Abstract
Purpose
RAS mutations occur in 12% to 27% of patients with acute myeloid leukemia (AML) and enhance sensitivity to cytarabine in vitro. We examined whether RAS mutations impact response to cytarabine in vivo.
Patients and Methods
One hundred eighty-five patients with AML achieving complete remission on Cancer and Leukemia Group B study 8525 and randomly assigned to one of three doses of cytarabine postremission were screened for RAS mutations. We assessed the impact of cytarabine dose on cumulative incidence of relapse (CIR) of patients with (mutRAS) and without (wild-type; wtRAS) RAS mutations.
Results
Thirty-four patients (18%) had RAS mutations. With 12.9 years median follow-up, the 10-year CIR was similar for mutRAS and wtRAS patients (65% v 73%; P = .31). However, mutRAS patients receiving high-dose cytarabine consolidation (HDAC; 3 g/m2 every 12 hours on days 1, 3, and 5 or 400 mg/m2/d × 5 days) had the lowest 10-year CIR, 45%, compared with 68% for wtRAS patients receiving HDAC and 80% and 100%, respectively, for wtRAS and mutRAS patients receiving low-dose cytarabine (LDAC; 100 mg/m2/d × 5 days; overall comparison, P < .001). Multivariable analysis revealed an interaction of cytarabine dose and RAS status (P = .06). After adjusting for this interaction and cytogenetics (core binding factor [CBF] AML v non-CBF AML), wtRAS patients receiving HDAC had lower relapse risk than wtRAS patients receiving LDAC (hazard ratio [HR] = 0.67; P = .04); however, mutRAS patients receiving HDAC had greater reduction in relapse risk (HR = 0.28; P = .002) compared with mutRAS patients treated with LDAC.
Conclusion
AML patients carrying mutRAS benefit from higher cytarabine doses more than wtRAS patients. This seems to be the first example of an activating oncogene mutation favorably modifying response to higher drug doses in AML.
INTRODUCTION
Activating mutations in the RAS proto-oncogenes occur frequently in many types of human cancer,1 including myelodysplastic syndromes2 and acute myeloid leukemia (AML).3-11 In de novo AML, between 12% and 27% of patients harbor RAS mutations.4-11 Moreover, in patients without RAS mutations, the RAS-dependent pathways are also frequently affected by relatively frequent mutations in other genes (ie, FLT3, KIT, and PDGFR).12 These mutations and those in RAS activate pro-proliferative and antiapoptotic signals critical for myeloid leukemogenesis and are often referred to as class I mutations.13 In addition, AML is often characterized by class II mutations that involve transcription factor signaling that impairs hematopoietic differentiation, frequently via gene fusions generated by balanced chromosomal rearrangements, such as RUNX1(AML1)-RUNX1T1(ETO) and t(8;21)(q22;q22), CBFB-MYH11 and inv(16) (p13q22)/t(16;16)(p13;q22), or PML-RARA and t(15;17)(q22;q12).12 Mouse models show that these fusion genes rarely cause overt leukemia on their own unless complemented by class I mutations.14
Although mutations in RAS are frequent in AML and seem to contribute to leukemogenesis in a subset of patients, their prognostic significance has not been firmly established. Some reports have suggested that patients with AML having RAS mutations have worse7-9 or similar4,5,10,11,15-17 clinical outcomes than patients carrying wild-type RAS genes, whereas others have found that mutations in RAS are associated with a more favorable prognosis.5,6 Although these conflicting results may stem from variation in the pretreatment features of patient populations analyzed in different series, they may also be related to differences in treatment regimens used.
AML is initially treated with induction chemotherapy, frequently consisting of standard-dosage cytarabine and an anthracycline (eg, daunorubicin). Patients entering complete remission are then treated with either chemotherapy or autologous or allogeneic stem-cell transplantation (SCT), depending on individual risk profiles. After publication of the Cancer and Leukemia Group B (CALGB) 8525 treatment trial, multicycle high-dose cytarabine has become preferential postinduction chemotherapy for patients not receiving SCT.18 Importantly, it seems that certain subsets of patients benefit from this therapy more than others. Indeed, several studies have shown that both t(8;21) and inv(16) sensitize AML blasts to high-dose cytarabine given as consolidation therapy.19-21 However, it is at present unknown whether other genetic alterations also influence response of patients with AML to treatment with high-dose cytarabine.
Intriguingly, in vitro data have suggested that mutant RAS proto-oncogenes may sensitize leukemia and carcinoma cells to cytarabine.22,23 Therefore, to determine whether RAS mutation status also influences response to cytarabine in vivo, we have analyzed retrospectively the outcome of patients with AML with and without RAS mutations enrolled onto a single treatment study, CALGB 8525. Our data show that the presence of RAS mutations sensitize AML cells to high-dose cytarabine therapy in vivo, suggesting that patients with AML having RAS mutations treated with chemotherapy alone should preferentially be administered high-dose cytarabine as postremission treatment.
