Abstract
Purpose
Diabetes is associated with lower risk of prostate cancer. Most men with diabetes are obese, and obesity is associated with greater prostate cancer mortality. Whether diabetes influences outcomes after prostate cancer diagnosis is unknown.
Patients and Methods
We assessed the relationship between prevalent diabetes and mortality using data from Radiation Therapy Oncology Group Protocol 92-02, a large randomized trial of men (N = 1,554) treated with radiation therapy and short-term versus long-term adjuvant goserelin for locally advanced prostate cancer. Regression and proportional hazard models were performed to evaluate relationships between prevalent diabetes and all-cause mortality, prostate cancer mortality, and non–prostate cancer mortality. Covariates included age, race, tumor stage, Gleason score, prostate-specific antigen, weight, and treatment arm.
Results
There were a total of 765 deaths; 210 (27%) were attributed to prostate cancer. In univariate analyses, prevalent diabetes was associated with greater all-cause mortality and non–prostate cancer mortality but not prostate cancer mortality. After controlling for other covariates, prevalent diabetes remained significantly associated with greater all-cause mortality and non–prostate cancer mortality (hazard ratio [HR] = 2.12; 95% CI, 1.69 to 2.66; P < .0001) but not prostate cancer mortality (HR = 0.80; 95% CI, 0.51 to 1.25; P = .34). In contrast, weight was associated with greater prostate cancer mortality (HR = 1.77; 95% CI, 1.22 to 2.55; P = .002) but not all-cause or non–prostate cancer mortality.
Conclusion
Weight but not prevalent diabetes is associated with greater prostate cancer mortality in men receiving combined modality treatment for locally advanced disease. These observations suggest that the association between obesity and greater prostate cancer mortality is mediated by mechanism(s) other than the characteristic metabolic alterations of diabetes.
INTRODUCTION
Diabetes is the fifth leading cause of death in the United States.1 Approximately 90% to 95% of individuals with diabetes have type 2 diabetes, characterized by insulin resistance.2 The risk of developing type 2 diabetes increases with age, obesity, and sedentary lifestyle. In the United States, approximately one in six men older than 60 years has diagnosed diabetes.3
Prevalent diabetes is associated with decreased prostate cancer incidence. In a recent meta-analysis of 19 studies, there was an inverse relationship between diabetes and prostate cancer diagnosis (relative risk = 0.84; 95% CI, 0.71 to 0.93).4 Most men with type 2 diabetes are obese.2 Overweight and obesity are associated with higher rates of prostate-specific antigen (PSA) recurrence after surgery or radiation therapy (RT) for early-stage disease.5-9 Overweight and obesity are also associated with greater prostate cancer mortality after combined-modality therapy for locally advanced disease.10 Several mechanisms have been put forward to explain the link between obesity and adverse prostate cancer outcomes, including elevated levels of insulin and insulin-like growth factors.11 Whether diabetes influences cancer-specific outcomes after prostate cancer diagnosis is unknown.
Radiation Therapy Oncology Group (RTOG) Protocol 92-02 was a large, randomized, controlled trial of short-term versus long-term adjuvant androgen deprivation therapy (ADT) with a gonadotropin-releasing hormone (GnRH) agonist in men receiving RT for locally advanced prostate cancer.12 We used data from RTOG 92-02 to evaluate the relationship between prevalent diabetes and prostate cancer mortality.
PATIENTS AND METHODS
The data used in this analysis were based on RTOG Protocol 92-02, a phase III trial designed to compare the effectiveness of long-term adjuvant ADT with goserelin, a GnRH agonist, versus short-term ADT administered in addition to standard external-beam RT in a population of men with locally advanced prostate cancer.12
Patient Eligibility
All patients had histologically confirmed adenocarcinoma of the prostate (clinical stage T2c to T4), with no involved lymph nodes in the common iliac or higher node chains, Karnofsky performance score ≥ 70, and pretreatment PSA level less than 150 ng/mL. Clinical stage was based on the 1992 American Joint Committee on Cancer TNM system. Patients with previous or concurrent cancers other than basal cell skin carcinoma were excluded. No prior therapy for prostate cancer was allowed. All patients provided written informed consent before study enrollment.
