Abstract
Purpose
Inflammatory processes have been implicated in the pathogenesis of both depression and cancer. Links between depressive symptoms, interleukin-6 (IL-6), and cortisol dysregulation have been demonstrated in cancer patients, but vegetative versus affective components of depression have been minimally examined. The objective of the current study was to examine associations between IL-6, diurnal cortisol rhythms, and facets of depression in epithelial ovarian cancer patients.
Patients and Methods
Patients awaiting surgery for a pelvic mass suspected for ovarian cancer completed questionnaires, collected salivary samples for 3 days presurgery, and gave a presurgical blood sample. Ascites was obtained during surgery. IL-6 was measured by enzyme-linked immunosorbent assay and cortisol by a chemiluminescence immunoassay. The final sample included 112 invasive ovarian cancer patients (86 advanced stage, 26 early stage) and 25 patients with tumors of low malignant potential (LMP).
Results
Advanced-stage ovarian cancer patients demonstrated elevations in vegetative and affective depressive symptoms, plasma IL-6, and the cortisol area under the curve (AUC) compared with patients with LMP tumors (all P < .05). Among invasive ovarian cancer patients, greater vegetative depression was related to elevated IL-6 in plasma (P = .008) and ascites (P = .024), but affective depression was unrelated to IL-6. Elevations in total depression (P = .026) and vegetative depression (P = .005) were also related to higher evening cortisol levels. Plasma IL-6 was related to greater afternoon and evening cortisol and cortisol AUC (all P values < .005).
Conclusion
These results demonstrate significant relationships between IL-6, cortisol, and vegetative depression, and may have implications for treatment of depression in ovarian cancer patients.
INTRODUCTION
Depression is common among cancer patients, approximately one third of whom report depressive symptoms around the time of diagnosis and up to one fourth of whom suffer from major depression.1,2 Ovarian cancer patients, who have the poorest survival rate among gynecologic cancer patients,3 show high rates of depression.4-6 Depression among cancer patients has frequently been attributed to the stress of a potentially life-threatening diagnosis and the difficulties of cancer treatment.7 However, several recent studies among cancer patients have found associations among depression, elevated levels of the proinflammatory cytokine interleukin-6 (IL-6), and/or dysregulation of the neuroendocrine hormone cortisol.8-11 Inflammation has been implicated in the pathogenesis of depression as well as cancer,12-14 and it has been proposed that tumor-derived inflammatory cytokines such as IL-6 may contribute to depression in cancer patients.8 However, there has been little systematic investigation of these relationships among cancer patients before potentially confounding treatment with surgery and chemotherapy.
IL-6 is a 21- to 28-kD protein, produced by multiple sources, that serves as a major regulatory cytokine in the human body.15 It is secreted at high levels by ovarian tumor cells and stimulates key processes in ovarian cancer growth and metastasis including angiogenesis, proliferation, attachment, migration, and invasion.16,17 In ovarian cancer, elevated IL-6 has been associated with larger tumors, faster tumor progression, decreased chemotherapy effectiveness, poorer clinical status, recurrence, and shorter survival.18-20
In healthy adults, elevated IL-6 has been associated with depressive symptoms21 and clinical depression,22,23 although at least one study with a healthy, nonclinically depressed sample, and a sample of hospitalized patients have failed to show these associations.24,25 IL-6 and other proinflammatory cytokines have profound effects on the CNS, inducing a syndrome of “sickness behaviors” characterized by anhedonia and vegetative symptoms including fatigue, malaise, anorexia, difficulty concentrating, reduced activity, sleep impairments, and disinterest in activities.26,27 Proinflammatory cytokines exert differential effects on affective and vegetative depression, with more prominent effects on vegetative symptoms.27 Affective and vegetative depressive symptoms are thought to occur via distinct mechanisms, with vegetative symptoms occurring significantly earlier than mood disturbance.14,27
Depressive symptoms are also associated with hypercortisolemia, downregulated glucocorticoid receptors, and general dysregulation of the hypothalamic pituitary adrenocortical (HPA) axis.28 With chronic stress and depression, the negative feedback system regulating cortisol may become impaired29 and diurnal cortisol rhythms altered, particularly with respect to evening cortisol.30-33 There is a well-characterized feedback loop whereby IL-6 stimulates HPA secretion of cortisol which exerts negative feedback on IL-6 for inflammatory control.23,27 Persistent inflammation is associated with HPA abnormalities and may contribute to the hypercortisolemia seen in depression.14,28 Cancer patients often demonstrate restricted and dysregulated cortisol rhythms.8,9,34
There has been minimal examination of components of depression (eg, affective v vegetative) that most strongly relate to IL-6 and cortisol abnormalities in cancer (described in Appendix, online only). The objectives of the current study were to contrast levels of IL-6, cortisol, and depressive symptoms according to severity of ovarian cancer, and to examine associations among IL-6, diurnal cortisol rhythms, and components of depression to shed light on unique mechanisms contributing to affective and vegetative depressive symptoms. Participants included two groups of ovarian cancer patients with invasive disease: early stage (I and II) and advanced stage (III and IV). Patients with ovarian tumors of low malignant potential (LMP) served as the comparison group. These patients had the same presurgical preparation as those found to have invasive cancer, but differed in having noninvasive tumors less likely to produce inflammatory and angiogenic cytokines such as IL-6.35,36 We hypothesized that (a) depression, IL-6, and cortisol dysregulation would be greatest in patients with advanced disease, and (b) depression, particularly vegetative depression, would be associated with elevated IL-6 and cortisol dysregulation among all invasive ovarian cancer patients.