PATIENTS AND METHODS
Patients
We studied 185 adult patients with a primary diagnosis of AML (excluding acute promyelocytic leukemia) who were enrolled onto the CALGB treatment trial 8525.18 CALGB 8525 was a study comparing the duration of complete remission and overall survival in patients treated postremission with high, intermediate, or standard doses of cytarabine.18 Only those patients for whom the RAS mutation status was determined, who had a successful cytogenetic and/or molecular genetic analysis of a pretreatment sample that allowed determination of whether patients had or did not have core binding factor (CBF) AML (ie, whether they were positive or negative for t(8;21)/RUNX1-RUNX1T1 and inv(16)/t(16;16)/CBFB-MYH11),24 achieved a complete remission, and were randomly assigned to one of three consolidation treatment arms on CALGB 8525 were eligible for inclusion in this study. There was no significant difference in outcome between the 185 patients included in the current analysis and those who were not included because of lack of available tissue (P > .68). Written, institutional review board–approved, protocol-specific informed consent was obtained when each patient entered the study.
Treatment
On CALGB 8525, patients 60 years of age or younger received induction chemotherapy of daunorubicin 45 mg/m2/d intravenously for 3 days and cytarabine 200 mg/m2/d as a continuous infusion for 7 days, whereas patients older than 60 years received daunorubicin 30 mg/m2/d intravenously for 3 days and cytarabine 200 mg/m2/d as a continuos infusion for 7 days. Those who attained a complete remission after one or two courses of induction therapy were randomly assigned to one of three postinduction arms that differed with regard to dose-intensity of cytarabine. These arms included four cycles of (1) 100 mg/m2 of cytarabine as a continuous infusion × 5 days, (2) 400 mg/m2 of cytarabine as a continuous infusion × 5 days, or (3) 3 g/m2 of cytarabine over 3 hours every 12 hours on days 1, 3, and 5. In each case, this was followed by maintenance treatment consisting of four monthly treatments with cytarabine (100 mg/m2 every 12 hours) for 5 days by subcutaneous injection and daunorubicin 45 mg/m2 on the first treatment day.18 Among the 185 patients included in the current analysis, there was no significant difference in cumulative incidence of relapse (CIR) between the 400-mg and 3-g cytarabine arms, whereas patients on the 100-mg arm had significantly higher CIR as compared with that of the patients on the 400-mg arm or those on the 3-g arm. Therefore, patients receiving postremission therapy on the 400-mg and 3-g cytarabine arms were combined into one high-dose cytarabine group (HDAC) for subsequent comparison of their CIR with that of patients who were in the 100-mg cytarabine arm, referred to as the low-dose cytarabine (LDAC) group. No patient received SCT in first complete remission.
DNA Extraction and RAS Mutations Detection
The genetic analyses were performed as part of CALGB companion protocols 8361 and 8765, which procured blood and bone marrow samples prospectively on patients entered on CALGB treatment studies. All molecular analyses were conducted in a blinded fashion on DNA extracted from cryopreserved cells taken at the time of diagnosis. Screening for RAS mutations was performed initially using single-strand conformation polymorphism analysis and a slot blot technique,6,25 and subsequently a denaturing high-performance liquid chromatography method11,26 and polymerase chain reaction enrichment assay based on PNA-mediated clamping.27 The inclusion of the positive and negative controls in all runs suggested similar performance by the two approaches. All suspected mutations identified by the screening techniques were confirmed by sequence analysis either directly or after subcloning.
Statistical Analysis
The purpose of the study was to determine whether mutations in the RAS proto-oncogenes influenced response to different doses of postremission cytarabine in vivo. The primary end point was CIR, with time calculated from date of complete remission until relapse. Patients alive without relapse were censored, whereas those who died without relapse were counted as a competing cause of failure. The secondary end point was survival, which was measured from the date of diagnosis until death or date last known alive, censoring for patients alive at last follow-up. Estimated probabilities for survival were calculated using the Kaplan-Meier method, and the log-rank test evaluated differences between survival distributions.
Fisher's exact and Wilcoxon rank sum tests compared, respectively, categoric and continuous variables. Estimates of CIR were calculated, and Gray's k-sample test28 was used to evaluate differences in relapse rates for the following variables: RAS mutation, age, sex, race, hemoglobin, platelets, WBC count, percentage of blood blasts, percentage of bone marrow blasts, cytogenetic group (CBF AML v non-CBF AML), spleen involvement, liver involvement, and consolidation treatment (LDAC v HDAC). Gray's method was constructed to build a multivariable CIR model using a limited backwards selection procedure.29 Variables significant at α = .20 from the univariable analyses were considered for multivariable analyses. Estimates for hazard ratios (HR) and corresponding 95% CIs were obtained for each significant prognostic factor. Adjusted CIR curves were generated using average covariate values from the multivariable CIR model.
An interaction term evaluating the differential effect of HDAC by RAS mutational status was included in the final multivariable CIR model and in the resultant adjusted CIR plots, supporting the primary hypothesis of the study. All analyses were performed by the CALGB Statistical Center.
RESULTS
Clinical Characteristics and Treatment
Mutations in RAS were detected in leukemia cells from 34 patients (18%), with the remaining 151 patients (82%) having wild-type RAS alleles. In Table 1, we compare pretreatment features of patients with and without RAS mutations. Patients with RAS mutations were more often nonwhite (P = .02) and had lower percentages of bone marrow blasts (P = .03). Other pretreatment characteristics were similarly distributed between the two groups (Table 1). In addition, there was no difference in the percentage of patients treated with LDAC or HDAC between the RAS mutated and RAS wild-type groups (Table 1).
Table 1.