Patient Evaluation
Pretreatment evaluation included medical history, assessment of sexual function, Karnofsky status evaluation, histologic evaluation, chest x-ray, and bone scan. Laboratory studies included CBC, AST, ALT, serum acid phosphatase, serum testosterone, alkaline phosphatase, PSA, and lymph node evaluation (lymphangiogram, computed tomography of the pelvis and abdomen, or exploratory laparotomy with lymph node sampling). Prevalent diabetes was ascertained by medical records and patient-reported past medical history and current medications.
Study Design
Random assignment was performed before any treatment was initiated. Patients were stratified by clinical stage (T2c v T3 v T4), pretreatment PSA level (≤ 30 v > 30 ng/mL), tumor grade, and nodal status. Gleason scores were provided by the institution whenever possible. The treatment allocation scheme described by Zelen13 was used because it balances for patient factors other than institution.
Treatment
All patients received external-beam RT to the whole pelvis followed by a boost to the prostate using a four-field technique with megavoltage machines (> 6 MV). The prostate dose ranged from 65 to 70 Gy at 1.8 to 2.0 Gy/d. The dose to the regional lymphatics ranged from 44 to 50 Gy.
All patients received short-term ADT consisting of 4 months (2 months before RT and 2 months concurrently until RT completion) of goserelin acetate (Zoladex; Zeneca Pharmaceutical, Wilmington, DE) 3.6 mg subcutaneously monthly and flutamide (Eulexin; Schering-Plough, Kenilworth, NJ) 250 mg by mouth three times daily. Patients were randomly assigned to receive no further therapy (arm I) or to receive adjuvant goserelin 3.6 mg subcutaneously monthly for an additional 24 months after the completion of RT (arm II). Subsequent ADT was allowed only with evidence of treatment failure.
Follow-Up
On completion of RT, follow-up was scheduled every 3 months during year 1, every 4 months during year 2, every 6 months from years 3 to 5, and annually thereafter. Each follow-up visit included a history, physical examination, Karnofsky performance status evaluation, sexual function assessment, liver function test, CBC, and PSA measurement. In addition, tumor status was evaluated, and toxicity was graded. Acid phosphatase and alkaline phosphatase were measured annually.
Study End Points
Cause of death was investigator defined. Source documentation for cause of death included death certificates and medical records. Prostate cancer–related death is defined as a death from prostate cancer or protocol treatment. Any other cause of death is considered non–prostate cancer related. Mortality was measured from the date of random assignment to the date of death or last follow-up.
Statistical Methods
χ2 test statistics were used to compare pretreatment characteristics of patients at study entry. The cumulative incidence method14 was used to estimate time to prostate cancer mortality and non–prostate cancer mortality because it specifically considers other competing causes of mortality. Gray's test statistic15 was used to compare cumulative incidence rates between groups categorized by prevalent diabetes and baseline weight. Univariate Cox proportional hazards regression analyses16 using the χ2 test were performed to evaluate the solitary effect of each variable on all-cause mortality. To analyze whether prevalent diabetes was independently associated with mortality while adjusting for other factors, a Cox proportional hazards regression model16 was used for all-cause mortality, and Fine and Gray’ regression model17 was used for prostate cancer mortality and non–prostate cancer mortality. Other covariates in the model included age (< 70 [reference level {RL}] v ≥ 70 years), race (black [RL] v white/other), Gleason score (2 to 6 [RL] v 7 to 10), tumor stage (T2 [RL] v T3 v T4), PSA (≤ 30 v > 30 ng/mL), weight (continuous or tertiles), and treatment (arm I [RL] v arm II). For the categoric variables, the cut points selected were made before the data were examined and were based on established strata. Unadjusted and adjusted hazard ratios (HRs) were calculated for all covariates using either the Cox proportional hazards model or Fine and Gray's regression model with associated 95% CIs and P values. All statistical tests were two-sided, and a P < .05 was considered statistically significant. SAS software (SAS Institute, Cary, NC) and R software were used for all statistical analyses.
RESULTS
Baseline Characteristics
Between June 1992 and April 1995, 1,551 eligible and assessable patients were enrolled onto the study. Seven hundred sixty-three patients were assigned to short-term ADT (arm I), and 758 patients were assigned to long-term adjuvant ADT (arm II). One patient assigned to arm II had unknown diabetes status and was excluded from the analyses. Pretreatment characteristics were similar between the treatment arms (Table 1). The median age was 70 years (range, 43 to 88 years). Two hundred ten patients (14%) had prevalent diabetes. More patients with prevalent diabetes, compared with patients without diabetes, were categorized as black or other race (24% v 15%, respectively; P = .001). Patients with diabetes, compared with those without diabetes, also had significantly greater body weight (median, 88.5 v 83.6 kg, respectively; P < .001) and tended to have a lower PSA (median, 18.4 v 20.5 ng/mL, respectively; P = .041).
