Abstract
Purpose
Two nonoperative approaches (one without fluorouracil) using induction chemotherapy and then definitive chemoradiotherapy developed at two centers were compared in patients with localized esophageal cancer (LEC). The primary end point was to assess whether any approach would achieve a ≥ 77.5% 1-year survival rate, surpassing the historical 66% rate from the Radiation Therapy Oncology Group (RTOG) protocol 9405.
Patients and Methods
In a multi-institutional cooperative group setting, patients with LEC who had unresectable cancer, were unwilling to undergo surgery, or were medically unfit for surgery were randomly assigned to receive either induction with fluorouracil, cisplatin, and paclitaxel and then fluorouracil plus paclitaxel with 50.4 Gy of radiation (arm A) or induction with paclitaxel plus cisplatin and then the same chemotherapy with 50.4 Gy of radiation (arm B). Safety and survival rates were assessed.
Results
A total of 84 patients were randomly assigned (arm A, n = 41; arm B, n = 43), and 72 were assessable (arm A, n = 37; arm B, n = 35). The median survival time was 28.7 months for patients in arm A and 14.9 months for patients in arm B (18.8 months for patients in RTOG 9405). The 1-year survival rate of 75.7% in arm A was close to, but did not meet or surpass, the 77.5% goal. The 2-year survival rate was 56% for arm A and 37% for arm B. Grade 3 (arm A = 54%, arm B = 43%) and grade 4 toxicities (arm A = 27%, arm B = 40%) were frequent. Treatment-related death occurred in 3% of patients in arm A and 6% of patients in arm B.
Conclusion
Both arms of RTOG 0113 were associated with high morbidity, and the study did not meet its 1-year survival end point.
INTRODUCTION
Esophageal cancer continues to be a major health burden worldwide.1,2 Obesity, gastroesophageal reflux, and Barrett's metaplasia may be responsible for a rapid increase in the rate ratio of adenocarcinoma of the esophagus over other cancers.3 The 5-year survival rates for esophageal cancer have remained less than 15% over decades, probably because of ineffective therapies and the detection of late-stage cancers.4 In North America, the use of preoperative chemoradiotherapy has been frequent despite the lack of convincing data to demonstrate its efficacy.4-9 Although a meta-analysis demonstrated a benefit for patients who received preoperative chemoradiotherapy compared with patients who did not,10 this matter is considered far from resolved.
Definitive chemoradiotherapy was established as an important therapy for patients with localized esophageal carcinoma.11 Thus, the approach of definitive chemoradiotherapy is appropriate for locally advanced cancer in patients who do not want surgery or in whom surgery is not possible as a result of technical or medical reasons. The issue of higher doses of radiation administered with concurrent chemotherapy was explored in the protocol RTOG 9504.12 In the RTOG 9504 trial, patients were randomly assigned to receive either 64.8 or 50.4 Gy with concurrent fluorouracil and cisplatin. The results demonstrated that the median survival time, 2-year survival rate, and locoregional failure rate were not different for the patients treated in the two arms. In addition, 11 treatment-related deaths occurred in the high-dose arm before the receipt of the intended high dose. Therefore, this study established 50.4 Gy as the standard dose of radiation to be administered concurrently with chemotherapy. The low-dose arm (50.4 Gy) formed the basis for the RTOG 0113 protocol.
When RTOG 0113 was conceived, there was considerable interest in paclitaxel for the treatment of esophageal cancer.13-15 Paclitaxel had been shown to be a potent radiation sensitizer,16,17 and paclitaxel-based chemoradiotherapy for localized esophageal carcinoma had been studied in human cancers.18 Two regimens developed in patients with localized esophageal cancer were of interest, a fluorouracil-based chemoradiotherapy developed at The University of Texas M. D. Anderson Cancer Center19 and a paclitaxel/cisplatin-based chemoradiotherapy developed at Memorial Sloan-Kettering Cancer Center and published later.20 Thus, RTOG 0113 was established to compare these two regimens (one with fluorouracil and one without, but both with paclitaxel) with the historical control from RTOG 9504. Here we report the cooperative group experience of this randomized phase II trial.