Pretreatment Characteristics, Treatment, and Clinical Outcome of Patients With and Without RAS Gene Mutations Among 185 Patients With Acute Myeloid Leukemia Studied
| Characteristic | Mutated RAS (n = 34)
|
Wild-Type RAS (n = 151)
|
P | ||
|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | ||
| Age, years | .48 | ||||
| Median | 42 | 43 | |||
| Range | 17-78 | 18-77 | |||
| > 60 | 5 | 15 | 34 | 23 | .36 |
| Male sex | 50 | 52 | .85 | ||
| Race | .02* | ||||
| White | 25 | 74 | 135 | 89 | |
| African American | 7 | 21 | 9 | 6 | |
| Other | 2 | 6 | 7 | 5 | |
| Hemoglobin, g/dL | .86 | ||||
| Median | 9.4 | 9.3 | |||
| Range | 5.3-12.5 | 4.5-14.2 | |||
| Platelet count, × 109/L | .59 | ||||
| Median | 53 | 51 | |||
| Range | 12-401 | 11-433 | |||
| WBC count, × 109/L | .63 | ||||
| Median | 36.1 | 29.7 | |||
| Range | 4-229 | 0.5-500.0 | |||
| Percentage of PB blasts | .40 | ||||
| Median | 72 | 70 | |||
| Range | 4-87 | 0-99 | |||
| Percentage of BM blasts | .03 | ||||
| Median | 68 | 75 | |||
| Range | 15-93 | 14-97 | |||
| FAB | |||||
| M0 | 0 | 0 | 1 | < 1 | |
| M1 | 1 | 3 | 37 | 25 | |
| M2 | 14 | 41 | 51 | 34 | |
| M4 | 8 | 24 | 31 | 21 | |
| M5 | 5 | 15 | 20 | 13 | |
| M6 | 1 | 3 | 2 | 1 | |
| Cytogenetic group | .47 | ||||
| CBF | 8 | 24 | 27 | 18 | |
| non-CBF | 26 | 76 | 124 | 82 | |
| Extramedullary involvement | |||||
| CNS | 0 | 0 | 2 | 1 | 1.00 |
| Hepatomegaly | 3 | 9 | 10 | 7 | .71 |
| Splenomegaly | 5 | 15 | 11 | 7 | .18 |
| Lymphadenopathy | 7 | 21 | 22 | 15 | .44 |
| Skin Infiltrates | 4 | 12 | 15 | 10 | .76 |
| Gingival hypertrophy | 5 | 15 | 24 | 16 | 1.00 |
| Consolidation treatment | 1.00 | ||||
| LDAC | 12 | 35 | 56 | 37 | |
| HDAC | 22 | 65 | 95 | 63 | |
| Relapse | 22 | 65 | 110 | 73 | .40 |
| Death in first CR | 3 | 9 | 9 | 6 | .46 |
| CIR† | .31 | ||||
| Median, years | 1.5 | 1.1 | |||
| % Relapsed, 10 years | 65 | 73 | |||
| 95% CI | 48 to 82 | 66 to 88 | |||
Abbreviations: PB, peripheral blood; BM, bone marrow; FAB, French-American-British classification; CBF, core binding factor; LDAC, low-dose cytarabine (cytarabine 100 mg/m2 as a continuous infusion × 5 days); HDAC, high-dose cytarabine (cytarabine 3 g/m2 by intravenous bolus over 3 hours every 12 hours on days 1, 3, and 5, or cytarabine 400 mg/m2/d as a continuous infusion × 5 days); CR, complete remission; CIR, cumulative incidence of relapse.
Comparison of white patients with nonwhite patients.
The median follow-up for patients alive (n = 55) is 12.9 years, ranging from 4.3 to 18.8 years.
Clinical Outcome
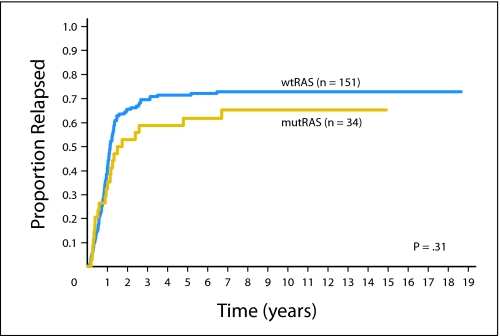
There was no significant difference in CIR when patients who had RAS mutations were compared with those who had wild-type RAS (Table 1 and Appendix Fig A1, online only). With a median follow-up of 12.9 years (range, 4.3 to 18.8 years), the estimated 10-year CIR for patients with RAS mutations was 65% (95% CI, 48% to 82%) compared with 73% (95% CI, 66% to 88%) for those with wild-type RAS (P = .31; Table 1).