Table 1.
Baseline Characteristics
| Characteristic | Patients With No Prevalent Diabetes (n = 1,310)
|
Patients With Prevalent Diabetes (n = 210)
|
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Treatment arm | |||||
| Arm I | 652 | 50 | 111 | 53 | .41 |
| Arm II | 658 | 50 | 99 | 47 | |
| Age, years | |||||
| < 70 | 579 | 44 | 101 | 48 | .29 |
| ≥ 70 | 731 | 56 | 109 | 52 | |
| Race | |||||
| White | 1,120 | 86 | 159 | 76 | .001 |
| Black | 157 | 12 | 40 | 19 | |
| Other | 33 | 3 | 11 | 5 | |
| Clinical stage | |||||
| T2 | 591 | 45 | 99 | 47 | .61 |
| T3 | 670 | 51 | 101 | 48 | |
| T4 | 49 | 4 | 10 | 5 | |
| Gleason score | |||||
| 2-6 | 499 | 38 | 83 | 40 | .51 |
| 7-10 | 721 | 55 | 117 | 56 | |
| Unknown | 90 | 7 | 10 | 5 | |
| PSA, ng/mL | |||||
| ≤ 30 | 867 | 66 | 154 | 73 | .041 |
| > 30 | 443 | 34 | 56 | 27 | |
| Median | 20.5 | 18.4 | |||
| Range | 0.1-250 | 0.9-228.4 | |||
| Weight, kg | |||||
| Median | 83.6 | 88.5 | < .0001 | ||
| Range | 48.6-173 | 47-131.3 | |||
| Unknown | 79 | 18 | |||
| Tertile 1 (< 78.2 kg) | 424 | 34 | 46 | 24 | < .0001 |
| Tertile 2 (78.2-89.5 kg) | 438 | 36 | 55 | 29 | |
| Tertile 3 (> 89.5 kg) | 369 | 30 | 91 | 47 | |
Abbreviation: PSA, prostate-specific antigen.
Mortality
The median follow-up time for all eligible patients was 8.1 years (range, 0.04 to 12.9 years). There were a total of 765 deaths; 210 deaths (27%) were related to prostate cancer.
Univariate Analyses
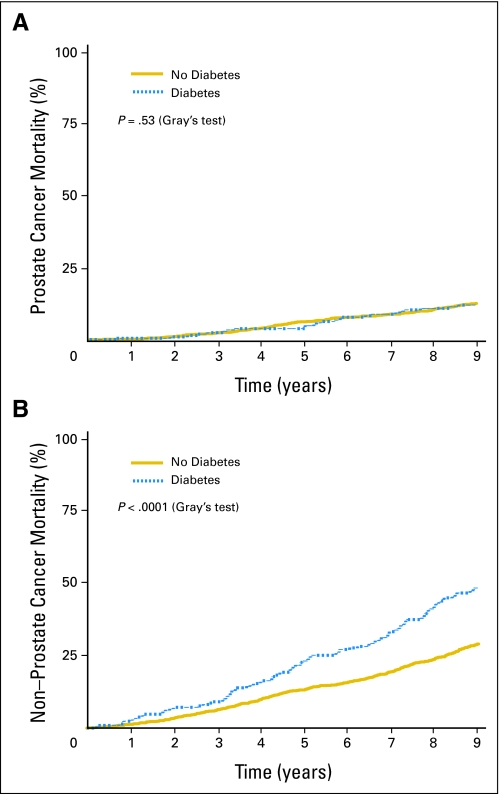
In univariate analyses, prevalent diabetes was significantly associated with greater all-cause and non–prostate cancer mortality but not prostate cancer mortality (Table 2). At 5 years, all-cause mortality was 27.0% in men with diabetes compared with 19.4% in men without diabetes; the unadjusted HR was 1.64 (95% CI, 1.37 to 1.97; P < .0001). Similarly, the 5-year non–prostate cancer mortality rate was 23.1% in men with diabetes compared with 13.2% in men without diabetes; the unadjusted HR was 1.88 (95% CI, 1.53 to 2.31; P < .0001). In contrast, prevalent diabetes was not associated with greater prostate cancer mortality (unadjusted HR = 1.07; 95% CI, 0.71 to 1.61; P = .74). Figure 1 displays prostate cancer mortality and non–prostate cancer mortality by presence or absence of prevalent diabetes.
