Abstract
Purpose
We examined the rate of increase in the body mass index (BMI; kg/m2) after final height attainment in survivors of acute lymphoblastic leukemia (ALL) and a noncancer comparison group.
Methods
Childhood Cancer Survivor Study (CCSS) is a retrospectively ascertained cohort study that prospectively tracks the health status of adults who were diagnosed with childhood cancer between 1970 and 1986 and a comparison group of siblings. Changes in BMI from baseline enrollment to time of completion of follow-up (mean interval, 7.8 years) were calculated for 1,451 ALL survivors (mean age, 32.3 years at follow-up) and 2,167 siblings of childhood cancer survivors (mean age, 35.9 years).
Results
The mean BMI of the CCSS sibling comparison group increased with age (women, 0.25 units/yr, 95% CI, 0.22 to 0.28 units; men, 0.23 units/yr, 95% CI, 0.20 to 0.25 units). Compared with CCSS siblings, ALL survivors who were treated with cranial radiation therapy (CRT) had a significantly greater increase in BMI (women, 0.41 units/yr, 95% CI, 0.37 to 0.45 units; men, 0.29 units/yr; 95% CI, 0.26 to 0.32 units). The rate of BMI increase was not significantly increased for ALL survivors who were treated with chemotherapy alone. Younger age at CRT exposure significantly modified risk.
Conclusion
CRT used in the treatment of childhood ALL is associated with a greater rate of increasing BMI, particularly among women treated with CRT during the first decade of life. Health care professionals should be aware of this risk and interventions to reduce or manage weight gain are essential in this high-risk population.
INTRODUCTION
Acute lymphoblastic leukemia (ALL), with a 10-year event-free survival rate exceeding 80%, accounts for approximately 25% of all childhood cancers.1 Despite advances in therapy, there exists a growing recognition of potential long-term health problems related to therapies for childhood cancer. We recently reported that by 30 years after a cancer diagnosis, 73% of survivors suffer from a chronic health condition, with 42% of these individuals having a severe or life-threatening disease or death owing to a chronic condition.2 Accordingly, risk factors for modifiable disease among survivors of childhood cancer need to be identified and addressed. Obesity is a well-described risk factor in the general population for the development of diabetes mellitus,3,4 hypertension,5 dyslipidemia,6 cardiovascular disease,7,8 and cancer.9,10 Indeed, among both men and women, the risk of death from all causes increases throughout the ranges of overweight and obesity.11,12 Given that both primary and secondary prevention of obesity are demonstrated to reduce morbidity and mortality,13 it is essential to identify those populations at greatest risk for developing obesity.
Over the past 20 years, several studies have suggested a role for the therapies used to treat childhood ALL in the increased risk of obesity observed among survivors.14-24 We previously reported findings from a cross-sectional analysis of data acquired from 1,765 adult survivors of childhood ALL at the time of enrollment onto the Childhood Cancer Survivor Study (CCSS).18 At that time, the mean age of the survivors was 24.1 years, with a mean interval from ALL diagnosis to study of 17.1 years. We identified cranial radiotherapy (CRT) in doses of 20 Gy or more as the primary risk factor for the increased prevalence of obesity noted among both men and women, with the highest risk observed in survivors treated at age younger than 4 years. In contrast with smaller cross-sectional reports,21,22 we did not detect an increased prevalence of obesity among survivors who were treated with chemotherapy alone or lower doses of CRT (10 to 19 Gy). To date, there exist no published data describing longitudinal changes and trends in body mass index (BMI) experienced by survivors of childhood ALL having attained their final height.
The present study was designed to investigate longitudinal changes in BMI with two specific aims. First, with nearly 8 additional years of elapsed time from our original study, we sought to determine whether the rate of weight gain during young adulthood was increased in ALL survivors when compared with a group of siblings of childhood cancer survivors. Second, we aimed to determine whether, with time, other subgroups of ALL survivors were at increased risk in addition to the previously identified group who were treated with CRT ≥ 20 Gy. Recognizing the lifetime morbidity associated with obesity, our long-term goal is to identify at-risk subpopulations of ALL survivors and develop targeted interventions aimed at prevention of obesity.
METHODS
CCSS
The methodology of the CCSS and a description of the study participants have previously been published in detail.25 Briefly, the CCSS cohort consists of survivors of specific childhood cancers (leukemia, brain tumors, Hodgkin's lymphoma, non-Hodgkin's lymphoma, renal tumors, neuroblastoma, soft tissue sarcomas, or bone tumors) who were diagnosed before the age of 21 years at one of 26 participating centers between 1970 and 1986 and who were alive at least 5 years from their original diagnosis. The eligible cohort consisted of 20,720 patients, of whom 14.6% were deemed to be lost to follow-up after execution of an intensive tracing protocol. Of the 17,703 patients who were successfully contacted, 14,372 patients (81.2%) enrolled onto the study. Comparisons of demographic and cancer-related characteristics of participants and nonparticipants did not demonstrate significant differences with regard to sex, cancer type, age at diagnosis, age when the cohort was assembled, and type of cancer treatment.25,26 To allow comparisons with a noncancer population, a random sample of participating survivors were asked to identify their nearest-age living sibling. Of 4,782 eligible siblings, 3,846 siblings (80.4%) participated. The study methodology was approved by the institutional review board of each of the participating institutions, and informed consent was obtained from each participant or his/her parent or guardian. Participating CCSS institutions are provided in the Appendix (online only). All questionnaires and medical record abstraction forms are available for review at www.stjude.org/ccss.
