Abstract
Purpose
Adjuvant chemotherapy for breast cancer (BC) may be associated with increased rates of bone loss and decreased bone mineral density (BMD) and may lead to premature osteoporosis and increased fracture risk. We examined whether zoledronic acid (ZA) prevents bone loss in premenopausal women receiving chemotherapy for early-stage BC.
Patients and Methods
This study is a randomized, double-blind, multicenter, phase III trial comparing ZA (4 mg intravenously every 3 months) versus placebo for 1 year. Premenopausal women underwent serial BMD measurements before initiating chemotherapy and at 6 and 12 months. The primary outcome was percent change in lumbar spine (LS) BMD at 6 months. Secondary outcomes were percent change at any BMD site and markers of bone turnover at 12 months. Linear mixed model analysis for repeated measures was performed.
Results
Of 101 women who were randomly assigned and completed baseline evaluation, 96 completed the 6-month evaluation, and 85 completed the 12-month evaluation. Baseline characteristics were comparable between the groups. Mean age was 42 years. Placebo was associated with significant decline in LS BMD at both 6 (2.4%) and 12 (4.1%) months. Similarly, total hip BMD declined by 0.8% at 6 months and 2.6% at 12 months. In contrast, BMD remained stable in ZA patients (P < .0001 compared with placebo).
Conclusion
Premenopausal women receiving chemotherapy for BC sustained significant bone loss at the LS and hip, whereas BMD remained stable in women who received ZA. Administration of ZA during the first year of chemotherapy is an effective and well-tolerated strategy for preventing bone loss.
INTRODUCTION
More than 3 million women living in the United States are breast cancer (BC) survivors.1 Because the number of women diagnosed with BC is increasing and the number who die each year has decreased,2 the number of survivors continues to increase.3 However, this improved survival does not come without costs.
For young women with early-stage BC, chemotherapy is often associated with either temporary or permanent cessation of menses.4 Thus, BC survivors are at risk for health consequences of premature estrogen deficiency such as osteoporosis.5-7 Small prospective studies have shown that bone loss ranges from 3% to 8% in the lumbar spine (LS) and 2% to 4% in the total hip (TH), with higher rates in those who develop amenorrhea.8,9 The higher rates of bone loss seem to translate into an increased risk of postmenopausal fractures. Data from the Women's Health Initiative Observational Study reported that postmenopausal survivors of BC have a 15% higher fracture risk than women without a history of BC.10
Oral clodronate and intravenous pamidronate are bisphosphonates that reduce the amount of bone loss associated with chemotherapy.8,11,12 However, with clodronate, a relatively weak bisphosphonate, significant LS bone loss (2.2%) persisted at 2 years. A more potent oral bisphosphonate, alendronate, is widely used for prevention and therapy of postmenopausal osteoporosis. However, alendronate is associated with GI adverse effects,13 which are of particular concern in women receiving chemotherapy. Intravenous zoledronic acid (ZA) prevents bone loss in premenopausal women receiving combined endocrine blockade therapy.14
The primary objective of this investigation was to study the efficacy of ZA, administered every 3 months, in reducing bone loss in premenopausal women with BC receiving chemotherapy. The secondary objectives were to evaluate of the effect of ZA on bone turnover markers, characterize the natural history of bone loss in a diverse patient population, and confirm the tolerability of ZA in combination with adjuvant chemotherapy.
PATIENTS AND METHODS
Patients
Patients were newly diagnosed premenopausal women with histologically proven, nonmetastatic BC. Premenopausal status was defined as last menstruation ≤ 6 months earlier or follicle-stimulating hormone less than 20 mU/L. Patients were enrolled after surgery but before initiating chemotherapy. The chemotherapeutic regimens were not dictated by study investigators. Exclusion criteria included T score of less than −2.0 at any site, fragility fracture, prior therapy with a bisphosphonate or calcitonin, LS anatomy precluding accurate bone mineral density (BMD) measurement of ≥ three lumbar vertebrae, serum creatinine ≥ 2 mg/dL, or pregnancy.
Protocol
After signing informed consent, patients were randomly assigned to either ZA 4 mg intravenously over 15 minutes every 3 months for 12 months or placebo. Treatment assignment was stratified by tumor hormone receptor status. On enrollment, information on tumor stage, history of fractures, reproductive and menstrual history, tobacco exposure, alcohol intake, and medications was collected. The baseline evaluation included a chemistry panel, intact parathyroid hormone, 25-hydroxyvitamin-D, bone-specific alkaline phosphatase (BSAP; a marker of bone formation), and serum C-telopeptide of type I collagen (CTX; a marker of bone resorption). All patients were provided with oral supplements containing calcium (1,000 mg) and vitamin D (400 to 800 U).