Table 2.
Univariate Analyses of Diabetes/Weight and Mortality
| Outcome and Covariate | No. of Patients | No. of Treatment Failures | 5-Year Failure Rate (%) | 95% CI (%) | Unadjusted Hazard Ratio | 95% CI | P |
|---|---|---|---|---|---|---|---|
| All-cause mortality | |||||||
| No diabetes | 1,310 | 625 | 19.4 | 17.3 to 21.6 | Reference | ||
| Diabetes | 210 | 140 | 27.0 | 21.0 to 33.0 | 1.64 | 1.37 to 1.97 | < .0001* |
| Prostate cancer mortality | |||||||
| No diabetes | 1,310 | 183 | 6.2 | 4.9 to 7.6 | Reference | ||
| Diabetes | 210 | 27 | 3.8 | 1.2 to 6.5 | 1.07 | 0.71 to 1.61 | .74† |
| Non–prostate cancer mortality | |||||||
| No diabetes | 1,310 | 442 | 13.2 | 11.3 to 15.0 | Reference | ||
| Diabetes | 210 | 113 | 23.1 | 17.4 to 28.9 | 1.88 | 1.53 to 2.31 | < .0001† |
| All-cause mortality | |||||||
| Weight: tertile 1 (< 78.2 kg) | 471 | 228 | 20.3 | 16.9 to 24.3 | Reference | ||
| Weight: tertile 2 (78.2-89.5 kg) | 493 | 250 | 21.9 | 18.4 to 26.0 | 1.07 | 0.89 to 1.28 | .47* |
| Weight: tertile 3 (> 89.5 kg) | 460 | 233 | 20.6 | 17.3 to 24.5 | 1.09 | 0.91 to 1.31 | .34* |
| Prostate cancer mortality | |||||||
| Weight: tertile 1 (< 78.2 kg) | 471 | 51 | 4.1 | 2.3 to 5.9 | Reference | ||
| Weight: tertile 2 (78.2-89.5 kg) | 493 | 65 | 6.6 | 4.4 to 8.8 | 1.24 | 0.86 to 1.79 | .24† |
| Weight: tertile 3 (> 89.5 kg) | 460 | 78 | 7.5 | 5.1 to 10.0 | 1.63 | 1.15 to 2.31 | .006† |
| Non–prostate cancer mortality | |||||||
| Weight: tertile 1 (< 78.2 kg) | 471 | 177 | 16.2 | 12.8 to 19.6 | Reference | ||
| Weight: tertile 2 (78.2-89.5 kg) | 493 | 185 | 14.0 | 10.9 to 17.1 | 0.99 | 0.81 to 1.21 | .93† |
| Weight: tertile 3 (> 89.5 kg) | 460 | 155 | 14.4 | 11.1 to 17.6 | 0.88 | 0.71 to 1.09 | .25† |
P value determined using χ2 test.
P value determined using Gray's test statistic.
Fig 1.
(A) Time to prostate cancer mortality and (B) non–prostate cancer mortality by prevalent diabetes.
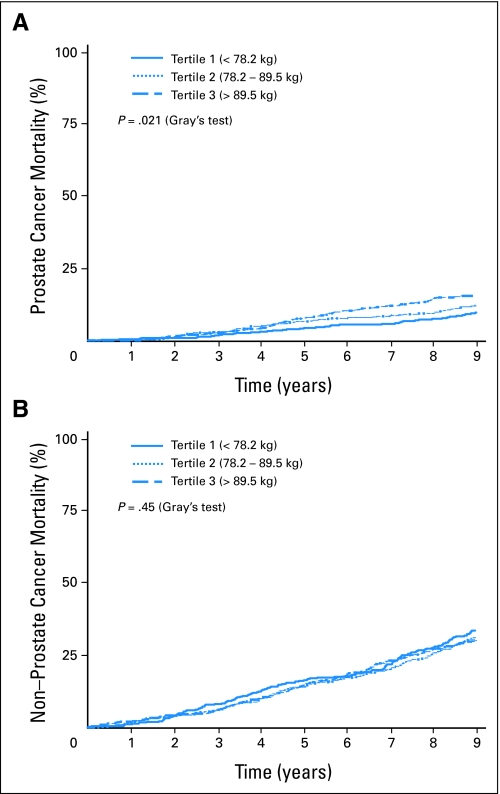
In contrast, baseline weight was associated with greater prostate cancer mortality but not all-cause mortality or non–prostate cancer mortality in univariate analyses (Table 2). At 5 years, prostate cancer mortality was 7.5% for men in the third tertile (weight > 89.5 kg) compared with 4.1% for men in first tertile (weight < 78.2 kg); the unadjusted HR was 1.63 (95% CI, 1.15 to 2.31; P < .006). Figure 2 displays prostate cancer mortality and non–prostate cancer mortality according to baseline weight.
