Abstract
Purpose
To evaluate the safety and the efficacy of imatinib in recurrent malignant gliomas.
Patients and Methods
This was a single-arm, phase II study. Eligible patients had recurrent glioma after prior radiotherapy with an enhancing lesion on magnetic resonance imaging. Three different histologic groups were studied: glioblastomas (GBM), pure/mixed (anaplastic) oligodendrogliomas (OD), and low-grade or anaplastic astrocytomas (A). Imatinib was started at a dose of 600 mg/d with dose escalation to 800 mg in case of no toxicity; during the trial this dose was increased to 800 mg/d with escalation to 1,000 mg/d. Trial design was one-stage Fleming; both an objective response and 6 months of progression-free survival (PFS) were considered a successful outcome to treatment.
Results
A total of 112 patients (51 patients with GBM, 25 patients with A, and 36 patients with OD) were enrolled. Imatinib was in general well tolerated. The median number of cycles was 2.0 (range, 1 to 43 cycles). Five patients had an objective partial response, including three patients with GBM; all had 6 months of PFS. The 6-month PFS rate was 16% (95% CI, 8.0% to 34.0%) in GBM, 4.0% (95% CI, 0.3% to 15.0%) in OD, and 9% (95% CI, 2.0% to 25.0%) in A. The exposure to imatinib was significantly lower in patients using enzyme-inducing antiepileptic drugs. The presence of ABCG2 point mutations were not correlated with pharmacokinetic findings. No somatic activating mutations of KIT or platelet-derived growth factor receptor–A or –B were found.
Conclusion
In the dose range of 600 to 1,000 mg/d, single-agent imatinib is well tolerated but has limited antitumor activity in patients with recurrent gliomas.
INTRODUCTION
Despite recent advances in the management of gliomas using temozolomide alone or in combination with radiotherapy for newly diagnosed tumors, treatment options for patients with recurrent astrocytomas and oligodendrogliomas remain limited. Ultimately, all patients develop progressive disease, which requires the development of more effective salvage regimens. In vivo and in vitro studies on glioma models have shown that tyrosine kinase receptors play an important role in glioma cell signal transduction. Activation of these pathways leads to increased cell proliferation, migration, and survival. The platelet derived growth factor receptor (PDGFR) pathway is one of these signaling pathways that seems to be involved in glioma. Although PDGFR mutations and amplification are rare, diffuse gliomas frequently overexpress both the ligands platelet-derived growth factor (PDGF) –A and/or PDGF-B and their receptors, which suggests the presence of autocrine loops.1-3 Levels of expression increase with increasing tumor grade, but PDGFR overexpression is already present in low-grade gliomas. Blocking of PDGF-mediated phosphorylation caused either apoptosis or growth inhibition in glioma cell lines.4,5 PDGF autocrine loops may be even more relevant for oligodendroglioma. Virtually all oligodendrogliomas express both PDGF ligands as well as both the PDGF-α and -β receptors.6-8 Both O-2A progenitor cells (which are assumed to be the precursor cells of oligodendrocytes and which may be the cells of origin of oligodendroglial tumors) and oligodendrogliomas express the combination of NG2 chondroitin proteoglycan sulfate and PDGF-α, a combination which is neither observed in glioblastoma (GBM) nor in normal brain.8,9 PDGF has a strong mitogenic effect on these glial progenitor cells.10 Moreover, induction of PDGF expression by intracranial gene transfer in mice induced the formation of tumors with an oligodendroglial phenotype in a transgenic mice model.11 These findings make the PDGF pathway a potential target in nearly all glioma subtypes.
Imatinib mesylate (imatinib) is a multitargeted tyrosine kinase inhibitor that blocks KIT, Bcr-Abl, and PDGFR in several in vitro and in vivo models.12,13 Imatinib displayed antitumor activity in PDGFR-dependent human GBM primary cell cultures.14 We therefore initiated a multicenter phase II study to evaluate the safety and the antitumor activity of imatinib in patients with progressive gliomas after prior radiotherapy. Translational research included correlative studies of PDGFR, KIT, and ABCG2 mutations/polymorphisms and imatinib pharmacokinetic (PK) measurements.
PATIENTS AND METHODS
Patient Population
Eligibility criteria were age ≥ 18 years, a progressive glioma after prior radiotherapy and documented by magnetic resonance imaging (MRI) or computed tomography (CT) made within 2 weeks from the start of treatment, stable or decreasing doses of corticosteroids for at least 2 weeks before baseline scan, Eastern Cooperative Oncology Group performance status ≤ 2, at least one bidimensionally measurable contrast-enhancing target lesion with at least one diameter of at least 2 cm, no brain irradiation in the last 3 months and no dosage of radiotherapy more than 65 Gy, no brain surgery within the last 3 months, and adequate bone marrow, renal, hepatic, and cardiac function. Patients requiring treatment with warfarin or any other anticoagulants and patients with unstable or active uncontrolled infections were not eligible. The study design was approved by the local and/or national ethic committee of all participating institutions, and all patients provided written informed consent according to International Congress on Harmonisation/Good Clinical Practice standards and national/local regulations.
