Abstract
Purpose
Radiation induces germ-cell mutations in experimental animals that result in adverse pregnancy outcomes, as does uterine damage caused by high-dose radiotherapy. We assessed the risks for spontaneous abortion and stillbirths among cancer survivors who received radiotherapy and subsequently became pregnant.
Patients and Methods
We identified 1,688 female survivors of childhood cancer in the Danish Cancer Registry. Radiation doses to the ovary and uterus were characterized as high to low. The pregnancy outcomes of survivors, 2,737 sisters, and 16,700 comparison women in the population were identified from nationwide registries. The proportions of pregnancies among survivors that resulted in a livebirth, stillbirth, or abortion were compared with the equivalent proportions among the two comparison groups, and proportion ratios (PRs) were computed with sisters as referent.
Results
More than 34,000 pregnancies were evaluated, 1,479 of which were among cancer survivors. No significant differences were seen between survivors and comparison women in the proportions of livebirths, stillbirths, or all types of abortions combined. Survivors, however, had a 23% excess risk for spontaneous abortion (PR, 1.23; 95% CI, 1.0 to 1.5), related primarily to prior radiation treatments (PR, 1.58; 95% CI, 1.2 to 2.2) and especially high-dose radiotherapy to the ovaries and uterus (PR, 2.8; 95% CI, 1.7 to 4.7).
Conclusion
The pregnancy outcomes of survivors were similar to those of comparison women. A slight excess risk for spontaneous abortion may have resulted from uterine damage after high-dose pelvic radiotherapy, consistent with previous studies, although radiation-induced germinal mutations or decreased hypothalamic-pituitary-ovarian function could not be ruled out.
INTRODUCTION
Today, the 5-year survival rate of childhood cancer approaches 75%,1 more and more childhood survivors of cancer reach reproductive age, and the possible increased risk of adverse pregnancy outcomes for such survivors is a mounting concern.2 Although several studies have indicated that childhood cancer survivors worry about the health of their offspring,3-5 only a few studies2 have addressed the reproductive hazards of the mutagenic treatments received by cancer survivors. The absence of such studies of an excess risk for cancer,6 abnormal karyotypes,7 or sex ratio alterations8 in the offspring of childhood cancer survivors might indicate that mutated germ cells of the survivor either do not mature or that abnormal zygotes or fetuses end up as spontaneous abortions or stillbirths.
We assessed the risks for abortions and stillbirths in female childhood cancer survivors, linking data for women identified in the Danish Cancer Registry to that of women in national reproductive outcome registers.
PATIENTS AND METHODS
Female Cancer Survivors
From the Danish Cancer Registry, we identified 5,508 persons born in 1950 through 1984 and alive on April 1, 1968, when the Central Population Register (CPR) was established, in whom cancer was diagnosed between 1950 and 1996 when they were younger than 20 years. Each cancer record includes the personal identification number, date of diagnosis, type of cancer, and whether radiotherapy was given.9 The identification number, which is unique to every Danish citizen, incorporates sex and date of birth and permits accurate linkage among registers. All members of the cohort were linked to the CPR for information on first-degree relatives, vital status, and migration. Of the 5,508 survivors, 3,963 patients (2,275 male patients and 1,688 female patients) survived until the onset of fertility (15 years) through 1999. The 1,688 female cancer survivors formed the survivor cohort.
Comparison Groups
From the CPR, we identified 5,745 siblings of the cancer survivors, using similar inclusion criteria. The subgroup of 2,737 sisters, who reached 15 years, formed the sister comparison group. In addition, a population comparison group of 16,700 women were randomly selected from the CPR on the basis of month and year of birth of survivors, in an approximate 10 to 1 selection.
Pregnancy Outcomes
During 1977 through 2003, 34,611 pregnancies of female cancer survivors and comparison women were identified, and pregnancy outcomes (livebirth, stillbirth, or abortion) were determined. We excluded 33 pregnancies that occurred before the date of diagnosis of cancer and 18 pregnancies that occurred within 9 months of that date, leaving 34,560 pregnancies for analysis.