At the time of enrollment (1995 to 1996 for most participants), a comprehensive baseline questionnaire was completed by the participant, and detailed medical information was abstracted from hospital records. Several subsequent questionnaires have been completed by the study participants, including a questionnaire completed in 2002 and 2003 (hereafter referred to as the 2002 to 2003 follow-up questionnaire). The mean time interval between these two surveys was 7.8 years. At both time points, respondents were asked to record their height and weight without shoes. Eligibility for this analysis was limited to those participants who completed both the baseline and the 2002 to 2003 follow-up questionnaires and for whom information regarding treatment for their original cancer was available.
Cancer Treatment Information
Information regarding original cancer diagnoses was obtained for all eligible cases from treating institutions. For all CCSS participants returning signed medical releases, information regarding primary cancer therapies was collected, including initial treatment and treatment for relapse. Qualitative information was abstracted from the medical record for 42 specific chemotherapeutic agents, for which quantitative dose information was abstracted on 22. Copies of radiation therapy records were obtained and centrally reviewed, including doses of CRT and craniospinal radiotherapy and total-body irradiation.
ALL Survivors and CCSS Sibling Comparison Group
A total of 1,809 ALL survivors were 18 years of age or older at the time of completion of the baseline questionnaire and remained alive at the time of completion of the 2002 to 2003 follow-up questionnaire. Complete chemotherapy, radiotherapy, height, and weight data from both baseline and 2002 to 2003 follow-up questionnaires were available for 1,451 of these 1,809 survivors.
Demographic and cancer treatment information for 1,451 adult survivors of childhood ALL is presented in Table 1. Mean age at time of the follow-up study was 32.3 years (range, 22 to 50 years). Mean age at initial cancer diagnosis was 7.7 years, with a mean interval time between diagnosis and completion of the follow-up study of 25.1 years (range, 16.3 to 34.3 years). Fifty-one percent of survivors were female; 86.5% were white, non-Hispanics.
Table 1.
Characteristics of Adult Survivors of Childhood ALL and Siblings of Childhood Cancer Survivors Who Completed Baseline and Follow-Up Questionnaires
| Variable | ALL Survivors (n = 1,451) | CCSS Siblings (n = 2,167) | P |
|---|---|---|---|
| Sex, % female | 51.1 | 53.7 | .1 |
| Race/ethnicity, % | |||
| White, NH | 86.5 | 92.9 | < .01 |
| Black, NH | 1.8 | 2.0 | |
| Hispanic/Latino | 4.4 | 2.6 | |
| Other | 7.3 | 2.5 | |
| Age at follow-up interview, years | |||
| Mean | 32.3 | 35.9 | < .01 |
| SD | 4.8 | 7.2 | |
| Median | 32.0 | 35.0 | |
| Range | 22-50 | 21-57 | |
| Age at cancer diagnosis, years | |||
| Mean | 7.7 | NA | |
| SD | 4.5 | ||
| Median | 6.3 | ||
| Range | 0.1-20.4 | ||
| Interval from diagnosis, years | |||
| Mean | 25.1 | NA | |
| SD | 4.1 | ||
| Median | 25.2 | ||
| Range | 16.3-34.3 | ||
| Chemotherapy,* % | NA | ||
| Cyclophosphamide | 46.9 | ||
| Daunorubicin | 25.0 | ||
| Dexamethasone | 10.1 | ||
| Doxorubicin | 27.9 | ||
| L-asparaginase | 89.5 | ||
| 6-mercaptopurine | 92.9 | ||
| Thioguanine | 12.8 | ||
| Etoposide | 5.5 | ||
| Chemotherapy without CRT, % | 23.0 | NA | |
| Chemotherapy with CRT, % | |||
| 10.0-19.9 Gy | 28.0 | ||
| 20.0-29.9 Gy | 43.6 | NA | |
| ≥ 30.0 Gy | 5.1 |
Abbreviations: ALL, acute lymphoblastic leukemia; CCSS, Childhood Cancer Survivor Study; NH, non-Hispanic; SD, standard deviation; NA, not applicable; CRT, cranial radiotherapy.
More than 97% were treated with methotrexate, vincristine, and prednisone.
The CCSS sibling comparison population consists of siblings of survivors from each of the different cancer groups, including ALL. Available for this analysis were 2,167 siblings of childhood cancer survivors, including 135 same-sex family pairs among ALL survivors included in this analysis. Mean age of these sibling controls at the time of the follow-up study (35.9 years) was greater than that of ALL survivors (32.3 years). A further comparison of the two groups revealed no significant sex differences but a higher percentage of white, non-Hispanics among siblings as compared with ALL survivors (92.9% v 86.5%; P < .01).
Participants who completed both baseline and 2002 to 2003 follow-up questionnaires were compared with nonparticipants (survivors and CCSS siblings who completed the baseline survey but were subsequently lost to follow-up or who did not participate in the follow-up survey). Specifically, a comparison of 1,451 participating survivors and 462 nonparticipating survivors revealed that nonparticipants were more likely to be men (54.8% v 48.9%; P < .03) or from ethnic or racial minority backgrounds (21.5% v 13.5%; P < .01). Also, nonparticipants were more likely to be younger at time of the baseline interview (23.6 years v 24.4 years; P < .01) and with a younger age at time of ALL diagnosis (7.1 years v 7.7 years; P = .02). Importantly, however, no statistically significant differences were detected between participants and nonparticipants regarding mean BMI at baseline, the prevalence of being overweight or obese at baseline, or ALL treatment category. Similarly, a comparison between 2,167 participating siblings and 852 nonparticipating siblings demonstrated that nonparticipants were more likely to be men (51.1% v 46.3%; P = .02) and from ethnic or minority backgrounds (10.6% v 7.1%; P < .01). No statistically significant differences in mean BMI or the prevalence of being overweight or obese at baseline were detected between participating and nonparticipating siblings.
