Abstract
Purpose
Identify prognostic factors that influence outcome after unrelated donor bone marrow transplantation in children with acute myeloid leukemia (AML).
Patients and Methods
Included are 268 patients (age ≤ 18 years) with AML in second complete remission (n = 142), relapse (n = 90), or primary induction failure (n = 36) at transplantation. All patients received bone marrow grafts from an unrelated donor and a myeloablative conditioning regimen. Cox regression models were constructed to identify risk factors that influence outcome after transplantation.
Results
In this analysis, the only risk factor that predicted leukemia recurrence and overall and leukemia-free survival was disease status at transplantation. The 5-year probabilities of leukemia-free survival were 45%, 20%, and 12% for patients who underwent transplantation at second complete remission, relapse, and primary induction failure, respectively. As expected, risk of acute but not chronic graft-versus-host disease (GVHD) was lower with T-cell–depleted bone marrow grafts; T-cell–depleted grafts were not associated with higher risks of leukemia recurrence. We observed similar risks of leukemia relapse in patients with and without acute and chronic GVHD.
Conclusion
Children who underwent transplantation in remission had a superior outcome compared with children who underwent transplantation during relapse or persistent disease. Nevertheless, 20% of children who underwent transplantation in relapse are long-term survivors, suggesting that unrelated donor bone marrow transplantation is an effective therapy in a significant proportion of children with recurrent or primary refractory AML.
INTRODUCTION
Eighty to ninety percent of children with acute myeloid leukemia (AML) treated on current chemotherapeutic trials achieve a complete remission.1-6 However, 30% to 40% of patients achieving a first remission experience relapse, and less than a third of these patients with recurrent leukemia survive long term.7-12 Although most reports identify the length of first remission as the best predictor of survival, others have reported that sex and French-American-British classification are predictors of achieving a second remission and long-term survival.7-12 Therapies for patients who experience relapse are variable and often include allogeneic hematopoietic stem-cell transplantation when a suitable donor is available. In this report, we sought to identify prognostic factors that influence outcome after unrelated donor bone marrow transplantation in children with AML who experience leukemia recurrence after achieving a first complete remission or who received transplantation for primary induction failure.
PATIENTS AND METHODS
Data Collection
The National Marrow Donor Program (NMDP) collects detailed demographic, disease, and transplantation characteristics and outcome data on all unrelated donor transplantations it facilitates in the United States. All patients are observed longitudinally, and computerized error checks, physician review of submitted data, and on-site audits of participating centers ensure data quality. The NMDP retrospectively obtained consent for data submission and study participation from surviving patients or their parent/legal guardian for transplantations it facilitated in the United States before 2002. Thereafter, informed consent was obtained prospectively. The Institutional Review Board of the NMDP waived consent for patients who had died before soliciting consent (transplantations facilitated before 2002). To overcome the bias caused by the inclusion of a proportion of surviving patients (those consenting) but all deceased recipients, and hence their over-representation, a sample of deceased patients was selected using a weighted randomized scheme that adjusts for over-representation of deceased patients in the consented cohort.13 This weighted randomized scheme was developed based on all survivors in the NMDP database. A logistic regression model was fit to identify the factors that predicted whether a patient had consented or not consented to use of data collected by the NMDP. This analysis found that the following factors were associated with the likelihood of a patient consenting: age, disease type, race, sex, cytomegalovirus serostatus, and country of transplantation (United States v not United States). Using estimated consenting probabilities from this model based on the characteristics of dead patients, the biased coin method of randomization was performed to determine which of the dead patients likely would have consented to participate had they been alive. Thus, this procedure includes the dead patients at the same probability as surviving patients who consented to participate. Approximately 13% of surviving patients declined to consent, and 12% of dead patients were deleted by the weighted randomized method. This method was tested several times, and on every occasion, the proportion of deleted dead patients was similar.
Inclusion Criteria
The study population includes 268 recipients of unrelated donor bone marrow transplantations performed in the United States between 1990 and 2003. Patients (age ≤ 18 years at transplantation) with AML who underwent transplantation in second complete remission or first or subsequent relapse and patients with primary induction failure are included. Complete remission was defined as neutrophil count more than 1.0 × 109/L; platelets more than 100 × 109/L; RBC transfusion independent; less than 5% blasts in bone marrow with absence of cells with Auer rods; normal maturation of the erythrocytic, granulocytic, and megakaryocytic series; and absence of extramedullary disease. All patients received bone marrow grafts and a myeloablative transplantation conditioning regimen. Patients who received an unrelated donor bone marrow transplantation in first complete remission, patients in third or subsequent remission, children with Down's syndrome, and recipients of peripheral-blood or umbilical cord blood grafts were excluded because risk factors were likely to vary in these groups.