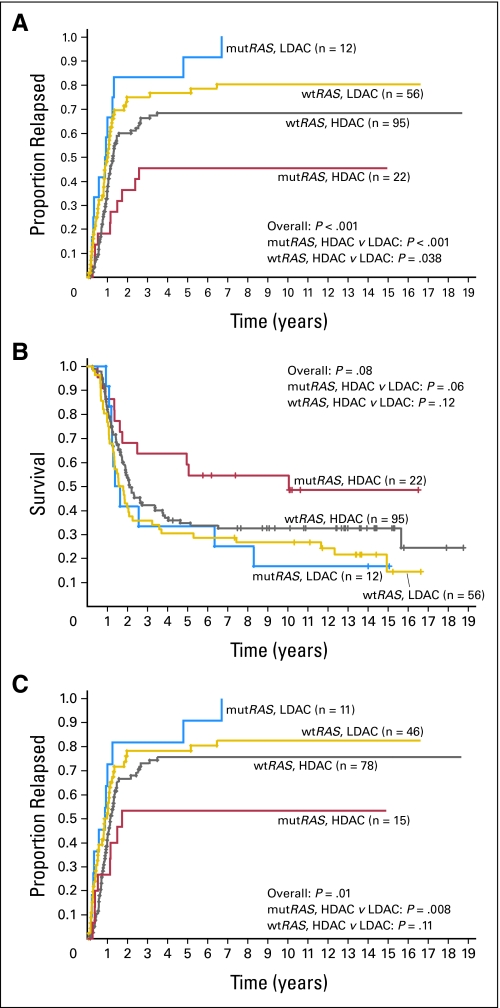
When both the RAS status and consolidation therapy were taken into account (Fig 1A), RAS-mutated patients treated with HDAC had a lower rate of relapse than RAS-mutated patients treated with LDAC (P < .001; 10-year CIR, 45% v 100%). Patients with wild-type RAS also benefited from HDAC, but to a lesser extent (P = .038; 10-year CIR, 68% v 80%). Among patients treated with HDAC, those with RAS mutations experienced relapse less frequently than patients with wild-type RAS (P = .05; 10-year CIR, 45% v 68%; Fig 1A). In contrast, among patients treated with LDAC, there was no significant difference in CIR between patients with and without RAS mutations (P = .20; 10-year CIR, all patients experienced relapse within 7 years v 80%). Similar results were also observed in a subset analysis of patients who had a normal karyotype. In this subset, patients with RAS mutations in the HDAC group experienced relapse at a lower rate than those with RAS mutations in the LDAC group (P = .02), whereas the effect of consolidation dose on outcome did not reach significance among patients with wild-type RAS (P = .13).
Fig 1.
Outcome of 185 patients with acute myeloid leukemia (AML) according to RAS mutation status (mutRAS, mutated RAS; wtRAS, wild-type RAS) and random assignment to consolidation treatment with high-dose cytarabine (HDAC) or low-dose cytarabine (LDAC). (A) Cumulative incidence of relapse of all patients included in this study. (B) Survival of all patients included in this study. (C) Cumulative incidence of relapse of patients with non–core binding factor AML (ie, those who did not harbor t(8;21)/RUNX1-RUNX1T1 or inv(16)/t(16;16)/CBFB-MYH11).
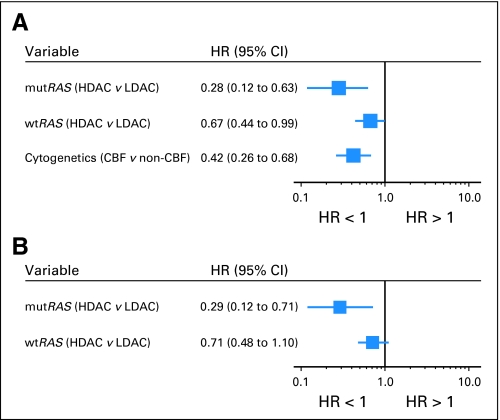
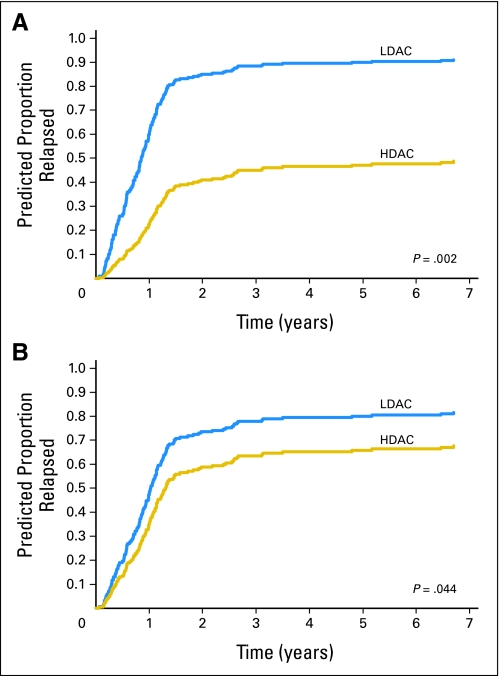
A trend for survival similar to that of CIR was also observed for the entire cohort of patients included in this analysis (Fig 1B). Among patients with mutated RAS, those in the HDAC group tended to have a better survival than patients in the LDAC group, whereas among patients with wild-type RAS, there was no substantial benefit from receiving HDAC. For multivariable analysis for CIR, RAS mutation (mutated v wild-type), consolidation therapy (HDAC v LDAC), percentage of bone marrow blasts, WBC count, cytogenetic group (CBF AML v non-CBF AML), and the interaction of RAS mutation and consolidation therapy fulfilled the inclusion criteria. The final multivariable model included RAS mutation status, cytogenetic group, consolidation therapy, and the interaction of RAS mutation and consolidation therapy. The difference in the consolidation dose of cytarabine had greater impact on patients with RAS mutations than on those with wild-type RAS (Table 2 and Fig 2A). In the same model, CBF status also predicted a lower CIR (Table 2 and Fig 2A). Once adjusting for CBF status (P < .001), among patients with mutated RAS, those in the HDAC group had a markedly lower relapse rate compared with those in the LDAC group (HR = 0.28; P = .002; Table 2 and Fig 3A). The impact of HDAC consolidation on CIR in patients with wild-type RAS was substantially less (HR = 0.67; P = .044; Table 2 and Fig 3B). Notably, the only group in which more than 50% of patients remained in remission was that with mutated RAS treated with HDAC.