A separate restricted random assignment list was prepared for each stratum at each site, using random permuted blocks. When a new patient was enrolled, the research pharmacy distributed study drug or placebo in unmarked blister packs. Study visits were scheduled at approximately 6, 9, 12, 18, 24, 36, and 52 weeks after random assignment. At each visit, patients were questioned about bone pain and toxicities.
The protocol was initially limited to Columbia University Medical Center (CUMC) and then was opened at eight additional sites to increase enrollment. Four of the eight additional sites contributed a total of 10 patients who were included in the analysis. The institutional review boards of the CUMC and each site approved the protocol.
Outcome Measurements
BMD.
BMD of the LS (L1 to L4), TH, and femoral neck (FN) was measured by dual-energy x-ray absorptiometry at random assignment and at 24, and 52 weeks using Hologic QDR 4500 bone densitometers (Hologic, Inc, Bedford, MA) in the array (fan beam) mode by radiology technicians certified by the International Society for Clinical Densitometry. An established program of instrument calibration and ongoing quality control to allow for accurate comparisons of BMD data was used. All instruments were calibrated before beginning the study with gold standard reference phantoms to read BMD within 1%. The subsequent calibration strategy included rescanning of the reference phantoms at 6-month intervals throughout the study. Patients were assessed on the same machine for each follow-up visit. All densitometry results were reviewed by a third party, and the investigator was notified if there was excessive bone loss (defined as > 8% decrease over 6 months).
Assays.
Fasting serum for CTX and BSAP was obtained before initiation of chemotherapy and at follow-up visits at 6, 12, 24, 36, and 52 weeks. Whenever possible, blood was collected in the morning after an overnight fast. Serum was aliquoted, frozen immediately, and stored at −70°C until batch analyses were undertaken. BSAP was measured using an immunoassay kit (Metra BAP; Quidel Corp, San Diego, CA), with low cross reactivity with the liver form of alkaline phosphatase (3% to 8%). Interassay variability was 8.6% at 13.7 U/L. The normal range in premenopausal women is 11.6 to 29.6 U/L. CTX was measured using a sandwich enzyme-linked immunosorbent assay (Serum Crosslaps; IDS Ltd, Fountain Hills, AZ) with an interassay variability of 11.1% at 0.408 ng/mL. Specimens were thawed once, processed batch-wise, and identified by a study number without reference to treatment assignment.
Statistical Analysis
The primary efficacy end points were percent change in LS BMD at 24 and 52 weeks after initiation of chemotherapy. This study was designed to detect a between-group difference of 3% change in LS BMD at 24 weeks assuming a pooled standard deviation of 4.5%, 90% power, and a 5% type I error rate. This was an intent-to-treat analysis. Secondary end points included percent change in TH and FN BMD and changes in CTX and BSAP at 24 and 52 weeks. The data were held by the investigators and analyzed using SAS (Version 9.1; SAS Institute, Cary, NC).
Baseline group differences were assessed using independent t tests for continuous measures and Fisher's exact test for categoric data. The effects of ZA or placebo on changes in BMD and biochemical measures were estimated with separate linear mixed models. In each model, group, time, and group-time interaction were entered as fixed effects; subject and error were entered as random effects; and an autoregressive covariance structure was used for the within-subject correlation. Means and standard deviations are presented for baseline measures, and means and SEs from model estimates are reported. P < .05 was considered statistically significant.