End Points
The primary outcomes studied were neutrophil and platelet recovery, acute and chronic graft-versus-host disease (GVHD), and early and overall mortality. Neutrophil recovery was defined as achieving an absolute neutrophil count of ≥ 0.5 × 109/L and platelets more than 20 × 109/L unsupported for 7 days. Failure to achieve an absolute neutrophil count of ≥ 0.5 × 109/L or a decline to less than 0.5 × 109/L after an initial recovery and without a subsequent recovery was considered graft failure. Incidence of grades 2, 3, and 4 acute GVHD and chronic GVHD were determined in all patients. Diagnosis of acute14 and chronic GVHD15 was based on local institutional criteria, with overall grade of acute GVHD assigned retrospectively by the NMDP based on stage of involvement reported for each individual organ. Any death occurring during continuous remission was defined as treatment-related mortality. Relapse was defined as morphologic leukemia recurrence at any site, and leukemia-free survival was defined as survival in a state of continuous complete remission.
Statistical Analysis
The probabilities of leukemia-free and overall survival were calculated using the Kaplan-Meier method.16 For analysis of survival, death from any cause was considered an event, and data on patients still alive were censored at date of last follow-up. For analysis of leukemia-free survival, leukemia relapse or death from any cause is considered an event, and patients were censored at last follow-up. The probabilities of neutrophil and platelet recovery, acute and chronic GVHD, transplantation-related mortality, and relapse were calculated with the use of the cumulative incidence function method.16 For neutrophil and platelet recovery and GVHD, death without the event (hematopoietic recovery or GVHD) was the competing event. Data on patients without an event were censored at last follow-up. For relapse, transplantation-related mortality was the competing event, and for transplantation-related mortality, relapse was the competing event. CIs were calculated using log transformation.
Cox regression models were built for analysis of risk factors for GVHD, transplantation-related mortality, relapse, treatment failure, and overall mortality.17 Multivariate models were built with the use of stepwise forward selection, with P ≤ .01 considered to indicate statistical significance. The variable for cytogenetic risk group did not attain the level of significance. Given the reported prognostic significance of cytogenetics in AML, analyses for transplantation-related mortality, relapse, treatment failure, and overall mortality were stratified by cytogenetic risk group. All variables met the proportional hazards assumptions. Variables considered in multivariate model building are listed in Table 1. We tested for an effect of transplantation center on outcome and found none.18 P values are two sided. Analyses were performed using SAS software, version 9.1 (SAS Institute, Cary, NC).
Table 1.
Patient, Disease, and Transplantation Characteristics by Disease Status at Transplantation
| Characteristic | Disease Status at Transplantation
|
|||||
|---|---|---|---|---|---|---|
| Second Complete Remission (n = 142)
|
First or Second Relapse* (n = 90)
|
Primary Induction Failure (n = 36)
|
||||
| No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Male sex | 74 | 52 | 49 | 54 | 17 | 42 |
| WBC count at diagnosis | ||||||
| ≤ 50 × 109/L | 75 | 53 | 54 | 60 | 24 | 67 |
| > 50-100 × 109/L | 26 | 18 | 11 | 12 | 3 | 8 |
| > 100 × 109/L | 17 | 12 | 11 | 12 | 8 | 22 |
| Unknown | 24 | 17 | 14 | 16 | 1 | 3 |