Eligible Histologies and Specific Study Requirements
Patients were enrolled onto the study according to the local pathologic diagnosis. Three different histologic subgroups were defined: recurrent GBM, recurrent low-grade astrocytoma and anaplastic astrocytoma (A), and recurrent low-grade or anaplastic oligodendroglioma or mixed (anaplastic) oligoastrocytoma (OD). Patients with GBM were eligible if they had received either no or no more than one prior chemotherapy regimen, either adjuvant or at administered at disease recurrence. Patients with A or OD were eligible if they had received one prior chemotherapy regimen, either adjuvant or administered at recurrence.
Imatinib Administration
At the start of the study, treatment consisted of imatinib at a once daily oral dose of 600 mg/d escalated to 800 mg/d (400 mg twice a day) in the absence of grade 2 or worse toxicity during the first 8 weeks of treatment. On the basis of initial safety data showing that imatinib was well tolerated in this patient population, the protocol was amended to increase the dose of imatinib to 800 mg/d (400 mg twice a day), with dose escalation to 1,000 mg/d (500 mg twice a day) after 8 weeks in the absence of grade 2 or worse toxicity. One cycle was defined as 4 weeks of imatinib. Treatment was continued until unacceptable toxicity or evidence of tumor progression.
Evaluation of Toxicity and Antitumor Activity
Patients were monitored for toxicity weekly for the first 8 weeks (in case of dose escalation, the first 8 weeks at the higher dose also required weekly monitoring). Toxicity was graded according to National Cancer Institute Common Toxicity Criteria version 2.0. Response to treatment was assessed every two cycles using MRI or CT scan according to Macdonald's criteria.15 The scans of all patients reportedly having an objective response (partial or complete response) or 6 months of progression-free survival (PFS) were centrally reviewed.
Study Design
This trial was an open-label, multicenter, phase II study. Because both an objective response (even if short lasting) and prolonged disease stabilization were considered to suggest activity, a combined primary end point (labeled success) was used, defined as either an objective response (partial or complete response) and/or 6 months of PFS. Secondary end points were duration of response, survival, and toxicity, as well as PK and pharmacodynamic analysis. The sample size was determined according to a one-stage Fleming design that was applied separately to each pathologic stratum.16 Imatinib was considered worthy of further investigations if success rates were obtained in 25%, 30%, and 30% for patients with GBM, A, and OD, respectively. With an α = 0.10 and β = 0.10, respectively 29, 24, and 24 eligible patients with GBM, A, and OD were required. When the dose was amended to a starting dose of 800 mg, the study was restarted.
Pharmacokinetic Analysis
A limited sampling strategy was used to investigate the exposure to imatinib and its main metabolite CGP74588 in patients with gliomas. At cycle 1, 8-mL blood samples were drawn on day 1, before the first imatinib intake, between 1 and 3 and 6 and 9 hours after first drug administration, then before the first intake at day 2. One additional sample was taken at day 1 of cycle 2 before imatinib to evaluate the steady-state. Samples were centrifuged at 4,000 × g for 15 minutes and stored at −20°C until analysis. Imatinib and CGP74588 concentrations were determined as described elsewhere using liquid chromatography coupled with tandem mass spectrometry. Maximum plasma concentration (Cmax), area under the serum concentration-time curve (AUC0-∞), and concentration ratio at steady-state are reported.
Molecular Diagnosis
Paraffin-embedded tumor samples were retrieved for pathology review (J.M.K.) and mutational analysis (M.C.H.) for mutations of KIT (exons 9, 11, 13, and 17) and PDGFRα (exons 12, 14, and 18) and PDGFRβ (exons 11 and 17) as previously described.17 Genotyping for the hypomorphic Q141K ABCG2 polymorphism was performed using tumor DNA and a Fluorescent Resonance Energy Transfer–based hybridization probes and melting curve analysis using a Roche LC480 instrument (Roche, Neuilly sur Seine, France; primer, probe, and polymerase chain reaction conditions available on request).18
Statistical Analysis
Demographic, response rate, safety, laboratory, PK, and pharmacodynamic data were analyzed using descriptive statistics. Kaplan-Meier technique was used to estimate medians and rates of PFS and overall survival at 6 months. PFS was measured from the day of registration to objective disease progression or death, whichever occurred first. Patients were considered as censored if they never experienced disease progression or death. The log-rank test was used for survival curves comparisons. Continuous measurements were summarized by means and coefficient of variation and compared using the Kruskall-Wallis test.