PATIENTS AND METHODS
Patient Eligibility
Patients with biopsy-proven squamous cell or adenocarcinoma of the thoracic or cervical esophagus or gastroesophageal junction with cancer that did not extend 2 cm beyond the stomach were eligible. Adequate bone marrow, liver, and renal function were required. In addition, it was necessary that patients have a caloric intake of ≥ 1,700 kCal/d and a Zubrod performance status of 0 or 1. Patients with clinical T1N1M0 or T2-4, N+/–, M0 were eligible. Patients deemed to have technically unresectable cancer, patients who refused to undergo surgery, or those considered medically unfit for surgery were eligible. Patients with tracheoesophageal fistula, evidence of metastatic cancer, lack of comprehension of the protocol, or inability to comply with the requirements of the protocol were not eligible for RTOG 0113.
Pretreatment Evaluation
All patients had a complete history and physical examination performed. They had an assessment of serum chemistry including magnesium level, carcinoembryonic antigen level, and CBC. Computed tomography of the chest and abdomen was obtained. Patients had an upper esophagogastroduodenoscopy with endoscopic ultrasonography. Bronchoscopy was performed when cancer was located less than 26 cm from the incisor. ECG was obtained in all patients. All patients provided an approved informed consent. Institutional review boards of the participating institutions approved this protocol before patient recruitment.
Therapy
Patients were randomly assigned to receive one of two therapies. Arm A (fluorouracil-based therapy) consisted of fluorouracil 700 mg/m2/24 h via an outpatient portable pump on days 1 through 5, cisplatin 15 mg/m2 on days 1 through 5, and paclitaxel 200 mg/m2 as a 24-hour infusion on day 1. Granulocyte colony-stimulating factor or pegfilgrastim was started or administered on day 6. This regimen was repeated once on day 29 provided patients had recovered to grade ≤ 1 of related toxicity and had no evidence of local progression by a radiographic or endoscopic technique. In case of local progression, patients directly proceeded to chemoradiation, but in case of metastatic progression, patients were taken off protocol treatment and observed for survival. During radiation, patients received fluorouracil 300 mg/m2 as continuous infusion for 96 hours (starting Monday morning and ending Friday during each of the 5 radiation therapy weeks) and paclitaxel 50 mg/m2 over 3 hours each of the five Mondays of the radiation weeks. Standard premedications were administered to prevent allergic reaction and significant nausea or vomiting as indicated. Dose modifications (only reductions) were implemented based on guidelines established in section 7 of the protocol (http://www.rtog.org/members/protocols/E0113/E0113.pdf).
Arm B (non–fluorouracil-based therapy) consisted of paclitaxel 175 mg/m2 over 3 hours followed by cisplatin 75 mg/m2 on day 1. This regimen was repeated once on day 21 provided patients had grade ≤ 1 of related toxicity and no evidence of local progression by a radiographic or endoscopic technique. In case of local progression, patients directly proceeded to chemoradiotherapy, but in case of metastatic progression, patients were taken off treatment and observed for survival. During radiation, patients received cisplatin 30 mg/m2 on days 1, 8, 15, 22, 29, and 36 and paclitaxel 60 mg/m2 as a continuous infusion over 96 hours on days 1, 8, 15, 22, 29, and 36. Standard premedications were administered to prevent allergic reaction and significant nausea or vomiting as indicated. Dose modifications (only reductions) were implemented based on guidelines already established.
Radiation therapy was administered using the three-dimensional planning technique. Daily fractions size was 1.8 Gy, and the total dose was 50.4 Gy delivered in 28 fractions. Megavoltage photon energy ≥ 6 MV was used. Computerized imaging was used to define the gross tumor volume (GTV). Locoregional lymph nodes were included in the clinical target volume (CTV). CTV was defined as having a 3-cm cephalad and caudad margin beyond GTV. The planning target volume, defined by the margin around CTV, was established. The planning target volume included up to a 2-cm margin around CTV. The superior and inferior margins were approximately 5 cm beyond the GTV. For cervical primaries, bilateral cervical lymph nodal regions were included. Every effort was made to reduce exposure to lungs, heart, spinal cord, kidney, and liver. Chemotherapy and acute radiotherapy toxicities were recorded by the National Cancer Institute Common Toxicity Criteria version 2.0 (http://ctep.info.nih.gov/). Late radiation therapy effects were recorded by the RTOG/EORTC Late Radiation Morbidity Scoring Schema (http://www.rtog.org/).