| FAB subtype | ||||||
| M0 | 1 | < 1 | — | — | — | — |
| M1 | 10 | 7 | 15 | 17 | 3 | 8 |
| M2 | 32 | 23 | 24 | 27 | 5 | 14 |
| M3 | 23 | 16 | 2 | 2 | — | — |
| M4 | 27 | 19 | 14 | 16 | 8 | 22 |
| M5 | 26 | 18 | 6 | 7 | 7 | 19 |
| M6 | 2 | 1 | 3 | 3 | 2 | 6 |
| M7 | 5 | 4 | 11 | 12 | 4 | 11 |
| Unknown | 16 | 10 | 15 | 17 | 7 | 19 |
| Extramedullary disease at diagnosis | ||||||
| Absent | 115 | 81 | 83 | 92 | 31 | 86 |
| CNS ± other sites | 20 | 14 | 4 | 4 | 2 | 6 |
| Other sites, not CNS | 7 | 5 | 3 | 3 | 3 | 8 |
| Cytogenetics† | ||||||
| Good risk | 31 | 22 | 13 | 14 | 2 | 6 |
| Intermediate risk | 72 | 51 | 47 | 52 | 16 | 44 |
| Poor risk | 10 | 7 | 6 | 7 | 10 | 28 |
| Unknown | 29 | 20 | 24 | 27 | 8 | 22 |
| Age at transplantation, years‡ | ||||||
| ≤ 5 | 50 | 35 | 27 | 30 | 17 | 47 |
| > 5-10 | 19 | 13 | 13 | 14 | 8 | 22 |
| > 10-15 | 41 | 29 | 28 | 31 | 6 | 17 |
| > 15-18 | 32 | 23 | 22 | 24 | 5 | 14 |
| Performance score | ||||||
| 90-100 | 110 | 77 | 59 | 66 | 24 | 67 |
| < 90 | 27 | 19 | 28 | 31 | 11 | 31 |
| Unknown | 5 | 4 | 3 | 3 | 1 | 3 |
| Duration of first complete remission | ||||||
| ≤ 12 months | 96 | 68 | 75 | 83 | 36 | 100 |
| > 12 months | 46 | 32 | 28 | 31 | — | — |
| Unknown | — | — | 1 | 1 | — | — |
| Conditioning regimen | ||||||
| Irradiation containing | 123 | 86 | 79 | 88 | 32 | 89 |
| Non–irradiation containing | 19 | 14 | 11 | 12 | 4 | 11 |
| GVHD prophylaxis | ||||||
| T-cell depletion | 50 | 35 | 37 | 41 | 14 | 39 |
| Cyclosporine + methotrexate | 70 | 49 | 44 | 49 | 19 | 53 |
| Cyclosporine ± other | 6 | 4 | 2 | 2 | 2 | 6 |
| Tacrolimus ± other | 14 | 10 | 7 | 8 | 1 | 3 |
| Methotrexate ± other | 2 | 1 | — | — | — | — |
| Donor-recipient sex match | ||||||
| Male to male | 47 | 33 | 31 | 34 | 10 | 28 |
| Male to female | 34 | 24 | 16 | 18 | 12 | 33 |
| Female to male | 27 | 19 | 18 | 20 | 7 | 19 |
| Female to female | 34 | 24 | 25 | 28 | 7 | 19 |
| Donor-recipient CMV serostatus | ||||||
| Donor negative/recipient negative | 54 | 38 | 37 | 41 | 16 | 44 |
| Donor positive/recipient negative | 25 | 18 | 16 | 18 | 5 | 14 |
| Donor negative/recipient positive | 29 | 20 | 18 | 20 | 6 | 17 |
| Donor positive/recipient positive | 31 | 22 | 15 | 17 | 8 | 22 |
| Unknown | 3 | 2 | 4 | 4 | 1 | 3 |
| Donor age, years | ||||||
| 18-30 | 42 | 30 | 22 | 24 | 9 | 25 |
| 31-40 | 58 | 41 | 35 | 39 | 11 | 31 |
| 41-50 | 35 | 25 | 26 | 29 | 12 | 33 |
| 51-60 | 7 | 5 | 7 | 8 | 4 | 11 |
| Donor-recipient HLA disparity | ||||||
| Matched§ | 42 | 30 | 22 | 24 | 13 | 36 |
| One locus mismatched‖ | 61 | 43 | 43 | 48 | 12 | 33 |
| > One locus mismatched¶ | 39 | 27 | 25 | 28 | 11 | 31 |
Abbreviations: FAB, French-American-British; GVHD, graft-versus-host disease; CMV, cytomegalovirus.
Sixty-six patients were in first relapse, 24 patients were in second relapse, and three patients were aplastic at transplantation.
Cytogenetic classification (Medical Research Council of the United Kingdom) was as follows: good risk: inv16/t(16;16)/del(16q), t(15;17), t(8;21) ± secondary abnormalities; intermediate risk: normal, 11q23 abn, +8, del(9q), del(7q), +21, +22, all others; and poor risk: del(5q)/−5,−7, abn(3q), t(9;22), t(6;9), complex karyotypes (≥ five unrelated abnormalities).
Nine patients were ≤ 1 year old at transplantation.
Matched: 66 patients were matched at HLA-A, HLA-B, HLA-C, and DRB1 (allele level); three were matched at HLA-A, HLA-B, and DRB1 (allele level) and HLA-C (low resolution); seven were matched at HLA-A, HLA-B, HLA-C (low resolution), and DRB1; and one was matched at HLA-A, HLA-B, and DRB1 (allele level, HLA-C typing not known).