RESULTS
Patient Characteristics
From March 2002 to August 2004, 112 patients (51 patients with GBM, 25 patients with A, and 36 patients with OD) were entered onto this study. Thirty patients were treated at the 600 mg/d starting dose level, and 82 patients were treated at the 800 mg/d starting dose level. Two patients were not eligible (one patient at the dose of 600 mg, one patient at 800 mg) because of a too-small baseline lesion; in one patient, baseline data were not made available. Baseline patient characteristics are listed in Table 1. No tumor material was received from 18 patients. The diagnosis of GBM was confirmed at review in 44 (92%) of 48 patients, diagnosis of an OD was confirmed in 13 (50%) of 26 patients, and diagnosis of an A was confirmed in nine (45%) of 20 patients. In case of discrepancy at review, usually another eligible histologic diagnosis was made. The median number of cycles was 2.0 (range, one to 43 cycles; Table 1). Dose reduction and cycle delay (mainly for nonhematologic toxicity) were performed in 11 (9.8%) and 36 patients (32.1%), respectively. At the 600-mg and 800-mg dose level, the dosage was increased in 15 and 16 patients (to 800 and 1,000 mg, respectively).
Table 1.
Patient Characteristics and Protocol Treatment Information
| Characteristic | No. of Patients
|
|
|---|---|---|
| Imatinib 600 mg | Imatinib 800 mg | |
| Sex | ||
| Male | 10 | 55 |
| Female | 20 | 24 |
| Age, years | ||
| Median | 52 | 46 |
| Range | 23-66 | 19-72 |
| ECOG performance status | ||
| 0, 1 | 24 | 70 |
| 2 | 6 | 10 |
| Prior chemotherapy | ||
| Adjuvant | 3 | 21 |
| For recurrence | 3 | 49 |
| Imatinib treatment | ||
| Patients with dose reduction | 5 | 6 |
| Patients with cycle delay | 12 | 24 |
| Patients with dose increase | 15 | 16 |
| Mean imatinib dose-intensity, cycle 1-2 | ||
| ≤ 70% | 2 | 0 |
| > 70% to ≤ 90% | 5 | 14 |
| > 90% to ≤ 110% | 22 | 66 |
| > 110% to ≤ 120% | 1 | 0 |
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Efficacy
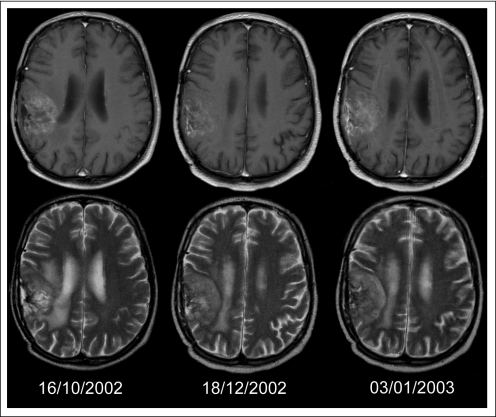
Table 2 lists the observed responses, PFS, and overall survival for all patients and the patients treated at 800 mg. At the dose level of 800 mg, and with the diagnosis according to the local pathologist, PFS at 6 months was observed in five (16%) of 31 patients with GBM, one (4%) of 27 patients with OD, and two (9%) of 22 patients with A. A partial response was observed in only two of the 80 patients treated at 800 mg. In some patients, decreased contrast enhancement was observed despite clinical deterioration, with T2-weighted images suggestive of tumor progression (Fig 1).
Table 2.
Outcome of Imatinib Treatment in All 110 Patients With Measurable Disease
| Variable | 800 mg
|
All (600 mg and 800 mg)
|
||||
|---|---|---|---|---|---|---|
| GBM | AOD/mixed OA | AA/rec LGA | GBM | AOD/mixed OA | AA/rec LGA | |
| All patients, n | 31 | 27 | 22 | 50 | 35 | 25 |
| Success, n | 20 | |||||
| No | 26 | 26 | 2 | 42 | 31 | 22 |
| Yes, 6 months of PFS or CR/PR | 5 | 1 | 8 | 4 | 3 | |
| 6-month PFS | 5 | 1 | 2 | 8 | 4 | 3 |
| PFS, months | ||||||
| Median | 1.7 | 1.8 | 1.7 | 1.8 | 1.9 | 1.8 |
| 95% CI | 1.6 to 2.3 | 1.7 to 2.3 | 1.2 to 2.1 | 1.7 to 2.3 | 1.8 to 3.2 | 1.3 to 2.1 |
| OS, months | ||||||
| Median | 5.2 | 4.8 | 4.9 | 5.9 | 5.3 | 5.0 |
| 95% CI | 3.8 to 7.8 | 2.8 to 7.7 | 3.1 to 9.7 | 4.2 to 7.8 | 3.7 to 10.9 | 3.1 to 9.7 |
| 6-month OS rate, % | ||||||
| Median | 48 | 32 | 45 | 50.0 | 42.9 | 48.0 |
| 95% CI | 30 to 64 | 17 to 51 | 24 to 64 | 35.6 to 62.8 | 26.4 to 58.3 | 27.8 to 65.6 |
| Best response | ||||||
| PR | ||||||
| No. | 1 | 0 | 1 | 3 | 1 | 1 |
| % | 3 | 0 | 5 | 6 | 3 | 4 |
| SD | ||||||
| No. | 8 | 4 | 3 | 13 | 9 | 5 |
| % | 26 | 15 | 14 | 26 | 25 | 20 |
Abbreviations: GBM, glioblastoma; AOD, anaplastic oligodendroglioma; OA, oligoastrocytoma; AA, anaplastic astrocytoma; rec LGA, recurrent low-grade astrocytoma; PFS, progression-free survival; CR, complete response; PR, partial response; OS, overall survival; SD, stable disease.