Outcome Measures
BMI was calculated from self-reported heights and weights for ALL survivors and CCSS siblings at baseline and follow-up. National Heart, Lung, and Blood Institute definitions for being overweight (BMI ≥ 25.0 to BMI < 30.0) or obese (BMI ≥ 30.0) were used.13
Statistical Analysis
Because of sex-specific differences in weight gain, all analyses were stratified by sex. Estimates were adjusted for race and attained age, the two variables that differed between ALL survivors and the CCSS siblings (Table 1). ALL treatment modalities were categorized into three groups: chemotherapy only, chemotherapy with CRT 10.0 to 19.9 Gy, and chemotherapy with CRT ≥ 20 Gy. Thirty-four (2.34%) of the survivors received total-body irradiation, including 18 survivors who had total-body irradiation after CRT and 16 survivors who had total-body irradiation without previous CRT.
A linear mixed model, adjusting for attained age and race, was used to model baseline and follow-up BMI and to estimate BMI change per year from baseline to follow-up for each ALL subgroup. Within-family correlation and within-individual correlation of BMI between baseline and follow-up were accounted for in the linear mixed model by an inclusion of two independent random intercepts for family and individual. All statistical analyses were performed using SAS version 9.1 (SAS Institute Inc, Cary, NC), and two-sided statistical inferences were used throughout the analyses.
RESULTS
Mean BMI and the prevalence of being overweight or obese at the time of baseline and follow-up time points, for both survivors of childhood ALL and CCSS siblings, are presented in Table 2. Survivors and CCSS siblings of both sexes gained weight between the time of baseline and follow-up, manifested by an increasing BMI and an increased likelihood of being overweight or obese. Adjusted for age and race, the female CCSS siblings experienced a mean increase in BMI of 0.25 units/yr (95% CI, 0.22 to 0.28 units; Table 3). The adjusted BMI increase for male CCSS siblings was 0.23 units/yr (95% CI, 0.20 to 0.25 units).
Table 2.
Mean BMI and Prevalence of Overweight and Obesity Among Survivors of Childhood ALL and CCSS Sibling Controls at Baseline and Follow-Up
| Characteristic | Baseline
|
Follow-Up
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | BMI
|
Overweight (%) | Obese (%) | No. | BMI
|
Overweight (%) | Obese (%) | |||
| Mean | SD | Mean | SD | |||||||
| Female study participants | ||||||||||
| CCSS siblings | 1164 | 24.6 | 5.5 | 19.6 | 15.0 | 1164 | 26.3 | 6.3 | 25.0 | 22.2 |
| ALL survivors | 742 | 25.0 | 5.6 | 22.4 | 16.7 | 742 | 27.9 | 6.9 | 28.8 | 31.7 |
| Therapy | ||||||||||
| Chemotherapy only | 186 | 23.6 | 4.9 | 18.8 | 9.1 | 186 | 25.6 | 5.7 | 22.0 | 19.9 |
| Chemotherapy + CRT 10-19 Gy | 195 | 24.3 | 5.2 | 20.5 | 12.8 | 195 | 27.9 | 6.7 | 30.8 | 31.8 |
| Chemotherapy + CRT ≥ 20 Gy | 359 | 26.1 | 5.9 | 25.1 | 22.8 | 359 | 29.3 | 7.3 | 31.5 | 37.9 |
| Male study participants | ||||||||||
| CCSS siblings | 1003 | 25.9 | 4.5 | 39.3 | 14.7 | 1003 | 27.4 | 4.9 | 44.2 | 22.5 |
| ALL survivors | 709 | 25.7 | 5.0 | 33.6 | 16.9 | 709 | 27.9 | 5.6 | 38.5 | 28.8 |
| Therapy | ||||||||||
| Chemotherapy only | 147 | 25.2 | 4.7 | 33.3 | 15.0 | 147 | 27.3 | 5.1 | 42.2 | 22.5 |
| Chemotherapy + CRT 10-19 Gy | 211 | 25.5 | 5.1 | 33.2 | 15.2 | 211 | 28.1 | 5.7 | 33.7 | 33.2 |
| Chemotherapy + CRT ≥ 20 Gy | 347 | 26.0 | 5.0 | 34.0 | 18.7 | 347 | 28.1 | 5.8 | 40.1 | 28.8 |
NOTE. Overweight is defined as BMI 25.0 to 29.9; obese is defined as BMI ≥ 30.
Abbreviations: BMI, body mass index; ALL, acute lymphoblastic leukemia; CCSS, Childhood Cancer Survivor Study; SD, standard deviation; CRT, cranial radiation therapy.
Table 3.