PATIENTS AND METHODS
Patients
Inclusion criteria.
Women older than 18 years with a newly diagnosed pelvic or abdominal mass suspected for ovarian cancer were potentially eligible for the study. Participation was confirmed after histologic diagnosis of a primary invasive epithelial ovarian, primary papillary peritoneal or fallopian tube malignant carcinoma, or an ovarian tumor of LMP. Patients with previous cancer history, primary cancer of another organ, nonepithelial ovarian malignant tumors, systemic steroid medication in the last 4 months, antidepressant medications, or comorbidities known to alter the immune response (eg, autoimmune disorders) were excluded. This study was approved by institutional review boards at the Universities of Iowa (Iowa City, Iowa) and Miami (Miami, Florida).
Sample characteristics.
Of 479 potentially eligible patients approached for study participation, 400 agreed to participate (83.5%). Subsequently, 165 patients were excluded following diagnosis with benign or nonovarian pathologies, 35 for antidepressant medications, and 23 for cancellation or rescheduling of surgery that precluded sample collection, neoadjuvant chemotherapy, or other exclusion criteria. Nineteen patients withdrew before surgery, mostly because of time constraints or emotional distress, and 21 did not fully complete questionnaires. The final sample included 112 invasive ovarian cancer patients (86 advanced stage; 26 early stage) and 25 patients with LMP tumors.
Procedure
Patients were recruited during a presurgical clinic visit and completed questionnaires between the initial visit and surgery. All assessments occurred before definitive knowledge of diagnosis and staging. Patients collected salivary cortisol samples four times daily (awakening, 30 minutes after awakening, 3-6 pm, and 8 pm–midnight) for the 3 days before surgery using salivettes (Sarstedt, Rommelsdorf, Germany). On the morning of surgery (between 6 am and noon) a 35-mL sample of peripheral venous blood was collected in heparinized vacutainer tubes (Becton Dickinson, Rutherford, NJ) before administration of preoperative medication or general anesthesia. Ascites was obtained from surgery. Blood and ascites were centrifuged at 1,126× g at 4°C for 15 minutes and frozen at −80°C until testing. Plasma was not obtained for 23 patients because of difficulty with venous access, change in surgical scheduling, or refusal of blood draw. Ascites was present in 57% of advanced- and 19% of early-stage patients. Medical information was abstracted from patient charts.
IL-6
Detection of IL-6 in plasma and ascites was performed by enzyme-linked immunosorbent assay (R&D Diagnostics, Minneapolis, MN), with results interpolated from the standard curve provided with the kit. The minimum detectable level is less than 0.7 pg/mL and interassay variability ranges from 3.3% to 6.4%. IL-6 samples below the sensitivity of the regular assay were quantitated with the R&D High-Sensitivity ELISA. IL-6 levels in plasma and ascites are highly correlated with tumor levels and are thought to represent the amount produced by tumor.37 IL-6 was not correlated with sampling time (r = 0.039, P = .72).
Cortisol
Salivary cortisol is considered a reliable measure of unbound, biologically active blood cortisol.38,39 Morning cortisol rises occur in conjunction with personal awakening time;40,41 thus, patients collected and recorded their first cortisol sample at their personal waking time. Self-report of collection time has been shown to be highly reliable.41 Assays were performed at the Technical University of Dresden, Germany. A commercial chemiluminescence immunoassay (IBL, Hamburg, Germany) was used, with a lower detection limit of 0.41 nmol/L. Inter- and intra-assay coefficients of variance are less than 10% across the expected range of cortisol levels.42 Patients with the most serious conditions frequently had surgery within 1 to 2 days after their clinic visit; these patients were often unable to collect salivary cortisol. Depression scores and IL-6 levels did not differ significantly among invasive ovarian patients who collected salivary samples and those who did not (P > 0.22).
Psychosocial Measures
The Center for Epidemiological Studies-Depression Scale (CES-D) is a validated 20-item measure on which subjects rate frequency of depressive symptoms over the previous week on a four-point scale ranging from 0 (rarely) to 3 (most or all of the time). Scores of 16 or higher have been associated with clinical depression.43,44 A four-factor structure has been identified for the CES-D with the following subscales: depressed affect, positive affect, vegetative symptoms, and interpersonal relations. These factors have been used independently to provide a more accurate picture of facets of depression, particularly for individuals with chronic illness.45 Patients also completed information about demographic characteristics and health behaviors such as sleep, caffeine, and smoking.