Fig 1.
A patient started treatment with imatinib in October 2002 for a progressive oligoastrocytoma after receiving 60 Gy of radiotherapy in 2001 and procarbazine, cyclophosphamide, and vincristine chemotherapy in 2002. At the first response assessment after two cycles, despite a slight increase of sensory deficits, T1-weighted contrast enhanced magnetic resonance imaging (MRI) suggested some decrease in enhancement and treatment was continued. One and a half weeks later, the patient was admitted because of increasing headache and further neurologic deterioration; T1-weighted contrast-enhanced MRI showed no new findings. However, close examination of T2-weighted images showed an increase of the tumor volume. Upper row: contrast-enhanced T1-weighted MRI; lower row, T2-weighted MRI on October 16, 2002 (baseline), December 18, 2002 (first response evaluation), and January 3, 2003 (at the time of admission).
Safety
Although the incidence of toxic events was slightly higher in patients receiving the dose of 800 mg/d as compared with those receiving 600 mg/d, treatment was well tolerated (Table 3). Hematologic toxicity mainly consisted of grade 1 to 2 neutropenia, with only five patients developing febrile grade 3 to 4 neutropenia at the dose of 800 mg/d. Mild to moderate increases in renal and hepatic functions were frequently reported in patients receiving imatinib concomitant with antiepileptic drugs. Grade 1 to 2 edema, primarily infra-orbital, was reported in approximately 40% of patients, regardless of the dose of imatinib.
Table 3.
Toxicity of Imatinib in Patients With Malignant Gliomas
| Toxicity | Imatinib 600 mg
|
Imatinib 800 mg
|
||||||
|---|---|---|---|---|---|---|---|---|
| Grade 1-2
|
Grade 3-4
|
Grade 1-2
|
Grade 3-4
|
|||||
| No. of Patients | % | No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Neutropenia | 6 | 20 | 2 | 6.6 | 13 | 16 | 9 | 11 |
| Febrile neutropenia | — | — | — | 5 | 6.2 | |||
| Thrombocytopenia | 2 | 6.6 | — | 8 | 9.8 | — | ||
| Anemia | 18 | 60 | 1 | 3.3 | 36 | 44.4 | 1 | 1.2 |
| Fatigue | 4 | 13.3 | 2 | 6.6 | 26 | 32.1 | 2 | 2.4 |
| Fever without neutropenia | — | — | 2 | 2.4 | — | |||
| Creatinine | 3 | 10 | — | 9 | 11 | — | ||
| Total bilirubin | 3 | 10 | — | 12 | 14.8 | — | ||
| ALT | 9 | 30 | 2 | 6.6 | 33 | 40.7 | 2 | 2.4 |
| AST | 11 | 36.6 | — | 19 | 23.4 | — | ||
| Alkaline phosphatase | 10 | 33.3 | — | 22 | 27.1 | 1 | 1.2 | |
| Albumin | 13 | 43.3 | 1 | 3.3 | 74 | 91.3 | 1 | 1.2 |
| Edema | 12 | 40 | — | 31 | 38.2 | 1 | 1.2 | |
| Hypotension | — | — | — | 1 | 1.2 | |||
| Skin toxicity* | 6 | 20 | — | 23 | 28.4 | 1 | 1.2 | |
| Diarrhea | 3 | 10 | — | 16 | 19.7 | — | ||
| Nausea | 15 | 50 | — | 23 | 28.4 | — | ||
| Vomiting | 11 | 36.6 | 1 | 3.3 | 13 | 16 | 1 | 1.2 |
| Stomatitis | — | — | 4 | 4.9 | — | |||
| Arthralgia and myalgia | 1 | 3.3 | — | 3 | 3.7 | — | ||
Skin toxicity included rash and dry skin.
