referent) | 1.0 | |||
| Race/ethnicity | ||||
| White, non-Hispanic (referent) | 1.0 | 1.0 | ||
| Hispanic | 0.6 | 0.3 to 1.2 | 0.3 | 0.1 to 1.5 |
| Black | 0.9 | 0.5 to 1.6 | 0.2 | 0.1 to 1.0 |
| Other | 1.1 | 0.7 to 1.6 | 2.2 | 0.9 to 5.4 |
| Age, years | ||||
| At diagnosis (per each year) | 1.02 | 0.98 to 1.05 | 0.99 | 0.94 to 1.04 |
| At interview (per each year) | 1.00 | 0.98 to 1.03 | 0.88 | 0.81 to 0.95† |
| Annual household income | ||||
| < $40,000 | 1.5 | 1.1 to 2.1† | 1.4 | 0.7 to 2.6 |
| $40,000-$79,000 | 1.2 | 0.9 to 1.6 | 1.1 | 0.7 to 2.0 |
| ≥ $80,000 (referent) | 1.0 | 1.0 | ||
| Unknown | 1.1 | 0.7 to 1.6 | 0.4 | 0.1 to 1.3 |
| Educational attainment | ||||
| < High school | 1.2 | 0.6 to 2.4 | 3.7 | 0.3 to 42.6 |
| High school graduate | 1.1 | 0.8 to 1.3 | 1.1 | 0.7 to 1.7 |
| College graduate (referent) | 1.0 | 1.0 | ||
| Employment status | ||||
| Unemployed | 0.7 | 0.5 to 1.1 | 1.0 | 0.4 to 2.3 |
| Employed‡ (referent) | 1.0 | 1.0 | ||
| Health insurance status | ||||
| No, United States | 2.0 | 1.2 to 3.2† | 1.5 | 0.6 to 4.1 |
| Yes, United States (referent) | 1.0 | 1.0 | ||
| Canadian resident | 1.7 | 1.1 to 2.8† | 1.1 | 0.5 to 2.7 |
| Poor emotional health | ||||
| No (referent) | 1.0 | 1.0 | ||
| Yes | 1.0 | 0.6 to 1.6 | 0.9 | 0.4 to 1.9 |
| Cancer-related anxiety | ||||
| None, a small amount (referent) | 1.0 | 1.0 | ||
| Moderate, a lot, extreme | 0.6 | 0.4 to 0.9† | 0.7 | 0.4 to 1.4 |
| Cancer-related pain | ||||
| None, a small amount (referent) | 1.0 | 1.0 | ||
| Moderate, a lot, extreme | 0.9 | 0.6 to 1.3 | 1.3 | 0.6 to 2.8 |
| Poor physical health | ||||
| No (referent) | 1.0 | 1.0 | ||
| Yes | 0.9 | 0.7 to 1.2 | 1.2 | 0.7 to 2.2 |
| Chronic disease status, grade | ||||
| 0, 1, 2 (referent) | 1.0 | 1.0 | ||
| 3, 4 | 0.8 | 0.7 to 1.1 | 1.2 | 0.7 to 1.9 |
Abbreviations: OR, odds ratio; NA, not applicable.
Adjusted for all other variables in the model.
Significant at P < .05.
Employed, student, or caring for home.
DISCUSSION
Only 17.8% of this cohort of 8,522 long-term survivors of childhood cancer reported a medical visit within the previous 2 years during which their health care provider specifically addressed the risks arising from their therapy. This low prevalence of risk-based care falls far below the goals advocated by the IOM.2 Strikingly, 88.8% of survivors had at least one medical visit during the study period, reflecting that access to medical care was not a barrier for most. Rather, the care that they received did not focus on their specific risks and strategies to ameliorate them. Fewer than 15% of survivors received medical care at a cancer center; most received their care from a primary care clinician in a community setting. However, survivors cared for in the community were less likely to report risk-based, survivor-focused care or to have received indicated echocardiography or mammography. Because more than 70% of survivors will develop one or more chronic conditions related to their prior therapy,3,4 this low rate of risk-based, survivor-focused care suggests multiple lost opportunities to prevent or expeditiously detect and treat these sequelae. Thus efforts must be focused on providing primary care clinicians with the education and resources needed to provide risk-based care to this group of patients.17
Previous studies have shown that key barriers to appropriate survivor care include a lack of patient and physician knowledge about the long-term risks of cancer therapy.18,19 Survivors are often unaware of the details of their cancer therapy, preventing them from seeking care focused on specific risks. One study has reported that among exposed survivors, fewer than one third recalled receiving anthracycline chemotherapy, which is associated with a risk of late cardiac toxicity.20,21 Similar knowledge deficits are likely in our study, as survivors who received anthracyclines were no more likely to get care than those who had not received anthracyclines. Only survivors who had received chest radiation were more likely to receive such care, suggesting that care-seeking behavior among those at risk for cardiac disease is influenced by radiation exposure, but not anthracycline therapy. This observation is particularly concerning given that approximately half of children treated for cancer will receive anthracycline chemotherapy. Similarly, survivors treated with low or moderate doses of alkylating agents, which have been associated with secondary leukemias22 and infertility,23-25 were no more likely to receive care than those not treated with these drugs.
