Abstract
Purpose
Evaluate the activity of everolimus (RAD001) in combination with octreotide long-acting repeatable (LAR) in patients with advanced low- to intermediate-grade neuroendocrine tumors.
Methods
Treatment consisted of RAD001 5 mg/d (30 patients) or 10 mg/d (30 patients) and octreotide LAR 30 mg every 28 days. Thirty carcinoid and 30 islet cell patients were enrolled.
Results
Intent-to-treat response rate was 20%. Per protocol, there were 13 with partial responses (22%), 42 with stable disease (SD; 70%), and five patients with progressive disease (8%). Overall median progression-free survival (PFS) was 60 weeks. Median PFS for patients with known SD at entry was longer than for those who had progressive disease (74 v 50 weeks; P < .01). Median overall survival has not been reached. One-, 2-, and 3-year survival rates were 83%, 81%, and 78%, respectively. Among 37 patients with elevated chromogranin A, 26 (70%) achieved normalization or more than 50% reduction. Most common toxicity was mild aphthous ulceration. Grade 3/4 toxicities occurring in ≥ 10% of patients included hypophosphatemia (11%), fatigue (11%), and diarrhea (11%). Treatment was associated with a dose-dependent rise in lactate dehydrogenase (LDH). Those with lower than 109 U/L rise in LDH at week 4 had shorter PFS (38 v 69 weeks; P = .01). Treatment was also associated with a decrease in proliferation marker Ki-67 among patients who underwent optional paired pre- and post-treatment biopsy (P = .04).
Conclusion
RAD001 at 5 or 10 mg/d was well tolerated in combination with octreotide LAR, with promising antitumor activity. Confirmatory studies are ongoing.
INTRODUCTION
Low- to intermediate-grade neuroendocrine tumors (LGNETs) originate from neuroendocrine cells scattered throughout the body. Oberndofer1 first coined the term carcinoid (or karzinoide) in 1907 to describe these tumors that have a more indolent clinical course compared with epithelial tumors from the same anatomic site. Today, carcinoid is typically used to describe LGNET arising outside the pancreas. Those arising from the pancreas or islet cell carcinoma are recognized to have a different genetic profile,2-4 more aggressive clinical course,5 and a different response pattern to cytotoxic chemotherapy.6,7 Despite their reputation for being rare, recent analyses from the Surveillance, Epidemiology, and End Results Program database suggest that the diagnosed incidence of LGNETs is rising.5
Mammalian target of rapamycin (mTOR) is a conserved serine/threonine kinase that regulates the cell cycle and metabolism in response to environmental factors. It mediates signaling transduction downstream of receptor tyrosine kinases. Evidence suggests that the mTOR pathway may be involved in the pathogenesis of neuroendocrine tumors. For example, tuberous sclerosis complex (TSC)1/2 is an inhibitor of mTOR that is present in normal neuroendocrine cells.8 Patients with defects in the TSC2 gene are known to develop islet cell tumors.9,10 The neurofibromatosis, (NF1) gene also regulates the activity of mTOR. The loss of NF1 leads to constitutive mTOR activation and is associated with the development of carcinoid tumors in the ampulla of Vater, duodenum, and mediastinum.11-14 Further, sporadic LGNETs are known to coexpress insulinlike growth factor (IGF)-1 and IGF-1 receptor (IGF-1R).15 Researchers have shown that IGF activates mTOR and increases cell proliferation.15 In contrast, mTOR inhibition suppresses neuroendocrine tumor growth.15,16 There is also rationale for combining octreotide and everolimus (RAD001), because the upregulation of upstream IGF pathway is thought to be a potential resistance mechanism for RAD001,17 while octreotide has been shown to reduce serum IGF-1 levels in patients with solid tumors.18
Most patients diagnosed with advanced LGNETs will eventually succumb to the disease. No new drug for the control of tumor growth has been approved in the past 3 decades. We studied the activity of RAD001, a novel oral inhibitor of mTOR, in combination with octreotide long-acting repeatable (LAR) in a phase II study in 60 patients with metastatic, unresectable LGNETs.
METHODS
Study Population
Eligible patients were ≥ 18 years old with measurable histologically confirmed metastatic or unresectable locoregional LGNETs. Prior treatment was per-mitted, including surgery (≥ 4 weeks), cytotoxic chemotherapy (≤ two prior regimens), radiation, interferon, therapy targeted to growth factor signaling, or oncogene products (> 30 days). Zubrod status ≤ 2, with adequate bone marrow, hepatic, and renal (granulocytes > 1,500/mm3, hemoglobin > 8 g/dL, platelets > 100,000/mm3, AST ≤ 2.5 × ULN, ALT ≤ 2.5 × ULN, bilirubin < 1.5 × ULN, creatinine < 1.5 mg/dL) function was required. Prior treatment with octreotide was allowed.