Mean Increase in BMI by Group
| Group | BMI Increase (units/yr) | 95% CI | P Compared With Siblings* |
|---|---|---|---|
| Female study participants | |||
| CCSS siblings | 0.25 | 0.22 to 0.28 | |
| ALL survivors | |||
| Chemotherapy only | 0.26 | 0.19 to 0.33 | .77 |
| Age at diagnosis 0-9 years | 0.28 | 0.20 to 0.37 | .41 |
| Age at diagnosis 10-20 years | 0.20 | 0.07 to 0.32 | .46 |
| Chemotherapy + CRT ≥ 10 Gy | 0.41 | 0.37 to 0.45 | < .01 |
| Age at diagnosis 0-9 years | 0.44 | 0.40 to 0.49 | < .01 |
| Age at diagnosis 10-20 years | 0.35 | 0.28 to 0.43 | .01 |
| Male study participants | |||
| CCSS siblings | 0.23 | 0.20 to 0.25 | |
| ALL survivors | |||
| Chemotherapy only | 0.26 | 0.21 to 0.31 | .32 |
| Age at diagnosis 0-9 years | 0.27 | 0.21 to 0.34 | .19 |
| Age at diagnosis 10-20 years | 0.22 | 0.12 to 0.32 | .88 |
| Chemotherapy + CRT ≥ 10 Gy | 0.29 | 0.26 to 0.32 | < .01 |
| Age at diagnosis 0-9 years | 0.30 | 0.27 to 0.33 | < .01 |
| Age at diagnosis 10-20 years | 0.27 | 0.21 to 0.33 | .19 |
Abbreviations: BMI, body mass index; CCSS, Childhood Cancer Survivor Study; ALL, acute lymphoblastic leukemia; CRT, cranial radiation therapy.
Adjusted for age at interview, race, and ethnicity.
Compared with the CCSS siblings, ALL survivors who were treated with chemotherapy alone did not have a significantly different BMI increase. In contrast, survivors who were treated with CRT in addition to chemotherapy had a significantly greater increase in BMI. For women, those treated with CRT had a mean BMI increase of 0.41 units/yr (95% CI, 0.37 to 0.45 units; P < .01). Men treated with CRT also had a significantly greater increase in mean BMI (0.29 units/yr; 95% CI, 0.26 to 0.32; P < .01) in comparison with male CCSS siblings, though not to the degree of increase seen in women.
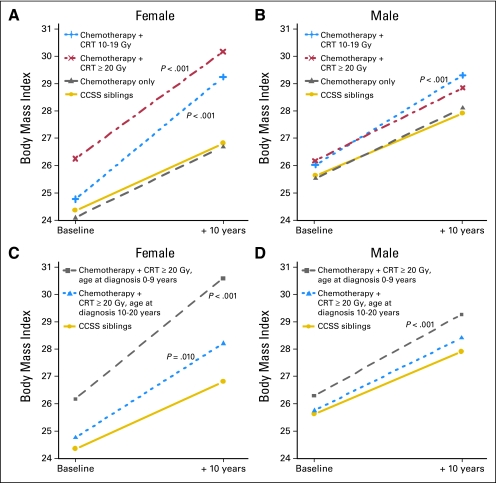
In comparison with the CCSS siblings, there was a significantly greater increase in BMI for women in both CRT groups. Importantly, the correlation between obesity and treatment with low to moderate dose CRT (10 to 19 Gy) is in contrast with our previous findings. This change is depicted in Figure 1A, where the 10-year model estimates are provided. At baseline enrollment onto the cohort, the BMI in women who were treated with CRT 10 to 19 Gy was not significantly different than that of the CCSS siblings. However, with aging, they experienced a much more rapid weight gain such that at follow-up, the BMI of those exposed to CRT 10 to 19 Gy approached the mean BMI of those women who were treated with moderate to high dose CRT (≥ 20 Gy). In men, the findings were similar but attenuated (Table 3 and Fig 1B).
Fig 1.
Model-based estimates of linear changes in mean body mass index between baseline and 10 years follow-up for (A) female Childhood Cancer Survivor Study (CCSS) sibling controls and adult survivors of childhood acute lymphoblastic leukemia (ALL) according to treatment; (B) male CCSS siblings and adult survivors according to treatment; (C) female CCSS sibling controls and adult survivors who received cranial radiation therapy (CRT) ≥ 20 Gy according to age at diagnosis of ALL; (D) male CCSS sibling controls and adult survivors who received CRT ≥ 20 Gy, according to age at diagnosis of ALL. The P values are from testing the equality of slope to the CCSS sibling controls (only those with P < .05 are shown).
Age at cancer diagnosis, and thus age at time of CRT, modified the outcome (Table 3; Figs 1C and 1D). For this part of the analysis, the differences between CRT 10 to 19 Gy and ≥ 20 Gy were minimal, and so these two groups were collapsed into a single group (CRT ≥ 10 Gy). Women who were treated with CRT ≥ 10 Gy before the age of 10 years had a mean BMI increase of 0.44 units/yr (95% CI, 0.40 to 0.49 units), whereas women who were treated between the ages of 10 and 20 years with CRT ≥ 10 Gy had a significantly smaller increase in BMI (0.35 units/yr, 95% CI, 0.40 to 0.49 units; P = .01) in comparison with the CCSS siblings. For men, only those who were treated before the age of 10 years with CRT ≥ 10 Gy had a greater increase in BMI in comparison with the CCSS siblings.
The modifying effect of dexamethasone treatment was also assessed. Of 1,451 ALL survivors, 146 survivors (10.1%) were treated with dexamethasone (68 female patients; 78 male patients). Dexamethasone did not significantly modify the outcomes for men (P = .92); women treated with dexamethasone had a nonsignificant smaller increase in BMI (P = .08).