One locus mismatched: 48 patients were mismatched at one locus considering allele-level typing at HLA-A, HLA-B, HLA-C, and DRB1; 13 were mismatched at one locus considering low-resolution typing at HLA-A, HLA-B, and HLA-C and matched at DRB1; 55 were matched (low resolution) at HLA-A and HLA-B and mismatched at one locus at DRB1, with data on HLA-C not known.
> One locus mismatched: 46 patients were mismatched at more than one locus considering allele-level typing at HLA-A, HLA-B, HLA-C, and DRB1; 25 were mismatched at one locus considering HLA-A, HLA-B, and DRB1, with HLA-C not known; four were mismatched at one locus considering low-resolution typing at HLA-A, HLA-B, and DRB1, with HLA-C not known.
RESULTS
Patient, disease, and transplantation characteristics by disease status at transplantation are listed in Table 1. Median age at transplantation was 10 years (range, < 1 to 18 years), and median time from diagnosis to transplantation was 13 months (range, < 1 to 88 months). Twenty patients (7%) had myelodysplastic syndrome that evolved to AML before transplantation. Fifty-three percent of patients were in second complete remission, 34% were in first or subsequent relapse at transplantation, and 13% had primary induction failure. Sixty-eight (76%) of 90 patients who underwent transplantation in relapse received chemotherapy before transplantation but did not achieve clinical remission. Most patients (87%) received total-body irradiation–containing conditioning regimens. All patients received bone marrow grafts, and 38% of bone marrow grafts were T-cell depleted. Median follow-up time of surviving patients is 5 years (range, 5 to 156 months).
Hematopoietic Recovery
Most patients achieved neutrophil and platelet recovery. The probability of neutrophil recovery at day 28 was 95% (95% CI, 83% to 98%), and the probability of platelet recovery at day 28 was 70% (95% CI, 64% to 76%).
GVHD
Grade 2 to 4 acute GVHD rates were lower after transplantation of T-cell–depleted bone marrow grafts (hazard ratio [HR] = 0.55; 95% CI, 0.37 to 0.82; P = .003); the probability of grade 2 to 4 acute GVHD at day 100 was 31% (95% CI, 22% to 40%) after transplantation of T-cell–depleted bone marrow compared with 52% (95% CI, 44% to 59%) after transplantation of non–T-cell–depleted grafts. In the current analysis, none of the factors tested was predictive for chronic GVHD. The 5-year probability of chronic GVHD was 34% (95% CI, 28% to 40%).
Transplantation-Related Mortality
Transplant-related mortality rates were higher in older patients (age 11 to 18 years) compared with those aged ≤ 10 years. The probabilities of early (day 100) and late (5-year) transplantation-related mortality in patients aged ≤ 10 years were 14% (95% CI, 9% to 21%) and 19% (95% CI, 12% to 26%), respectively; the corresponding probabilities in older patients were 25% (95% CI, 18% to 32%) and 41% (95% CI, 33% to 50%), respectively.
Relapse
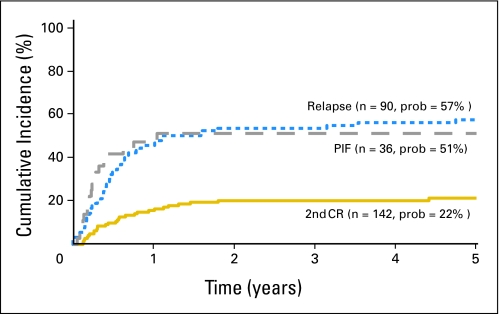
Relapse rates were higher in patients who underwent transplantation in first or second relapse and at primary induction failure compared with patients who underwent transplantation in second complete remission, stratified for cytogenetic risk (Table 2). Rates were similar when transplantation was performed at relapse or primary induction failure (HR = 1.33; 95% CI, 0.73 to 2.40; P = .355). The 5-year probabilities of leukemia recurrence were 22% (95% CI, 15% to 29%), 57% (95% CI, 46% to 67%), and 51% (95% CI, 34% to 66%) for patients who underwent transplantation in second complete remission, relapse, and primary induction failure, respectively (Fig 1). The duration of first complete remission was not associated with leukemia recurrence after transplantation (HR = 1.61; 95% CI, 0.91 to 2.86; P = .106). In the current study, we observed similar rates of relapse in patients with and without acute and chronic GVHD (data not shown).
Table 2.