Statistical Analyses
Distributions were examined for outliers and non-normality. IL-6 data were normalized by logarithmic transformation. Cortisol data were examined for sampling time outliers and then for cortisol value outliers. Acceptable sampling times were determined to fit the maximum number of participants, while retaining homogeneity. First morning cortisol samples were excluded if they were outside the window of 4 to 9 am. Afternoon values from 3 to 6 pm and evening values from 8 pm to midnight were included in analyses. Cortisol values more than four standard deviations from the mean for any time point were classified as outliers and replaced with the highest acceptable value as performed previously.46 Mean cortisol values for each patient at each time point were calculated over the 3 collection days. Data were then normalized using natural log transformations. Area under the curve (AUC) over 24 hours was calculated using the trapezoidal formula.
Between-group differences for continuous variables were tested by one-way analyses of variance (ANOVAs) and differences in categoric variables were tested using χ2 analyses. Univariate analyses of covariance (ANCOVAs) adjusting for age were performed to test whether depression, IL-6, and cortisol (AUC) levels differed between the three groups. Post hoc tests comparing each pair of groups were conducted after significant ANCOVAs. All ANCOVA models used two-sided tests of significance with Bonferroni-adjusted P values. An adjusted P value of less than .05 was considered significant. Because only two patients with LMP tumors and five early-stage patients had ascites, between-group analyses for ascites IL-6 were not conducted. Linear mixed-models adjusting for age examined whether change in cortisol over time varied by group.47 ANCOVAs then examined between-group differences at each time point. Multiple regression models adjusting for age and stage were conducted to examine relationships between depression, IL-6, and cortisol among invasive ovarian cancer patients.
RESULTS
Demographic Information
Demographic and clinical characteristics are shown in Table 1. Patients with LMP tumors were significantly younger than patients with advanced-stage disease [F(1,134) = 8.87, P = .009]; therefore, age was included as a covariate in between-group analyses. There were no significant differences among the three groups with regard to smoking status, use of alcohol, caffeine, hours of sleep over the previous night or week, exercise frequency, income, or education (all P > .12). Among invasive ovarian cancer patients, stage was associated with plasma IL-6 (r = .23, P = .025), age and stage were significantly associated with cortisol levels for at least one cortisol time point (P < .05), and caffeine was associated with lower afternoon cortisol (r = −033, P = .033). Age and stage were therefore used as covariates for all regression analyses; analyses with afternoon cortisol adjusted for caffeine. No other potential covariate was significantly associated with IL-6 or cortisol values (P > .06).
Table 1.
Demographic Characteristics of Sample
| Measure | Patients With LMP Tumors | Early-Stage Ovarian Cancer Patients | Advanced-Stage Ovarian Cancer Patients |
|---|---|---|---|
| Age, years | |||
| No. of patients | 25 | 26 | 86 |
| Mean | 51.24 | 55.60 | 60.22* |
| Standard deviation | 19.80 | 10.31 | 11.65 |
| Range | 23-82 | 38-78 | 29-81 |
| Education, % | |||
| No. of patients | 25 | 26 | 84 |
| Less than high school | 12.0 | 0.0 | 2.4 |
| Some high school | 12.0 | 3.9 | 8.3 |
| High school graduate | 28.0 | 23.0 | 29.8 |
| Trade school/some college | 16.0 | 57.6 | 29.8 |
| College graduate | 28.0 | 11.6 | 21.4 |
| Postgraduate | 4.0 | 3.9 | 8.3 |
| Income, % | |||
| No. of patients | 22 | 22 | 73 |
| < $10,000 | 18.0 | 13.6 | 11.0 |
| $10,001-$20,000 | 31.9 | 13.6 | 12.3 |
| $20,001-$30,000 | 9.2 | 9.2 | 20.5 |
| $30,001-$40,000 | 4.5 | 18.2 | 19.2 |
| $40,001-$60,000 | 31.9 | 22.7 | 24.7 |
| $60,001-$80,000 | 4.5 | 4.5 | 9.6 |
| > $80,000 | 0.0 | 18.2 | 2.7 |
| Race, % | |||
| No. of patients | 25 | 26 | 86 |
| American Indian/Alaskan Native | 4.0 | 7.7 | 1.2 |
| Asian/Pacific Islander | 4.0 | 3.8 | 1.2 |
| African American | 4.0 | 7.7 | 1.2 |
| White | 92.0 | 80.8 | 96.4 |
| Ethnicity, % | |||
| No. of patients | 25 | 26 | 86 |
| Hispanic | 4.0 | 11.5 | 7.0 |
| Non-Hispanic | 96.0 | 88.5 | 93.0 |
| Stage, % | |||
| No. of patients | 25 | 26 | 86 |
| I | 65.4 | ||
| II | 34.6 | ||
| III | 81.4 | ||
| IV | 18.6 | ||
| Grade, % | |||
| No. of patients | 0 | 21 | 65 |
| 1 | 38.1 | 4.6 | |
| 2 | 23.8 | 21.5 | |
| 3 | 38.1 | 73.9 | |
| Tumor histology, % | |||
| No. of patients | 25 | 26 | 86 |
| Serous | 24.0 | 50.0 | 81.4 |
| Endometrioid | 4.0 | 30.9 | 5.8 |
| Clear cell | 0.0 | 3.9 | 1.2 |
| Mucinous | 32.0 | 7.7 | 2.3 |
| Other | 40.0 | 7.7 | 9.3 |
Abbreviation: LMP, low malignant potential.