Effective methods of contraception were required for patients with reproductive potential. Pregnant and lactating females were excluded, as were patients with uncontrolled diabetes, serious infections, uncontrolled nonmalignant medical conditions, and medical conditions whose control may be jeopardized by complications of therapy. Patients with previous malignancy were eligible if disease free for longer than 5 years. All patients gave informed consent, the protocol was approved by the institutional review committee of the M. D. Anderson Cancer Center, and the study followed the Declaration of Helsinki and good clinical practice guidelines.
Treatment and Study Evaluations
Patients received intramuscular octreotide LAR 30 mg every 28 days and oral RAD001 either 5 mg/d (patients 1 to 30) or 10 mg/d (patients 31 to 60). One treatment course was 28 days. Patients could remain on study until disease progression or completion of 12 courses, which could be extended if felt to be beneficial by the treating physician.
Pretreatment and on-study evaluations included history, physical examination, and laboratory tests. Tumor was measured by multiphasic CT scans or MRI at baseline and every three courses. Response Evaluation Criteria in Solid Tumors (RECIST) were used to assess the type of response,19 and National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0 (CTCAE) were used to assess toxicity (www.cancer.gov).
RAD001 was discontinued for CTCAE grade 3 or 4 hematologic or nonhematologic toxicity and resumed at the same dose after recovery to grade 0 or 1, or the patient was removed from the study if recovery did not occur within 2 weeks. If grade 3 or 4 toxicity recurred, RAD001 was again held until recovery to grade 0 or 1 and then resumed, but with a reduction in one dose level to 5 mg every other day (cohort 1) or 5 mg/d (cohort 2). If grade 3 or 4 toxicity recurred with 5 mg every other day, the patient was removed from study. Grade 1 or 2 hypertriglyceridemia was treated with lipid-lowering agents at the investigator's discretion. Patients with grade 3 or 4 hypertriglyceridemia discontinued RAD001 for 1 week, triglyceride-lowering agents were initiated, and RAD001 resumed if the level decreased to grade ≤ 2 within 2 weeks. Subsequent occurrences of grade 3/4 hypertriglyceridemia were managed with treatment interruptions and dose reductions as described. If discontinuation of RAD001 for more than 28 continuous days was required for any reason, the patient was removed from the study.
Octreotide LAR dose reductions in 10-mg increments were allowed for CTCAE grade 2, 3, or 4 bradycardia and other CTCAE grade 3 or 4 toxicities attributed to octreotide LAR. The next scheduled dose was held until recovery to grade 0 or 1. A maximum of 8 weeks was allowed for recovery, and patients continued RAD001 during this period. In the absence of recovery, the patient permanently discontinued depot octreotide but was allowed to continue RAD001 therapy.
Statistical Considerations
Sixty assessable patients (30 carcinoid and 30 islet cell) were treated in two strata. Patients with multiple endocrine neoplasia type 1 syndrome were enrolled in the islet cell strata. A two-stage design allowed for early stopping. A progression-free survival (PFS) rate at week 12 lower than 75% was considered unacceptable, whereas a PFS rate at week 12 ≥ 90% was considered acceptable. If 14 or fewer of the first 18 assessable patients enrolled are alive and progression free after 12 weeks of treatment, the study would terminate. The probability of early termination was 69%. In fact, 17 of the first 18 assessable patients enrolled were progression free after 12 weeks, therefore additional 42 patients were enrolled.
PFS and overall survival (OS) were measured from the date of study entry. Chromogranin A (CGA) response was evaluated among patients with elevated CGA at baseline and defined by either a ≥ 50% reduction or normalization of CGA. All statistical calculations were performed using SPSS 14.0 (SPSS Inc, Chicago, IL). Differences were considered significant when the two-sided P value was ≤ .05.
RESULTS
Patient Population
Between February 2005 and July 2006, 67 patients gave informed consent and were screened for study entry. Three were ineligible based on laboratory measurements. Three discontinued therapy early due to toxicity. One patient discontinued therapy due to need for surgery. Therefore, 60 patients were assessable for response. Thirty-five patients had foregut primary, 16 had midgut primary, three had hindgut primary, one had renal primary, and five had unknown primary. Patient and tumor characteristics are included in Table 1.
Table 1.