Follow-Up Evaluations
Approximately 42 days after the completion of therapy, patients underwent complete history and physical examinations, CBCs, chemistry including magnesium levels, chest radiograph, computed tomography evaluation, and endoscopic evaluation. Patients were then observed every 4 months during the first year and then every 6 months for 2 additional years and then on a yearly basis. Investigations during follow-up included the above mentioned studies, as indicated.
Data Collection and Verification
All data are housed at the RTOG Headquarters and analyzed by its statistical group. A variety of forms (chemotherapy and radiotherapy) developed by RTOG were used to collect data on a specified schedule. Data on all forms were verified for accuracy by the RTOG data managers. Clinicians, representing medical oncology or radiation oncology, reviewed the finalized chemotherapy and radiotherapy forms. Data were analyzed after clinicians’ review.
Statistical Considerations
Patients were stratified according to weight loss (< 10% v ≥ 10%), length of the lesion (≤ 5 v > 5 cm), and histology (squamous cell carcinoma v adenocarcinoma). The primary end point of the study was 1-year overall survival for patients treated by each regimen. Secondary end points included treatment completion and safety. On the basis of a 1-year survival rate of 60% from the RTOG esophageal database, it was decided that either of the two arms would be of interest for study in a phase III trial if the 1-year survival rate was ≥ 77.5%. Thirty-eight assessable patients for each treatment were needed to test this hypothesis, which corresponded to a hazard reduction of 50%, with a one-sided type I error of 0.05% and 80% power.21 A 10% adjustment for data attrition resulted in a sample size of 84 patients. The permuted block randomization method was used with patient factors balanced according to the method described by Zelen.22 Early stopping rules for therapy tolerance (80% of patients in a given arm receive at least three chemotherapy cycles and 90% of the radiation dose) and grade 5 (fatal) toxicity caused by chemotherapy and radiation therapy were evaluated with a significance level of P = .05 after the first 25 assessable patients in each arm were assessed. Failure for overall survival was death as a result of any cause, and time to overall survival was measured from date of study entry to date of death. Overall survival was estimated using the Kaplan-Meier method,23 and each treatment arm was compared with the 50.4-Gy arm of RTOG 9405 using the log-rank test24 at a significance level of P = .05.
RESULTS
Thirty-six RTOG institutions accrued 84 patients between April 2001 and April 2005; 72 patients were assessable. On arm A, two patients were ineligible, and two patients did not receive any protocol treatment. On arm B, there were six ineligible patients, one patient did not receive any protocol treatment, and one patient withdrew consent.
Patient Characteristics
The patient characteristics by the two groups are listed in Table 1. The groups were well balanced for all characteristics assessed. As anticipated, the patient population was predominantly comprised of white men (n = 51) and women (n = 14).
Table 1.