Significantly different than LMP at P = .009.
Depressive Symptoms
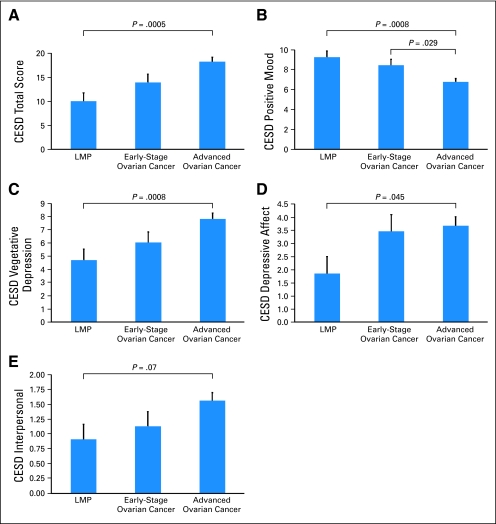
Patients with advanced-stage disease reported significantly greater total depressive symptoms (CES-D total) than patients with LMP tumors [F(1,133) = 15.02, P = .0005; omnibus tests are show in Appendix Table A1, online only). Early-stage patients did not differ significantly in total depression from the other two groups, (P > .08; Fig 1A) Significantly more advanced-stage patients (57%) than patients with LMP tumors (28%) had CES-D scores in the range of clinical depression [χ2(111) = 6.51, P = .011]. Early-stage patients did not significantly differ from the other two groups in the proportion of clinically depressed patients (38%; P > .09). Advanced-stage patients reported significantly less positive mood than patients with early-stage disease [F(1,133) = 6.91, P = .029], or LMP tumors [F(1,133) = 13.92, P = .0008], who did not differ from each other (P = .92). Advanced-stage patients reported significantly greater vegetative depression [F(1,133) = 9.49, P = .008] and depressed mood [F(1,133) = 6.08, P = .045] but no difference in interpersonal difficulties [F(1,133) = 5.26, P = .07] compared with patients with LMP tumors. Early-stage patients did not differ significantly in these facets of depression from the other two groups (P > .15; Figs 1B-1E).
Fig 1.
Age-adjusted means (and SE bars) for Center for Epidemiologic Studies-Depression Scale (CES-D) among advanced- and early-stage invasive ovarian cancer patients and patients with tumors of low malignant potential (LMP). (A) CES-D total, (B) positive mood subscale, (C) vegetative depression subscale, (D) depressive mood subscale, and (E) depressive interaction subscale. All significance levels are Bonferroni adjusted.
IL-6
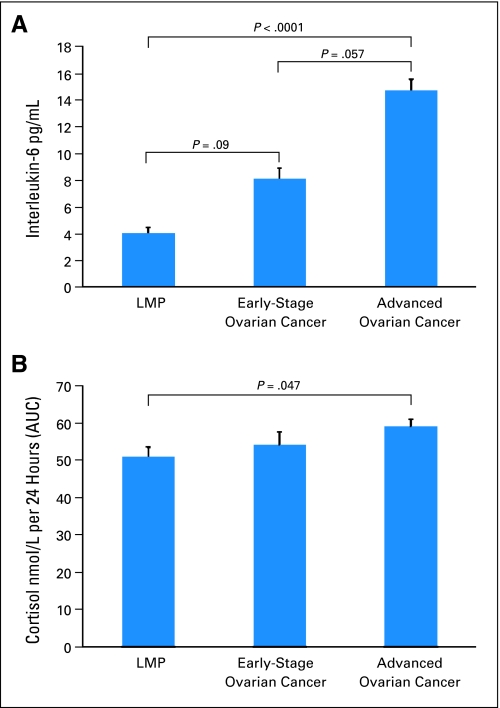
Plasma IL-6 levels among advanced-stage patients (M = 14.72 ± 8.36 pg/mL) were substantially elevated above a cutoff (3.19 pg/mL) previously associated with greater all-cause mortality in community-dwelling elders,48 and were significantly higher than those of patients with LMP tumors [F(1,111) = 23.79, P < .0001]. IL-6 levels of early-stage patients were between levels of the two other groups [early v advanced stage, F(1,111) = 5.67, P = .057; early stage v LMP, F(1,111) = 4.85, P = .09; Fig 2A]. Mean ascites IL-6 was profoundly elevated in both groups of invasive cancer patients who did not differ significantly from each other (P = .81; Table 2).