Patient Characteristics
| Characteristic | RAD001 (mg)
|
Overall
|
||||
|---|---|---|---|---|---|---|
| 5
|
10
|
|||||
| No. | % | No. | % | No. | % | |
| No. of patients | 30 | 30 | 60 | |||
| Sex | ||||||
| Female | 16 | 53 | 13 | 43 | 29 | 48 |
| Male | 14 | 47 | 17 | 57 | 31 | 52 |
| Race | ||||||
| African-American or Asian | 2 | 7 | 1 | 3 | 3 | 5 |
| Hispanic | 3 | 10 | 3 | 10 | 6 | 10 |
| White | 25 | 83 | 25 | 83 | 50 | 81 |
| Mean age, years | 53 | 57 | 55 | |||
| Standard deviation | 11 | 10 | 11 | |||
| Disease at entry | ||||||
| PD | 20 | 67 | 19 | 63 | 39 | 65 |
| SD | 8 | 27 | 8 | 27 | 16 | 27 |
| Unknown | 2 | 7 | 3 | 10 | 5 | 8 |
| Prior therapy | ||||||
| Octreotide | 19 | 63 | 19 | 63 | 38 | 63 |
| Chemotherapy | 13 | 43 | 12 | 40 | 25 | 42 |
| Interferon | 3 | 10 | 6 | 20 | 9 | 15 |
| Bevacizumab | 3 | 10 | 3 | 10 | 6 | 10 |
| HAE | 2 | 6 | 3 | 10 | 5 | 8 |
| Primary site | ||||||
| Foregut | ||||||
| Gastric | 1* | 3 | 1 | 2 | ||
| Lung | 4 | 13 | 4 | 7 | ||
| Thymus | 1 | 3 | 1 | 2 | ||
| Pancreas | 13 | 43 | 16 | 53 | 29 | 48 |
| Midgut | ||||||
| Small intestine | 7 | 23 | 9 | 30 | 16 | 27 |
| Hindgut | ||||||
| Rectum | 1 | 3 | 2 | 7 | 3 | 5 |
| Other | ||||||
| Renal | 1 | 3 | 1 | 2 | ||
| Unknown | 3 | 10 | 2 | 7 | 5 | 8 |
Abbreviations: RAD001, everolimus; PD, progressive disease; SD, stable disease; HAE, hepatic artery embolization.
Patient with multiple endocrine neoplasia type 1 syndrome enrolled in islet cell group with bulky gastric carcinoid and metastatic gastrinoma of unknown primary origin.
Tumor and Biochemical Changes
Using intent-to-treat analysis method, overall response rate was 20%. In the per protocol population, we observed 13 confirmed partial responses (PRs; 22%), 42 patients with stable disease (SD; 70%), and five with progressive disease (PD; 8%). Among 30 carcinoid patients, there were five confirmed PRs (17%), 24 SDs (80%), and one PD (3%). Among 30 islet cell patients, there were eight PRs (27%), 18 SDs (60%), and four PDs (13%). Stratified by RAD001 dose, in the 5-mg cohort there were four PRs (13%), 22 SDs (73%), and four PDs (13%); in the 10-mg cohort, there were nine PRs (30%), 20 SDs (67%), and one PD (3%). There were proportionately more carcinoid patients treated at 5 mg and more islet cell patients treated at 10 mg.
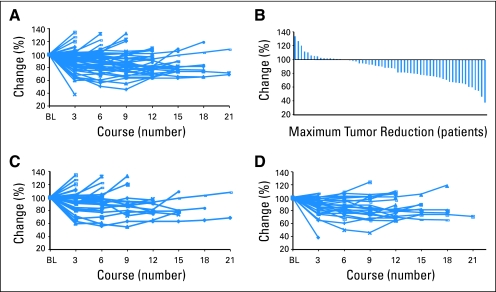
Plots of serial tumor measurements over time and a waterfall plot of best response to better characterize antitumor activity show that the majority of patients had some degree of tumor reduction (Fig 1). Further, response and maximal tumor shrinkage may take time to develop, with some patients experiencing continued tumor shrinkage more than 12 months after initiation of therapy.
Fig 1.
Serial tumor measurements. Percentage change in the sum of target lesion diameters by course for (A) all patients and (C) for those in the 5-mg and (D) 10-mg cohorts. (B) The percentage change as the best response in each patient is shown as a waterfall plot. Maximum tumor reduction was achieved after several courses of therapy in many patients. BL, baseline.
Thirty-seven patients had elevated CGA at study entry. Thirteen were octreotide naïve, and 24 had received prior octreotide. Among these, 26 achieved a CGA response. The response rates among patients who either had prior octreotide or were octreotide-naïve were 67% and 77%, respectively (odds ratio, 1.7; 95% CI, 0.4 to 7.8).