Livebirths and stillbirths, the latter defined as an infant with a gestational age of 28 weeks or more who showed no sign of life, were identified by linkage to the CPR (started in 1968) and the Medical Birth Register (started in 1973), and early neonatal deaths (death within the first 7 days of life) were identified from the Danish Cause-of-Death Register. Information on induced abortions was obtained from the Register of Induced Abortions (1977 through 1994) and, after 1994, from the National Hospital Register (started in 1977). Information on other types of abortions was obtained from the Hospital Register for the full period. The Hospital Register contains information for virtually every nonpsychiatric hospital admission in Denmark since 1977, including discharge diagnoses and surgical procedures.10,11 Since 1995, data on outpatients and emergency room patients have also been included. Abortions were categorized as spontaneous, induced, ectopic pregnancies, hydatiform moles, and other (primarily nonhydatidiform mole and missed abortion). Information on surgical evacuation was obtained to validate spontaneous abortions identified from the Hospital Register. A prior study of childhood cancer survivors had indicated that spontaneous abortions during the early weeks of pregnancy might be associated with cranial irradiation, possibly through disruption of the hypothalamic-pituitary-ovarian axis.12 To evaluate this possibility further, we obtained the medical records for those children who had cranial irradiation in our study and abstracted the gestational weeks that miscarriage occurred. In addition, indications for second-trimester abortions were sought in the relevant registers and by reviewing the medical records.
Radiation Dose Estimation
For survivors treated with radiotherapy, doses to the ovary, uterus, and pituitary gland were characterized. Four categories of radiation dose were used: low, low or medium, medium or high, and high. Dose categories were assigned to each organ by a highly experienced medical physics team (M.S.) based on the type of cancer being treated, treatment procedures for specific tumors during time, and assumptions of the gonadal proximity to the radiation field, taking into consideration the topography of the tumor (above or below the diaphragm). The categories “low or medium” and “medium or high” were used to group cases in which the exposure of the organ was uncertain as a result of lack of treatment details. For primary cancer treatment extending below the diaphragm, the dose to the ovary and uterus was estimated to be medium to high, ranging between 1 and 40 Gy. For cancer treatment above the diaphragm only, including treatment for retinoblastoma or brain tumors, the dose to the ovary and uterus was estimated to be between 0.01 and 1 Gy. The dose to the pituitary gland was estimated to be high during radiation for brain tumors and leukemia treated with cranial irradiation, typically between 5 and 50 Gy, but low for tumors located below the diaphragm, including treatment for Wilms’ and gonadal tumors, ranging from 0.01 to 0.1 Gy.
Statistical Analysis
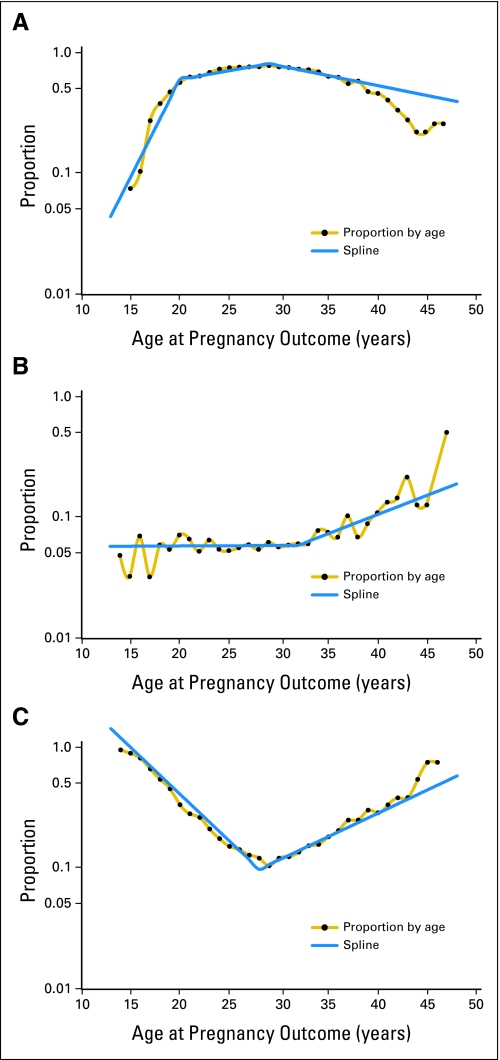
The proportions of pregnancies among female survivors that resulted in a livebirth, a stillbirth, or an abortion were compared with the equivalent proportions among sisters and the population comparisons. Proportion ratios (PRs) were calculated, with sisters as the referent. For each outcome, PRs were estimated by use of a log-linear regression model under the assumption that the outcome was binomially distributed. The generalized estimating equations method was used to accommodate correlation between pregnancy outcomes in the same woman, and 95% CIs were calculated by use of empirical variance estimates.13 For most analyses, the PRs were adjusted for the age of the mother at pregnancy, modeled by linear splines, as indicated by the solid lines in Figure 1, and for calendar time linearly.
Fig 1.