DISCUSSION
From this large and diverse population of North American survivors of childhood ALL, who were a mean age of 32 years at follow-up, we report not only an increased risk of obesity, but a significantly greater rate of change in BMI over time, as compared with a noncancer comparison population. To illustrate this BMI increase, consider, for example, a 25-year-old female CCSS sibling who had a height of 1.63 meters (64.2 inches), a weight of 63.8 kg (140.7 pounds), and a BMI of 24.0 at baseline. With a 0.25 unit/yr increase, or 2.5 units over 10 years, at the age of 35 years, she would have a BMI of 26.5, or weight gain of 6.6 kg (14.5 pounds). In contrast, a female survivor who was treated with CRT and had the same BMI at baseline as the CCSS sibling would gain 10.9 kg (24.0 pounds) over 10 years, with a mean BMI increase of 0.41 units/yr. Indeed, the group with the greatest increase in mean BMI was women who were treated with CRT, particularly those who were diagnosed with their cancer before the age of 10 years. Of this latter group, by follow-up, 61.9% were overweight or obese (29.7% overweight; 32.2% obese). The other novel finding of this study is that low to moderate dose CRT (10 to 19 Gy) is associated with an increased risk of obesity in young adulthood, in contrast with our previous report18 that suggested otherwise. Again, this finding was most evident in women. Encouragingly, we did not find an excess weight gain in survivors who were treated with chemotherapy alone in comparison with the CCSS siblings.
This differential effect of CRT on female patients highlights the particularly elevated risk of adverse health outcomes faced by women in comparison with men who received the same therapies. A list of such outcomes is now documented to include neurocognitive impairment,25,26 earlier onset of puberty,27,28 and reduced final height.29 Although mechanisms underlying the differential impact of CRT on women remain unclear, some investigators hypothesize that it may be related to more rapid brain growth experienced by women relative to men during early childhood.25,30
In both men and women treated with CRT, the BMI increase per year was modified by age at CRT exposure. Previously considered mechanisms include the impact of CRT at a young age on growth hormone secretion; growth hormone deficiency is associated with an increase in percent body fat.31 CRT received at a young age may also blunt hypothalamic leptin sensitivity and thereby alter the body's response to leptin and the regulation of body weight, metabolism, and reproductive function.32 Of particular interest in this regard, we previously reported that a hypothalamic leptin receptor polymorphism is associated with obesity in women who were treated with CRT at a young age.33 We have also found that CRT is associated with physical inactivity, particularly in women, and thus may contribute to this excess weight gain.34 Interestingly, adults with mild-to-moderate mental retardation seem to have an increased prevalence of physical inactivity and obesity.35 Whether or not mild-to-moderate cognitive dysfunction after CRT contributes to the obesity and physical inactivity seen among ALL survivors has not been formally studied and warrants further investigation.
We did not find a significant association between treatment with chemotherapy alone (without CRT) and risk of obesity or change in BMI over time. With the more frequent use of dexamethasone in contemporary therapy, some have suggested that this may lead to an increased risk of obesity. Though limited by small numbers, we did not find that dexamethasone exposure increased the prevalence of obesity or the rate of BMI increase. Likewise, other recent studies have not reported a differential impact of dexamethasone on rates of obesity experienced by survivors of childhood ALL.22,36
When interpreting the findings of this study, it is important to recognize some limitations. Individuals who did not participate in the follow-up interview included higher percentages of men, minorities, and survivors who were younger at the time of ALL diagnosis. Additionally, we used self-reported heights and weights to determine BMI, which may be subject to bias and imprecision. However, similar measurements of height and weight, including those self-reported by study participants, have been found to be generally accurate and do not contribute importantly to errors in BMI calculation.37 Furthermore, we assume that measurement imprecision and reporting biases, to the extent they did occur, were similar for survivors and CCSS siblings.38,39 Lastly, with contemporary therapy, only approximately 5% to 25% of patients with ALL, primarily those with high-risk disease, are treated with CRT. However, based on the incidence of ALL and the evolution of therapy, ALL survivors who were treated with CRT represent approximately 10% of all current pediatric cancer survivors.
In summary, CRT is associated with significantly greater rates of BMI gain over time compared with that observed in CCSS siblings and ALL survivors treated with chemotherapy alone. This association is observed in both male and female survivors, with the association being most pronounced among women who received CRT during the first decade of life. ALL survivors also face significantly increased risks of cardiovascular disease,40-43 lipid abnormalities,41 osteoporosis,44,45 and all-cause mortality.46 Given that the vast majority of adult survivors of childhood ALL are not observed at a cancer center but rather receive most of their care at a primary care physician's office,47 it is imperative that primary health care professionals become familiar with these risks. Shortly after completing CRT, ALL survivors and their families should receive counseling on evidence-based lifestyle practices that can help prevent later-in-life obesity. Such advice should be consistent with the lifestyle practice recommendations of the World Cancer Research Fund (www.dietandcancerreport.org), the American Cancer Society,48 and the Dietary Guidelines for Americans (http://www.health.gov/DietaryGuidelines/).