Pharmacokinetic Parameters
Pharmacokinetic data were obtained for 80 patients, allowing an accurate determination of Cmax, AUC, and the steady-state concentration ratio for imatinib and its main metabolite CGP74588. Overall, there was considerable interpatient variability. At the doses of 600 and 800 mg, Cmax for imatinib was 1,366 ng/mL (49%) and 1,335 ng/mL, respectively; Cmax of CGP74588 was 300 ng/mL and 234 ng/mL, respectively. At the doses of 600 and 800 mg, AUC0-∞ of imatinib were 771 ng · h/mL and 1,089 ng · h/mL, respectively; and AUC0-∞ of CGP74588 were 172 ng · h/mL and 199 ng · h/mL, respectively. Concentration ratios at steady-state were 3.9 and 2.5 for imatinib at the dose of 600 and 800 mg, respectively. Concentration ratios at steady-state were 4.4 and 3.6 for CGP74588 at the dose of 600 and 800 mg, respectively. Cmax and AUC0-∞ were significantly reduced (P = .0019 and P ≤ .0001) in patients taking enzyme-inducing antiepileptic drugs (EIAEDs; Table 4). No effect of EIAEDs was observed on concentration ratio at steady-state and on the PK parameters of CGP74588. PFS was not affected by dosing of imatinib nor by any PK parameters.
Table 4.
Pharmacokinetic Parameters for STI571 and its Main Metabolite, CGP74588, in Patients Not Taking Antiepileptic Drugs and Patients Taking Either EIAEDs or Non-EIAEDs
| Parameter | No. | Median | Minimum Observed Value | Maximum Observed Value | P (df = 2) All Patients |
|---|---|---|---|---|---|
| Cmax, STI571, ng/mL | |||||
| No antiepileptic drugs | 18 | 1595 | 533 | 3930 | .0019 |
| EIAEDs | 37 | 861 | 35 | 2960 | |
| Non-EIAEDs | 25 | 1270 | 78 | 4620 | |
| Cmax, CGP74588, ng/mL | |||||
| No antiepileptic drugs | 18 | 225 | 49 | 1170 | .35 |
| EIAEDs | 37 | 198 | 18 | 892 | |
| Non-EIAEDs | 25 | 166 | 11 | 570 | |
| AUC, STI571, ng·h/mL | |||||
| No antiepileptic drugs | 18 | 1263 | 314 | 2574 | < .0001 |
| EIAEDs | 37 | 614 | 247 | 1611 | |
| Non-EIAEDs | 25 | 1139 | 192 | 3021 | |
| AUC, CGP74588, ng·h/mL | |||||
| No antiepileptic drugs | 18 | 174 | 34 | 942 | .31 |
| EIAEDs | 37 | 152 | 59 | 529 | |
| Non-EIAEDs | 25 | 160 | 22 | 552 | |
| RAP29, STI571 | |||||
| No antiepileptic drugs | 10 | 1.87 | 0.68 | 16.44 | .99 |
| EIAEDs | 28 | 1.85 | 0.62 | 6.58 | |
| Non-EIAEDs | 15 | 1.80 | 0.88 | 24.38 | |
| RAP29, CGP74588 | |||||
| No antiepileptic drugs | 10 | 2.75 | 1.48 | 9.70 | .87 |
| EIAEDs | 28 | 2.65 | 1.23 | 6.46 | |
| Non-EIAEDs | 15 | 2.25 | 0.92 | 39.60 |
Abbreviations: EIAEDs, enzyme-inducing antiepileptic drugs; Non-EIAEDs, non–enzyme-inducing AEDs; Cmax, maximum concentration; AUC, area under the curve; RAP, concentration at steady state (day 29 before taking imatinib).
Molecular Analysis
Tumor samples allowing molecular analysis were available for 70 patients. Among 69 assessable specimens, no somatic activating mutations of KIT, PDGFRα, or PDGFRβ were detectable. PDGFRα single nucleotide polymorphisms (SNPs) were observed in the intron of exon 11 in one patient, in exon 14 in one patient (K666K), and in exon 18 in seven patients (SNP V824V). PFS was not correlated with PDGFRα SNPs. In 69 patients, material was available for the assessment of ABCG2 point mutations. Twelve patients had the Q141K exon 5 SNP. No correlation was found between the presence of ABCG2 point mutations and any of the pharmacokinetic parameters (data not shown).