Proportion of pregnancies resulting in a livebirth, spontaneous or induced abortion (on a logarithmic scale) by age of mother at pregnancy in the combined group of sisters and population comparison women. Solid lines are splines as applied in the age adjustment of risk estimates.
For spontaneous abortions among cancer survivors, estimates according to type of childhood cancer (12 categories14), age at diagnosis (< 1, 2 to 4, 5 to 9, 10 to 14, and 15 to 19 years), year of diagnosis (quartiles according to number of abortions), radiotherapy (yes, no), estimated organ dose, and time since cancer diagnosis (linear) were calculated. In a subanalysis of women for whom a full reproductive history was available (approximately 40% of women), we adjusted for previous stillbirths or spontaneous abortions.
RESULTS
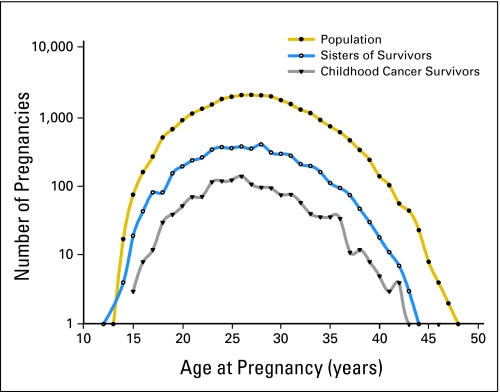
Between 1977 and 2003, 1,479 pregnancies with 1,497 fetuses were reported among 1,688 cancer survivors, for an average of 0.9 pregnancies per woman (Table 1). In the same period, 5,092 pregnancies with 5,139 fetuses were reported among the 2,737 sisters (average, 1.9 pregnancies per sister), and 27,989 pregnancies with 28,286 fetuses were reported among the 16,700 population comparison women (average, 1.7 pregnancies per woman). Of the 1,688 childhood cancer survivors, 649 women (38%) had at least one pregnancy during follow-up, compared with 1,944 sisters (71%) and 11,257 women (67%) in the population comparison group. The average number of pregnancies per women who were ever pregnant was 2.3 for survivors, 2.6 for sisters, and 2.5 for population controls. Moreover, the distribution of pregnancies among survivors by age at pregnancy was remarkably close to that in the comparison groups (Fig 2).
Table 1.
Pregnancies, Pregnancy Outcomes, and Estimated PRs Among Childhood Cancer Survivors, Sisters, and a Population Comparison Group, With Sisters As Referent
| Study Group | Size of Cohort | Pregnancies | Fetuses* | Livebirths
|
All Abortions†
|
Stillbirths
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | PR‡ | 95% CI | No. | % | PR‡ | 95% CI | No. | % | PR§ | 95% CI | ||||
| Survivors | 1,688 | 1,479 | 1,497 | 1,022 | 69.1 | 0.97 | 0.94 to 1.01 | 470 | 31.8 | 1.06 | 0.97 to 1.15 | 5 | 0.3 | 1.1 | 0.4 to 2.9 |
| Sisters | 2,737 | 5,092 | 5,139 | 3,574 | 70.2 | 1 | Referent | 1,548 | 30.4 | 1 | Referent | 17 | 0.3 | 1 | Referent |
| Population comparisons | 16,700 | 27,989 | 28,286 | 19,535 | 69.8 | 0.98 | 0.96 to 1.00 | 8,657 | 30.9 | 1.03 | 0.99 to 1.08 | 94 | 0.3 | 1.1 | 0.6 to 1.8 |
Abbreviation: PR, proportion ratio.
Multiple births were included in the analyses. For example, twins born alive were counted as one livebirth, whereas a twin who was stillborn would be counted as stillborn and the other as liveborn.
Including spontaneous abortions, induced abortions, ectopic pregnancies, hydatidiform moles, and other, primarily nonhydatidiform mole and missed abortions.
Adjusted for age of mother at pregnancy (linear spline) and calendar time (linear).
Unadjusted estimate.
Fig 2.
Log-frequency distribution of pregnancies among female cancer survivors and sisters and population comparison women, by age at pregnancy.
The proportions of pregnancies that resulted in a livebirth were 69.1% among survivors, 70.2% among sisters, and 69.8% among population comparisons (Table 1). With the proportion of liveborn infants among sisters as referent, the age- and calendar year-adjusted PRs were 0.97 for survivors and 0.98 for population comparison women, neither of which was significantly different from that for sisters. The proportion of pregnancies that ended in abortion was slightly but nonsignificantly higher (31.8%) among survivors than among sisters (30.4%) and population comparisons (30.9%). The adjusted PR among survivors was 1.06, but with a lower 95% CI of 0.97. The proportion of stillbirths among survivors (0.3%) was similar to those in the comparison groups. Only three early neonatal deaths were observed among infants of survivors (0.3%), whereas there were 12 among infants of siblings (0.3%) and 63 among infants of population comparisons (0.3%; not shown).