Multivariate Analysis of Transplantation Outcome: Treatment-Related Mortality, Leukemia Relapse, Treatment Failure (relapse or death, inverse of leukemia-free survival), and Overall Mortality Stratified by Cytogenetic Risk Group
| Outcome and Disease Status at Transplantation | No. of Patients | Total No. of Patients Assessable | Hazard Ratio | 95% CI | P |
|---|---|---|---|---|---|
| Transplantation-related mortality | |||||
| ≤ 10 years old at transplantation | 24 | 134 | 1.00 | ||
| > 10-18 years old at transplantation | 55 | 134 | 2.20 | 1.35 to 3.60 | .002 |
| Relapse | |||||
| Second complete remission | 29 | 142 | 1.00 | < .001* | |
| Primary induction failure | 18 | 36 | 4.33 | 2.28 to 8.19 | < .0001 |
| First or subsequent relapse | 51 | 90 | 3.41 | 2.15 to 5.40 | < .0001 |
| Treatment failure | |||||
| Second complete remission | 75 | 142 | 1.00 | < .001* | |
| Primary induction failure | 31 | 36 | 2.59 | 1.64 to 4.09 | < .001 |
| First or subsequent relapse | 71 | 90 | 1.77 | 1.27 to 2.45 | .001 |
| Overall mortality | |||||
| Second complete remission | 72 | 142 | 1.00 | .004* | |
| Primary induction failure | 29 | 36 | 2.10 | 1.32 to 3.34 | .002 |
| First or subsequent relapse | 62 | 90 | 1.46 | 1.03 to 2.06 | .032 |
NOTE. The variable for cytogenetic risk group was nonproportional in the model for overall mortality; therefore, multivariate models for treatment-related mortality, relapse, treatment failure, and overall mortality were stratified by cytogenetic risk group. The following categories were collapsed because there were no differences between groups: disease status at transplantation: first relapse and second relapse; and age at transplantation: ≤ 5 and > 5-10 years and > 10-15 and > 15-18 years.
Two df test.
Fig 1.
The 5-year probabilities of leukemia relapse after unrelated donor bone marrow transplantation by disease status at transplantation. PIF, primary induction failure; CR, complete remission.
Leukemia-Free Survival
Treatment failure rates (relapse or death; inverse of leukemia-free survival) were higher in patients who underwent transplantation in first or second relapse and primary induction failure, stratified for cytogenetic risk (Table 2). Failure rates were similar when transplantation was performed in primary induction failure and in relapse (HR = 1.56; 95% CI, 0.98 to 2.48; P = .059). Treatment failure rates were not associated with duration of first complete remission (HR = 1.43; 95% CI, 0.95 to 2.13; P = .084). The 5-year probabilities of leukemia-free survival were 45% (95% CI, 37% to 54%), 20% (95% CI, 13% to 30%), and 12% (95% CI, 3% to 26%) for patients who underwent transplantation in second clinical remission, relapse, and primary induction failure, respectively.
Overall Survival
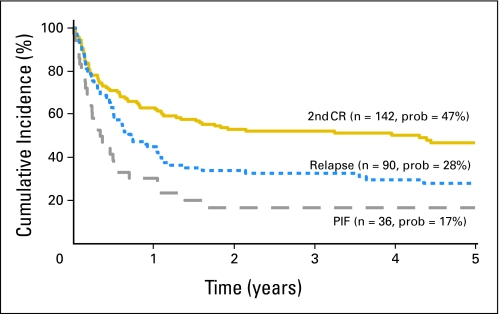
One hundred sixty-three patients died; overall mortality rates were higher in patients who underwent transplantation in primary induction failure compared with patients who underwent transplantation in second complete remission, stratified for cytogenetic risk (Table 2). The 5-year probabilities of overall survival were 47% (95% CI, 38% to 55%), 28% (95% CI, 19% to 38%), and 17% (95% CI, 7% to 32%) for patients who underwent transplantation in second complete remission, relapse, and primary induction failure, respectively (Fig 2). Mortality rates were similar in patients who underwent transplantation in primary induction failure compared with patients in relapse (HR = 1.48; 95% CI, 0.91 to 2.39; P = .111). Recurrent leukemia was the cause of death in 85 of 163 patients who died. Other causes of mortality included GVHD (n = 18), infection (n = 30), adult respiratory distress syndrome or interstitial pneumonitis (n = 9), hemorrhage (n = 8), organ failure (n = 11), and graft failure (n = 2).
Fig 2.
The 5-year probabilities of overall survival after unrelated donor bone marrow transplantation by disease status at transplantation. PIF, primary induction failure; CR, complete remission.