Fig 2.
Means and (SE bars) for (A) plasma interleukin-6 (IL-6) (pg/mL) and (B) cortisol area under the curve (AUC) in advanced- and early-stage invasive ovarian cancer patients and in patients with tumors of low malignant potential (LMP). All significance levels are Bonferroni adjusted.
Table 2.
Age-Adjusted Means of Psychosocial and Physiological Measures
| Measure | No. of Patients
|
Patients With LMP Tumors
|
Early-Stage Ovarian Cancer Patients
|
Advanced-Stage Ovarian Cancer Patients
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| LMP | Early | Advanced | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |
| CES-D | |||||||||
| Total score | 25 | 26 | 86 | 10.40 | 6.92 to 13.88 | 13.96 | 10.61 to 17.31 | 18.23* | 16.38 to 20.09 |
| Vegetative | 25 | 26 | 86 | 4.90 | 3.28 to 6.54 | 6.05 | 4.48 to 7.62 | 7.83† | 6.96 to 8.70 |
| Positive | 25 | 26 | 86 | 9.27 | 8.11 to 10.44 | 8.46 | 7.34 to 9.58 | 6.75*†‡ | 6.12 to 7.37 |
| Depressed mood | 25 | 26 | 86 | 1.86 | 0.58 to 3.13 | 3.46 | 2.23 to 4.69 | 3.67§ | 3.00 to 4.35 |
| Interpersonal | 25 | 26 | 86 | 0.92 | 0.42 to 1.42 | 1.14 | 0.66 to 1.62 | 1.59 | 1.32 to 1.85 |
| IL-6 pg/mL | |||||||||
| Plasma | 20 | 23 | 72 | 4.07 | 2.57 to 6.31 | 8.13 | 5.25 to 12.45 | 14.45‖ | 11.48 to 18.62 |
| Ascites | 2 | 5 | 50 | 1169.49 | 246.60 to 5,546.26 | 3,854.78 | 1,442.11 to 10,303.86 | 3451.43 | 2,511.88 to 4,709.77 |
| Cortisol, nmol/L | |||||||||
| am | 18 | 12 | 36 | 14.15 | 10.39 to 19.11 | 17.64 | 12.00 to 24.58 | 20.49 | 15.99 to 24.53 |
| am +30 | 17 | 13 | 34 | 19.53 | 15.41 to 24.78 | 21.63 | 16.49 to 28.36 | 26.58 | 22.44 to 31.50 |
| pm | 18 | 13 | 32 | 6.30 | 5.03 to 7.89 | 6.69 | 5.11 to 8.77 | 8.10 | 6.82 to 9.63 |
| Night | 19 | 14 | 34 | 4.91 | 3.39 to 7.09 | 7.24 | 4.71 to 11.09 | 6.31 | 4.75 to 8.30 |
| AUC | 17 | 10 | 30 | 51.06 | 45.98 to 56.12 | 54.28 | 47.52 to 61.03 | 59.16¶ | 55.26 to 63.05 |
Abbreviations: LMP, low malignant potential; CES-D, Center for Epidemiological Studies-Depression Scale; AUC, area under the curve; ES, effect size.
Significantly different from LMP at P ≤ .001, ES = .10.
Significantly different from LMP at P < .01, ES = 0.067.
Significantly different from early stage at P < .05, ES = 0.05.
Significantly different from LMP at P < .05, ES = 0.04.
Significantly different from LMP at P ≤ .001, ES = 0.18.
Significantly different from LMP at P < .05, ES = 0.11. All significance levels are Bonferroni adjusted.
Cortisol
Salivary cortisol levels in all patients were elevated above population norms at each assessment, with evening levels in invasive patients approximately three times healthy population norms.39 The cortisol AUC was significantly higher among advanced-stage patients than among patients with LMP tumors [F(1,53) = 6.24, P = .047] but early-stage patients did not differ from the other two groups (P > .50; Fig 2B). Diurnal cortisol levels did not differ significantly among the three groups at any time point (P > .06) or over the day (P > .73; Table 2).