Details of subtypes of abortions are listed in Table 2. The small increase in risk for all abortions among survivors was due mainly to a marginally significant 23% increase in risk for spontaneous abortion, equal to 20 extra abortions among survivors. Further adjustment for prior history of adverse pregnancy outcomes among women with a complete reproductive history did not change the estimate. We observed no measurable effect on the risk for spontaneous abortion of the survivors’ age at cancer diagnosis, the calendar year at diagnosis, or time after diagnosis. More than 70% of women hospitalized for a spontaneous abortion had undergone surgical evacuation, confirming the validity of our approach to identify this outcome based on hospital discharge records. Since 1995, the proportion of spontaneous abortions registered as an outpatient or emergency room visit only (ie, the very early abortions) was significantly lower in survivors than in sisters (14% v 32%; P = .01) and lower than in population comparisons (14% v 28%; P = .03).
Table 2.
Various Types of Abortions and Estimated PRs Among Childhood Cancer Survivors, Sisters, and a Population Comparison Group, With Sisters As Referent
| Study Group | Spontaneous Abortion (643; DO03)*
|
Induced Abortion (640-642; DO04-06)*
|
Ectopic Pregnancy (631; DO00)*
|
Hydatidiform Mole (631.90, 634.29, 634.60, 634.69, 645.0; DO01)*
|
Other Types (634.61, 645.1, 645.2; DO02)*†
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | PR‡ | 95% CI | No. | % | PR‡ | 95% CI | No. | % | PR§ | 95% CI | No. | % | PR§ | 95% CI | No. | % | PR§ | 95% CI | |
| Survivors | 109 | 7.4 | 1.23 | 1.00 to 1.52 | 292 | 19.7 | 1.08 | 0.96 to 1.22 | 17 | 1.1 | 0.9 | 0.5 to 1.5 | 3 | 0.2 | 1.0 | 0.2 to 5.0 | 49 | 3.3 | 0.8 | 0.6 to 1.1 |
| Sisters | 304 | 6.0 | 1 | Referent | 961 | 18.9 | 1 | Referent | 66 | 1.3 | 1 | Referent | 10 | 0.2 | 1 | Referent | 207 | 4.1 | 1 | Referent |
| Population comparisons | 1,718 | 6.1 | 0.98 | 0.87 to 1.11 | 5,505 | 19.7 | 1.07 | 1.01 to 1.14 | 433 | 1.5 | 1.2 | 0.9 to 1.6 | 34 | 0.1 | 0.6 | 0.3 to 1.3 | 967 | 3.5 | 0.8 | 0.7 to 1.0 |
Abbreviation: PR, proportion ratio.
Discharge diagnoses were coded according to a modified version of the International Classification of Diseases, eighth revision (ICD-8),15 until 1993, and thereafter to ICD-10.16
Primarily nonhydatidiform mole and missed abortion.
Adjusted for age of mother at pregnancy (linear spline) and calendar time (linear).
Unadjusted estimate.
The prevalence of induced and other types of abortions among cancer survivors was not significantly different from that among comparisons (Table 2). Only two second-trimester pregnancy terminations for fetal abnormality (one fetus with a neural tube defect and one with an abdominal wall defect) were registered among cancer survivors (0.7% of all induced abortions), in contrast to nine among sisters (0.9%) and 45 among the population comparisons (0.8%) (not shown).
Table 3 shows that 38% of survivors became pregnant at least once, ranging from 25% to 62% for survivors of leukemia and retinoblastoma, respectively. Sisters and the population comparison group had essentially the same risk of spontaneous abortion. A marginally significant increased risk for spontaneous abortion was seen among cancer survivors (PR, 1.23; 95% CI, 1.00 to 1.52), related primarily to prior radiation treatments (PR, 1.58; 95% CI, 1.15 to 2.17). Three-fold increased risks were also seen among survivors of Wilms’ tumor (PR, 3.0; 95% CI, 1.6 to 5.5, based on 10 abortions in seven women) and among survivors of malignant germ-cell neoplasms (PR, 2.7; 95% CI, 1.4 to 5.2, based on eight abortions in seven women). All seven survivors of Wilms’ tumor and only one of the seven survivors of a germ-cell neoplasm had received radiotherapy. Four of the 14 women treated for either Wilms’ tumor or germ-cell neoplasm had at least one livebirth.