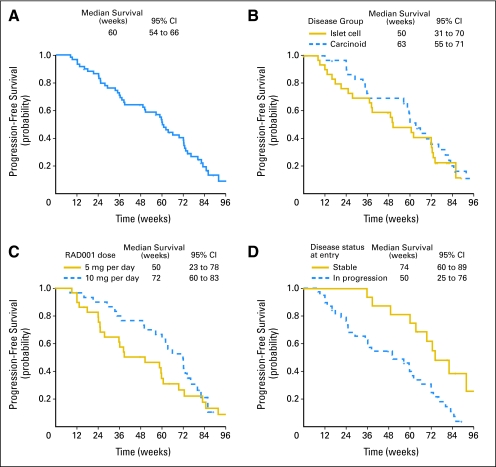
PFS and OS
The overall median PFS of patients treated with octreotide LAR and RAD001 was 60 weeks (95% CI, 54 to 66 weeks; Fig 2A). Stratified by tumor group (Fig 2B), median PFS of patients with carcinoid and islet cell tumors was 63 weeks (95% CI, 55 to 71 weeks) and 50 weeks (95% CI, 31 to 70 weeks), respectively (HR, 1.2; 95% CI, 0.7 to 2.2). By dose level (Fig 2C), median PFS of patients treated with 5 and 10 mg of RAD001 was 50 weeks (95% CI, 23 to 78 weeks) and 72 weeks (95% CI, 60 to 83 weeks) respectively, (HR, 1.5; 95% CI, 0.8 to 2.6). Prior octreotide therapy had no impact on median PFS (60 weeks in both groups). Disease status at time of study entry (by tumor measurements) did affect PFS (Fig 2D; HR, 2.9; 95% CI, 1.4 to 6.1); median PFS for patients with known SD at entry was 74 weeks (95% CI, 60 to 89 weeks), whereas median PFS for those in progression was 50 weeks (95% CI, 25 to 76 weeks). When tumor type, dose level, prior octreotide use, and disease status at time of study entry were analyzed in a Cox proportional hazard model, RAD001 dose of 10 mg was associated with superior PFS (HR, 0.5; 95% CI, 0.3 to 0.98), and progression at study entry was associated with shorter PFS (HR, 3.3; 95% CI, 1.5 to 7.2). However, the study was not prospectively powered for these comparisons. These analyses should be considered exploratory.
Fig 2.
Kaplan-Meier analysis of progression-free survival (PFS). (A) For all 60 patients, 6- and 12-month PFS rates were 80% and 59%. (B) PFS by disease subgroups. Six- and 12-month PFS rates were 86% and 69% for the carcinod group, and were 73% and 48% for the islet cell group (P = .46). (C) PFS by dose group. Six- and 12-month PFS rates were 68% and 46% for the 5-mg group and were 90% and 70% for 10-mg group (P = .19). (D) PFS by disease status at entry. Six- and 12-month PFS rates were 68% and 49% for those with progressive (n = 39) and were 100% and 81% for those with stable disease (n = 16) at study entry (P = .01). RAD001, everolimus.
Median OS for the study has not been reached. The 1-, 2-, and 3-year survival rates estimated by the Kaplan-Meier method were 83%, 81%, and 78%, respectively. Patients with known progression at entry had inferior median survival (31 months) compared with those known to be stable (not reached; P = .02).
Safety
Safety was monitored by patient diary and laboratory measurements. Treatment with the combination of RAD001 at 5 or 10 mg per day and octreotide LAR 30 mg every 4 weeks was feasible and generally well tolerated. Common hematologic adverse events attributed to RAD001 included mild grade 1/2 thrombocytopenia (30%) and leukopenia (48%). Grade 3 thrombocytopenia occurred in 3% in the 5-mg cohort and 6% in the 10-mg cohort. Grade 3/4 leukopenia occurred in 3% treated in the 5-mg cohort and in 6% in the 10-mg cohort. Hematologic toxicities were more likely to occur among patients with pre-existing cytopenia at time of study entry. Other common laboratory abnormalities included grade 1/2 hyperglycemia (61%), hypertriglyceridemia (44%), and hypophosphatemia (21%). Grade 3/4 hyperglycemia (9%), hypertriglyceridemia (3%), and hypophosphatemia (11%) were less common.
The most common nonhematologic adverse event was aphthous ulceration, which is graded under CTCAE as stomatitis. These grade 1/2 events generally appearing as one to two foci of oral ulceration that often resolved without intervention, occurred in 61% of treated patients. Grade 1/2 rash was also common and occurred in 59% of the patients. Grade 3 aphthous ulcerations occurred in 8% of patients, and grade 3 rash occurred in 5% of the patients.
A less common but potentially more serious adverse event associated with mTOR inhibitors is pneumonitis. We did not observe any pneumonitis at the 5-mg dose level. However, at the 10-mg dose level, three patients (9%) experienced grade 2 pneumonitis and one patient experienced grade 3 pneumonitis. A variety of supportive measures were used, including a brief treatment break and steroids. Treatment for the single patient with grade 3 pneumonitis included a treatment break followed by a dose reduction to 5 mg per day.
Grade 3/4 adverse events by dose level are summarized in Table 2. In all, eight patients (one from the 5-mg cohort and seven from the 10-mg cohort) required a dose reduction for adverse events. Two each were for aphthous ulceration, leukopenia, and hypophosphatemia, and one each was for thrombocytopenia and pneumonitis. Final doses tolerated were 5 mg per day in five patients and 5 mg every other day in three.
Table 2.