Fig 2.
(A) Time to prostate cancer mortality and (B) non–prostate cancer mortality according to baseline weight in tertiles.
Multivariate Analyses
After controlling for age, race, Gleason score, tumor stage, PSA, treatment arm, and weight, prevalent diabetes was significantly associated with greater all-cause mortality (HR = 1.77; 95% CI, 1.45 to 2.16; P < .0001) and non–prostate cancer mortality (HR = 2.12; 95% CI, 1.69 to 2.66; P < .0001; Table 3). In contrast, prevalent diabetes was not associated with greater prostate cancer mortality (HR = 0.80; 95% CI, 0.51 to 1.26; P = .34). Age, tumor stage, Gleason score, weight, and treatment arm were significantly associated with prostate cancer mortality. Higher weight was significantly associated with greater prostate cancer mortality (HR = 1.77; 95% CI, 1.22 to 2.55; P = .002 for third tertile v first tertile). When assessed as a continuous variable (kilograms), weight remained significantly associated with greater prostate cancer mortality (HR = 1.01; 95% CI, 1.00 to 1.02; P = .008). Weight was not significantly associated with all-cause mortality or non–prostate cancer mortality (Table 3).
Table 3.
Multivariate Proportional Hazard Models
| Outcome and Covariate | Hazard Ratio | 95% CI | P |
|---|---|---|---|
| All-cause mortality | |||
| Age < 70 v ≥ 70 years | 1.57 | 1.34 to 1.84 | < .0001 |
| Race: black v other | 1.07 | 0.84 to 1.37 | .57 |
| Tumor stage | |||
| < T3 | — | — | |
| T3 | 1.11 | 0.95 to 1.30 | .20 |
| T4 | 1.79 | 1.25 to 2.57 | .002 |
| Gleason sum: 2-6 v 7-10 | 1.41 | 1.20 to 1.66 | < .0001 |
| Prostate-specific antigen: ≤ 30 v > 30 ng/mL | 1.02 | 0.86 to 1.20 | .83 |
| Treatment arm: I v II | 0.86 | 0.74 to 1.01 | .06 |
| Diabetes: no v yes | 1.77 | 1.45 to 2.16 | < .0001 |
| Weight | |||
| Tertile 1 (< 78.2 kg) | — | — | |
| Tertile 2 (78.2-89.5 kg) | 1.10 | 0.92 to 1.33 | .30 |
| Tertile 3 (> 89.5 kg) | 1.14 | 0.94 to 1.38 | .20 |
| Prostate cancer mortality | |||
| Age < 70 v ≥ 70 years | 0.65 | 0.48 to 0.87 | .004 |
| Race: black v other | 1.27 | 0.80 to 2.02 | .31 |
| Tumor stage | |||
| < T3 | — | — | |
| T3 | 1.44 | 1.05 to 1.98 | .02 |
| T4 | 3.96 | 2.28 to 6.88 | < .0001 |
| Gleason sum: 2-6 v 7-10 | 1.88 | 1.35 to 2.63 | .0002 |
| Prostate-specific antigen: ≤ 30 v > 30 ng/mL | 1.25 | 0.93 to 1.68 | .14 |
| Treatment arm: I v II | 0.63 | 0.47 to 0.85 | .002 |
| Diabetes: no v yes | 0.80 | 0.51 to 1.25 | .32 |
| Weight | |||
| Tertile 1 (< 78.2 kg) | — | — | |
| Tertile 2 (78.2-89.5 kg) | 1.25 | 0.85 to 1.84 | .26 |
| Tertile 3 (> 89.5 kg) | 1.77 | 1.22 to 2.55 | .002 |
| Non–prostate cancer mortality | |||
| Age < 70 v ≥ 70 years | 2.14 | 1.75 to 2.60 | < .0001 |
| Race: black v other | 1.00 | 0.76 to 1.33 | .99 |
| Tumor stage | |||
| < T3 | — | — | |
| T3 | 0.93 | 0.78 to 1.12 | .47 |
| T4 | 0.86 | 0.52 to 1.43 | .56 |
| Gleason sum: 2-6 v 7-10 | 1.16 | 0.96 to 1.39 | .12 |
| Prostate-specific antigen: ≤ 30 v > 30 ng/mL | 0.94 | 0.78 to 1.15 | .57 |
| Treatment arm: I v II | 1.05 | 0.88 to 1.26 | .57 |
| Diabetes: no v yes | 2.12 | 1.69 to 2.66 | < .0001 |
| Weight | |||
| Tertile 1 (< 78.2 kg) | — | — | |
| Tertile 2 (78.2-89.5 kg) | 1.02 | 0.83 to 1.25 | .86 |
| Tertile 3 (> 89.5 kg) | 0.85 | 0.67 to 1.07 | .17 |