Forty-one percent of females at increased risk for breast cancer reported a mammogram within the prior 2 years, despite evidence that by 45 years of age, 20% of women treated with chest radiation as children will develop breast cancer.26 Only 28% of survivors at risk for a cardiomyopathy reported an echocardiogram. Self-report of mammography has been demonstrated to be valid in several studies, with 81% to 97% congruence between patient self-report and medical record audits.27-30 There are no published data on the validity of self-report of echocardiograms. Although patients who received their care at a cancer center had higher rates of appropriate surveillance, many patients treated at these specialized centers did not undergo the recommended tests. Whether the failure to perform a recommended echocardiogram in 47% and a mammogram in 38% of patients seen at a cancer center was due to lack of insurance coverage, inadequate physician knowledge, or an active decision not to heed the guidelines cannot be deduced from this study. The provision of a comprehensive survivorship care plan to all survivors, as recommended by the IOM,2,7 may increase the number of survivors who receive recommended surveillance tests such as echocardiography or breast imaging.
As noted in studies of health care use in other diseases, male patients, the uninsured, and those with lower household incomes31 are particularly vulnerable, because they are at greater risk of receiving no medical care at all. Almost 30% of uninsured survivors had not received medical care in the previous 2 years, compared with 10% of insured survivors. In comparison, in a study of 1,718 survivors of adult cancer that used data from the National Health Interview Survey (1998 and 2000 surveys), 45.1% of uninsured survivors reported not getting needed medical care within the preceding year because of concerns about the cost of that care; in contrast, 16.7% of publicly insured and 4.4% of privately insured survivors did not receive needed care.32 Among survivors in our study who reported some form of care, those who had developed sequelae of their prior therapy (such as pain, anxiety, poorer physical health, or a severe chronic physical condition) were more likely to report a visit related to their previous malignancy, suggesting that these individuals may have been seeking care for extant symptoms. However, because the incidence of serious chronic health conditions increases as survivors age,3,4 even survivors who have not developed late effects may benefit from care focused on prevention and early detection.17
Several methodologic limitations should be considered when interpreting the results. First, data were obtained from self-reports. We cannot examine whether survivors’ impressions about the purpose and content of their medical visit was concordant with their caregivers’ intentions. Second, the results are derived from 8,522 of 11,114 eligible survivors. This potential for selection bias is compounded by the observation that CCSS participants are a select group of survivors, likely better educated about the potential late effects of their cancer therapy than nonparticipants. Thus this study likely overestimates the proportion of survivors who receive appropriate care, and the poor compliance with recommended care demonstrated here is probably a best-case scenario. Third, this cohort of survivors received their therapy from 1970 to 1986. Caution should be exercised in generalizing to patients treated more recently. It is plausible that patients treated in the current era are better informed about the long-term risks of their therapy. The CCSS is currently recruiting a cohort of survivors treated from 1987 to 1999 to examine such questions.