National Cancer Institute Common Terminology Criteria for Adverse Events Version 3.0 Grade 3 and 4 Adverse Events
| Toxicity | RAD001 (mg)
|
All
|
||||
|---|---|---|---|---|---|---|
| 5
|
10
|
|||||
| No | % | No | % | No | % | |
| No. of patients | 34 | 32 | 64 | |||
| Hematologic | ||||||
| Thrombocytopenia | 1 | 3 | 2 | 6 | 3 | 5 |
| Anemia | 1 | 3 | 1 | 3 | 2 | 3 |
| Leukopenia | 1 | 3 | 2 | 6 | 3 | 5 |
| Leukocytosis | 1 | 3 | 1 | 2 | ||
| Biochemical | ||||||
| Hyperglycemia | 1 | 3 | 5 | 16 | 6 | 9 |
| Hypertriglyceridemia | 2 | 6 | 2 | 3 | ||
| Hypoglycemia | 2 | 6 | 2 | 3 | ||
| Elevated AST | 1 | 3 | 2 | 6 | 3 | 5 |
| Hypophosphatemia | 2 | 6 | 5 | 16 | 7 | 11 |
| Elevated ALT | 3 | 9 | 3 | 5 | ||
| Hypokalemia | 1 | 3 | 1 | 3 | 2 | 3 |
| Hyperbilirubinemia | 1 | 3 | 1 | 2 | ||
| Other | ||||||
| Apthous ulcers* | 2 | 6 | 3 | 9 | 5 | 8 |
| Fatigue | 5 | 15 | 2 | 6 | 7 | 11 |
| Nausea | 1 | 3 | 1 | 2 | ||
| Nail changes | 1 | 3 | 1 | 2 | ||
| Diarrhea | 3 | 9 | 4 | 13 | 7 | 11 |
| Pain | 2 | 6 | 2 | 3 | ||
| Rash | 2 | 6 | 1 | 3 | 3 | 5 |
| Abdominal pain | 2 | 6 | 2 | 2 | ||
| Pruritus | 1 | 3 | 1 | 2 | ||
| Dysgeusia | 1 | 3 | 1 | 2 | ||
| Pneumonitis | 1 | 3 | 1 | 2 | ||
Abbreviation: RAD001, everolimus.
Graded as stomatitis under National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0.
Laboratory Findings
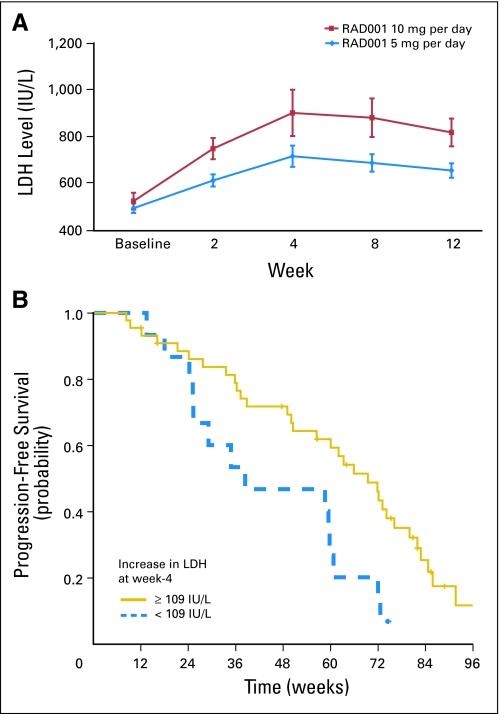
There was a consistent rise in plasma lactate dehydrogenase (LDH) after treatment with RAD001, although some of the increase was subtle, for example, rising from low normal to high normal. This was confirmed by paired comparison of serial LDH measurements. The mean pretreatment LDH was 510 U/L and rose to 680, 810, 785, and 738 U/L at weeks 2, 4, 8, and 12, respectively. Compared with baseline, these differences were statistically significant by paired sample t-test (P < .01 in all comparisons), as was the increase from week 2 to week 4 (P < .01); peak LDH increase occurred at week 4.
Comparison of serial LDH measurements by dosing cohort (Fig 3) showed no significant differences in baseline LDH (P = .47), but higher LDH values were observed in the 10-mg cohort at week 2 (P = .1), week 4 (P = .09), week 8 (P = .04), and week 12 (P = .02).
Fig 3.
(A) Changes in mean (± standard deviation) plasma lactate dehydrogenase (LDH) levels during treatment for the 5-mg and 10-mg dose groups. (B) Kaplan-Meier analysis of progression-free survival (PFS) for patients in the lowest (< 109 U/L) and higher three quartiles (≥ 109 U/L) for changes in plasma LDH levels during treatment. Patients in the higher three quartiles had a significantly longer PFS than the 25% with the lowest increases in plasma LDH during treatment (P = .01). RAD001, everolimus.
Changes in LDH values did not correlate with RECIST response but did correlate with PFS. Analyses were performed by separating the patients into four quartiles ranking their absolute LDH changes at week 4. Median PFS by quartiles was 38, 69, 62, and 66 weeks. Those in the lowest quartile (LDH increase < 109 U/L), compared with those in the higher three quartiles as a group (≥ 109 U/L increase), had a significantly shorter PFS (P = .01). All LDH measurements were performed at M. D. Anderson Cancer Center (normal range, 313 to 618 U/L).