DISCUSSION
Sibling donor transplantation in first remission has been used for many years as a primary approach to treatment of AML, with survival rates of 60% to 70% reported from many centers and cooperative groups.2,3,5,6 Improvements in risk stratification on the basis of genetic abnormalities in leukemic blasts and more effective chemotherapy protocols now allow the identification of subgroups of children with AML for whom transplantation is deemed unnecessary in first remission because cure with chemotherapy is equally likely.2 In parallel with interest in limiting use of transplantation in children with a good prognosis, there is increased interest in investigating whether unrelated donor transplantation can improve outcomes for children with particularly poor prognoses, such as those with primary induction failure and those who relapse after a first remission.11,19,20 In this study, we have explored outcomes in a large group of children receiving unrelated donor transplantations facilitated by the NMDP in the United States to determine how successful this therapy is in rescuing children for whom chemotherapy has been ineffective and to identify risk factors that predict a good outcome after transplantation.
The majority of children included in this study underwent transplantation in second remission. Overall, outcomes were encouraging, with almost half of the children receiving transplantation in second complete remission surviving 5 years later and significant numbers of survivors among the children with refractory disease receiving transplantation. In the current report, the only risk factor that predicted relapse, overall survival, and leukemia-free survival was disease status at the time of transplantation, with children who underwent transplantation in second complete remission having superior outcomes to children who underwent transplantation in relapse or with primary refractory disease. Despite this, 28% of children who underwent transplantation in relapse and 17% of children who underwent transplantation with primary induction failure were alive 5 years after transplantation, suggesting that transplantation can cure at least some children with the most resistant disease. It is perhaps surprising that length of first complete remission did not predict outcome in our study. This may be a reflection of the demographics of the patients, the majority of whom had experienced relapse early, with first complete remission of less than 12 months, limiting statistical power to look at this risk factor.
Children receiving transplantation in remission clearly had a superior outcome to those who underwent transplantation in relapse. It is commonly debated whether it is preferable to perform transplantation in children identified in early relapse immediately or to pursue reinduction chemotherapy and attempt to achieve a second remission before performing transplantation. Although our data show better disease control in children who underwent transplantation in remission, these patients had chemotherapy-sensitive disease and would, therefore, be expected to have better outcomes. Importantly, 76% of the patients who underwent transplantation in relapse had received chemotherapy but did not achieve remission; however, these patients had a 5-year probability of overall survival of 28%. This result suggests that transplantation is worthwhile in this group of particularly difficult patients. Our data are unable to definitively answer the question of the efficacy of immediate transplantation without an attempt at reinduction because there were only 22 such patients in this study. However, the 5-year leukemia-free survival rate was 25% in this group, which is similar to the rate in the group for whom reinduction was attempted. Most of the 22 patients reported good performance scores (90 to 100) despite a high tumor burden; nine patients had peripheral blasts, 10 patients had marrow blast counts of more than 10%, and three patients had marrow blast counts of 5% to 10%. We did not observe differences in leukemia-free survival rates after transplantation for patients in first relapse and second relapse, but there were only 24 patients in the latter group, and our inability to observe differences may be explained by the relatively small number of patients (5-year leukemia-free survival rates of 19% and 22%, respectively).
The importance of adverse cytogenetics was challenging to assess in this group. Our analysis failed to show a significant effect of intermediate- or poor-risk cytogenetics on leukemia recurrence, leukemia-free survival, or overall survival. This may be explained by the fact that patients with recurrent leukemia have high-risk disease, and consequently, the relevance of cytogenetics is limited by the relatively small sample size of approximately 260 patients. Data on cytogenetics were not available for approximately 23% of patients, which is a limitation that occurs when using data reported to an observational database and when transplantations are performed over a 10-year period because cytogenetic testing was not routinely performed during the early years. We adjusted for this limitation by stratifying all analysis of risk factors for transplantation outcome by cytogenetic risk group given the prognostic importance of cytogenetics for this disease.
Almost one third of grafts in this study were T-cell depleted. Although T-cell depletion reduced acute GVHD rates, treatment-related mortality was unchanged, as were leukemia-free and overall survival and relapse, indicating a neutral effect of T-cell depletion on overall outcome. As reported by others, we did not observe lower chronic GVHD rates after T-cell–depleted transplantations.21 Age and WBC count at diagnosis were not associated with transplantation outcome after a first relapse. This is similar to observations by Webb et al11 on outcome for children with relapsed AML after treatment on the Medical Research Council AML 10 trial at diagnosis. We did not observe a graft-versus-leukemia effect in our cohort. This may be explained by the inclusion of patients who received T-cell–depleted grafts (38% of patients) and patients with high tumor burden (47% of patients underwent transplantation in relapse or primary induction failure).