In summary, despite a significant risk of late effects after cancer therapy, the majority of adult survivors of childhood cancer do not receive regular medical care focused on their long-term risks. Only a minority of survivors at the highest risk for developing cardiac dysfunction or breast cancer receive recommended surveillance tests. The majority of survivors receive medical care in a community setting where, they are less likely to receive risk-based care or recommended screening tests. Given the rapidly expanding population of childhood cancer survivors, it is imperative that efforts be focused on educating both survivors and the health care providers who will care for them as adults about the importance of regular, risk-based medical care.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Paul C. Nathan, Mark L. Greenberg, Kirsten K. Ness, Melissa M. Hudson, James G. Gurney, Sarah S. Donaldson, Leslie L. Robison, Kevin C. Oeffinger
Administrative support: Ann C. Mertens
Provision of study materials or patients: Melissa M. Hudson, Ann C. Mertens
Collection and assembly of data: Melissa M. Hudson, Ann C. Mertens, Sarah S. Donaldson, Wendy M. Leisenring, Leslie L. Robison
Data analysis and interpretation: Paul C. Nathan, Mark L. Greenberg, Kirsten K. Ness, Melissa M. Hudson, Martin C. Mahoney, James G. Gurney, Sarah S. Donaldson, Wendy M. Leisenring, Kevin C. Oeffinger
Manuscript writing: Paul C. Nathan, Mark L. Greenberg, Kirsten K. Ness, Kevin C. Oeffinger
Final approval of manuscript: Paul C. Nathan, Mark L. Greenberg, Kirsten K. Ness, Melissa M. Hudson, Ann C. Mertens, Martin C. Mahoney, James G. Gurney, Sarah S. Donaldson, Wendy M. Leisenring, Leslie L. Robison, Kevin C. Oeffinger
Appendix
The Childhood Cancer Survivor Study (CCSS) is a collaborative, multi-institutional project, funded as a resource by the National Cancer Institute, of individuals who survived 5 or more years after diagnosis of childhood cancer. CCSS is a retrospectively ascertained cohort of 20,720 childhood cancer survivors diagnosed before age 21 years between 1970 and 1986, in addition to approximately 4,000 siblings of survivors who serve as a control group. The cohort was assembled through the efforts of 26 participating clinical research centers in the United States and Canada. The study is currently funded by a U24 resource grant (National Cancer Institute Grant No. U24 CA55727) awarded to St Jude Children's Research Hospital. Currently, we are in the process of expanding the cohort to include an additional 14,000 childhood cancer survivors diagnosed before age 21 between 1987 and 1999. For information on how to access and use the CCSS resource, visit www.stjude.org/ccss.
Table A1.
Childhood Cancer Survivor Study (CCSS) Institutions and Investigators
| Institution | Investigators |
|---|---|
| St Jude Children's Research Hospital, Memphis, TN | Leslie L. Robison, PhD,‖‡ Melissa Hudson, MD*‡ |
| Greg Armstrong, MD‡ | |
| Children's Health Care-Minneapolis, MN | Joanna Perkins, MD,* Maura O’Leary, MD† |
| Children's Hospital and Medical Center, Seattle, WA | Debra Friedman, MD, MPH,* Thomas Pendergrass, MD† |
| Children's Hospital, Denver, CO | Brian Greffe, MD,* Lorrie Odom, MD† |
| Children's Hospital, Los Angeles, CA | Kathy Ruccione, RN, MPH* |
| Children's Hospital, Oklahoma City, OK | John Mulvihill, MD‡ |
| Children's Hospital of Philadelphia, PA | Jill Ginsberg, MD,* Anna Meadows, MD‡ |
| Children's Hospital of Pittsburgh, PA | Jean Tersak, MD,* A. Kim Ritchey, MD,† Julie Blatt, MD† |
| Children's National Medical Center, Washington, DC | Gregory Reaman, MD,* Roger Packer, MD‡ |
| Cincinnati Children's Hospital Medical Center, Cincinnati, OH | Stella Davies, MD, PhD‡ |
| City of Hope, Los Angeles, CA | Smita Bhatia, MD* |
| Columbus Children's Hospital, Columbus, OH | Amanda Termuhlen, MD,* Frederick Ruymann, MD,† Stephen Qualman, MD,‡ Sue Hammond, MD‡ |
| Dana-Farber Cancer Institute, Boston, MA | Lisa Diller, MD,* Holcombe Grier, MD,† Frederick Li, MD§ |
| Emory University , Atlanta, GA | Lillian Meacham, MD,* Ann Mertens, PhD‡ |
| Fred Hutchinson Cancer Research Center, Seattle, WA | Wendy Leisenring, ScD,*‡ John Potter, MD, PhD†‡ |
| Hospital for Sick Children, Toronto, Ontario, Canada | Mark Greenberg, MB, ChB,* Paul C. Nathan, MD, MSc‡ |
| International Epidemiology Institute, Rockville, MD | John Boice, ScD‡ |