We considered the possibility that our results may vary by treatment arm. To address this issue, we performed additional analyses for each of the treatment arms. Similar results were observed in univariate and multivariate analyses for both the short-term and long-term ADT groups. Specifically, prevalent diabetes was associated with significantly greater all-cause and non–prostate cancer mortality but not prostate cancer mortality in each treatment arm (data not shown).
Treatment Effect by Prevalent Diabetes
In the primary analyses of RTOG 92-02, long-term goserelin treatment improved cancer-specific survival compared with short-term goserelin.12 We conducted subset analyses to address whether this improvement in cancer-specific mortality varied with prevalent diabetes. In multivariate analyses of men without prevalent diabetes, long-term goserelin treatment was significantly associated with lower risk of prostate cancer mortality (HR = 0.65; 95% CI, 0.47 to 0.89; P = .008). In the smaller subset of men with prevalent diabetes, long-term goserelin was not significantly associated with prostate cancer mortality (HR = 0.41; 95% CI, 0.17 to 1.01; P = .053), although the HR was similar or lower to that observed for men without prevalent diabetes. Long-term goserelin treatment was not associated with risk for non–prostate cancer mortality in either men with prevalent diabetes (HR = 1.09; 95% CI, 0.72 to 1.99; P = .68) or men without diabetes (HR = 1.05; 95% CI, 0.86 to 1.28; P = .61). Long-term goserelin treatment was also not associated with risk for all-cause mortality in either men with prevalent diabetes (HR = 0.81; 95% CI, 0.56 to 1.19; P = .29) or men without diabetes (HR = 0.87; 95% CI, 0.73 to 1.03; P = .10).
DISCUSSION
Using data from a large, multicenter, prospective, randomized controlled trial with long follow-up, we found that greater body weight but not prevalent diabetes was significantly associated with prostate cancer mortality after treatment for locally advanced disease. In contrast, prevalent diabetes, but not weight, was significantly associated with greater all-cause mortality and non–prostate cancer mortality.
Prior explanations for the inverse relationship between diabetes and prostate cancer diagnosis have centered on the metabolic and hormonal changes associated with diabetes. However, a recent genome-wide association scan demonstrated that a common variant on chromosome 17 confers increased prostate cancer risk and protection against type 2 diabetes.18 This observation suggests that a genetic variation may functionally impact one or more hormonal or metabolic pathways throughout an individual's lifetime and incidentally modulate the risks of developing prostate cancer and diabetes later in life.18 Our finding that prevalent diabetes is not associated with cancer-specific mortality after combined-modality therapy predicts that this genetic variation impacts risk for prostate cancer diagnosis but not cancer progression after treatment.
Most men with type 2 diabetes are obese.2 Overweight and obesity are associated with higher rates of PSA recurrence after surgery or RT for early-stage disease.5-9 A recent study also reported that overweight and obesity are associated with greater prostate cancer mortality after combined-modality therapy for locally advanced disease.10 Consistent with that observation, we found that greater body weight was significantly associated with greater prostate cancer mortality.