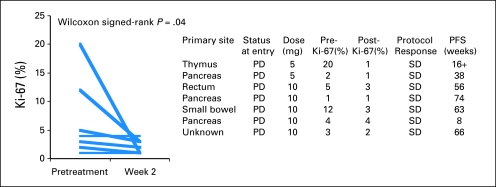
Tumor Ki-67 Proliferation
Some patients underwent image-guided core needle biopsy of metastatic tumor before treatment initiation and after 2 weeks of therapy. Sufficient tumor tissue was available in seven patients for Ki-67 analyses in a blinded manner (Fig 4). Five of seven patients had a decrease in Ki-67 labeling. In two patients, Ki-67 labeling was unchanged. Mean tumor Ki-67 decreased from 6.7% (± 2.6) to 2.1% (± 0.5). The decrease was statistically significant (P = .04) by Wilcoxon signed rank test.
Fig 4.
Changes in Ki-67 measurements. Patients consenting to optional biopsy had prospective sampling of metastases by image guided core needle biopsy at baseline and week 2. Mean tumor Ki-67 decreased from 6.7% (± 2.6) to 2.1% (± 0.5). The decrease was statistically significant (P = .04) by Wilcoxon signed rank test. PFS, progression-free survival, PD, progressive disease; SD, stable disease.
DISCUSSION
In this open-label, phase II study of the novel oral mTOR inhibitor RAD001, we observed promising antitumor activity as assessed by RECIST response rate and PFS duration for patients with advanced LGNET. In our study, RAD001 had antitumor activity against a wide spectrum of LGNETs, with confirmed PRs among LGNETs originating from the stomach, pancreas, small bowel, kidney, and unknown primary site. PFS also compared favorably with those recently reported in phase II and III studies with chemotherapy,20 bortezomib,21 imatinib,22 sorafenib,23 sunitinib,24 and bevaci-zumab.25 Similarly, the median PFS of 50 weeks (11.6 months) for the islet cell group compared favorably with results obtained with sunitinib24 and sorafenib.23
Observed antitumor activity was also supported by changes in biomarkers. Increased CGA among patients with LGNETs is associated with poor PFS26 and survival.27 Preclinical studies have shown that mTOR inhibition can inhibit CGA secretion and tumor growth.15 Consistent with this finding, we observed high rates of CGA responses. Ki-67 staining is an immunohistochemical marker of proliferation that is prognostic of outcome and has been proposed as a grading criteria for neuroendocrine tumors.28 Consistent with our finding of disease stabilization and minor responses in a majority of patients with RAD001, we also observed decreases in Ki-67 labeling among patients who consented to prospective serial tumor biopsy.
Our finding of a dose- and time-dependent rise in plasma LDH and the association of this rise with PFS duration suggest that LDH may be a potentially useful pharmacokinetic and pharmacodynamic marker. While the exact cause for this increase is not known, a rise in LDH in association with mTOR inhibition has also been reported after organ transplantation. There are a number of possible explanations for the increase in LDH. For example, tumor hypoxia has been linked to high serum LDH,29-31 and mTOR inhibition has been shown to inhibit angiogenesis and may induce hypoxia.32,33 Alternatively, the increase in LDH could be related to release after death of tumor cells or hematopoietic cells. In either case, the variations in the amount of LDH elevation likely reflect the contribution of two factors. First, interpatient variability in concurrent medications and genomic polymorphisms in drug metabolism may lead to variability in RAD001 pharmacokinetics, leading to some patients having a lower plasma concentration of RAD001. Second, it is possible that genomic polymorphisms in the PI3 kinase, AKT, and mTOR pathways may lead to variability in the sensitivity of the pathway to RAD001.
In ongoing confirmatory phase II and III studies with RAD001 in LGNETs, we are obtaining serial measurements of LDH isoenzymes to confirm and further refine our observations. In an exploratory study using perfusion computed tomography scans to measure the effect of treatment on tumor blood flow, we will explore the antiangiogenic properties of RAD001 or bevacizumab alone followed by the combination of both drugs, providing a unique opportunity to correlate changes in LDH (and its isoenzymes) with changes in tumor blood flow.
All patients received a combination of RAD001 with octreotide LAR in our study. It is unlikely that the antitumor activity seen would have been observed with octreotide alone, because the tumor response rate for octreotide in the historical literature is only 2% to 3%.34 Also, a significant number of patients had received prior octreotide. However, whether octreotide contributed to the activity of RAD001 in a synergistic fashion is not clear from this single-arm study.