Table 2.
Final Multivariable Model for Cumulative Incidence of Relapse
| Variable | Hazard Ratio | 95% CI | P |
|---|---|---|---|
| Interaction of RAS status and consolidation | .06 | ||
| Mutated RAS | |||
| HDAC v LDAC | 0.28 | 0.12 to 0.63 | .002 |
| Wild-type RAS | |||
| HDAC v LDAC | 0.67 | 0.44 to 0.99 | .044 |
| Cytogenetic group | |||
| CBF AML v non-CBF AML | 0.42 | 0.26 to 0.68 | < .001 |
NOTE. Variables considered for model inclusion were RAS mutation status (wild-type v mutated), WBC, % bone marrow blasts, cytogenetic group, consolidation therapy group (HDAC v LDAC), and the RAS mutation-consolidation therapy interaction term.
Abbreviations: HDAC, high-dose cytarabine; LDAC, low-dose cytarabine; CBF, core binding factor; AML, acute myeloid leukemia.
Fig 2.
Forest plots summarizing the multivariable models for cumulative incidence of relapse of (A) all 185 patients with acute myeloid leukemia (AML) and (B) 150 patients with non–core binding factor AML. The hazard ratios (HRs) with 95% CIs for each variable in the model are shown. HRs less than 1 indicate lower risk for relapse for the first category listed. mutRAS, mutated RAS; wtRAS, wild-type RAS; HDAC, high-dose cytarabine; LDAC, low-dose cytarabine.
Fig 3.
Predicted cumulative incidence of relapse of patients with acute myeloid leukemia receiving high-dose cytarabine (HDAC) and low-dose cytarabine (LDAC) postremission therapy according to RAS mutation status (mutated or wild-type) adjusting for core binding factor status. (A) Patients with RAS mutation. Model was based on 22 patients on the HDAC arm and 12 patients on the LDAC arm. (B) Patients with wild-type RAS. Model was based on 95 patients on the HDAC arm and 56 patients on the LDAC arm.
Because CBF status was predictive of a lower CIR in addition to postremission cytarabine dose (Table 2 and Fig 2A), we also performed an analysis restricted to patients with non-CBF AML (ie, those who did not harbor t(8;21)/RUNX1-RUNX1T1 or inv(16)/t(16;16)/CBFB-MYH11). Consistent with the results of our overall analysis, the patients with non-CBF AML with mutated RAS treated with HDAC had the lowest CIR (Fig 1C). In multivariable analysis (Table 3 and Fig 2B), these patients had a significantly lower relapse rate than the patients with non-CBF AML with mutated RAS who were randomly assigned to LDAC (HR = 0.29; P = .008). The favorable impact of HDAC on CIR was no longer statistically significant in patients with non-CBF AML with wild-type RAS (HR = 0.71; P = .13).
Table 3.
Final Multivariable Model for Cumulative Incidence of Relapse for Non-CBF AML Cases
| Variable | Hazard Ratio | 95% CI | P |
|---|---|---|---|
| Interaction of RAS status and consolidation | .08 | ||
| Mutated RAS | |||
| HDAC v LDAC | 0.29 | 0.12 to 0.71 | .008 |
| Wild-type RAS | |||
| HDAC v LDAC | 0.71 | 0.48 to 1.1 | .13 |
NOTE. Variables considered for model inclusion were RAS mutation status (wild-type v mutated), WBC, spleen involvement, consolidation therapy group (HDAC v LDAC), and the RAS mutation-consolidation therapy interaction term.
Abbreviations: CBF, core binding factor; AML, acute myeloid leukemia; HDAC, high-dose cytarabine; LDAC, low-dose cytarabine.
DISCUSSION
Earlier studies of the prognostic significance of mutations in RAS in AML have yielded contradictory results. Some studies reported that patients with RAS mutations had improved overall survival,5,6 whereas others found that these patients had worse complete remission rates,8 overall survival,7-9 and disease-free survival7 than those with wild-type RAS. In contrast, several studies found no significant differences in outcome, including complete remission rates,4,7,10,11,15,16 overall survival,4,10,11,15-17 disease-free survival,8,10,11,16,17 event-free survival,6,17 or relapse-free survival,15 between patients with and without RAS mutations. Notably, in most of these studies, the type of postremission treatment was not taken into account in the analysis of clinical outcome.4-7,9-11,15-17
In the current analysis of adult patients with primary AML, all of whom were enrolled on the same treatment protocol, achieved complete remission, and have prolonged follow-up, there was no significant difference in outcome between patients with and without RAS mutations, which was similar to the findings of most previous studies.6,8,10,11,15-17 However, when we considered both the RAS status and consolidation therapy, our analysis revealed for the first time that the impact of RAS mutations on the risk of relapse in adult AML depends on the type of postremission chemotherapy. Although therapy with HDAC resulted in a lower CIR both in patients with and without RAS mutations, its benefit was much more pronounced in patients with mutated RAS. With HDAC, patients with mutated RAS had a substantial reduction in relapse risk relative to those with wild-type RAS. Only 45% of patients with mutated RAS experienced relapse. In contrast, all patients with mutated RAS in the LDAC group experienced relapse, all but two patients within 16 months after achievement of a complete remission. It is important to underscore that although this was an intent-to-treat analysis, the actual amount of cytarabine administered to patients in each of the consolidation arms was not different between the mutated RAS and wild-type RAS groups (not shown).