RESULTS
Baseline Characteristics of the Study Population
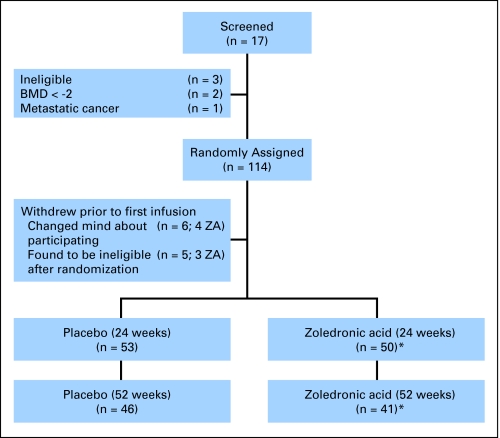
Of the 117 patients who consented to participate, three were ineligible, 114 were randomly assigned, and 103 completed the baseline evaluation. Five patients (all in the placebo group) withdrew before the 24-week assessment, and 11 patients withdrew between the 24-week and 52-week assessments (four receiving placebo and six receiving ZA). In addition, two patients were not included in the analyses because baseline and follow-up BMDs were measured on different densitometers (Fig 1). Baseline characteristics of the participants are listed in Table 1. The mean age (42 ± 6 years) and mean body mass index (26 ± 5 kg/m2) did not differ between groups. The groups were similar with regard to stage, treatment duration, hormone receptor status, and race. The population was racially/ethnically diverse, with 51% non-Hispanic white patients, 35% Hispanic patients, 12% African American patients, and 2% Asian patients. The groups were well balanced with respect to menstrual history and risk factors for osteoporosis. Patients in the placebo group reported slightly more calcium supplement use. Tamoxifen use and aromatase inhibitor use were similar between the groups.
Fig 1.
CONSORT diagram of patient participation and drop out throughout the study period. Drop out resulted from patients not wanting to come back for follow-up assessments. (*) Two patients on zoledronic acid (ZA) arm were excluded from analysis because bone mineral density (BMD) analysis was performed on two different dual-energy x-ray absorptiometry densitometers.
Table 1.
Baseline Characteristics of Study Participants
| Characteristic | Placebo
|
Zoledronic Acid
|
||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| BMD, g/cm2 | ||||
| LS | ||||
| Mean | 1.083 | 1.055 | ||
| SD | 0.14 | 0.12 | ||
| FN | ||||
| Mean | 0.881 | 0.851 | ||
| SD | 0.14 | 0.12 | ||
| TH | ||||
| Mean | 0.990 | 0.960 | ||
| SD | 0.14 | 0.12 | ||
| z score | ||||
| LS | ||||
| Mean | 0.25 | 0.15 | ||
| SD | 1.20 | 0.90 | ||
| FN | ||||
| Mean | 0.11 | 0.01 | ||
| SD | 0.92 | 0.98 | ||
| TH | ||||
| Mean | 0.25 | 0.17 | ||
| SD | 0.92 | 0.91 | ||
| Age, years | ||||
| Mean | 42 | 43 | ||
| SD | 6 | 6 | ||
| BMI, kg/m2 | ||||
| Mean | 25.3 | 27.2 | ||
| SD | 4.3 | 5.5 | ||
| Disease stage | ||||
| I | 19 | 38 | 13 | 29 |
| II | 27 | 54 | 30 | 67 |
| III | 4 | 8 | 2 | 4 |
| Stratification factors | ||||
| Hormone receptor positive | 37 | 62 | 37 | 69 |
| Hormonal therapy (after treatment) | ||||
| Aromatase inhibitor | 10 | 27 | 9 | 25 |
| Tamoxifen | 25 | 63 | 26 | 75 |
| GnRH agonist/oophorectomy | 0 | 0 | 0 | 0 |
| Chemotherapy | ||||
| 4 cycles | 10 | 19 | 9 | 19 |
| AC | 8 | 15 | 6 | 13 |
| T | 1 | 2 | 2 | 4 |
| 6-8 cycles | 43 | 81 | 39 | 81 |
| ACT | 38 | 70 | 31 | 65 |
| CMF | 3 | 5 | 4 | 8 |
| CAF | 4 | 7 | 5 | 10 |
| Regular menses at random assignment | 49 | 91 | 43 | 91 |
| Hysterectomy | 2 | 4 | 3 | 7 |
| Menses during treatment | ||||
| Stopped and did not return by 52 weeks | 37 | 69 | 35 | 73 |
| Stopped and had ≥ 1 by 52 weeks | 17 | 31 | 13 | 27 |
| Oral contraceptives (ever) | 21 | 42 | 16 | 42 |
| Tobacco (ever) | 19 | 38 | 14 | 37 |
| No. of pack years | ||||
| Mean | 8.2 | 7.8 | ||
| SD | 1.9 | 1.6 | ||
| Family history of osteoporosis | 12 | 26 | 11 | 29 |
| Exercise | ||||
| Current | 17 | 35 | 13 | 34 |
| Past | 33 | 69 | 19 | 53 |
| Calcium supplements | 22 | 42 | 14 | 29* |
| Vitamin D | 20 | 38 | 14 | 27* |
| Multivitamin | 13 | 25 | 12 | 25 |
| Race | ||||
| White | 27 | 46 | 30 | 56 |
| Hispanic | 20 | 34 | 19 | 35 |
| Black | 10 | 17 | 4 | 8 |
| Asian | 2 | 3 | 1 | 2 |
Abbreviations: BMD, bone mineral density; LS, lumbar spine; SD, standard deviation; FN, femoral neck; TH, total hip; BMI, body mass index; GnRH, gonadotropin-releasing hormone; A, doxorubicin; C, cyclophosphamide; T, paclitaxel; M, methotrexate; F, fluorouracil.