| Mayo Clinic, Rochester, MN | Vilmarie Rodriguez, MD,* W. Anthony Smithson, MD,† Gerald Gilchrist, MD† |
| Memorial Sloan-Kettering Cancer Center, New York, NY | Charles Sklar, MD,*‡ Kevin Oeffinger, MD‡ |
| Miller Children's Hospital, Long Beach, CA | Jerry Finklestein, MD† |
| National Cancer Institute, Bethesda, MD | Barry Anderson, MD,‡ Peter Inskip, ScD‡ |
| Riley Hospital for Children, Indianapolis, IN | Terry A. Vik, MD,* Robert Weetman, MD† |
| Roswell Park Cancer Institute, Buffalo, NY | Daniel M. Green, MD,*‡ |
| St Louis Children's Hospital, MO | Robert Hayashi, MD,* Teresa Vietti, MD† |
| Stanford University School of Medicine, Stanford, CA | Neyssa Marina, MD,* Sarah S. Donaldson, MD,‡ Michael P. Link, MD† |
| Texas Children's Hospital, Houston, TX | Zoann Dreyer, MD* |
| University of Alabama, Birmingham, AL | Kimberly Whelan, MD, MSPH,* Jane Sande, MD,† Roger Berkow, MD† |
| University of Alberta, Edmonton, Alberta, Canada | Yutaka Yasui, PhD‡ |
| University of California–Los Angeles, CA | Jacqueline Casillas, MD, MSHS,* Lonnie Zeltzer, MD†‡ |
| University of California–San Francisco, CA | Robert Goldsby, MD,* Arthur Ablin, MD† |
| University of Michigan, Ann Arbor, MI | Raymond Hutchinson, MD* |
| University of Minnesota, Minneapolis, MN | Joseph Neglia, MD, MPH‡* |
| University of Southern California | Dennis Deapen, DrPH‡ |
| University of Washington, Seattle, WA | Norman Breslow, PhD‡ |
| University of Texas–Southwestern Medical Center at Dallas, TX | Dan Bowers, MD,* Gail Tomlinson, MD,† George R. Buchanan, MD† |
| University of Texas M.D. Anderson Cancer Center, Houston, TX | Louise Strong, MD,*‡ Marilyn Stovall, MPH, PhD‡ |
Institutional Principal Investigator.
Former Institutional Principal Investigator.
Member, CCSS Steering Committee.
Former Member, CCSS Steering Committee.
Project Principal Investigator (U24 CA55727).
Supported by Grant No. U24-CA-55727 (L.L. Robison, principal investigator) from the United States Department of Health and Human Services, funding to the University of Minnesota from the Children's Cancer Research Fund, and funding to St Jude Children's Research Hospital from the American Lebanese Syrian Associated Charities.
Presented in part at the 43rd Annual Meeting of the American Society of Clinical Oncology, June 1-5, 2007, Chicago, IL.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Ries LA, Harkins D, Krapcho M, et al: SEER Cancer Statistics Review, 1975-2003. Bethesda, MD, National Cancer Institute, 2006
- 2.Hewitt M, Weiner SL, Simone JV: Childhood Cancer Survivorship: Improving Care and Quality of Life. Washington, DC, National Academies Press, 2003 [PubMed]
- 3.Geenen MM, Cardous-Ubbink MC, Kremer LC, et al: Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. JAMA 297:2705-2715, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Oeffinger KC, Mertens AC, Sklar CA, et al: Chronic health conditions in adult survivors of childhood cancer. N Engl J Med 355:1572-1582, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Mertens AC, Yasui Y, Neglia JP, et al: Late mortality experience in five-year survivors of childhood and adolescent cancer: The Childhood Cancer Survivor Study. J Clin Oncol 19:3163-3172, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Möller TR, Garwicz S, Barlow L, et al: Decreasing late mortality among 5-year survivors of cancer in childhood and adolescence: A population-based study in the Nordic countries. J Clin Oncol 19:3173-3181, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Hewitt M, Greenfield S, Stovall E: From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC, National Academies Press, 2005
- 8.Robison LL, Mertens AC, Boice JD, et al: Study design and cohort characteristics of the Childhood Cancer Survivor Study: A multi-institutional collaborative project. Med Pediatr Oncol 38:229-239, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Mertens AC, Walls RS, Taylor L, et al: Characteristics of childhood cancer survivors predicted their successful tracing. J Clin Epidemiol 57:933-944, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Landier W, Bhatia S, Eshelman DA, et al: Development of risk-based guidelines for pediatric cancer survivors: The Children's Oncology Group Long-Term Follow-Up Guidelines from the Children's Oncology Group Late Effects Committee and Nursing Discipline. J Clin Oncol 22:4979-4990, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Skinner R, Wallace WHB, Levitt GA (eds): Therapy Based Long Term Follow Up (ed 2). Leicester, United Kingdom, United Kingdom Children's Cancer Study Group: Late Effects Group, 2005