Table 3.
Risk for Spontaneous Abortion Among Childhood Cancer Survivors by Type of Cancer and Radiotherapy, With Sisters As Referent
| Stratification Criteria | No. of Women | Women Who Became Pregnant
|
No. of Pregnancies | Spontaneous Abortions
|
PR* | 95% CI | ||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. of Abortions | No. of Women | |||||
| Sisters | 2,737 | 1,944 | 71 | 5,092 | 304 | 261 | 1 | Referent |
| Population comparisons | 16,700 | 11,257 | 67 | 27,989 | 1,718 | 1,454 | 0.98 | 0.87 to 1.11 |
| Survivors of childhood cancer | 1,688 | 649 | 38 | 1,479 | 109 | 99 | 1.23 | 1.00 to 1.52 |
| Type of cancer | ||||||||
| Leukemias | 292 | 72 | 25 | 158 | 10 | 10 | 1.2 | 0.7 to 2.0 |
| Lymphomas | 222 | 93 | 42 | 205 | 15 | 14 | 1.2 | 0.8 to 2.0 |
| CNS neoplasms | 434 | 148 | 34 | 323 | 25 | 23 | 1.3 | 0.9 to 1.9 |
| Sympathetic nervous system tumors | 53 | 17 | 32 | 41 | 4 | 4 | 1.7 | 0.8 to 4.0 |
| Retinoblastoma | 58 | 36 | 62 | 101 | 8 | 7 | 1.3 | 0.7 to 2.4 |
| Wilms’ and other renal tumors | 76 | 25 | 33 | 58 | 10 | 7 | 3.0 | 1.6 to 5.5 |
| Malignant bone tumors | 82 | 25 | 30 | 56 | 2 | 2 | 0.6 | 0.2 to 2.2 |
| Soft-tissue sarcomas | 107 | 50 | 47 | 121 | 8 | 8 | 1.1 | 0.6 to 2.0 |
| Germ-cell, trophoblastic, and other gonadal neoplasms | 83 | 25 | 30 | 48 | 8 | 7† | 2.7 | 1.4 to 5.2 |
| Carcinomas and other malignant epithelial neoplasms | 252 | 149 | 59 | 346 | 16 | 15 | 0.8 | 0.5 to 1.2 |
| Other and unspecified malignant neoplasms | 24 | 9 | 38 | 22 | 3 | 2 | 2.4 | 0.9 to 6.6 |
| Radiotherapy‡ | ||||||||
| No | 1,082 | 448 | 41 | 1,006 | 63 | 60 | 1.06 | 0.82 to 1.36 |
| Yes | 578 | 192 | 33 | 457 | 44 | 37 | 1.58 | 1.15 to 2.17 |
Abbreviation: PR, proportion ratio.
Adjusted for age of mother at pregnancy (linear spline) and calendar time (linear).
One survivor with an uterine choriocarcinoma, one with ovarian cystadenocarcinoma, and five with ovarian germ-cell tumors.
Information on radiotherapy was not available for 28 survivors.
For 154 of the 192 survivors who became pregnant after treatment with radiation, we were able to characterize the level of irradiation to organs important for normal reproduction. With sisters as referent, the risk for spontaneous abortion was particularly increased among the 11 survivors who had received high-dose irradiation to the ovary and uterus (PR, 2.8; 95% CI, 1.7 to 4.7; Table 4). Interestingly, seven of these 11 survivors succeeded in having at least one liveborn child. The risk for spontaneous abortion was also significantly increased among 13 survivors who had received high-dose irradiation to the pituitary gland (1.8; 95% CI, 1.1 to 3.0), 10 of whom had at least one livebirth.
Table 4.
Risk for Spontaneous Abortion by Estimated Level of Irradiation to the Ovary and Uterus and Pituitary Gland Among Survivors of Childhood Cancer, With Sisters As Referent
| Level of Irradiation
|
No. of Women Who Became Pregnant | No. of Pregnancies | Spontaneous Abortions
|
PR* | 95% CI | ||
|---|---|---|---|---|---|---|---|
| Ovary and Uterus | Pituitary Gland | No. of Abortions | No. of Women | ||||
| Low† | Low† | 59 | 152 | 7 | 6 | 0.8 | 0.3 to 1.7 |
| Low† | High‡ | 60 | 137 | 15 | 13§ | 1.8 | 1.1 to 3.0 |
| High‡ | Low† | 35 | 86 | 14 | 11‖ | 2.8 | 1.7 to 4.7 |
Abbreviation: PR, proportion ratio.
Adjusted for age of mother at pregnancy (linear spline) and calendar time (linear).