P < .05.
Baseline LS, FN, and TH BMDs, expressed both as g/cm2 and z scores, were normal and comparable in both groups (Table 1). In addition, baseline BSAP and CTX were similar between groups. The percentage of patients with LS z scores between −1.0 and −2.0 at baseline was 9% in the placebo group and 11% in the ZA group.
Change in BMD
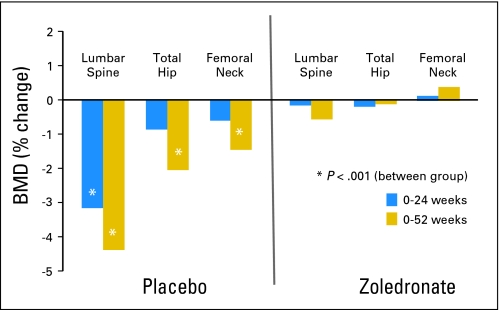
As presented in Table 2 and Figure 2, LS, FN, and TH BMD remained stable in the ZA group at 24 and 52 weeks. For women randomly assigned to placebo, however, there was a significant decrease in LS BMD at both 24 (−3.2%) and 52 weeks (−4.4%). At 52 weeks, TH and FN BMD had also decreased significantly from baseline by −2.1% and −1.5%, respectively. The percent change in LS BMD at 52 weeks was greater in the patients enrolled at CUMC (−5.6%). The percentage of patients with z scores less than −1.0 at 52 weeks increased from 9% to 14% in the ZA group and from 11% to 24% in the placebo group. By 52 weeks, 29% of the patients had a 5% loss of BMD at one of the three sites tested. Of these patients, 17 (61%) were in the placebo group, and 11 (39%) were in the ZA group. In the placebo group, change in BMD was not correlated with type of chemotherapy, baseline BMD, or permanence of menstrual cessation.
Table 2.
Absolute BMD and Percent Change from Baseline in Patients Randomly Assigned to ZA or Placebo at 24 and 52 Weeks
| Site | BMD (g/cm2)
|
% Change in BMD
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline
|
24 Weeks
|
52 Weeks
|
24 Weeks
|
52 Weeks
|
P* | ||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SEM | Mean | SEM | ||
| Lumbar spine | |||||||||||
| Placebo | 1.083 | 0.14 | 1.041† | 0.14 | 1.044†‡ | 0.15 | −2.98§ | 0.44 | −4.39§ | 0.45 | .0001 |
| ZA | 1.058 | 0.12 | 1.055 | 0.11 | 1.063 | 0.11 | −0.03 | 0.44 | −0.6 | 0.49 | .0001 |
| Femoral neck | |||||||||||
| Placebo | 0.881 | 0.14 | 0.874 | 0.14 | 0.873 | 0.15 | −0.60 | 0.55 | −1.50§ | 0.56 | .13 |
| ZA | 0.851 | 0.12 | 0.845 | 0.12 | 0.861 | 0.13 | 0.2 | 0.57 | 0.4 | 0.63 | .21 |
| Total hip | |||||||||||
| Placebo | 0.990 | 0.14 | 0.979† | 0.14 | 0.983†‡ | 0.14 | −0.86 | 0.35 | −2.08§ | 0.36 | .02 |
| ZA | 0.960 | 0.12 | 0.958 | 0.11 | 0.975 | 0.11 | −0.19 | 0.36 | −0.12 | 0.39 | .02 |
Abbreviations: BMD, bone mineral density; ZA, zoledronic acid; SD, standard deviation.
P value for fixed effect of group above and P value for fixed effect of group-time interaction below.
P < .05 for within-group change from baseline.
P < .05 for within-group change from week 24.
P < .05 compared between treatment groups at specific times.
Fig 2.
Percent change in bone mineral density (BMD) at the lumbar spine (LS), total hip (TH), and femoral neck (FN) 24 and 52 weeks from baseline in women treated with zoledronic acid or placebo. Percent change in LS BMD in the placebo group was −3.5 and −5.6 at 24 and 52 weeks, respectively, in the subset of patients at the primary enrollment site.