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Charles A. Sklar, Ann C. Mertens, Yutaka Yasui, Leslie L. Robison, Kevin C. Oeffinger
Financial support: Ann C. Mertens, Leslie L. Robison, Kevin C. Oeffinger
Administrative support: Charles A. Sklar, Ann C. Mertens, Leslie L. Robison
Provision of study materials or patients: Ann C. Mertens, Marilyn A. Stovall, Leslie L. Robison
Collection and assembly of data: Qi Liu, Ann C. Mertens, Marilyn A. Stovall, Yutaka Yasui, Leslie L. Robison, Kevin C. Oeffinger
Data analysis and interpretation: Edward G. Garmey, Qi Liu, Charles A. Sklar, Lillian R. Meacham, Ann C. Mertens, Marilyn A. Stovall, Yutaka Yasui, Leslie L. Robison, Kevin C. Oeffinger
Manuscript writing: Edward G. Garmey, Qi Liu, Charles A. Sklar, Lillian R. Meacham, Ann C. Mertens, Marilyn A. Stovall, Yutaka Yasui, Leslie L. Robison, Kevin C. Oeffinger
Final approval of manuscript: Edward G. Garmey, Qi Liu, Charles A. Sklar, Lillian R. Meacham, Ann C. Mertens, Marilyn A. Stovall, Yutaka Yasui, Leslie L. Robison, Kevin C. Oeffinger
Appendix
The Childhood Cancer Survivor Study (CCSS) is a collaborative, multi-institutional project, funded as a resource by the National Cancer Institute, of individuals who survived 5 or more years after diagnosis of childhood cancer.
CCSS is a retrospectively ascertained cohort of 20,346 childhood cancer survivors diagnosed before age 21 years between 1970 and 1986 and approximately 4,000 siblings of survivors, who serve as a control group. The cohort was assembled through the efforts of 26 participating clinical research centers in the United States and Canada. The study is currently funded by a U24 resource grant (National Cancer Institute Grant No. U24 CA55727) awarded to St Jude Children's Research Hospital. Currently, we are in the process of expanding the cohort to include an additional 14,000 childhood cancer survivors diagnosed before age 21 years between 1987 and 1999. For information on how to access and use the CCSS resource, visit www.stjude.org/ccss.
Table A1.
Childhood Cancer Survivor Study Institutions and Investigators
| Institution | Investigators |
|---|---|
| St Jude Children's Research Hospital, Memphis, TN | Leslie L. Robison, PhD,‖‡ Melissa Hudson, MD,*‡ Greg Armstrong, MD‡ |
| Children's Health Care-Minneapolis, MN | Joanna Perkins, MD,* Maura O'Leary, MD† |
| Children's Hospital and Medical Center, Seattle, WA | Debra Friedman, MD, MPH,* Thomas Pendergrass, MD† |
| Children's Hospital, Denver, CO | Brian Greffe, MD,* Lorrie Odom, MD† |
| Children's Hospital, Los Angeles, CA | Kathy Ruccione, RN, MPH* |
| Children's Hospital, Oklahoma City, OK | John Mulvihill, MD‡ |
| Children's Hospital of Philadelphia, Philadelphia, PA | Jill Ginsberg, MD,* Anna Meadows, MD‡ |
| Children's Hospital of Pittsburgh, Pittsburgh, PA | Jean Tersak, MD,* A. Kim Ritchey, MD,† Julie Blatt, MD† |
| Children's National Medical Center, Washington, DC | Gregory Reaman, MD,* Roger Packer, MD‡ |
| Cincinnati Children's Hospital Medical Center, Cincinnati, OH | Stella Davies, MD, PhD‡ |
| City of Hope, Los Angeles, CA | Smita Bhatia, MD* |
| Columbus Children's Hospital, Columbus, OH | Amanda Termuhlen, MD,* Frederick Ruymann, MD,† Stephen Qualman, MD,‡ Sue Hammond, MD‡ |
| Dana-Farber Cancer Institute, Boston, MA | Lisa Diller, MD,* Holcombe Grier, MD,† Frederick Li, MD§ |
| Emory University, Atlanta, GA | Lillian Meacham, MD,* Ann Mertens, PhD‡ |
| Fred Hutchinson Cancer Research Center, Seattle, WA | Wendy Leisenring, ScD,*‡ John Potter, MD, PhD†‡ |
| Hospital for Sick Children, Toronto, ON, Canada | Mark Greenberg, MBChB,* Paul C. Nathan, MD† |
| International Epidemiology Institute, Rockville, MD | John Boice, ScD‡ |
| Mayo Clinic, Rochester, MN | Vilmarie Rodriguez, MD,* W. Anthony Smithson, MD,† Gerald Gilchrist, MD† |
| Memorial Sloan-Kettering Cancer Center, New York, NY | Charles Sklar, MD,*‡ Kevin Oeffinger, MD‡ |
| Miller Children's Hospital, Long Beach, CA | Jerry Finklestein, MD† |
| National Cancer Institute, Bethesda, MD | Barry Anderson, MD,‡ Peter Inskip, ScD‡ |
| Riley Hospital for Children, Indianapolis, IN | Terry A. Vik, MD,* Robert Weetman, MD† |
| Roswell Park Cancer Institute, Buffalo, NY | Daniel M. Green, MD*‡ |
| St Louis Children's Hospital, St Louis, MO | Robert Hayashi, MD,* Teresa Vietti, MD† |
| Stanford University School of Medicine, Stanford, CA | Neyssa Marina, MD,* Sarah S. Donaldson, MD,‡ Michael P. Link, MD† |
| Texas Children's Hospital, Houston, TX | Zoann Dreyer, MD* |
| University of Alabama, Birmingham, AL | Kimberly Whelan, MD, MSPH,* Jane Sande, MD,† Roger Berkow, MD† |
| University of Alberta, Edmonton, AB, Canada | Yutaka Yasui, PhD‡ |
| University of California, Los Angeles, CA | Jacqueline Casillas, MD, MSHS,* Lonnie Zeltzer, MD†‡ |
| University of California, San Francisco, CA | Robert Goldsby, MD,* Arthur Ablin, MD† |
| University of Michigan, Ann Arbor, MI | Raymond Hutchinson, MD* |
| University of Minnesota, Minneapolis, MN | Joseph Neglia, MD, MPH‡* |
| University of Southern California, Los Angeles, CA | Dennis Deapen, DrPH‡ |
| University of Washington, Seattle, WA | Norman Breslow, PhD‡ |
| University of Texas Southwestern Medical Center, Dallas, TX | Dan Bowers, MD,* Gail Tomlinson, MD,† George R. Buchanan, MD† |
| University of Texas M. D. Anderson Cancer Center, Houston, TX | Louise Strong, MD,*‡ Marilyn Stovall, MPH, PhD‡ |
Institutional Principal Investigator.