Our study showed promising activity for the combination of depot octreotide and RAD001 in LGNET, which is being confirmed in a program of RAD001 in Advanced Neuroendocrine Tumors studies, which includes one phase II and two ongoing phase III studies involving close to 1,000 patients.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: James C. Yao, Novartis Pharmaceuticals (C); Carmen Jacobs, Novartis Pharmaceuticals (C) Stock Ownership: None Honoraria: Carmen Jacobs, Novartis Pharmaceuticals Research Funding: Alexandria T. Phan, Novartis Pharmaceuticals; Robert A. Wolff, Genentech, Sanofi-aventis, Eli Lilly & Co; Funda Meric-Bernstam, Novartis Pharmaceuticals, Wyeth Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: James C. Yao, Kenneth Hess, Funda Meric-Bernstam
Administrative support: James C. Yao, Carmen Jacobs
Provision of study materials or patients: James C. Yao, Alexandria T. Phan, David Z. Chang, Robert A. Wolff, Sanjay Gupta, Carmen Jacobs, Jeannette E. Mares, Andrea N. Landgraf, Asif Rashid
Collection and assembly of data: James C. Yao, Alexandria T. Phan, David Z. Chang, Robert A. Wolff, Carmen Jacobs, Asif Rashid
Data analysis and interpretation: James C. Yao, Kenneth Hess, Andrea N. Landgraf, Asif Rashid, Funda Meric-Bernstam
Manuscript writing: James C. Yao
Final approval of manuscript: James C. Yao, Alexandria T. Phan, David Z. Chang, Robert A. Wolff, Kenneth Hess, Sanjay Gupta, Carmen Jacobs, Jeannette E. Mares, Andrea N. Landgraf, Asif Rashid, Funda Meric-Bernstam
Supplementary Material
Supported in part by a grant from Novartis Oncology (East Hanover, NJ) and Grant No. R01 CA112199 from the National Institutes of Health.
Presented in part at the 42nd Annual Meeting of the American Society of Clinical Oncology, June 2-6, 2006, Atlanta, GA; Chemotherapy Foundation Symposium, November 8-11, New York, NY; 4th Annual European Neuroendocrine Tumor Society Conference, March 15-17, 2007, Barcelona, Spain; 43rd Annual Meeting of the American Society of Clinical Oncology, June 2-5, 2007, Chicago, IL; and at the 9th World Congress on Gastrointestinal Cancer, June 27-30, 2008, Barcelona, Spain.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Obendorfer S: Carcinoid of the small intestine [in German]. Frankf Z Pathol 1:425-429, 1907 [Google Scholar]
- 2.Kim do H, Nagano Y, Choi IS, et al: Allelic alterations in well-differentiated neuroendocrine tumors (carcinoid tumors) identified by genome-wide single nucleotide polymorphism analysis and comparison with pancreatic endocrine tumors. Genes Chromosomes Cancer 47:84-92, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Choi IS, Estecio MR, Nagano Y, et al: Hypomethylation of LINE-1 and Alu in well-differentiated neuroendocrine tumors (pancreatic endocrine tumors and carcinoid tumors). Mod Pathol 20:802-810, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Wang GG, Yao JC, Worah S, et al: Comparison of genetic alterations in neuroendocrine tumors: Frequent loss of chromosome 18 in ileal carcinoid tumors. Mod Pathol 18:1079-1087, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Modlin IM, Lye KD, Kidd M: A 5-decade analysis of 13,715 carcinoid tumors. Cancer 97:934-959, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Kouvaraki MA, Ajani JA, Hoff P, et al: Fluorouracil, doxorubicin, and streptozocin in the treatment of patients with locally advanced and metastatic pancreatic endocrine carcinomas. J Clin Oncol 22:4762-4771, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Kulke MH, Stuart K, Enzinger PC, et al: Phase II study of temozolomide and thalidomide in patients with metastatic neuroendocrine tumors. J Clin Oncol 24:401-406, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Plank TL, Logginidou H, Klein-Szanto A, et al: The expression of hamartin, the product of the TSC1 gene, in normal human tissues and in TSC1- and TSC2-linked angiomyolipomas. Mod Pathol 12:539-545, 1999 [PubMed] [Google Scholar]
- 9.Verhoef S, van Diemen-Steenvoorde R, Akkersdijk WL, et al: Malignant pancreatic tumour within the spectrum of tuberous sclerosis complex in childhood. Eur J Pediatr 158:284-287, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Eledrisi MS, Stuart CA, Alshanti M: Insulinoma in a patient with tuberous sclerosis: Is there an association? Endocr Pract 8:109-112, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Johannessen CM, Reczek EE, James MF, et al: The NF1 tumor suppressor critically regulates TSC2 and mTOR. Proc Natl Acad Sci U S A 102:8573-8578, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan CC, Hall RI, Semeraro D, et al: Ampullary somatostatinoma associated with von Recklinghausen's neurofibromatosis presenting as obstructive jaundice. Eur J Surg Oncol 22:298-301, 1996 [DOI] [PubMed] [Google Scholar]