Patient Characteristics
| Characteristic | Fluorouracil-Based Arm (n = 37)
|
Non–Fluorouracil-Based Arm (n = 35)
|
||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| Age, years | ||||
| Median | 61 | 66 | ||
| Range | 41-80 | 28-77 | ||
| Weight loss in last 6 months | ||||
| < 10% | 25 | 68 | 22 | 63 |
| ≥ 10% | 12 | 32 | 12 | 34 |
| Unknown | 0 | 0 | 1 | 3 |
| Sex | ||||
| Male | 28 | 76 | 28 | 80 |
| Female | 9 | 24 | 7 | 20 |
| Tumor size, cm | ||||
| ≤ 5 | 23 | 62 | 22 | 63 |
| > 5 | 14 | 38 | 13 | 37 |
| Zubrod performance status | ||||
| 0 | 19 | 51 | 19 | 54 |
| 1 | 18 | 49 | 16 | 46 |
| Histology | ||||
| Squamous cell | 13 | 35 | 12 | 34 |
| Adenocarcinoma | 24 | 65 | 23 | 66 |
| Extent of dysphagia | ||||
| Asymptomatic | 5 | 14 | 4 | 11 |
| Symptomatic: unrestricted diet | 14 | 38 | 11 | 31 |
| Symptomatic: soft foods only | 13 | 35 | 14 | 40 |
| Symptomatic: liquids only | 3 | 8 | 5 | 14 |
| Cannot swallow | 2 | 5 | 1 | 3 |
| Asymptomatic/symptomatic: unrestricted diet | 19 | 51 | 15 | 43 |
| Symptomatic: soft foods/liquids only/cannot swallow | 18 | 49 | 20 | 57 |
| Primary T classification | ||||
| T1: invasion of lamina propria or submucosa | 1 | 3 | 0 | 0 |
| T2: invasion of muscular propria | 7 | 19 | 11 | 31 |
| T3: invasion of adventia | 27 | 73 | 21 | 60 |
| T4: invasion of adjacent structures | 2 | 5 | 1 | 3 |
| TX | 0 | 0 | 2 | 6 |
| T1/T2 | 8 | 22 | 11 | 33 |
| T3/T4 | 29 | 78 | 22 | 67 |
Treatment Characteristics
Among 94% of patients reviewed for chemotherapy, 91% had chemotherapy administered according to the protocol or with acceptable variation in arm A, and 94% had chemotherapy administered according to the protocol or with acceptable variation in arm B. All patients’ records were reviewed for radiation therapy administration, and 81% of patients in arm A and 83% of patients in arm B received radiation therapy according to the protocol or with acceptable variation.
Survival
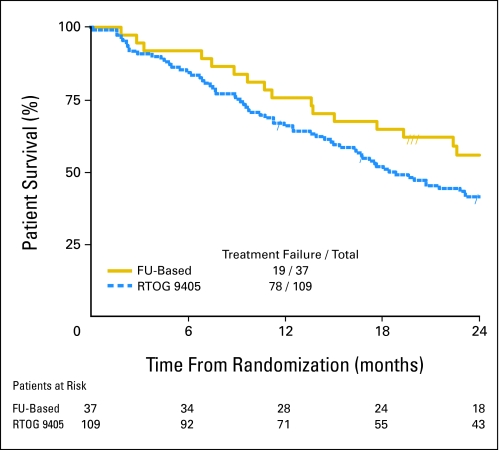
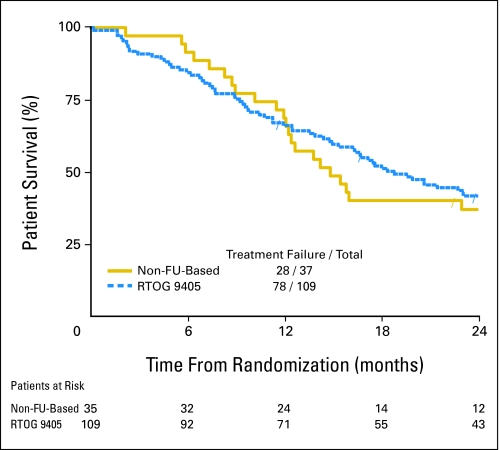
At 1 year, 76% of patients in arm A (fluorouracil-based therapy) and 69% of patients in arm B (no fluorouracil) were alive. Neither arm achieved the hypothesized 1-year survival rate of at least 77.5%. At 2-years, 56% of patients in arm A and 37% of patients in arm B were alive. The median survival time of patients was 29 months (95% CI, 18 months to not calculable) in arm A and 15 months (95% CI, 12 to 26 months) in arm B. The median survival time of the historical arm12 (50.4 Gy with fluorouracil plus cisplatin in RTOG 9405) was 19 months (95% CI, 15 to 25 months). Neither arm A (P = .103; Fig 1) nor arm B (P = .165; Fig 2) showed a statistically significant improvement in overall survival when compared with the 50.4-Gy arm of RTOG 9405. More details are listed in Table 2. The median follow-up time is 34 months for the 16 patients who are still alive in arm A and 39 months for the eight patients who are still alive in arm B.