Markers of Bone Turnover
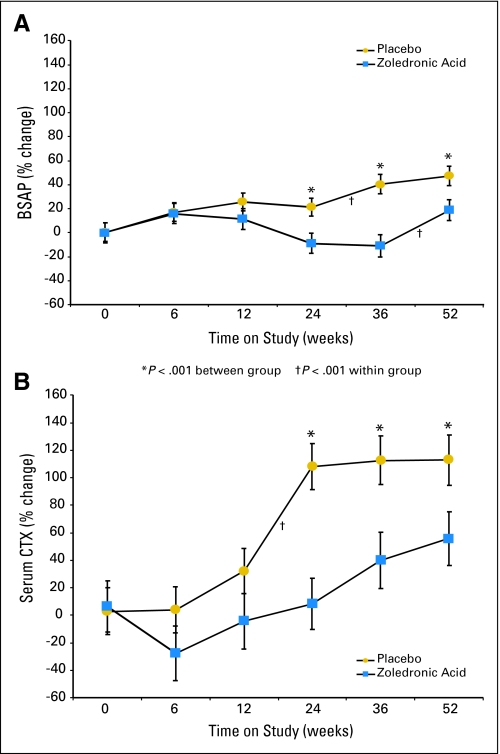
Markers of bone formation (BSAP) and bone resorption (CTX) differed significantly between groups at 24 and 52 weeks (Fig 3). In the placebo group, serum BSAP increased 24% above baseline at 6, 12, and 24 weeks, with a further increase to 47% above baseline at 52 weeks. Similarly, serum CTX increased 30% above baseline at 12 weeks, doubled by 24 weeks, and remained markedly elevated. In the ZA group, serum BSAP increased by 16% at 6 weeks, with a gradual decline thereafter. There was an initial 26% decline in serum CTX between baseline and 6 weeks. By 12 weeks, serum CTX had returned to baseline, and by 52 weeks, it was 56% above baseline. The amount of suppression of both BSAP and CTX in the ZA group decreased between 24 and 52 weeks.
Fig 3.
Mean percent change from baseline to 52 weeks in (A) bone-specific alkaline phosphatase (BSAP), a marker of bone formation, and (B) C-telopeptide of type I collagen (CTX), a marker of bone resorption. (*) P < .001 between groups. (†) P < .001 within groups.
Toxicity
Treatment was well tolerated. There were no serious adverse events reported. No patients had a change in renal function or reported osteonecrosis of the jaw. As can be seen in Table 3, the percentage of patients who experienced adverse events was similar between the groups, with the exception of eye discomfort, which was significantly more common in patients treated with ZA versus placebo (47% v 25%, respectively; P < .01). No patient had cancer recurrence during the 52-week study period.
Table 3.
Adverse Events Reported in Each of the Study Groups
| Adverse Event | Placebo
|
Zoledronic Acid
|
||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| Fever | 10 | 20 | 11 | 21 |
| Flu-like symptoms | 21 | 40 | 21 | 48 |
| Myalgia | 24 | 65 | 30 | 63 |
| Arthralgia | 32 | 62 | 34 | 71 |
| Muscle stiffness | 24 | 46 | 25 | 52 |
| Bone pain | 22 | 42 | 20 | 42 |
| Reflux | 14 | 27 | 19 | 40 |
| Headache | 31 | 60 | 24 | 50 |
| Fatigue | 29 | 56 | 24 | 50 |
| Eye discomfort | 13 | 25 | 22 | 47* |
| Insomnia | 32 | 62 | 26 | 54 |
P < .05.
DISCUSSION
This study confirms that premenopausal women with BC experience significant bone loss during the first year after initiating chemotherapy. The rate of bone loss was greatest at the LS, with a decrease of 4.4% by 1 year. Significant, although less severe, losses were observed at the FN and TH. The bone loss was accompanied by highly significant increases in both bone turnover markers (BSAP and CTX), indicating an increase in bone remodeling activity. The intravenous administration of 4 mg of ZA every 3 months completely prevented the bone loss, either suppressed or attenuated the increase in bone turnover markers, and was well tolerated.