- 13.van Basten JP, van Hoek B, de Bruine A, et al: Ampullary carcinoid and neurofibromatosis: Case report and review of the literature. Neth J Med 44:202-206, 1994 [PubMed] [Google Scholar]
- 14.Yoshida A, Hatanaka S, Ohi Y, et al: Von Recklinghausen's disease associated with somatostatin-rich duodenal carcinoid (somatostatinoma), medullary thyroid carcinoma and diffuse adrenal medullary hyperplasia. Acta Pathol Jpn 41:847-856, 1991 [DOI] [PubMed] [Google Scholar]
- 15.von Wichert G, Jehle PM, Hoeflich A, et al: Insulin-like growth factor-I is an autocrine regulator of chromogranin A secretion and growth in human neuroendocrine tumor cells. Cancer Res 60:4573-4581, 2000 [PubMed] [Google Scholar]
- 16.Moreno A, Akcakanat A, Munsell MF, et al: Antitumor activity of rapamycin and octreotide as single agents or in combination in neuroendocrine tumors. Endocr Relat Cancer 15:257-266, 2008 [DOI] [PubMed] [Google Scholar]
- 17.O'Reilly KE, Rojo F, She QB, et al: MTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res 66:1500-1508, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pollak MN, Polychronakos C, Guyda H: Somatostatin analogue SMS 201-995 reduces serum IGF-I levels in patients with neoplasms potentially dependent on IGF-I. Anticancer Res 9:889-891, 1989 [PubMed] [Google Scholar]
- 19.Therasse P, Arbuck SG, Eisenhauer EA, et al: New guidelines to evaluate the response to treatment in solid tumors: European Organisation for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205-216, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Rivera VM, Tang H, Metcalf CA III, et al: Anti-proliferative activity of the mTOR inhibitor AP23573 in combination with cytotoxic and targeted agents. AACR Meeting 45:896a, 2004. (abstr 3887) [Google Scholar]
- 21.Shah MH, Young D, Kindler HL, et al: Phase II study of the proteasome inhibitor bortezomib (PS-341) in patients with metastatic neuroendocrine tumors. Clin Cancer Res 10:6111-6118, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Yao JC, Zhang JX, Rashid A, et al: Clinical and In vitro studies of imatinib in advanced carcinoid tumors. Clin Cancer Res 13:234-240, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Hobday TJ, Rubin J, Holen K, et al: MC044h, a phase II trial of sorafenib in patients (pts) with metastatic neuroendocrine tumors (NET): A phase II consortium (P2C) study. J Clin Oncol 25:4504, 2007 [Google Scholar]
- 24.Kulke MH, Lenz HJ, Meropol NJ, et al: A phase 2 study to evaluate the efficacy and safety of SU11248 in patients (pts) with unresectable neuroendocrine tumors (NETs). J Clin Oncol 23:310s, 2005 [Google Scholar]
- 25.Yao JC, Phan A, Hoff PM, et al: Targeting vascular endothelial growth factor in advanced carcinoid tumor: A random assignment phase II study of depot octreotide with bevacizumab and pegylated interferon alpha-2b. J Clin Oncol 26:1316-1323, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Jensen EH, Kvols L, McLoughlin JM, et al: Biomarkers predict outcomes following cytoreductive surgery for hepatic metastases from functional carcinoid tumors. Ann Surg Oncol 14:780-785, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Janson ET, Holmberg L, Stridsberg M, et al: Carcinoid tumors: Analysis of prognostic factors and survival in 301 patients from a referral center. Ann Oncol 8:685-690, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Rindi G, Kloppel G, Couvelard A, et al: TNM staging of midgut and hindgut (neuro) endocrine tumors: A consensus proposal including a grading system. Virchows Arch 451:757-762, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Azuma M, Shi M, Danenberg KD, et al: Serum lactate dehydrogenase levels and glycolysis significantly correlate with tumor VEGFA and VEGFR expression in metastatic CRC patients. Pharmacogenomics 8:1705-1713, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Lukacova S, Sorensen BS, Alsner J, et al: The impact of hypoxia on the activity of lactate dehydrogenase in two different pre-clinical tumour models. Acta Oncol:1-7, 2007 [DOI] [PubMed] [Google Scholar]
- 31.van der Poel HG, Hanrahan C, Zhong H, et al: Rapamycin induces Smad activity in prostate cancer cell lines. Urol Res 30:380-386, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Lang SA, Klein D, Koehl G, et al: Blocking mTOR by rapamycin reduces HIF-1{alpha} function in human gastric cancer cells in vitro and inhibits angiogenesis and tumor growth in vivo. AACR Meeting 46:265, 2005. (abstr 1136) [Google Scholar]
- 33.Del Bufalo D, Ciuffreda L, Trisciuoglio D, et al: Antiangiogenic potential of the mammalian target of rapamycin inhibitor temsirolimus. Cancer Res 66:5549-5554, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Schnirer II, Yao JC, Ajani JA: Carcinoid: A comprehensive review. Acta Oncol 42:672-692, 2003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.