Fig 1.
Kaplan-Meier survival plots for overall survival of the fluorouracil (FU)–based arm versus the 50.4-Gy arm of the Radiation Therapy Oncology Group (RTOG) 9405 protocol.
Fig 2.
Kaplan-Meier survival plots for overall survival of the non–fluorouracil (FU)–based arm versus the 50.4-Gy arm of the Radiation Therapy Oncology Group (RTOG) 9405 protocol.
Table 2.
Overall Survival of the Two Arms and Historical Control
| Measure | Fluorouracil-Based Arm
|
Non–Fluorouracil-Based Arm
|
RTOG 9405 Arm 2
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % Alive | 95% CI (%) | Cumulative Deaths (No.) | No. of Patients at Risk | % Alive | 95% CI (%) | Cumulative Deaths (No.) | No. of Patients at Risk | % Alive | 95% CI (%) | Cumulative Deaths (No.) | No. of Patients at Risk | |
| Months | ||||||||||||
| 0 | 100.0 | 0 | 37 | 100.0 | 0 | 35 | 100.0 | 0 | 109 | |||
| 6 | 91.9 | 76.9 to 97.3 | 3 | 34 | 91.4 | 75.7 to 97.2 | 3 | 32 | 84.4 | 76.1 to 90.0 | 17 | 92 |
| 12 | 75.7 | 58.5 to 86.5 | 9 | 28 | 68.6 | 50.5 to 81.2 | 12 | 24 | 66.0 | 56.3 to 74.1 | 37 | 71 |
| 18 | 64.9 | 47.3 to 77.9 | 13 | 24 | 40.0 | 24.0 to 55.5 | 22 | 14 | 52.0 | 42.2 to 60.9 | 52 | 55 |
| 24 | 55.9 | 38.4 to 70.3 | 15 | 18 | 36.9 | 21.4 to 52.5 | 24 | 12 | 41.6 | 32.2 to 50.7 | 63 | 43 |
| Total deaths | 21 | 27 | 78 | |||||||||
| Median survival time, months | 28.7 | 14.9 | 18.8 | |||||||||
| 95% CI | 17.7 to NC | 12.1 to 26.4 | 15.4 to 25.1 | |||||||||
| P* | .1038 | .1648 | ||||||||||
Abbreviations: RTOG, Radiation Therapy Oncology Group; NC, not calculable.
Treatment comparisons versus RTOG 9405 arm 2 historical control (one-sided log-rank test).
Safety
Chemotherapy and acute radiotherapy toxicities are listed in Table 3. Grade 3 toxicity occurred in 54% of patients in arm A and 40% of patients in arm B. Grade 4 toxicity occurred in 27% of patients in arm A and 40% of patients in arm B. GI grade 3 or 4 toxicities were similar in both arms (arm A = 54% and arm B = 60%). Febrile neutropenia occurred in 22% of arm A patients and 17% of arm B patients. Grade 3 or 4 myelotoxicity occurred in 38% of patients in arm A and 69% of patients in arm B.
Table 3.