The results of our study are consistent with others showing that bisphosphonates prevent bone loss during and after chemotherapy for BC.8,12,15-17 Delmas et al15 evaluated an unconventional cyclic regimen of risedronate in women who had entered menopause after chemotherapy. Greenspan et al16 evaluated weekly oral risedronate in patients who had completed chemotherapy, on average, 3 years before random assignment. In both studies, the patients were older and had lower baseline BMD than the patients in our study, and the rate of loss in the placebo group was less than we observed, perhaps because the most rapid phase of bone loss may have occurred before random assignment. In both studies, BMD remained stable or increased in the risedronate-treated groups. Two bisphosphonate studies have been published in which premenopausal women were randomly assigned before initiation of chemotherapy. In both studies, the amount of bone loss in the placebo arm was comparable to our results. Saarto et al11 randomly assigned women to clodronate 1,600 mg or placebo. At 1 year, BMD declined by only 1% in the clodronate-treated women. Clodronate, however, is not available in the United States and is associated with GI adverse effects. The second smaller study randomly assigned 40 premenopausal Lebanese women to either intravenous pamidronate 60 mg every 3 months or placebo.8 In the placebo arm, LS BMD declined by 3.2% by 1 year and by 4% in the group that became amenorrheic. This loss was prevented with pamidronate. However, bone turnover markers did not differ between the groups. In our study, the majority of women entered menopause, and those who did not had alterations in their menstrual cycle. Therefore, we did not see a difference in bone loss between these groups.
Aromatase inhibitors are increasingly used to prevent BC recurrence in postmenopausal women. Unfortunately, these drugs also accelerate bone loss and are associated with increased risk of fracture.18,19 In premenopausal women receiving combined endocrine therapy with gonadotropin-releasing hormone agonists and aromatase inhibitors, bone loss is dramatically increased and is greater with longer duration of treatment. For example, in a study combining goserelin and anastrozole, the mean percent decrease in BMD was 6% over the first year and 17.4% over 3 years. However, much of this loss was reversed when the hormonal therapy was stopped and menstruation resumed.14,20 In contrast, few patients undergoing chemotherapy regain normal menses, although this may change as the treatment of BC evolves.
Bisphosphonates act by inhibiting osteoclast-mediated bone resorption. Their administration is associated with suppression of markers of bone resorption and, secondarily, of bone formation. Consistent with this mechanism of action, we observed that bone turnover markers increased in the placebo group and were significantly lower in the ZA group at most points. In this regard, our results differ from the study of Fuleihan et al,8 possibly because of the greater potency and longer duration of action of ZA than pamidronate. A single infusion of ZA has been shown to suppress markers of bone resorption for up to 1 year in women with postmenopausal osteoporosis.21,22 However, despite the difference we observed between the treatment groups, we noted that ZA, although administered at a relatively high dose compared with that used for postmenopausal osteoporosis, did not completely prevent an increase in serum CTX, which was 50% above baseline at 1 year. Although it is possible that the acute estrogen deficiency that accompanied chemotherapy may have opposed the effects of ZA on the osteoclast, we regard that as unlikely.
Although our results are consistent with other studies in demonstrating that premenopausal women who undergo chemotherapy for BC sustain significant bone loss and that the bone loss can be prevented by bisphosphonates, the question of whether it is necessary to prevent this relatively acute and possibly self-limited bone loss remains unclear. Low BMD and biochemical evidence of increased bone resorption (CTX) have been shown to be independent risk factors for fracture in elderly women.23 However, the relationship between BMD fracture risk is much different in younger women than in older women.24 In general, younger women are at much lower short-term risk of fracture than elderly women, even when BMD is comparably low. In this regard, the women in this study were young and had BMD z scores greater than −2.0, and thus, their BMD was within the expected range for age, both before and after chemotherapy.25 When compared with the WHO criteria for diagnosis of osteoporosis in postmenopausal white women,26 at baseline, 17% had low bone mass or osteopenia with T scores between −1.0 and −2.5. No patient fractured or developed osteoporosis.