Former Institutional Principal Investigator.
Member, Childhood Cancer Survivor Study Steering Committee.
Former Member, Childhood Cancer Survivor Study Steering Committee.
Project Principal Investigator (Grant No. U24 CA55727).
Supported by Grant No. U24-CA-55727 (L.L.R., principal investigator) and Grant No. R01-CA-100474 (K.C.O., principal investigator) from the United States Department of Health and Human Services, funding to the University of Minnesota from the Children's Cancer Research Fund, and funding to St Jude Children's Research Hospital from the American Lebanese Syrian Associated Charities.
K.C.O. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The funding organizations had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Ries LAG HD, Krapcho M, et al: SEER cancer statistics review, 1975-2003. Bethesda, MD, National Cancer Institute, 2006
- 2.Oeffinger KC, Mertens AC, Sklar CA, et al: Chronic health conditions in adult survivors of childhood cancer. N Engl J Med 355:1572-1582, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Chan JM, Rimm EB, Colditz GA, et al: Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care 17:961-969, 1994 [DOI] [PubMed] [Google Scholar]
- 4.Colditz GA, Willett WC, Rotnitzky A, et al: Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med 122:481-486, 1995 [DOI] [PubMed] [Google Scholar]
- 5.Vasan RS, Larson MG, Leip EP, et al: Assessment of frequency of progression to hypertension in non-hypertensive participants in the Framingham Heart Study: A cohort study. Lancet 358:1682-1686, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Gostynski M, Gutzwiller F, Kuulasmaa K, et al: Analysis of the relationship between total cholesterol, age, body mass index among males and females in the WHO MONICA Project. Int J Obes Relat Metab Disord 28:1082-1090, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Poirier P, Giles TD, Bray GA, et al: Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss—An update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 113:898-918, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Rosengren A, Wedel H, Wilhelmsen L: Body weight and weight gain during adult life in men in relation to coronary heart disease and mortality. A prospective population study. Eur Heart J 20:269-277, 1999 [PubMed] [Google Scholar]
- 9.Calle EE, Kaaks R: Overweight, obesity and cancer: Epidemiological evidence and proposed mechanisms. Nat Rev Cancer 4:579-591, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Pan SY, Johnson KC, Ugnat AM, et al: Association of obesity and cancer risk in Canada. Am J Epidemiol 159:259-268, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Calle EE, Rodriguez C, Walker-Thurmond K, et al: Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 348:1625-1638, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Gu D, He J, Duan X, et al: Body weight and mortality among men and women in China. JAMA 295:776-783, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report—National Institutes of Health. Obes Res 6:51S–209S, 1998. (suppl 2) [PubMed] [Google Scholar]
- 14.Dalton VK, Rue M, Silverman LB, et al: Height and weight in children treated for acute lymphoblastic leukemia: Relationship to CNS treatment. J Clin Oncol 21:2953-2960, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Jarfelt M, Lannering B, Bosaeus I, et al: Body composition in young adult survivors of childhood acute lymphoblastic leukaemia. Eur J Endocrinol 153:81-89, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Mayer EI, Reuter M, Dopfer RE, et al: Energy expenditure, energy intake and prevalence of obesity after therapy for acute lymphoblastic leukemia during childhood. Horm Res 53:193-199, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Nysom K, Holm K, Michaelsen KF, et al: Degree of fatness after treatment for acute lymphoblastic leukemia in childhood. J Clin Endocrinol Metab 84:4591-4596, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Oeffinger KC, Mertens AC, Sklar CA, et al: Obesity in adult survivors of childhood acute lymphoblastic leukemia: A report from the Childhood Cancer Survivor Study. J Clin Oncol 21:1359-1365, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Razzouk BI, Rose SR, Hongeng S, et al: Obesity in survivors of childhood acute lymphoblastic leukemia and lymphoma. J Clin Oncol 25:1183-1189, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Reilly JJ, Ventham JC, Newell J, et al: Risk factors for excess weight gain in children treated for acute lymphoblastic leukaemia. Int J Obes Relat Metab Disord 24:1537-1541, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Sklar CA, Mertens AC, Walter A, et al: Changes in body mass index and prevalence of overweight in survivors of childhood acute lymphoblastic leukemia: Role of cranial irradiation. Med Pediatr Oncol 35:91-95, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Van Dongen-Melman JE, Hokken-Koelega AC, Hahlen K, et al: Obesity after successful treatment of acute lymphoblastic leukemia in childhood. Pediatr Res 38:86-90, 1995 [DOI] [PubMed] [Google Scholar]
- 23.Craig F, Leiper AD, Stanhope R, et al: Sexually dimorphic and radiation dose dependent effect of cranial irradiation on body mass index. Arch Dis Child 81:500-504, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warner JT, Evans WD, Webb DK, et al: Body composition of long-term survivors of acute lymphoblastic leukaemia. Med Pediatr Oncol 38:165-172, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Bleyer WA, Fallavollita J, Robison L, et al: Influence of age, sex, and concurrent intrathecal methotrexate therapy on intellectual function after cranial irradiation during childhood: A report from the Children's Cancer Study Group. Pediatr Hematol Oncol 7:329-338, 1990 [DOI] [PubMed] [Google Scholar]