Includes dose category “low or medium.”
Includes dose category “medium or high.”
Eight survivors of CNS tumors, three of leukemia, and two of retinoblastoma.
Seven survivors of Wilms’ tumors, two of soft-tissue sarcomas, one of malignant lymphoma, and one of gonadal tumor.
DISCUSSION
Our population-based cohort study of 1,688 female childhood cancer survivors in Denmark relied on previously collected data contained in population and health registries that spanned 54 years. Cancer survivors who wanted and were able to become pregnant adopted reproductive habits similar to that of other Danish women and had, on average, the same number of children. Moreover, the overall distribution of pregnancies by type of outcome was similar to that of sisters and of population comparisons, indicating that any effects of cancer treatment were likely to be small among the survivors who desired and were able to become pregnant. It is recognized, however, that many survivors become infertile because of the curative therapies received (ie, ovarian dysfunction can be caused by radiotherapy to the pelvis and by systemic chemotherapy).17,18
The risk for spontaneous abortion was marginally increased among survivors overall and especially among those treated with radiation. Three-fold increased risks among survivors of Wilms’ tumor and of malignant germ-cell neoplasms were noteworthy, although they were based on small numbers. The association with Wilms’ tumor might be related to high-dose irradiation to the ovaries and uterus, whereas the association with germ-cell neoplasms might be related to previous intra-abdominal surgery, leading to reduced chances of subsequent fetal growth and development. It is possible that nonsterilizing doses of radiation to the ovaries may cause genetic damage to the ovum, which might result in higher rates of spontaneous abortion or stillbirths,19 although there is little evidence to support this supposition.20 The increased risk for abortion and stillbirths might, however, be due to radiation-induced atrophic damage to the uterus, which could adversely affect implantation of the embryo and maintenance of pregnancy.19 This direct effect of pelvic radiation on uterine function has been reported in several previous studies.21-23 Our study did not allow us to judge which of these mechanisms is most likely to be involved; however, studies of preterm births indicate that uterine radiation dose is the causal factor in early births and low birth weight.24 Although the numbers in our study were small, we saw no increased risk for stillbirths among survivors, and we observed only two second-trimester pregnancy terminations for fetal abnormality. It should be noted, however, that the definition of stillbirth differs in Denmark and the United States. In Denmark, the gestation must last 28 weeks before a fetal death is classified as a stillbirth, whereas a gestation must last only 20 weeks in the United States. Thus many of the spontaneous abortions in Denmark would be classified as stillbirths in the United States. The rate of early neonatal deaths among liveborn children was similar in all cohorts.
The Childhood Cancer Survivor Study (CCSS) in the United States12 reported on the outcomes of 4,029 pregnancies in 1,915 female cancer survivors who were ever pregnant and of 1,903 pregnancies in a subsample of sisters based on self-reports on a mailed questionnaire. Similar to our findings, they reported higher, although not statistically significant, risks for spontaneous abortion among survivors who were given high-dose irradiation to the ovaries and uterus, measured as survivors whose ovaries were in or near the radiotherapy field, in comparison with survivors who did not undergo radiation and with sisters. In contrast to our findings, no excess risk for spontaneous abortions was reported for survivors of Wilms’ tumor.
In the CCSS investigation, significantly more spontaneous abortions, defined as a miscarriage occurring before 20 weeks of gestational age, were reported by the survivors of CNS tumors than among sisters (PR, 1.65; 95% CI, 1.2 to 2.3). In our study, the risk for spontaneous abortion was also significantly increased among the 13 survivors who had received high-dose irradiation to the pituitary gland. As progesterone is essential for successful implantation and maintenance of pregnancy, inadequate progesterone secretion by the corpus luteum is likely to affect the outcome of pregnancy.25 Furthermore, short luteal phases have been associated with reduced fertility and early miscarriage.26 Bath et al27 showed that cranial irradiation had an adverse effect on the hypothalamic-pituitary-ovarian axis and that the length of the luteal phase was significantly shorter in 12 female survivors of childhood acute lymphoblastic leukemia treated with chemotherapy and cranial irradiation at a total dose of 18 to 24 Gy than in 16 controls. The CCSS12 found an increased risk for self-reported spontaneous abortions among cohort members who received cranial irradiation, but only for spontaneous abortions that occurred after 12 or more weeks of gestation (ie, after the onset of placental endocrine function). In our study, most of the spontaneous abortions after high radiation doses to the pituitary occurred before week 12, but most of these women also had at least one livebirth, suggesting that if hypothalamic-pituitary-ovarian function was affected, it was probably only modestly.