Depressive Symptoms, IL-6, and Cortisol Among Invasive Ovarian Patients
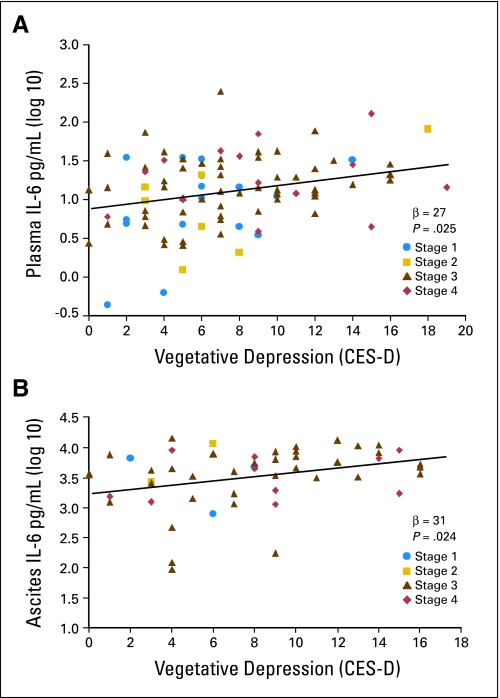
Multiple regression analyses examined relationships between IL-6 and facets of depression in early- and advanced-stage patients. Because their regression slopes did not differ significantly, both groups of invasive ovarian cancer patients were combined in analyses, adjusting for age and stage. Invasive ovarian cancer patients with greater vegetative depression had higher IL-6 in both plasma (β = .27, P = .008, effect size [ES] = .07) and ascites (β = .31, P = .024, ES = .093; Figs 3A and 3B). Elevations in total depression (β = .33, P = .026, ES = .10) and vegetative depression (β = .43, P = .005, ES = .17) were related to higher evening cortisol, and vegetative depression was also related to higher afternoon cortisol (β = .29, P = .04, ES = .08). Other facets of depression and total depression were not significantly related to IL-6 or cortisol at any time point, or to the cortisol AUC (P > .15).
Fig 3.
Vegetative depression and interleukin-6 (IL-6; pg/mL) in (A) peripheral blood (β = .27, P = .008) and (B) ascites (β = .31, P = .024) in invasive ovarian cancer patients. Stage 1 (circle), stage 2 (square), stage 3 (triangle), stage 4 (diamond). All analyses adjust for age and cancer stage. Regression line is representative of all stages of disease.
Plasma IL-6 was related to greater evening cortisol (β = .48, P < .0003, ES = .21), afternoon cortisol (β = .58, P < .0001, ES = .25), and cortisol AUC (β = .49, P = .003, ES = .22). Ascites IL-6 was marginally associated with greater evening cortisol (β = .45, P = .056, ES = .17) but not to other cortisol values.
DISCUSSION
This study extends previous work by documenting elevations of IL-6 and both affective and vegetative depressive symptoms in advanced-stage ovarian cancer patients presurgery. Early-stage patients generally had levels of IL-6 and depressive symptoms that were greater than those observed in LMP patients but lower than those in patients with advanced disease. Among patients with invasive disease, only the vegetative component of depression was linked with IL-6 and evening cortisol. Aspects of depression related to affect were not associated with either IL-6 or cortisol, suggesting that different mechanisms may underlie affective versus vegetative depression in these patients. Elevated IL-6 was also related to greater disturbances in the diurnal cortisol rhythm among invasive ovarian cancer patients, with elevated plasma and ascites IL-6 related to higher evening cortisol, and plasma IL-6 also related to higher afternoon cortisol and cortisol AUC. IL-6 means among advanced-stage patients were greater than a cutoff of 10.9 pg/mL previously associated with depression in metastatic cancer patients.9 The present results are consistent with the “proinflammatory cytokine theory of depression” in suggesting that pathophysiologic elevations in circulating inflammatory mediators may drive the appearance of depressive symptomatology via cytokine regulation of CNS function.14
Several possible mechanisms may contribute to depression in invasive ovarian cancer patients, all of which may operate simultaneously. Because patients with more advanced cancers demonstrated the greatest vegetative symptoms, it is possible that physical symptoms secondary to the bulk of the tumor, including bowel difficulties and distention, may contribute to vegetative symptoms. Additionally, although assessments of depressive symptoms were made before patients knew their diagnosis and prognosis, ovarian cancer has been associated with elevated depression,4-6 and concerns about ovarian cancer may have contributed to elevated depressive symptoms.
It is also possible that elevated levels of tumor-derived IL-6 directly contribute to the development of “sickness behaviors” that overlap with symptoms of vegetative depression,26 although the extent of independent effects by IL-6 relative to other cytokines is not clear.49 Proinflammatory cytokines influence the CNS via several direct pathways, including passage through regions of permeability of the blood-brain barrier and stimulation of afferent fibers in the vagus nerve. These fibers relay information to specific brain nuclei with subsequent downstream effects on multiple central processes including induction of cytokines, neurotransmitters, stimulation of the HPA axis, and development of sickness behaviors.26-28,50 Relationships between IL-6 and vegetative depression accompanied by the absence of associations between affective depression and IL-6 are consistent with the possibility that inflammatory mechanisms may contribute to vegetative symptoms,27 whereas other mechanisms may underlie affective symptoms of depression.
There are also well-established links between the HPA axis and depression.12,27,51 Chronic inflammation can induce glucocorticoid resistance52,53 and lead to a hyperactive HPA axis along with suppressed negative feedback.27 The resultant HPA dysregulation and high levels of cortisol may contribute to depression,50 providing an indirect pathway between IL-6 and depression.