DISCUSSION
Oral imatinib doses of 600 and 800 mg daily with dose escalation to 1,000 mg/d in case of no significant toxicity was well tolerated in patients with malignant gliomas, with a slightly increased incidence of mild to moderate toxicity in patients receiving higher imatinib doses. Despite the sporadic objective responses and prolonged tumor stabilization that were observed, the overall response rate and 6-month PFS rate achieved are insufficient to suggest that imatinib has clinically useful activity in any subtype of diffuse gliomas when given as a single agent. Somewhat unexpectedly, the few sustained objective responses were mainly observed in patients with GBM, with 16% PFS at 6 months. Although currently a 6-month PFS rate of less than 15% is considered as the benchmark indicating a negative trial in recurrent high-grade glioma, previous negative studies from the European Organisation for Research and Treatment of Cancer in recurrent GBM using classical cytotoxic agents and using similar inclusion criteria consistently observed 6-month PFS rates well below 10%.19-22 Although this suggests that imatinib has some low level of activity in GBM, this was not confirmed in a North American study on 35 patients with GBM.23 In that study, only a 3% 6-month PFS rate was noted, together with a 10% 6-month PFS rate in 15 recurrent grade 3 tumors. Recent phase II studies reported interesting activity of imatinib combined with hydroxyurea, a ribonucleoside diphosphate reductase inhibitor, in patients with recurrent GBM.24,25 A large phase III trial, however, has not been able to confirm these findings.26 The North American study observed intratumoral hemorrhages in patients with GBM treated at 800 mg, although all occurred in the setting of progressive disease.23 A phase I study on pediatric brainstem gliomas and recurrent malignant gliomas also observed intratumoral hemorrhages.27 In the present study, no clinically significant hemorrhages were observed.
The outcome of the central pathology review in our study was similar to that of other studies in glioma: a high rate of confirmation of locally diagnosed GBMs but frequent discrepancies in the diagnosis of ODs and As. However, at central review, enough patients with the various glioma subtypes were left to conclude that sufficient single-agent activity of imatinib was present in none of the glioma subtypes.
Imatinib is metabolized by the CYP4503A4, with the N-demethylation of the piperazine 4-nitrogen producing its main metabolite CGP74588. Because many patients with brain tumors receive EIAEDs that may modify the PK profile of imatinib, the exposure to imatinib and its main metabolite CGP74588 was investigated using a limited sampling strategy. Overall, exposure to imatinib was similar in patients receiving 600 and 800 mg imatinib, possibly as a result of the large interpatient variability at daily dosage greater than 600 mg. The use of EIAEDs significantly influenced the PK profile of imatinib, with an approximately 50% reduction of its Cmax and AUC. Still, no correlation between exposure and toxicity was detectable nor between PK exposure or used imatinib dosage (600 v 800 mg) and antitumor activity (data not shown). In a phase I dose-escalation study, other investigators have shown that glioma patients using EIAEDs may receive up to 1,200 mg/d of imatinib without developing dose-limiting toxicity.23 In that study, plasma exposure of imatinib was reduced by approximately 68% in patients receiving EIAEDs. The presence of ABCG2 point mutations were not correlated with PK findings. This confirms the previous findings that common genetic variants in this gene have only a limited impact on the PK of imatinib.28
As expected, all analyzed tumors were shown to express wild-type PDGFRs at the time of initial surgery. A drawback of our approach is that no samples collected at the time of recurrence were investigated. Our data confirm that somatic mutations of PDGFRs are infrequent in gliomas. Expression of PDGFR and its ligands using immunohistochemistry was not attempted because of its modest reliability in archival paraffin-embedded tissue samples. Despite the upregulation of PDGF signaling pathways in many gliomas, the lack of significant imatinib activity in our study and those of others may reflect a lack of importance of PDGFR signaling for the survival and growth of human gliomas. An insufficient exposure of the tumor cells to imatinib could be another explanation for the lack of activity in glioma. Imatinib is a substrate for the P-glycoprotein efflux pump, limiting the passage of imatinib over an intact blood-brain barrier.29 Alternatively, sufficient levels of target inhibition may not have been achieved. These latter considerations are a problem for nearly all recently investigated targeted agents in gliomas. Most of these trials failed, but only a few studies explored whether the intended intratumoral target inhibition was indeed achieved. A recent study that investigated GBM samples before and after exposure to imatinib demonstrated inhibition of AKT and MAPK activity, but no significant effect on PDGFRA/B or KIT. In some patients, a persistent phosphorylation level of PDGFRB was detected despite imatinib treatment, which may imply an imatinib-resistant activation mechanism of PDGFRB.30 To answer these pivotal questions on drug penetration and target inhibition, investigations of new targeted treatments in gliomas need to integrate molecular analyses into the design of early trials (phase 0 trials).
In this study, we also observed pseudo improvements, characterized by a decrease of gadolinium enhancement on MRI, despite the fact that the patient was clinically deteriorating. This may be due to the expression of PDGFR on both endothelial cells and pericytes, leading to a normalization of abnormal vessel permeability or to changes in regional cerebral blood volume without a real antitumor effect. Similar though more pronounced observations have recently been made in studies on agents interfering with vascular endothelial growth factor signaling pathways (bevacizumab, AZD2171).31,32 This suggests that classical T1-weighted and contrast-enhanced MRI alone may not be the best way to monitor responsiveness to agents interfering with the PDGF and vascular endothelial growth factor signaling pathways.