Our results are consistent with in vitro data showing that mutations in RAS render tumor cell lines derived from AML and non–small-cell lung and colon carcinomas more sensitive to certain cytotoxic drugs, such as cytarabine or topoisomerase II inhibitors.22,30 Koo et al23 have demonstrated that cells harboring an activated RAS oncogene fail to arrest in the S phase of the cell cycle in response to cytarabine treatment and that this results in their apoptotic death. In contrast, tumor cells with wild-type RAS genes undergo marked S-phase growth arrest on exposure to cytarabine that is reversible once the drug is removed. The authors concluded that the presence of a RAS mutation may change cellular response to cytarabine from cytostatic to cytotoxic, most likely because of altered cellular checkpoint functions in response to cytarabine.23 Recent studies provide experimental evidence that mutated RAS not only induces proliferation, apoptosis, senescence, or differentiation (depending on the cellular context in which it is expressed),31 but it may also induce a DNA damage checkpoint response.32-34 These results provide biologic plausibility to our clinical observations.
Concordant with our data, Illmer et al11 have recently shown that among younger (<60 years) patients with AML receiving high-dose cytarabine as induction therapy, activated RAS proteins predicted for a significantly higher response rate and longer overall survival. Additionally, two groups have reported promising response rates with regimens containing high-dose cytarabine in patients with advanced pancreatic carcinoma, a tumor where up to 90% of the patients show RAS mutations.35,36
In summary, the current study demonstrates that patients with primary AML harboring RAS mutations treated with HDAC as postremission therapy are significantly less likely to experience relapse than patients treated with LDAC. Furthermore, among patients receiving HDAC, those with RAS mutations also had a lower risk of relapse than patients with the wild-type RAS. These results suggest that mutations in the RAS gene constitute a novel molecular marker potentially useful in the clinic to identify patients who would optimally benefit from consolidation with HDAC. To date, risk-adapted stratification to HDAC postremission therapy in CALGB protocols has been performed on the basis of cytogenetic and molecular genetic detection of t(8;21)/RUNX1-RUNX1T1 and or inv(16)/CBFB-MYH11. If our findings are confirmed, testing for RAS mutations could become useful, in addition to CBF-AML detection, for risk-adapted stratification to HDAC postremission treatment in adults with de novo AML treated with chemotherapy alone. Because RAS mutations seem to be the first example of activating oncogene mutations that sensitize AML blasts to higher doses of cell-cycle phase-specific chemotherapeutic agent, further studies are needed to elucidate the molecular mechanisms of this phenomenon.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Andreas Neubauer, Edison T. Liu, Clara D. Bloomfield
Financial support: Christian Thiede, Edison T. Liu, Clara D. Bloomfield
Administrative support: Clara D. Bloomfield
Provision of study materials or patients: Robert J. Mayer, Richard A. Larson, Clara D. Bloomfield
Collection and assembly of data: Andreas Neubauer, Christian Thiede, Peter Paschka, Edison T. Liu
Data analysis and interpretation: Andreas Neubauer, Kati Maharry, Krzysztof Mrózek, Christian Thiede, Guido Marcucci, Clara D. Bloomfield
Manuscript writing: Andreas Neubauer, Kati Maharry, Krzysztof Mrózek, Clara D. Bloomfield
Final approval of manuscript: Andreas Neubauer, Kati Maharry, Krzysztof Mrózek, Christian Thiede, Guido Marcucci, Peter Paschka, Robert J. Mayer, Richard A. Larson, Edison T. Liu, Clara D. Bloomfield
Acknowledgments
We thank Donna Bucci of the CALGB Leukemia Tissue Bank at Ohio State University Comprehensive Cancer Center, Columbus, OH, for sample processing and storage services.
Appendix
The following Cancer and Leukemia Group B institutions, principal investigators, and cytogeneticists participated in this study: Weill Medical College of Cornell University, New York, NY: John Leonard and Ram S. Verma (Grant No. CA07968); Wake Forest University School of Medicine, Winston-Salem, NC: David D. Hurd, P. Nagesh Rao and Mark J. Pettenati (Grant No. CA03927); University of Iowa Hospitals, Iowa City, IA: Gerald H. Clamon and Shivanand R. Patil (Grant No. CA47642); Duke University Medical Center, Durham, NC: Jeffrey Crawford and Sandra H. Bigner (Grant No. CA47577); Walter Reed Army Medical Center, Washington, DC: Thomas Reid and Rawatmal B. Surana (Grant No. CA26806); Roswell Park Cancer Institute, Buffalo, NY: Ellis G. Levine and AnneMarie W. Block (Grant No. CA02599); Dartmouth Medical School, Lebanon, NH: Marc S. Ernstoff and Doris H. Wurster-Hill (Grant No. CA04326); University of Maryland Cancer Center, Baltimore, MD: Martin J. Edelman, Joseph R. Testa and Stuart Schwartz (Grant No. CA31983); Rhode Island Hospital, Providence, RI: William Sikov and Teresita Padre-Mendoza (Grant No. CA08025); University of Minnesota, Minneapolis, MN: Bruce A. Peterson and Diane C. Arthur (Grant