Few would argue against initiating preventive antiresorptive therapy in older postmenopausal women with BC who are already at high short-term risk of fracture. However, initiating bisphosphonate therapy in young women who are at low short-term risk of fractures, with the goal of preventing acute bone loss that may result in future fractures, may not be necessary, beneficial, or cost effective. However, the statistically significant bone loss seen in untreated women, particularly at the LS, and the number of women who had more than 5% loss at one BMD site raise concerns for their future bone health because bone loss continues over time27 and may be exacerbated by subsequent hormonal therapies to prevent cancer recurrence.18 Moreover, because chemotherapy-induced ovarian dysfunction results in onset of menopause approximately 10 years earlier than average,6 most BC survivors are estrogen deficient for a longer time period than women who undergo natural menopause.28 Extrapolating over the 5- to 10-year treatment period for BC, women may sustain more than a 20% bone loss. This will undoubtedly increase their risk of postmenopausal fracture, as well as lower the age at which such fractures occur. Thus, a cogent case could be made that preventing this predictable and possibly severe bone loss could have significant long-term benefit. It remains unclear when to intervene.29
This study has several limitations. First, although ZA administered every 3 months maintains bone density, such frequent dosing may not be necessary. The expansion of the study to external sites resulted in a higher than expected ineligibility rate, poorer patient retention, and questions about the quality of the BMD data. Accrual was slow because patients had difficulty deciding whether to participate before initiating chemotherapy, and dropout was higher than expected for the same reason. This should inform future studies assessing survivorship issues in young women with BC.
In summary, we have shown that ZA prevented bone loss, reduced serum markers of bone turnover, and was well tolerated in premenopausal women with BC undergoing chemotherapy. The results have important implications for the growing number of young BC survivors who will enter menopause early and experience both a prolonged period of estrogen deficiency and an increased long-term risk of osteoporotic fracture. Questions remain regarding the optimal time to initiate bisphosphonates, the appropriate dose and duration of therapy, and the potential for preemptive intervention to reduce future fractures. It is unclear whether these questions can ever be answered because such studies would necessarily be of long duration. However, given the number of women who experience significant and substantial bone loss, the standard of care for premenopausal women should at least include BMD measurements starting before initiation of chemotherapy and continuing at regular intervals thereafter.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: Dawn L. Hershman, Novartis Pharmaceuticals; Elizabeth Shane, Novartis Pharmaceuticals; Does your study have any NIH funding? Yes Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Dawn L. Hershman, Donald J. McMahon, Elizabeth Shane
Financial support: Dawn L. Hershman
Administrative support: Dawn L. Hershman, Dinaz Irani, Gina Cucchiara, Lois Brafman, Elizabeth Shane
Provision of study materials or patients: Dawn L. Hershman, Katherine D. Crew, Lois Brafman
Collection and assembly of data: Dawn L. Hershman, Donald J. McMahon, Serge Cremers, Dinaz Irani, Gina Cucchiara, Lois Brafman, Elizabeth Shane
Data analysis and interpretation: Dawn L. Hershman, Donald J. McMahon, Katherine D. Crew, Serge Cremers, Elizabeth Shane
Manuscript writing: Dawn L. Hershman, Donald J. McMahon, Elizabeth Shane
Final approval of manuscript: Dawn L. Hershman, Donald J. McMahon, Katherine D. Crew, Serge Cremers, Dinaz Irani, Gina Cucchiara, Lois Brafman, Elizabeth Shane
published online ahead of print at www.jco.org on August 18, 2008
Supported by a K07 Award from the National Cancer Institute (Grant No. CA95597) (D.L.H.), a career development award from American Society of Clinical Oncology (D.L.H.), a K24 Award from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (AR 052665) (E.S.), and Novartis Pharmaceuticals Corporation.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Hewitt M, Breen N, Devesa S: Cancer prevalence and survivorship issues: Analyses of the 1992 National Health Interview Survey. J Natl Cancer Inst 91:1480-1486, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Wingo PA, Ries LA, Parker SL, et al: Long-term cancer patient survival in the United States. Cancer Epidemiol Biomarkers Prev 7:271-282, 1998 [PubMed] [Google Scholar]