In summary, using doses ranging from 600 to 1,000 mg/d, imatinib had an acceptable safety profile but no clinically significant activity in patients with recurrent diffuse gliomas, although marginal activity was observed in GBM. On the basis of these results, further studies using single-agent imatinib at doses of 600 to 800 mg/d are not warranted in patients with malignant gliomas.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Roger Stupp, Novartis Pharmaceuticals (C); Michael C. Heinrich, Novartis Pharmaceuticals (C), Molecular MD (U); Martin J. van den Bent, Novartis Pharmaceuticals (C) Stock Ownership: Michael C. Heinrich, Molecular MD Honoraria: Roger Stupp, Novartis Pharmaceuticals; Michael C. Heinrich, Novartis Pharmaceuticals; Martin J. van den Bent, Novartis Pharmaceuticals Research Funding: Michael C. Heinrich, Novartis Pharmaceuticals; Martin J. van den Bent, Novartis Pharmaceuticals Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Eric Raymond, Denis Lacombe, Martin J. van den Bent
Administrative support: Roger Stupp, Denis Lacombe, Martin J. van den Bent
Provision of study materials or patients: Eric Raymond, Alba A. Brandes, Christian Dittrich, Pierre Fumoleau, Bruno Coudert, Paul M.J. Clement, Marc Frenay, Roy Rampling, Roger Stupp, Johan M. Kros, Martin J. van den Bent
Collection and assembly of data: Eric Raymond, Alba A. Brandes, Christian Dittrich, Roy Rampling, Roger Stupp, Michael C. Heinrich, Martin J. van den Bent
Data analysis and interpretation: Eric Raymond, Michael C. Heinrich, Thierry Gorlia, Denis Lacombe, Martin J. van den Bent
Manuscript writing: Eric Raymond, Alba A. Brandes, Pierre Fumoleau, Paul M.J. Clement, Roger Stupp, Michael C. Heinrich, Thierry Gorlia, Martin J. van den Bent
Final approval of manuscript: Eric Raymond, Alba A. Brandes, Christian Dittrich, Pierre Fumoleau, Bruno Coudert, Paul M.J. Clement, Marc Frenay, Roy Rampling, Roger Stupp, Johan M. Kros, Michael C. Heinrich, Thierry Gorlia, Denis Lacombe, Martin J. van den Bent
Acknowledgments
We thank Thea Kalebic and Zariana Nikolova of Novartis Pharma AG for their logistical support.
Supported by the European Organisation for Research and Treatment of Cancer Charitable Trust and Grants No. 5U10 CA11488-32 through 5U10 CA11488-34 from the National Cancer Institute (Bethesda, MD). Additional funding was provided by Novartis Pharma AG and a Veterans Affairs Merit Review Grant (M.C.H.). The imatinib used in this study was provided free of charge by Novartis Pharma AG.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Hermanson M, Funa K, Koopman J, et al: Association of loss of heterozygosity on chromosome 17p with high platelet-derived growth factor α receptor expression in human gliomas. Cancer Res 56:164-171, 1996 [PubMed] [Google Scholar]
- 2.Guha A, Dashner K, Black PM, et al: Expression of PDGF and PDGF receptor in human astrocytoma operation specimen supports the existence of an autocrine loop. Int J Cancer 60:168-173, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Smith JS, Wang X-Y, Qian J, et al: Amplification of the platelet -derived growth factor receptor-A (PDGFRA) gene occurs in oligodendrogliomas with grade IV anaplastic features. J Neuropathol Exp Neurol 59:495-503, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Chin LS, Murray SF, Zitnay KM, et al: K252a inhibits proliferation of glioma cells by blocking platelet-derived growth factor signal transduction. Clin Cancer Res 3:771-776, 1997 [PubMed] [Google Scholar]
- 5.Vassbotn FS, Östmann A, Langeland N, et al: Autocrine platelet-derived growth factor autocrine pathway drives the transformed phenotype of a human glioblastoma cell line. J Cell Physiol 158:381-389, 1994 [DOI] [PubMed] [Google Scholar]
- 6.Di Rocco F, Carroll RS, Zhang J, et al: Platelet-derived growth factor and its receptor expression in human oligodendrogliomas. Neurosurgery 42:341-346, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Robinson SW, Cohen M, Prayson R, et al: Constitutive expression of growth-related oncogene and it receptor in oligodendrogliomas. Neurosurgery 48:864-874, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Shoshan Y, Nishiyama A, Chang A, et al: Expression of oligodendrocyte progenitor cell antigens by gliomas: Implications for the histogenesis of brain tumors. Proc Natl Acad Sci U S A 96:10361-10366, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raff MC, Miller RH, Noble M: A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on the culture medium. Nature 303:390-396, 1983 [DOI] [PubMed] [Google Scholar]