This study represents the largest series of children receiving unrelated donor bone marrow transplantation for AML currently in the literature. The strengths of the study are its large size and high-quality audited data. The limitations of the study are its retrospective nature, the heterogeneity inevitable in registry studies describing aggregate outcomes of transplantations performed at multiple centers, and our inability to compare transplantation outcomes to those after chemotherapy alone in a similar group of patients. We did not observe a significant correlation between year of transplantation, HLA mismatch, and survival, and this may be explained by the relatively few patients who received allele-matched bone marrow grafts in this report. Larger studies in unrelated donor bone marrow transplantation clearly demonstrate the negative effect of HLA mismatch on survival, and matching between donor and recipient using allele-level typing at HLA-A, HLA-B, HLA-C, and DRB1 represents the current standard of care.22 Despite these limitations, these data indicate that unrelated donor bone marrow transplantation is an effective therapy for a significant proportion of children with recurrent or refractory AML who are unlikely to be cured with chemotherapy alone.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Nancy J. Bunin, Stella M. Davies, Richard Aplenc, Bruce M. Camitta, Kenneth B. DeSantes, Rakesh K. Goyal, Neena Kapoor, Nancy A. Kernan, Joseph Rosenthal, Franklin O. Smith, Mary Eapen
Collection and assembly of data: Mary Eapen
Data analysis and interpretation: Nancy J. Bunin, Stella M. Davies, Richard Aplenc, Bruce M. Camitta, Kenneth B. DeSantes, Rakesh K. Goyal, Neena Kapoor, Nancy A. Kernan, Joseph Rosenthal, Franklin O. Smith, Mary Eapen
Manuscript writing: Nancy J. Bunin, Stella M. Davies, Bruce M. Camitta, Franklin O. Smith, Mary Eapen
Final approval of manuscript: Nancy J. Bunin, Stella M. Davies, Richard Aplenc, Bruce M. Camitta, Kenneth B. DeSantes, Rakesh K. Goyal, Neena Kapoor, Nancy A. Kernan, Joseph Rosenthal, Franklin O. Smith, Mary Eapen
Acknowledgments
Supported by Public Health Service Grant No. U24-CA76518 from the National Cancer Institute, the National Institute of Allergy and Infectious Diseases, and the National Heart, Lung and Blood Institute; Office of Naval Research; Health Resources and Services Administration (Department of Health and Human Services); and grants from AABB; Aetna; American International Group, Inc; American Society for Blood and Marrow Transplantation; Amgen, Inc; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US, Inc; Baxter International, Inc; Bayer HealthCare Pharmaceuticals; BioOne Corporation; BloodCenter of Wisconsin; Blue Cross and Blue Shield Association; Bone Marrow Foundation; Bristol-Myers Squibb Company; Cangene Corporation; Celgene Corporation; CellGenix, GmbH; Cerus Corporation; Cubist Pharmaceuticals; Cylex Inc; CytoTherm; DOR BioPharma, Inc; Dynal Biotech, an Invitrogen Company; EKR Therapeutics; Enzon Pharmaceuticals, Inc; Gambro BCT, Inc; Gamida Cell, Ltd; Genzyme Corporation; Gift of Life Bone Marrow Foundation; GlaxoSmithKline, Inc.; Histogenetics, Inc; HKS Medical Information Systems; Hospira, Inc; Infectious Diseases Society of America; Kiadis Pharma; Kirin Brewery Co, Ltd; Merck & Company; The Medical College of Wisconsin; MGI Pharma, Inc; Millennium Pharmaceuticals, Inc; Miller Pharmacal Group; Milliman USA, Inc; Miltenyi Biotec, Inc; MultiPlan, Inc; National Marrow Donor Program; Nature Publishing Group; Oncology Nursing Society; Osiris Therapeutics, Inc; Pall Life Sciences; PDL BioPharma, Inc; Pfizer Inc; Pharmion Corporation; Roche Laboratories; Schering Plough Corporation; Society for Healthcare Epidemiology of America; StemCyte, Inc; StemSoft Software, Inc; SuperGen, Inc; Sysmex; Teva Pharmaceutical Industries; The Marrow Foundation; THERAKOS, Inc; University of Colorado Cord Blood Bank; ViaCell, Inc; Vidacare Corporation; ViraCor Laboratories; ViroPharma, Inc; and Wellpoint, Inc.