Several mechanisms have been proposed to explain the link between obesity and adverse prostate cancer outcomes including changes in gonadal steroid levels, alterations in adipocytokines, and hyperinsulinemia/insulin resistance.11 In a xenograft model of prostate cancer, diet-induced hyperinsulinemia was associated with increased tumor growth.19 Elevated fasting plasma insulin and other components of the metabolic syndrome were associated with greater prostate cancer mortality in a single-center cohort study of 320 men (54 deaths) with clinical stage T2-3 prostate cancer.20 In contrast, the convincing absence of an association between diabetes and prostate cancer death after treatment for locally advanced disease in our large multicenter prospective study suggests that the characteristic metabolic alterations of type 2 diabetes, including insulin resistance, are not the mechanism(s) responsible for the link between obesity and cancer-specific mortality. Consistent with our results, an observational study reported that diabetes is not associated with risk for disease recurrence after RT for prostate cancer.21
A recent large claims-based analysis using Surveillance, Epidemiology and End Results–Medicare data demonstrated a significant association between GnRH agonists and a greater risk for incident diabetes, coronary heart disease, and hospital admission for myocardial infarction in men with prostate cancer.22 These observations have raised concerns about the potential impact of GnRH agonists on noncancer mortality and the optimal duration of adjuvant treatment in men with comorbid medical conditions. In multivariate analyses, we found no evidence that long-term goserelin conferred a greater risk for all-cause mortality or non–prostate cancer mortality in men with prevalent diabetes compared with nondiabetic men. Our results also suggest that the improvement in prostate cancer mortality with long-term goserelin was similar for both diabetic and nondiabetic men. Taken together, these results suggest that prevalent diabetes should not influence decisions about duration of adjuvant GnRH agonist therapy in men receiving RT for locally advance prostate cancer.
Our study has substantial strengths. The study was large with 1,521 patients, had a median follow-up time of greater than 8 years, and had a total of 765 deaths. Nonetheless, the study may have been underpowered to detect a small effect of diabetes on prostate cancer mortality. Approximately 14% of patients had prevalent diabetes compared with the 16% prevalence of diagnosed diabetes in the general population of US men older than 60 years.3 Diabetes was ascertained by medical history. In ambulatory adults, there is substantial agreement between self-report and medical record data for diabetes and other chronic medical conditions.23 In the general population, diabetes is associated with significantly greater all-cause mortality.24,25 The robust association between prevalent diabetes and greater all-cause mortality in our patients also suggests that ascertainment of diabetes by self-report was reliable. The study lacked other detailed information about diabetes including disease duration, form of medical therapy, and glycemic control. Body mass index is commonly used to identify obesity and provides an acceptable approximation of total body fat for most patients.26 Because information about patient height was not available, however, we used weight rather than body mass index to characterize body composition.
In summary, we found that weight, but not prevalent diabetes, is associated with prostate cancer mortality in men undergoing combined-modality therapy for locally advanced prostate cancer. In contrast, prevalent diabetes, but not weight, was significantly associated with greater all-cause and non–prostate cancer mortality. These observations suggest that the greater prostate cancer mortality observed in obese men is mediated by mechanism(s) other than the metabolic alterations associated with diabetes.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Matthew R. Smith
Collection and assembly of data: Matthew R. Smith
Data analysis and interpretation: Matthew R. Smith, Kyounghwa Bae, Jason A. Efstathiou, William U. Shipley
Manuscript writing: Matthew R. Smith, Kyounghwa Bae, Jason A. Efstathiou, Gerald E. Hanks, Miljenko V. Pilepich, Howard M. Sandler, William U. Shipley
Final approval of manuscript: Matthew R. Smith, Kyounghwa Bae, Jason A. Efstathiou, Gerald E. Hanks, Miljenko V. Pilepich, Howard M. Sandler, William U. Shipley
Supported by Grants No. RTOG U10 CA21661, CCOP U10 CA37422, and Stat U10 CA32115 from the National Cancer Institute. M.R.S. is supported by an NIH K24 Midcareer Investigator Award (K24 CA121990) and competitive research awards from the Prostate Cancer Foundation.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Jemal A, Ward E, Hao Y, et al: Trends in the leading causes of death in the United States, 1970-2002. JAMA 294:1255-1259, 2005 [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association: Diagnosis and classification of diabetes mellitus. Diabetes Care 30:S42-S47, 2007. (suppl) [DOI] [PubMed] [Google Scholar]