- 26.Christie D, Leiper AD, Chessells JM, et al: Intellectual performance after presymptomatic cranial radiotherapy for leukaemia: Effects of age and sex. Arch Dis Child 73:136-140, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oberfield SE, Soranno D, Nirenberg A, et al: Age at onset of puberty following high-dose central nervous system radiation therapy. Arch Pediatr Adolesc Med 150:589-592, 1996 [DOI] [PubMed] [Google Scholar]
- 28.Quigley C, Cowell C, Jimenez M, et al: Normal or early development of puberty despite gonadal damage in children treated for acute lymphoblastic leukemia. N Engl J Med 321:143-151, 1989 [DOI] [PubMed] [Google Scholar]
- 29.Sklar C, Mertens A, Walter A, et al: Final height after treatment for childhood acute lymphoblastic leukemia: Comparison of no cranial irradiation with 1800 and 2400 centigrays of cranial irradiation. J Pediatr 123:59-64, 1993 [DOI] [PubMed] [Google Scholar]
- 30.Armstrong GT, Sklar CA, Hudson MM, et al: Long-term health status in adult survivors of childhood cancer: Does gender matter? J Clin Oncol 25:4477-4489, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Carroll PV, Christ ER, Bengtsson BA, et al: Growth hormone deficiency in adulthood and the effects of growth hormone replacement: A review—Growth Hormone Research Society Scientific Committee. J Clin Endocrinol Metab 83:382-395, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Ahima RS, Saper CB, Flier JS, et al: Leptin regulation of neuroendocrine systems. Front Neuroendocrinol 21:263-307, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Ross JA, Oeffinger KC, Davies SM, et al: Genetic variation in the leptin receptor gene and obesity in survivors of childhood acute lymphoblastic leukemia: A report from the Childhood Cancer Survivor Study. J Clin Oncol 22:3558-3562, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Florin TA, Fryer GE, Miyoshi T, et al: Physical inactivity in adult survivors of childhood acute lymphoblastic leukemia: A report from the childhood cancer survivor study. Cancer Epidemiol Biomarkers Prev 16:1356-1363, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Draheim CC: Cardiovascular disease prevalence and risk factors of persons with mental retardation. Ment Retard Dev Disabil Res Rev 12:3-12, 2006 [DOI] [PubMed] [Google Scholar]
- 36.van Beek RD, de Muinck Keizer-Schrama SM, Hakvoort-Cammel FG, et al: No difference between prednisolone and dexamethasone treatment in bone mineral density and growth in long term survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer 46:88-93, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Stevens J, Keil JE, Waid LR, et al: Accuracy of current, 4-year, and 28-year self-reported body weight in an elderly population. Am J Epidemiol 132:1156-1163, 1990 [DOI] [PubMed] [Google Scholar]
- 38.Aronne LJ, Segal KR: Adiposity and fat distribution outcome measures: Assessment and clinical implications. Obes Res 10:14S-21S, 2002. (suppl 1) [DOI] [PubMed] [Google Scholar]
- 39.Misra A, Garg A, Abate N, et al: Relationship of anterior and posterior subcutaneous abdominal fat to insulin sensitivity in nondiabetic men. Obes Res 5:93-99, 1997 [DOI] [PubMed] [Google Scholar]
- 40.Kourti M, Tragiannidis A, Makedou A, et al: Metabolic syndrome in children and adolescents with acute lymphoblastic leukemia after the completion of chemotherapy. J Pediatr Hematol Oncol 27:499-501, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Oeffinger KC, Buchanan GR, Eshelman DA, et al: Cardiovascular risk factors in young adult survivors of childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol 23:424-430, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Talvensaari KK, Lanning M, Tapanainen P, et al: Long-term survivors of childhood cancer have an increased risk of manifesting the metabolic syndrome. J Clin Endocrinol Metab 81:3051-3055, 1996 [DOI] [PubMed] [Google Scholar]
- 43.Gurney JG, Ness KK, Sibley SD, et al: Metabolic syndrome and growth hormone deficiency in adult survivors of childhood acute lymphoblastic leukemia. Cancer 107:1303-1312, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Brennan BM, Rahim A, Adams JA, et al: Reduced bone mineral density in young adults following cure of acute lymphoblastic leukaemia in childhood. Br J Cancer 79:1859-1863, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davies JH, Evans BA, Jenney ME, et al: Skeletal morbidity in childhood acute lymphoblastic leukaemia. Clin Endocrinol (Oxf) 63:1-9, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Mertens AC, Yasui Y, Neglia JP, et al: Late mortality experience in five-year survivors of childhood and adolescent cancer: The Childhood Cancer Survivor Study. J Clin Oncol 19:3163-3172, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Oeffinger KC, Mertens AC, Hudson MM, et al: Health care of young adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Ann Fam Med 2:61-70, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kushi LH, Byers T, Doyle C, et al: American Cancer Society Guidelines on Nutrition and Physical Activity for cancer prevention: Reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin 56:254-281, 2006 [DOI] [PubMed] [Google Scholar]