- 3.Edwards BK, Brown ML, Wingo PA, et al: Annual report to the nation on the status of cancer, 1975-2002, featuring population-based trends in cancer treatment. J Natl Cancer Inst 97:1407-1427, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Bines J, Oleske DM, Cobleigh MA: Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J Clin Oncol 14:1718-1729, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Kanis JA, McCloskey EV, Powles T, et al: A high incidence of vertebral fracture in women with breast cancer. Br J Cancer 79:1179-1181, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruning PF, Pit MJ, de Jong-Bakker M, et al: Bone mineral density after adjuvant chemotherapy for premenopausal breast cancer. Br J Cancer 61:308-310, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Headley JA, Theriault RL, LeBlanc AD, et al: Pilot study of bone mineral density in breast cancer patients treated with adjuvant chemotherapy. Cancer Invest 16:6-11, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Fuleihan Gel-H, Salamoun M, Mourad YA, et al: Pamidronate in the prevention of chemotherapy-induced bone loss in premenopausal women with breast cancer: A randomized controlled trial. J Clin Endocrinol Metab 90:3209-3214, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Shapiro CL, Manola J, Leboff M: Ovarian failure after adjuvant chemotherapy is associated with rapid bone loss in women with early-stage breast cancer. J Clin Oncol 19:3306-3311, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Chen Z, Maricic M, Bassford TL, et al: Fracture risk among breast cancer survivors: Results from the Women's Health Initiative Observational Study. Arch Intern Med 165:552-558, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Saarto T, Blomqvist C, Valimaki M, et al: Chemical castration induced by adjuvant cyclophosphamide, methotrexate, and fluorouracil chemotherapy causes rapid bone loss that is reduced by clodronate: A randomized study in premenopausal breast cancer patients. J Clin Oncol 15:1341-1347, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Saarto T, Blomqvist C, Valimaki M, et al: Clodronate improves bone mineral density in post-menopausal breast cancer patients treated with adjuvant antioestrogens. Br J Cancer 75:602-605, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonnick S, Saag KG, Kiel DP, et al: Comparison of weekly treatment of postmenopausal osteoporosis with alendronate versus risedronate over two years. J Clin Endocrinol Metab 91:2631-2637, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Gnant MF, Mlineritsch B, Luschin-Ebengreuth G, et al: Zoledronic acid prevents cancer treatment-induced bone loss in premenopausal women receiving adjuvant endocrine therapy for hormone-responsive breast cancer: A report from the Austrian Breast and Colorectal Cancer Study Group. J Clin Oncol 25:820-828, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Delmas PD, Balena R, Confravreux E, et al: Bisphosphonate risedronate prevents bone loss in women with artificial menopause due to chemotherapy of breast cancer: A double-blind, placebo-controlled study. J Clin Oncol 15:955-962, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Greenspan SL, Bhattacharya RK, Sereika SM, et al: Prevention of bone loss in survivors of breast cancer: A randomized, double-blind, placebo-controlled clinical trial. J Clin Endocrinol Metab 92:131-136, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Powles TJ, McCloskey E, Paterson AH, et al: Oral clodronate and reduction in loss of bone mineral density in women with operable primary breast cancer. J Natl Cancer Inst 90:704-708, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Howell A, Cuzick J, Baum M, et al: Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet 365:60-62, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Goss PE, Ingle JN, Martino S, et al: Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: Updated findings from NCIC CTG MA.17. J Natl Cancer Inst 97:1262-1271, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Gnant MF, Mlineritsch B, Luschin-Ebengreuth G, et al: Bone mineral density at 5 years after diagnosis in premenopausal patients with endocrine-responsive breast cancer, after 3 years of adjuvant endocrine treatment with goserelin and tamoxifen or anastrozole or both treatments in combination with zoledronic acid. 30th Annual San Antonio Breast Cancer Symposium, San Antonio, TX, December 13-16, 2007. (abstr)
- 21.Black DM, Delmas PD, Eastell R, et al: Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 356:1809-1822, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Reid IR, Miller P, Lyles K, et al: Comparison of a single infusion of zoledronic acid with risedronate for Paget's disease. N Engl J Med 353:898-908, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Garnero P, Hausherr E, Chapuy MC, et al: Markers of bone resorption predict hip fracture in elderly women: The EPIDOS Prospective Study. J Bone Miner Res 11:1531-1538, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Hui SL, Slemenda CW, Johnston CC Jr: Age and bone mass as predictors of fracture in a prospective study. J Clin Invest 81:1804-1809, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hans D, Downs RW Jr, Duboeuf F, et al: Skeletal sites for osteoporosis diagnosis: The 2005 ISCD Official Positions. J Clin Densitom 9:15-21, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Kanis JA: Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: Synopsis of a WHO report—WHO Study Group. Osteoporos Int 4:368-381, 1994 [DOI] [PubMed] [Google Scholar]
- 27.Fogelman I, Blake GM, Blamey R, et al: Bone mineral density in premenopausal women treated for node-positive early breast cancer with 2 years of goserelin or 6 months of cyclophosphamide, methotrexate and 5-fluorouracil (CMF). Osteoporos Int 14:1001-1006, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Marshall D, Johnell O, Wedel H: Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 312:1254-1259, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gralow JR: Bone density in breast cancer: When to intervene? J Clin Oncol 25:3194-3197, 2007 [DOI] [PubMed] [Google Scholar]