- 3.Cowie CC, Rust KF, Byrd-Holt DD, et al: Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health and Nutrition Examination Survey 1999-2002. Diabetes Care 29:1263-1268, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Kasper JS, Giovannucci E: A meta-analysis of diabetes mellitus and the risk of prostate cancer. Cancer Epidemiol Biomarkers Prev 15:2056-2062, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Amling CL, Riffenburgh RH, Sun L, et al: Pathologic variables and recurrence rates as related to obesity and race in men with prostate cancer undergoing radical prostatectomy. J Clin Oncol 22:439-445, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Freedland SJ, Aronson WJ, Kane CJ, et al: Impact of obesity on biochemical control after radical prostatectomy for clinically localized prostate cancer: A report by the Shared Equal Access Regional Cancer Hospital database study group. J Clin Oncol 22:446-453, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Freedland SJ, Grubb KA, Yiu SK, et al: Obesity and risk of biochemical progression following radical prostatectomy at a tertiary care referral center. J Urol 174:919-922, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Strom SS, Wang X, Pettaway CA, et al: Obesity, weight gain, and risk of biochemical failure among prostate cancer patients following prostatectomy. Clin Cancer Res 11:6889-6894, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Strom SS, Kamat AM, Gruschkus SK, et al: Influence of obesity on biochemical and clinical failure after external-beam radiotherapy for localized prostate cancer. Cancer 107:631-639, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Efstathiou JA, Bae K, Shipley WU, et al: Obesity and mortality in men with locally advanced prostate cancer: Analysis of RTOG 85-31. Cancer 110:2691-2699, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freedland SJ, Platz EA: Obesity and prostate cancer: Making sense out of apparently conflicting data. Epidemiol Rev 29:88-97, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Hanks GE, Pajak TF, Porter A, et al: Phase III trial of long-term adjuvant androgen deprivation after neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate: The Radiation Therapy Oncology Group Protocol 92-02. J Clin Oncol 21:3972-3978, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Zelen M: The randomization and stratification of patients to clinical trials. J Chronic Dis 27:365-375, 1974 [DOI] [PubMed] [Google Scholar]
- 14.Kalbfleisch J, Prentice R: The Statistical Analysis of Failure Time Data (ed 2). New York, NY, John Wiley & Sons, 2002
- 15.Gray RJ: A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 16:1141-1154, 1988 [Google Scholar]
- 16.Cox DR: Regression models and life tables. J R Stat Soc B 34:187-229, 1972 [Google Scholar]
- 17.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94:496-509, 1999 [Google Scholar]
- 18.Gudmundsson J, Sulem P, Steinthorsdottir V, et al: Two variants on chromosome 17 confer prostate cancer risk, and the one in TCF2 protects against type 2 diabetes. Nat Genet 39:977-983, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Venkateswaran V, Haddad AQ, Fleshner NE, et al: Association of diet-induced hyperinsulinemia with accelerated growth of prostate cancer (LNCaP) xenografts. J Natl Cancer Inst 99:1793-1800, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Hammarsten J, Hogstedt B: Hyperinsulinaemia: A prospective risk factor for lethal clinical prostate cancer. Eur J Cancer 41:2887-2895, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Chan JM, Latini DM, Cowan J, et al: History of diabetes, clinical features of prostate cancer, and prostate cancer recurrence-data from CaPSURE (United States). Cancer Causes Control 16:789-797, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Keating NL, O'Malley AJ, Smith MR: Diabetes and cardiovascular disease during androgen deprivation for prostate cancer. J Clin Oncol 24:4448-4456, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Okura Y, Urban LH, Mahoney DW, et al: Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol 57:1096-1103, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Gu K, Cowie CC, Harris MI: Mortality in adults with and without diabetes in a national cohort of the U.S. population, 1971-1993. Diabetes Care 21:1138-1145, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Gregg EW, Gu Q, Cheng YJ, et al: Mortality trends in men and women with diabetes, 1971 to 2000. Ann Intern Med 147:149-155, 2007 [DOI] [PubMed] [Google Scholar]
- 26.National Institutes of Health: Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report. Obes Res 6:51S-209S, 1998. (suppl 2) [PubMed] [Google Scholar]