Chemotherapy and Acute Radiotherapy Toxicity
| Toxicity | Fluorouracil-Based Arm (n = 37)Grade (No. of patients)
|
Non–Fluorouracil-Based Arm (n = 35)Grade (No. of patients)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | |
| Allergy/immunology | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Auditory/hearing | 1 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 |
| Blood/bone marrow | 4 | 17 | 5 | 9 | 0 | 5 | 5 | 14 | 10 | 0 |
| Cardiovascular, arrhythmia | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 |
| Cardiovascular, general | 2 | 3 | 4 | 2 | 0 | 4 | 3 | 2 | 1 | 0 |
| Constitutional symptoms | 10 | 12 | 9 | 0 | 0 | 6 | 16 | 6 | 3 | 0 |
| Dermatology/skin | 9 | 17 | 1 | 0 | 0 | 5 | 14 | 0 | 0 | 0 |
| GI | 5 | 12 | 18 | 2 | 0 | 4 | 8 | 15 | 6 | 0 |
| Hemorrhage | 1 | 0 | 1 | 0 | 1 | 5 | 0 | 0 | 0 | 0 |
| Hepatic | 8 | 1 | 2 | 0 | 0 | 5 | 5 | 0 | 1 | 0 |
| Infection/febrile neutropenia | 0 | 1 | 8 | 0 | 0 | 2 | 4 | 5 | 0 | 1 |
| Lymphatics | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Metabolic/laboratory | 8 | 7 | 5 | 2 | 0 | 5 | 2 | 8 | 1 | 0 |
| Musculoskeletal | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 |
| Neurology | 7 | 4 | 4 | 0 | 0 | 10 | 4 | 4 | 0 | 0 |
| Ocular/visual | 2 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 |
| Pain | 12 | 6 | 1 | 0 | 0 | 5 | 8 | 4 | 0 | 0 |
| Pulmonary | 4 | 6 | 1 | 0 | 0 | 2 | 3 | 1 | 0 | 0 |
| Renal/genitourinary | 5 | 2 | 0 | 0 | 0 | 4 | 3 | 0 | 0 | 0 |
| Worst nonhematologic | 1 | 6 | 25 | 4 | 1 | 1 | 8 | 17 | 8 | 1 |
| % of patients | 3 | 16 | 68 | 11 | 3 | 3 | 23 | 49 | 23 | 3 |
| Worst overall | 0 | 6 | 20 | 10 | 1 | 0 | 5 | 15 | 14 | 1 |
| % of patients | 0 | 16 | 54 | 27 | 3 | 0 | 14 | 43 | 40 | 3 |
Late grade 3 or 4 radiation toxicities are listed in Table 4 and occurred in 8% of patients in arm A and 12% of patients in arm B. The majority of late radiation toxicities were related to esophageal injury. There was one treatment-related death in arm A (GI hemorrhage during the concurrent phase) and two treatment-related deaths in arm B (neutropenic sepsis after completion of induction chemotherapy and upper GI bleed 6 months after treatment completion).
Table 4.
Late Radiotherapy Toxicity
| Toxicity | Fluorouracil-Based Arm (n = 37)Grade (No. of patients)
|
Non–Fluorouracil-Based Arm (n = 34)Grade (No. of patients)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 5 | |
| Bone | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Esophagus | 9 | 1 | 2 | 1 | 5 | 3 | 3 | 0 | 1 |
| Larynx | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lung | 5 | 1 | 0 | 0 | 3 | 1 | 1 | 1 | 0 |
| Skin | 2 | 0 | 0 | 0 | 1 | 4 | 0 | 0 | 0 |
| Other | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Worst overall | 11 | 4 | 2 | 1 | 5 | 6 | 3 | 1 | 1 |
| % of patients | 30 | 11 | 5 | 3 | 15 | 18 | 9 | 3 | 3 |
DISCUSSION
Localized carcinoma of the esophagus is often treated with preoperative chemoradiotherapy25; however, when patients have unresectable cancer (T4 or locoregional IVB), an inoperable condition as a result of significant comorbid conditions, or a desire not to undergo surgery, then definitive chemoradiotherapy is an appropriate alternative to preoperative chemoradiotherapy followed by surgery.26-28 In addition, for patients with cervical esophageal carcinoma and those with high thoracic squamous cell carcinoma, definitive chemoradiation might be a preferred option.26-28 The outcome of patients who are treated with combined-modality therapy remains poor and needs considerable improvement.25 In the mid-1990s, there was considerable interest in paclitaxel, and this led to investigations of paclitaxel-based combinations.19,20 The investigators at the Memorial Sloan-Kettering Cancer Center had developed non–fluorouracil-based chemoradiotherapy for esophageal cancer20 because of the concerns over and a demonstrated high rate of stomatitis in a multi-institutional study.13 Thus, one of the end points of this study was to assess whether the fluorouracil-based strategy (induction chemotherapy and chemoradiotherapy) was as well tolerated as the non–fluorouracil-based strategy. One goal was to select one of these two strategies for a future phase III nonoperative chemoradiotherapy trial. A phase II comparative trial was designed in which the efficacy (as defined by 1-year survival rate) was to be compared with the historical control (low-dose arm of the previous RTOG study, RTOG 9405). The chosen historical control had a 1-year survival rate of 66%, and it was felt that if one or both arms of the current trial (RTOG 0113) could achieve ≥ 77.5% 1-year survival rate, then consideration might be given to such treatment in the development of a future phase III trial.