No. CA16450); University of Missouri/Ellis Fischel Cancer Center, Columbia, MO: Michael C. Perry, Judith H. Miles and Jeffrey R. Sawyer (Grant No. CA12046); Dana-Farber Cancer Institute, Boston, MA: Eric P. Winer and Ramana Tantravahi (Grant No. CA32291); University of Alabama at Birmingham: Robert Diasio and Andrew J. Carroll (Grant No. CA47545); University of California, San Diego, CA: Barbara A. Parker and Renée Bernstein (Grant No. CA11789); Massachusetts General Hospital, Boston, MA: Jeffrey W. Clark and Leonard L. Atkins (Grant No. CA 12,449); Washington University School of Medicine, St. Louis, MO: Nancy L. Bartlett and Michael S. Watson (Grant No. CA77440); State University of New York Upstate Medical University, Syracuse, NY: Stephen L. Graziano, Lawrence P. Gordon and Constance K. Stein (Grant No. CA21060); McGill Department of Oncology, Montreal, Quebec: J.L. Hutchison and Jacqueline Emond (Grant No. CA31809); University of Massachusetts Medical Center, Worcester, MA: William W. Walsh and Philip L. Townes (Grant No. CA37135); University of North Carolina, Chapel Hill, NC: Thomas Shea and Kathleen W. Rao (Grant No. CA47559); Christiana Care Health System, Inc, Newark, DE: Stephen S. Grubbs and Digamber S. Borgaonkar (Grant No. CA45418); Minneapolis VA Medical Center, Minneapolis, MN: Vicki A. Morrison and Sugandhi A. Tharapel (Grant No. CA47555); University of Cincinnati Medical Center, Cincinnati, OH: Orlando J. Martelo and Ashok K. Srivastava; Columbia-Presbyterian Medical Center, New York, NY: Rose R. Ellison and Dorothy Warburton (Grant No. CA12011); Eastern Maine Medical Center, Bangor, ME: Harvey M. Segal and Laurent J. Beauregard (Grant No. CA35406); State University of New York Maimonides Medical Center, Brooklyn, NY: Sameer Rafla and Ram S. Verma (Grant No. CA25119); Mount Sinai School of Medicine, New York, NY: Lewis R. Silverman and Vesna Najfeld (Grant No. CA04457); North Shore-Long Island Jewish Health System, Manhasset, NY: Daniel R. Budman and Prasad R.K. Koduru (Grant No. CA35279); and Southern Nevada Cancer Research Foundation Community Clinical Oncology Program, Las Vegas, NV: John Ellerton (Grant No. CA35421).
Fig A1.
Cumulative incidence of relapse of 185 patients with acute myeloid leukemia according to RAS mutation status (mutRAS, mutated RAS; wtRAS, wild-type RAS).
published online ahead of print at www.jco.org on June 16, 2008.
Supported in part by a Grant Transregio 17 (to A.N.) from the Deutsche Forschungsgemeinschaft; Grants No. CA77658, CA101140, CA114725, CA31946, and CA16058 from the National Cancer Institute, Bethesda, MD; and The Coleman Leukemia Research Foundation.
Presented in part at the 41st Annual Meeting of the American Society of Clinical Oncology, May 13-17, 2005, Orlando, FL (abstr 6514).
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Bos JL: RAS oncogenes in human cancer: A review. Cancer Res 49:4682-4689, 1989. [Erratum: Cancer Res 50:1352, 1990] [PubMed] [Google Scholar]
- 2.Paquette RL, Landaw EM, Pierre RV, et al: N-RAS mutations are associated with poor prognosis and increased risk of leukemia in myelodysplastic syndrome. Blood 82:590-599, 1993 [PubMed] [Google Scholar]
- 3.Bos JL, Verlaan-de Vries M, van der Eb AJ, et al: Mutations in N-RAS predominate in acute myeloid leukemia. Blood 69:1237-1241, 1987 [PubMed] [Google Scholar]
- 4.Radich JP, Kopecky KJ, Willman CL, et al: N-RAS mutations in adult de novo acute myelogenous leukemia: Prevalence and clinical significance. Blood 76:801-807, 1990 [PubMed] [Google Scholar]
- 5.Coghlan DW, Morley AA, Matthews JP, et al: The incidence and prognostic significance of mutations in codon 13 of the N-RAS gene in acute myeloid leukemia. Leukemia 8:1682-1687, 1994 [PubMed] [Google Scholar]
- 6.Neubauer A, Dodge RK, George SL, et al: Prognostic importance of mutations in the RAS proto-oncogenes in de novo acute myeloid leukemia. Blood 83:1603-1611, 1994 [PubMed] [Google Scholar]
- 7.De Melo MB, Lorand-Metze I, Lima CSP, et al: N-RAS gene point mutations in Brazilian acute myelogenous leukemia patients correlate with a poor prognosis. Leuk Lymphoma 24:309-317, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Kiyoi H, Naoe T, Nakano Y, et al: Prognostic implication of FLT3 and N-RAS gene mutations in acute myeloid leukemia. Blood 93:3074-3080, 1999 [PubMed] [Google Scholar]
- 9.Meshinchi S, Stirewalt DL, Alonzo TA, et al: Activating mutations of RTK/ras signal transduction pathway in pediatric acute myeloid leukemia. Blood 102:1474-1479, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Ritter M, Kim TD, Lisske P, et al: Prognostic significance of N-RAS and K-RAS mutations in 232 patients with acute myeloid leukemia. Haematologica 89:1397-1399, 2004 [PubMed] [Google Scholar]