The strengths of our study include the cohort design and the population-based approach, with unbiased ascertainment and validation of cancer cases through a search in a nationwide cancer register, unbiased identification of comparison women from a national population register, and unbiased ascertainment of pregnancy outcomes from nationwide health registers. The various systems of records had been in existence for many decades, starting as early as 1950 and through 2003. We were able to identify women with spontaneous abortions leading to hospitalization and, since 1995, also women with early abortions examined as outpatients only. Fatal germ-cell mutations, however, might lead to extremely early abortions that are not even noticed by the women themselves and, therefore, are never reported or registered. The clinical impact of such early abortions is of lower consequence than those requiring hospitalization or a hospital visit. Although we might have missed such early abortions, we have captured those abortions with more serious consequences (ie, women hospitalized for a spontaneous abortion for whom more than 70% had undergone surgical evacuation).
The analysis of irradiated survivors was limited by having only a relatively rough characterization of organ doses. The approximate gonadal dose categories were validated against more accurate doses estimated based on the actual radiotherapy schemes28 for a subset of overlapping 39 female survivors of CNS neoplasms, leukemia, and lymphomas also enrolled in the parent ongoing study (www.gcct.org). Only one discrepancy was found. Thus we believe that our approximate dose categories are of sufficient validity that results have not been distorted by the absence of complete dosimetry information. We were unable to examine the risk for spontaneous abortion owing to chemotherapeutic agents because the Cancer Registry records were incomplete. Nevertheless, no significant risk was observed among nonirradiated survivors of leukemia, who likely received intense chemotherapy administrations, and previous studies have found no association between chemotherapy and adverse pregnancy outcomes.12,21,22,29 We, therefore, believe that our conclusions regarding radiation treatments are not likely to be invalid.
In conclusion, overall pregnancy outcomes in female childhood cancer survivors who were able to become pregnant were similar to those of sisters and of population comparisons. The slight excess risk for spontaneous abortions observed among survivors might be the result of uterine damage after radiotherapy as previously reported in studies of low birth weight or, conceivably, radiation-induced germinal mutations from ovarian exposure or decreased hypothalamic-pituitary-ovarian function from cranial radiotherapy.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Jeanette F. Winther, John D. Boice Jr, Kirsten Frederiksen, Jørgen H. Olsen
Financial support: John D. Boice Jr
Collection and assembly of data: Jeanette F. Winther, Marilyn Stovall
Data analysis and interpretation: Jeanette F. Winther, John D. Boice Jr, Anne Louise Svendsen, Kirsten Frederiksen, Jørgen H. Olsen
Manuscript writing: Jeanette F. Winther, John D. Boice Jr, Anne Louise Svendsen, Jørgen H. Olsen
Final approval of manuscript: Jeanette F. Winther, John D. Boice Jr, Anne Louise Svendsen, Kirsten Frederiksen, Marilyn Stovall, Jørgen H. Olsen
Supplementary Material
Acknowledgments
We thank data manager Andrea Bautz, Institute of Cancer Epidemiology, for preparing the data set for analysis, and Catherine Kasper and Rita Weathers, The University of Texas M. D. Anderson Cancer Center, Houston, TX, for their contribution to the dose estimates.
Supported in part by the Danish Cancer Society, the International Epidemiology Institute (IEI) through a contract with Westlakes Research Institute (agreement 01/12/99 DC), and a grant from the National Cancer Institute (Grant No. CA104666).