The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the US Government.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Creutzig U, Zimmermann M, Ritter J, et al: Treatment strategies and long-term results in paediatric patients treated in four consecutive AML-BFM trials. Leukemia 19:2030-2042, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Gibson BE, Wheatley K, Hann IM, et al: Treatment strategy and long-term results in paediatric patients treated in consecutive UK AML trials. Leukemia 19:2130-2138, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Ravindranath Y, Chang M, Steuber CP, et al: Pediatric Oncology Group (POG) studies of acute myeloid leukemia (AML): A review of four consecutive childhood AML trials conducted between 1981 and 2000. Leukemia 19:2101-2116, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Ribeiro RC, Razzouk BI, Pounds S, et al: Successive clinical trials for childhood acute myeloid leukemia at St Jude Children's Research Hospital, from 1980 to 2000. Leukemia 19:2125-2129, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Smith FO, Alonzo TA, Gerbing RB, et al: Long-term results of children with acute myeloid leukemia: A report of three consecutive phase III trials by the Children's Cancer Group: CCG 251, CCG 213 and CCG 2891. Leukemia 19:2054-2062, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Woods WG, Neudorf S, Gold S, et al: A comparison of allogeneic bone marrow transplantation, autologous bone marrow transplantation, and aggressive chemotherapy in children with acute myeloid leukemia in remission. Blood 97:56-62, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Aladjidi N, Auvrignon A, Leblanc T, et al: Outcome in children with relapsed acute myeloid leukemia after initial treatment with the French Leucemie Aique Myeloide Enfant (LAME) 89/91 protocol of the French Society of Pediatric Hematology and Immunology. J Clin Oncol 21:4377-4385, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Johnston DL, Alonzo TA, Gerbing RB, et al: Risk factors and therapy for isolated central nervous system relapse of pediatric acute myeloid leukemia. J Clin Oncol 23:9172-9178, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Rubnitz JE, Razzouk BI, Lensing S, et al: Prognostic factors and outcome of recurrence in childhood acute myeloid leukemia. Cancer 109:157-163, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Stahnke K, Boos J, Bender-Gotze C, et al: Duration of first remission predicts remission rates and long-term survival in children with relapsed acute myelogenous leukemia. Leukemia 12:1534-1538, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Webb DK, Wheatley K, Harrison G, et al: Outcome for children with relapsed acute myeloid leukaemia following initial therapy in the Medical Research Council (MRC) AML 10 trial: MRC Childhood Leukaemia Working Party. Leukemia 13:25-31, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Wells RJ, Adams MT, Alonzo TA, et al: Mitoxantrone and cytarabine induction, high-dose cytarabine, and etoposide intensification for pediatric patients with relapsed or refractory acute myeloid leukemia: Children's Cancer Group Study 2951. J Clin Oncol 21:2940-2947, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Farag SS, Bacigalupo A, Eapen M, et al: The effect of KIR ligand incompatibility on the outcome of unrelated donor transplantation: A report from the Center for International Blood and Marrow Transplant Research, the European Blood and Marrow Transplant Registry, and the Dutch Registry. Biol Blood Marrow Transplant 12:876-884, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Przepiorka D, Weisdorf D, Martin P, et al: 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 15:825-828, 1995 [PubMed] [Google Scholar]
- 15.Flowers ME, Kansu E, Sullivan KM: Pathophysiology and treatment of graft-versus-host disease. Hematol Oncol Clin North Am 13:1091-1112, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Klein JP, Moeschberger ML: Survival Analysis: Techniques of Censored and Truncated Data (ed 2). New York, NY, Springer-Verlag, 2003
- 17.Cox DR: Regression models and life tables. J R Stat Soc B 34:187-200, 1972 [Google Scholar]
- 18.Andersen PK, Klein JP, Zhang MJ: Testing for centre effects in multi-centre survival studies: A Monte Carlo comparison of fixed and random effects tests. Stat Med 18:1489-1500, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Eapen M, Rubinstein P, Zhang MJ, et al: Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: A comparison study. Lancet 369:1947-1954, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Locatelli F, Labopin M, Ortega J, et al: Factors influencing outcome and incidence of long-term complications in children who underwent autologous stem cell transplantation for acute myeloid leukemia in first complete remission. Blood 101:1611-1619, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Wagner JE, Thompson JS, Carter SL, et al: Effect of graft-versus-host disease prophylaxis on 3-year disease-free survival in recipients of unrelated donor bone marrow (T-Cell Depletion Trial): A multi-centre, randomised phase II-III trial. Lancet 366:733-741, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Lee SJ, Klein J, Haagenson M, et al: High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood 110:4576-4583, 2007 [DOI] [PubMed] [Google Scholar]