- 11.Illmer T, Thiede C, Fredersdorf A, et al: Activation of the RAS pathway is predictive for a chemosensitive phenotype of acute myelogenous leukemia blasts. Clin Cancer Res 11:3217-3224, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Reilly JT: Pathogenesis of acute myeloid leukaemia and inv(16)(p13;q22): A paradigm for understanding leukaemogenesis? Br J Haematol 128:18-34, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Kelly L, Clark J, Gilliland DG: Comprehensive genotypic analysis of leukemia: Clinical and therapeutic implications. Curr Opin Oncol 14:10-18, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Chan IT, Gilliland DG: Oncogenic K-RAS in mouse models of myeloproliferative disease and acute myeloid leukemia. Cell Cycle 3:536-537, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Stirewalt DL, Kopecky KJ, Meshinchi S, et al: FLT3, RAS, and TP53 mutations in elderly patients with acute myeloid leukemia. Blood 97:3589-3595, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Bowen DT, Frew ME, Hills R, et al: RAS mutation in acute myeloid leukemia is associated with distinct cytogenetic subgroups but does not influence outcome in patients younger than 60 years. Blood 106:2113-2119, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Bacher U, Haferlach T, Schoch C, et al: Implications of NRAS mutations in AML: A study of 2502 patients. Blood 107:3847-3853, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Mayer RJ, Davis RB, Schiffer CA, et al: Intensive postremission chemotherapy in adults with acute myeloid leukemia. N Engl J Med 331:896-903, 1994 [DOI] [PubMed] [Google Scholar]
- 19.Bloomfield CD, Lawrence D, Byrd JC, et al: Frequency of prolonged remission duration after high-dose cytarabine intensification in acute myeloid leukemia varies by cytogenetic subtype. Cancer Res 58:4173-4179, 1998 [PubMed] [Google Scholar]
- 20.Byrd JC, Dodge RK, Carroll A, et al: Patients with t(8;21)(q22;q22) and acute myeloid leukemia have superior failure-free and overall survival when repetitive cycles of high-dose cytarabine are administered. J Clin Oncol 17:3767-3775, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Byrd JC, Ruppert AS, Mrózek K, et al: Repetitive cycles of high-dose cytarabine benefit patients with acute myeloid leukemia and inv(16)(p13q22) or t(16;16)(p13;q22): Results from CALGB 8461. J Clin Oncol 22:1087-1094, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Koo H-M, Monks A, Mikheev A, et al: Enhanced sensitivity to 1-β-D-arabinofuranosylcytosine and topoisomerase II inhibitors in tumor cell lines harboring activated RAS oncogenes. Cancer Res 56:5211-5216, 1996 [PubMed] [Google Scholar]
- 23.Koo H-M, McWilliams MJ, Alvord WG, et al: RAS oncogene-induced sensitization to 1-β-D-arabinofuranosylcytosine. Cancer Res 59:6057-6062, 1999 [PubMed] [Google Scholar]
- 24.Mrózek K, Prior TW, Edwards C, et al: Comparison of cytogenetic and molecular genetic detection of t(8;21) and inv(16) in a prospective series of adults with de novo acute myeloid leukemia: A Cancer and Leukemia Group B study. J Clin Oncol 19:2482-2492, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Neubauer A, He M, Schmidt CA, et al: Genetic alterations in the p53 gene in the blast crisis of chronic myelogenous leukemia: Analysis by polymerase chain reaction based techniques. Leukemia 7:593-600, 1993 [PubMed] [Google Scholar]
- 26.Bowen DT, Frew ME, Rollinson S, et al: CYP1A1*2B (Val) allele is overrepresented in a subgroup of acute myeloid leukemia patients with poor-risk karyotype associated with NRAS mutation, but not associated with FLT3 internal tandem duplication. Blood 101:2770-2774, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Thiede C, Bayerdörffer E, Blasczyk R, et al: Simple and sensitive detection of mutations in the ras proto-oncogenes using PNA-mediated PCR clamping. Nucleic Acids Res 24:983-984, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gray RJ: A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 16:1141-1154, 1988 [Google Scholar]
- 29.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94:496-509, 1999 [Google Scholar]
- 30.Koo H-M, Gray-Goodrich M, Kohlhagen G, et al: The RAS oncogene-mediated sensitization of human cells to topoisomerase II inhibitor-induced apoptosis. J Natl Cancer Inst 91:236-244, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Downward J: Targeting RAS signaling pathways in cancer therapy. Nat Rev Cancer 3:11-22, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Di Micco R, Fumagalli M, Cicalese A, et al: Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature 444:638-642, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Knauf JA, Ouyang B, Knudsen ES, et al: Oncogenic RAS induces accelerated transition through G2/M and promotes defects in the G2 DNA damage and mitotic spindle checkpoints. J Biol Chem 281:3800-3809, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Fikaris AJ, Lewis AE, Abulaiti A, et al: Ras triggers ataxia-telangiectasia-mutated and Rad-3-related activation and apoptosis through sustained mitogenic signaling. J Biol Chem 281:34759-34767, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Dougherty JB, Kelsen D, Kemeny N, et al: Advanced pancreatic cancer: A phase I-II trial of cisplatin, high-dose cytarabine, and caffeine. J Natl Cancer Inst 81:1735-1738, 1989 [DOI] [PubMed] [Google Scholar]
- 36.Ahmed S, Vaitkevicius VK, Zalupski MM, et al: Cisplatin, cytarabine, caffeine, and continuously infused 5-fluorouracil (PACE) in the treatment of advanced pancreatic carcinoma: A phase II study. Am J Clin Oncol 23:420-424, 2000 [DOI] [PubMed] [Google Scholar]