The results of RTOG 0113 demonstrate that although such intense therapies are feasible in a multi-institutional setting, they are associated with considerable morbidity (> 80% rate of grade 3 or 4 toxicity). Also, neither of the two arms achieved the desired 1-year survival mark. Therefore, we do not recommend that either of these two arms become the experimental arm of a future nonoperative esophageal cancer chemoradiotherapy trial. This trial was not designed to statistically compare the efficacy of the two randomized treatment arms. However, some of these results are intriguing. The fluorouracil-based arm approached the desired 1-year survival rate of 77.5% (75%; median survival time, 29 months; 2-year survival rate, 56%), but the non–fluorouracil-based arm did not (1-year survival rate, 69%; median survival time, 15 months; 2-year survival rate, 37%). In addition, the fluorouracil-based arm resulted in less grade 4 toxicity and less treatment-related mortality compared with the non–fluorouracil-based arm. The fluorouracil-based arm also did not result in the anticipated higher rate of esophagitis compared with the non–fluorouracil-based arm. Given the efficacy and safety data, it may be that fluorouracil should continue to be incorporated in the treatment of localized esophageal cancer.
In conclusion, in RTOG 0113, neither of the two treatments proved to be sufficiently superior to the historical control of RTOG 9405 to warrant further investigation. Both arms resulted in an unacceptably high level of morbidity. Studies are currently underway to test the hypothesis that the addition of a biologic agent to chemotherapy and radiation would improve the outcome for patients with esophageal cancer. Future trials should also focus on developing a greater understanding of the molecular biology of esophageal cancer and patient genetics to guide individualized therapy.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Jaffer A. Ajani, Ritsuko Komaki, David P. Kelsen, Bruce D. Minsky
Administrative support: Jaffer A. Ajani, Kathryn Winter, David P. Kelsen, Christopher G. Willett
Provision of study materials or patients: Ritsuko Komaki, David P. Kelsen, Bruce D. Minsky, Zhongxing Liao, Jeffrey Bradley, Mitchel Fromm, David Hornback
Collection and assembly of data: Kathryn Winter
Data analysis and interpretation: Jaffer A. Ajani, Kathryn Winter
Manuscript writing: Jaffer A. Ajani, Kathryn Winter, Ritsuko Komaki, David P. Kelsen, Bruce D. Minsky, Zhongxing Liao, Jeffrey Bradley, Mitchel Fromm, David Hornback, Christopher G. Willett
Final approval of manuscript: Jaffer A. Ajani
published online ahead of print at www.jco.org on June 23, 2008
Supported by Grant Nos. CA21661, CA37422, and 32115 from the National Cancer Institute.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al: Cancer statistics, 2007. CA Cancer J Clin 57:43-66, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, et al: Global cancer statistics, 2002. CA Cancer J Clin 55:74-108, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Pohl H, Welch HG: The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst 97:142-146, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Enzinger PC, Mayer R: Esophageal cancer. N Engl J Med 349:2241-2252, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Bosset JF, Gignoux M, Triboulet JP, et al: Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. N Engl J Med 337:161-167, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Burmeister BH, Smithers BM, Gebski V, et al: Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: A randomised controlled phase III trial. Lancet Oncol 6:659-668, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Kelsen DP: Multimodality therapy of local regional esophageal cancer. Semin Oncol 32:S6-S10, 2005. (suppl 9) [DOI] [PubMed] [Google Scholar]