- 10.van Heyningen P, Calver AR, Richardson WD: Control of progenitor cell number by mitogen supply and demand. Curr Biol 11:232-241, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Dai C, Celestino JC, Okada Y, et al: PDGF autocrine stimulation dedifferentiates cultured astrocytes and induces oligodendrogliomas and oligoastrocytomas from neural progenitors and astrocytes in vivo. Genes Devel 15:1913-1925, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verweij J, van OA, Blay JY, et al: Imatinib mesylate (STI-571 Glivec, Gleevec) is an active agent for gastrointestinal stromal tumours, but does not yield responses in other soft-tissue sarcomas that are unselected for a molecular target: Results from an EORTC Soft Tissue and Bone Sarcoma Group phase II study. Eur J Cancer 39:2006-2011, 2003 [PubMed] [Google Scholar]
- 13.Druker BJ, Talpaz M, Resta DJ, et al: Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med 344:1031-1037, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Hägerstrand D, Hesselager G, Achterberg S, et al: Characterization of an imatinib-sensitive subset of high-grade human glioma cultures. Oncogene 25:4913-4922, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Macdonald DR, Cascino TL, Schold SC, et al: Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277-1280, 1990 [DOI] [PubMed] [Google Scholar]
- 16.Fleming TR: One-sample multiple testing procedures for phase II clinical trials. Biometrics 38:143-151, 1982 [PubMed] [Google Scholar]
- 17.Heinrich MC, McArthur GA, Demetri GD, et al: Clinical and molecular studies of the effect of imatinib on advanced aggressive fibromatosis (desmoid tumor). J Clin Oncol 24:1195-1203, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Li J, Cusatis G, Brahmer J, et al: Association of variant ABCG2 and the pharmacokinetics of epidermal growth factor receptor tyrosine kinase inhibitors in cancer patients. Cancer Biol Ther 6:432-438, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Wong ET, Hess KR, Gleason MJ, et al: Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol 17:2572-2578, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Twelves CJ, Campone M, Coudert B, et al: Phase II study of XR5000 (DACA) administered as a 120-h infusion in patients with recurrent glioblastoma multiforme. Ann Oncol 13:777-780, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Raymond E, Campone M, Stupp R, et al: Multicentre phase II and pharmacokinetic study of RFS2000 (9-nitro-campothecin) administered orally 5 days a week in patients with glioblastoma multiforme. Eur J Cancer 38:1348-1350, 2002 [DOI] [PubMed] [Google Scholar]
- 22.van den Bent MJ, Grisold W, Frappaz D, et al: European Organization for Research and Treatment of Cancer (EORTC) open label phase II study on glufosfamide administered as a 60-minute infusion every three weeks in recurrent glioblastoma multiforme. Ann Oncol 14:1732-1734, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Wen PY, Yung WK, Lamborn KR, et al: Phase I/II study of imatinib mesylate for recurrent malignant gliomas: North American Brain Tumor Consortium Study 99-08. Clin Cancer Res 12:4899-4907, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Reardon D, Friedman A, Herndon JE, et al: Phase II trial of imatinib mesylate plus hydroxyurea in the treatment of patients with malignant glioma. Neuro Oncol 6:381, 2004. (abstr TA-47) [Google Scholar]
- 25.Dresemann G: Imatinib and hydroxyurea in pretreated progressive glioblastoma multiforme: A patient series. Ann Oncol 16:1702-1708, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Dresemann G, Rosenthal M, Höffken K, et al: Imatinib plus hydroxyurea versus hydroxyurea in progressive glioblastoma (GBM): An international open label randomised phase III study (AMBROSIA study). Neuro Oncol 9:519, 2007. (abstr MA-17) [Google Scholar]
- 27.Pollack IF, Jakacki RI, Blaney SM, et al: Phase I trial of imatinib in children with newly diagnosed brainstem and recurrent malignant gliomas: A Pediatric Brain Tumor Consortium report. Neuro Oncol 9:145-160, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gardner ER, Burger H, van Schaik RH, et al: Association of enzyme and transporter genotypes with the pharmacokinetics of imatinib. Clin Pharmacol Ther 80:192-201, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Dai H, Marbach P, Lemaire M, et al: Distribution of STI-571 to the brain is limited by P-Glycoprotein-mediated efflux. J Pharmacol Exp Ther 304:1085-1092, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Razis E, Selviaridis P, Fletcher J, et al: Biochemical evidence of tumor response and measurable levels of the drug in glioblastoma tissue from patients treated with imatinib. J Clin Oncol 25:80s, 2007. (suppl; abstr 2023) [Google Scholar]
- 31.Vredenburgh JJ, Desjardins A, Herndon JE, et al: Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res 13:1253-1259, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Batchelor TT, Sorensen AG, di TE, et al: AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 11:83-95, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]