- 12.Scottish Collegiate Guidelines Network: Long term follow up of survivors of childhood cancer: A national clinical guideline. http://www.sign.ac.uk/guidelines/fulltext/76/index.html
- 13.Derogatis LR: BSI-18 Administration, Scoring and Procedures Manual. Minneapolis, MN, National Computer Systems, 2000
- 14.Hudson MM, Mertens AC, Yasui Y, et al: Health status of adult long-term survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. JAMA 290:1583-1592, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Ware JE Jr, Sherbourne CD: The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care 30:473-483, 1992 [PubMed] [Google Scholar]
- 16.Stokes ME, Davis CS, Koch GG: Categorical Data Analysis Using the SAS System (ed 2). Cary, NC, SAS Institute Inc, 2000
- 17.Oeffinger KC, Robison LL: Childhood cancer survivors, late effects, and a new model for understanding survivorship. JAMA 297:2762-2764, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Mertens AC, Cotter KL, Foster BM, et al: Improving health care for adult survivors of childhood cancer: Recommendations from a delphi panel of health policy experts. Health Policy 69:169-178, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Zebrack BJ, Eshelman DA, Hudson MM, et al: Health care for childhood cancer survivors: Insights and perspectives from a Delphi panel of young adult survivors of childhood cancer. Cancer 100:843-850, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Kadan-Lottick NS, Robison LL, Gurney JG, et al: Childhood cancer survivors’ knowledge about their past diagnosis and treatment: Childhood Cancer Survivor Study. JAMA 287:1832-1839, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Kremer LC, van Dalen EC, Offringa M, et al: Frequency and risk factors of anthracycline-induced clinical heart failure in children: A systematic review. Ann Oncol 13:503-512, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Bhatia S, Krailo MD, Chen Z, et al: Therapy-related myelodysplasia and acute myeloid leukemia after Ewing sarcoma and primitive neuroectodermal tumor of bone: A report from the Children's Oncology Group. Blood 109:46-51, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nicholson HS, Byrne J: Fertility and pregnancy after treatment for cancer during childhood or adolescence. Cancer 71:3392-3399, 1993 [DOI] [PubMed] [Google Scholar]
- 24.Byrne J, Fears TR, Gail MH, et al: Early menopause in long-term survivors of cancer during adolescence. Am J Obstet Gynecol 166:788-793, 1992 [DOI] [PubMed] [Google Scholar]
- 25.Sklar CA, Mertens AC, Mitby P, et al: Premature menopause in survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. J Natl Cancer Inst 98:890-896, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Bhatia S, Yasui Y, Robison LL, et al: High risk of subsequent neoplasms continues with extended follow-up of childhood Hodgkin's disease: Report from the Late Effects Study Group. J Clin Oncol 21:4386-4394, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Zapka JG, Bigelow C, Hurley T, et al: Mammography use among sociodemographically diverse women: The accuracy of self-report. Am J Public Health 86:1016-1021, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.King ES, Rimer BK, Trock B, et al: How valid are mammography self-reports? Am J Public Health 80:1386-1388, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Puleo E, Zapka JG, Goins KV, et al: Recommendations for care related to follow-up of abnormal cancer screening tests: Accuracy of patient report. Eval Health Prof 28:310-327, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Brown JB, Adams ME: Patients as reliable reporters of medical care process: Recall of ambulatory encounter events. Med Care 30:400-411, 1992 [DOI] [PubMed] [Google Scholar]
- 31.Woolf SH: Future health consequences of the current decline in US household income. JAMA 298:1931-1933, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Sabatino SA, Coates RJ, Uhler RJ, et al: Health insurance coverage and cost barriers to needed medical care among U.S. adult cancer survivors age < 65 years. Cancer 106:2466-2475, 2006 [DOI] [PubMed] [Google Scholar]