- 8.Urba SG, Orringer MB, Turrisi A, et al: Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol 19:305-313, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Tepper J, Krasna MJ, Niedzwiecki D, et al: Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 26:1086-1092, 2008. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrive&db=PubMed&dopt=Citation&list_uids=18309943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gebski V, Burmeister B, Smithers BM, et al: Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: A meta-analysis. Lancet Oncol 8:226-234, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Cooper JS, Guo MD, Herskovic A, et al: Chemoradiotherapy of locally advanced esophageal cancer: Long-term follow-up of a prospective randomized trial (RTOG 85-01)—Radiation Therapy Oncology Group. JAMA 281:1623-1627, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Minsky BD, Pajak TF, Ginsberg RJ, et al: INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: High-dose versus standard-dose radiation therapy. J Clin Oncol 20:1167-1174, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Ilson DH, Ajani J, Bhalla K, et al: Phase II trial of paclitaxel, fluorouracil, and cisplatin in patients with advanced carcinoma of the esophagus. J Clin Oncol 16:1826-1834, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Ajani JA, Ilson DH, Kelsen DP: Paclitaxel in the treatment of patients with upper gastrointestinal carcinomas. Semin Oncol 23:55-58, 1996 [PubMed] [Google Scholar]
- 15.Ajani JA, Ilson DH, Daugherty K, et al: Activity of Taxol in patients with squamous cell carcinoma and adenocarcinoma of the esophagus. J Natl Cancer Inst 86:1086-1091, 1994 [DOI] [PubMed] [Google Scholar]
- 16.Sinclair WK, Morton RA: X-ray sensitivity during the cell generation cycle of cultured Chinese hamster cells. Radiat Res 29:450-474, 1966 [PubMed] [Google Scholar]
- 17.Terasima T, Tolmachi LJ: X-ray sensitivity and DNA synthesis in synchronized populations of HeLa cells. Science 140:490-492, 1963 [DOI] [PubMed] [Google Scholar]
- 18.Choy H, Akerley H, Safran H, et al: Phase I trial of outpatient weekly paclitaxel and concurrent radiation therapy for advanced non-small cell lung cancer. J Clin Oncol 12:2682-2686, 1994 [DOI] [PubMed] [Google Scholar]
- 19.Schnirer II, Komaki R, Yao JC, et al: Pilot study of concurrent 5-fluorouracil/paclitaxel plus radiotherapy in patients with carcinoma of the esophagus and gastroesophageal junction. Am J Clin Oncol 24:91-95, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Brenner B, Ilson DH, Minsky BD, et al: Phase I trial of combined-modality therapy for localized esophageal cancer: Escalating doses of continuous-infusion paclitaxel with cisplatin and concurrent radiation therapy. J Clin Oncol 22:45-52, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Dixon DO, Simon R: Sample size considerations for studies comparing survival curves using historical controls. J Clin Epidemiol 41:1209-1213, 1988 [DOI] [PubMed] [Google Scholar]
- 22.Zelen M: The randomization and stratification of patients to clinical trials. J Chron Dis 27:365-375, 1974 [DOI] [PubMed] [Google Scholar]
- 23.Kaplan EL, Meier P: Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457-481, 1958 [Google Scholar]
- 24.Mantel N: Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 50:163-170, 1966 [PubMed] [Google Scholar]
- 25.Kleinberg L, Forastiere AA: Chemoradiation in the management of esophageal cancer. J Clin Oncol 25:4110-4117, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Urba S: Combined modality therapy of esophageal cancer: Standard of care? Surg Oncol Clin N Am 11:377-386, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Ajani J, Bekaii-Saab T, D'Amico TA, et al: Esophageal Cancer Clinical Practice Guidelines. J Natl Compr Canc Netw 4:328-347, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Das P, Fukami N, Ajani JA: Combined modality therapy of localized gastric and esophageal cancers. J Natl Compr Canc Netw 4:375-382, 2006 [DOI] [PubMed] [Google Scholar]