The excessive production of IL-6 by ovarian carcinomas may set up a chronic proinflammatory state, eliciting sickness behaviors in the CNS and hypersecretion and dysregulation of the HPA axis, both contributing to depressive symptomatology.27,52 Because of extremely high levels of tumor-secreted IL-6, particularly in ascites, secreted cortisol may be inadequate to suppress IL-6. Ovarian tumor cells contain glucocorticoid receptors54 which are downregulated by dexamethasone;55 if such downregulation occurs in response to chronically elevated cortisol, it could potentially interrupt the negative feedback loop in the tumor microenvironment.
Alternatively, depression may contribute to enhanced IL-6 secretion. Depression has been associated with systemic elevations in norepinephrine.56-59 Norepinephrine stimulation is known to enhance IL-6 secretion by ovarian tumor cells in vitro,60 potentially setting up a positive feedback loop for IL-6 in the tumor microenvironment. It is also possible that all of these pathways may operate simultaneously.
These findings are correlational and thus limit causal inferences. We are currently using an experimental animal model of ovarian cancer to further understand these issues. To accommodate surgical scheduling and limit circadian variability of IL-6, blood sampling was performed between 6 am and noon. IL-6 was not related to blood sampling time, suggesting minimal circadian contribution to variability. Some patients were missing one of the physiological variables, particularly cortisol values; this may have contributed to loss of power, and these findings should be interpreted with caution.
Our findings provide a new understanding of relationships between an important proinflammatory cytokine (IL-6), cortisol, and depressive symptoms in ovarian cancer. Moreover, these results raise intriguing questions regarding whether tumor IL-6 production contributes to vegetative depression in ovarian cancer. Further mechanistic work is needed to clarify such questions and may offer hope for novel pharmacologic treatments for vegetative depression in ovarian cancer.61
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Susan K. Lutgendorf, Anil K. Sood, David M. Lubaroff
Financial support: Susan K. Lutgendorf
Administrative support: Susan K. Lutgendorf, Frank Penedo, David M. Lubaroff
Provision of study materials or patients: Susan K. Lutgendorf, Frank Penedo, Koen DeGeest, Joseph A. Lucci
Collection and assembly of data: Susan K. Lutgendorf, Aliza Z. Weinrib, Frank Penedo, Patrick J. Henderson, David M. Lubaroff
Data analysis and interpretation: Susan K. Lutgendorf, Aliza Z. Weinrib, Frank Penedo, Daniel Russell, Koen DeGeest, Sandra E. Sephton, Nicholas Rohleder, Steve W. Cole, Anil K. Sood, David M. Lubaroff
Manuscript writing: Susan K. Lutgendorf, Aliza Z. Weinrib, Frank Penedo, Daniel Russell, Koen DeGeest, Erin S. Costanzo, Sandra E. Sephton, Nicholas Rohleder, Steve W. Cole, Anil K. Sood, David M. Lubaroff
Final approval of manuscript: Susan K. Lutgendorf, Aliza Z. Weinrib, Frank Penedo, Daniel Russell, Koen DeGeest, Erin S. Costanzo, Patrick J. Henderson, Sandra E. Sephton, Nicholas Rohleder, Joseph A. Lucci, Steve W. Cole, Anil K. Sood, David M. Lubaroff
Acknowledgments
We thank Heena Maiseri, Cecilia Torres, Vanessa Lehner, Stephanie McGinn, Kelsey Flaten, Erin Johnson, and Elizabeth Ohsann for assistance in patient recruitment; Mark Doobay and Benjamin Green for laboratory assistance; Emily Schlitter and Madeline Gereau for assistance with data; Barrie Anderson, MD, Joel Sorosky, MD, David Bender, MD, Michael Goodheart MD, and Thomas Beukers, MD, for assistance in identifying eligible patients; and Bridget Zimmerman, PhD, for statistical consultation.