Presented in part at the 9th International Conference on Long-Term Complications of Treatment of Children and Adolescents for Cancer, June 9-10, 2006, Niagara-on-The-Lake, Ontario, Canada.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Hawkins MM: Long-term survivors of childhood cancers: What knowledge have we gained? Nat Clin Pract Oncol 1:26-31, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Boice JD Jr, Tawn EJ, Winther JF, et al: Genetic effects of radiotherapy for childhood cancer. Health Phys 85:65-80, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Schover LR, Rybicki LA, Martin BA, et al: Having children after cancer: A pilot survey of survivors’ attitudes and experiences. Cancer 86:697-709, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Langeveld NE, Ubbink MC, Last BF, et al: Educational achievement, employment and living situation in long-term young adult survivors of childhood cancer in the Netherlands. Psychooncology 12:213-225, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Zebrack BJ, Casillas J, Nohr L, et al: Fertility issues for young adult survivors of childhood cancer. Psychooncology 13:689-699, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Sankila R, Olsen JH, Anderson H, et al: Risk of cancer among offspring of childhood-cancer survivors: Association of the Nordic Cancer Registries and the Nordic Society of Paediatric Haematology and Oncology. N Engl J Med 338:1339-1344, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Winther JF, Boice JD Jr, Mulvihill JJ, et al: Chromosomal abnormalities among offspring of childhood-cancer survivors in Denmark: A population-based study. Am J Hum Genet 74:1282-1285, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winther JF, Boice JD Jr, Thomsen BL, et al: Sex ratio among offspring of childhood cancer survivors treated with radiotherapy. Br J Cancer 88:382-387, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Storm HH, Michelsen EV, Clemmensen IH, et al: The Danish Cancer Registry: History, content, quality and use. Dan Med Bull 44:535-539, 1997 [PubMed] [Google Scholar]
- 10.Danish National Board of Health: The Activity in the Hospital Care System 1979 (in Danish). Copenhagen, Denmark, Danish National Board of Health, 1981
- 11.Andersen TF, Madsen M, Jørgensen J, et al: The Danish National Hospital Register: A valuable source of data for modern health sciences. Dan Med Bull 46:263-268, 1999 [PubMed] [Google Scholar]
- 12.Green DM, Whitton JA, Stovall M, et al: Pregnancy outcome of female survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Am J Obstet Gynecol 187:1070-1080, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Liang KY, Zeger SL: Longitudinal data analysis using generalized linear models. Biometrika 73:13-22, 1986 [Google Scholar]
- 14.Birch JM, Marsden HB: A classification scheme for childhood cancer. Int J Cancer 40:620-624, 1987 [DOI] [PubMed] [Google Scholar]
- 15.Danish National Board of Health: Danish Classification of Diseases, 8th revision (in Danish). Copenhagen, Denmark, Danish National Board of Health, 1986
- 16.Danish National Board of Health: Danish Classification of Diseases, 10th revision (in Danish). Copenhagen, Denmark, Danish National Board of Health, 1993
- 17.Larsen EC, Müller J, Schmiegelow K, et al: Reduced ovarian function in long-term survivors of radiation- and chemotherapy-treated childhood cancer. J Clin Endocrinol Metab 88:5307-5314, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Edgar AB, Wallace WH: Pregnancy in women who had cancer in childhood. Eur J Cancer 43:1890-1894, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Kalapurakal JA, Peterson S, Peabody EM, et al: Pregnancy outcomes after abdominal irradiation that included or excluded the pelvis in childhood Wilms tumor survivors: A report from the National Wilms Tumor Study. Int J Radiat Oncol Biol Phys 58:1364-1368, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Committee on Medical Aspects of Radiation in the Environment (COMARE): Eighth report. Review of pregnancy outcomes following preconceptional exposure to radiation. Chilton, United Kingdom, National Radiological Protection Board, 2004
- 21.Green DM, Fine WE, Li FP: Offspring of patients treated for unilateral Wilms’ tumor in childhood. Cancer 49:2285-2288, 1982 [DOI] [PubMed] [Google Scholar]
- 22.Li FP, Gimbrere K, Gelber RD, et al: Outcome of pregnancy in survivors of Wilms’ tumor. JAMA 257:216-219, 1987 [PubMed] [Google Scholar]
- 23.Critchley HO, Wallace WH, Shalet SM, et al: Abdominal irradiation in childhood: The potential for pregnancy. Br J Obstet Gynaecol 99:392-394, 1992 [DOI] [PubMed] [Google Scholar]
- 24.Signorello LB, Cohen SS, Bosetti C, et al: Female survivors of childhood cancer: Preterm birth and low birth weight among their children. J Natl Cancer Inst 98:1453-1461, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arredondo F, Noble LS: Endocrinology of recurrent pregnancy loss. Semin Reprod Med 24:33-39, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Soules MR, McLachlan RI, Ek M, et al: Luteal phase deficiency: Characterization of reproductive hormones over the menstrual cycle. J Clin Endocrinol Metab 69:804-812, 1989 [DOI] [PubMed] [Google Scholar]
- 27.Bath LE, Anderson RA, Critchley HO, et al: Hypothalamic-pituitary-ovarian dysfunction after prepubertal chemotherapy and cranial irradiation for acute leukaemia. Hum Reprod 16:1838-1844, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Stovall M, Donaldson SS, Weathers RE, et al: Genetic effects of radiotherapy for childhood cancer: Gonadal dose reconstruction. Int J Radiat Oncol Biol Phys 60:542-552, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Hawkins MM, Smith RA: Pregnancy outcomes in childhood cancer survivors: Probable effects of abdominal irradiation. Int J Cancer 43:399-402, 1989 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.