Appendix
Depression is a multifaceted construct including components such as neurovegetative and somatic symptoms as well as psychological symptoms that include mood and cognitive alterations. Low positive and high negative affect are thought to be independent components of depression, resulting from different biobehavioral mechanisms (Watson D, Wiese D, Vaidya J, et al. J Personality Social Psychol 76:820-838, 1999). The Center for Epidemiological Studies Depression scale has a stable four-factor measurement structure that identifies four dimensions of depressive symptoms: positive mood, negative mood, vegetative symptoms, and interpersonal symptoms (such as feeling like a failure, etc; Sheehan TJ, Fifield J, Reisine S, et al. J Personality Assess 64:507-521, 1995). In depression induced by inflammatory cytokines, neurovegetative and somatic symptoms such as fatigue, anorexia, pain, reduction in movement, and sleep disorders predominate, and tend to have an early onset. Psychological symptoms of depression occur later (Capuron L, Gumnick JF, Musselman DL, et al. Neuropsychopharmacology 26:643-652, 2002; Capuron L, Dantzer R. Brain Behav Immun 7:S119-S124, 2001; Capuron L, Ravaud A, Dantzer R. J Clin Oncol 18:2143-2151, 2001; Dantzer R, O'Connor JC, Freund GG, et al. Nat Rev Neurosci 9:46-57, 2008). We have previously reported that the vegetative symptom of fatigue was related to elevated IL-6 in plasma and ascites in advanced-stage presurgical ovarian cancer patients, and that patients with a self-reported history of depression had higher presurgical IL-6 (Costanzo ES, Lutgendorf SK, Sood AK. Cancer 104:305-313, 2005). Understanding which components of depression are related to an inflammatory cytokine such as IL-6 sheds light on potential etiology of symptoms. Thus, if IL-6 is related to vegetative depression but not to affective depression, this would be consistent with an interpretation that this inflammatory cytokine may contribute to the symptoms of vegetative depression, whereas another mechanism may underlie the affective component of depression. Because in ovarian cancer IL-6 is largely tumor derived (Burger RA, Grosen EA, Ioli GR, et al. Spontaneous release of interleukin-6 by primary cultures of lymphoid and tumor cell populations purified from human ovarian carcinoma. J Interferon Cytokine Res 5:255-260, 1995), the implication of this finding is that a tumor-derived product is driving the depression.
Table A1.
Summary of Between-Group Analyses
| Measure | df | Omnibus ANOVA
|
df of Post hoc Tests | LMP v Early-Stage Patients
|
LMP v Advanced-Stage Patients
|
Early-Stage v Advanced-Stage Patients
|
||||
|---|---|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | F | P | |||
| CES-D | 2,133 | 8.31 | < .0001 | 1,133 | 2.15 | .42 | 15.02 | .0005 | 4.83 | .08 |
| Total score | 2,133 | 5.48 | .005 | 1,133 | 1.01 | .96 | 9.49 | .008 | 3.81 | .16 |
| Vegetative | 2,133 | 8.48 | < .0001 | 1,133 | 1.00 | .92 | 13.92 | .0008 | 6.91 | .029 |
| Positive | 2,133 | 3.09 | .049 | 1,133 | 3.23 | .23 | 6.08 | .045 | 0.10 | .99 |
| Depressed mood | 2,133 | 3.18 | .045 | 1,133 | 0.40 | .99 | 5.26 | .07 | 2.54 | .33 |
| IL-6 pg/mL (plasma) | 2,111 | 12.62 | < .0001 | 1,111 | 4.85 | .09 | 23.79 | < .0001 | 5.67 | .057 |
| Cortisol, nmol/L | ||||||||||
| am | 2,62 | 1.69 | .19 | 1,62 | 0.81 | .99 | 3.37 | .21 | 0.35 | .99 |
| am +30 | 2,60 | 2.36 | .10 | 1,60 | 0.32 | .99 | 4.34 | .12 | 1.63 | .63 |
| pm | 2,59 | 1.71 | .19 | 1,59 | 0.13 | .99 | 3.10 | .24 | 1.36 | .75 |
| Night | 2,63 | 1.05 | .36 | 1,63 | 1.93 | .51 | 1.12 | .87 | 0.30 | .99 |
| AUC | 2,53 | 3.18 | .05 | 1,53 | 0.60 | .99 | 6.24 | .048 | 1.50 | .69 |
NOTE. P values of post hoc tests are Bonferroni corrected. A P value of .99 is given if the Bonferroni adjustment would have made it greater than 1.00.
Abbreviations: ANOVA, analysis of variance; LMP, low malignant potential; CES-D, Center for Epidemiological Studies-Depression Scale; IL-6, interleukin-6; AUC, area under the curve.
published online ahead of print at www.jco.org on September 8, 2008.
Supported in part by Grants No. CA88293 and CA104825 (S.K.L.) from the National Cancer Institute.
Presented in part at the Annual Meeting of the Psychoneuroimmunology Research Society, May 30-June 2, 2007, Arcachon, France.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Massie MJ: Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr 32:57-71, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Spoletini I, Gianni W, Repetto L, et al: Depression and cancer: An unexplored and unresolved emergent issue in elderly patients. Crit Rev Oncol Hematol 65:143-155, 2008 [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society: Cancer Facts and Figures. Atlanta, GA, American Cancer Society, 2008
- 4.Bodurka-Bevers D, Basen-Engquist K, Camark CL, et al: Depression, anxiety, and quality of life in patients with epithelial ovarian cancer. Gyn Oncol 78:302-308, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Norton TR, Manne SL, Rubin S, et al: Prevalence and predictors of psychological distress among women with ovarian cancer. J Clin Oncol 22:919-926, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Hipkins J, Whitworth M, Tarrier N, et al: Social support, anxiety and depression after chemotherapy for ovarian cancer: A prospective study. Br J Health Psychol 9:569-581, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Spiegel D, Giese-Davis J: Depression and cancer: Mechanisms and disease progression. Biol Psychiatry 54:269-282, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Musselman DL, Miller AH, Porter MR, et al: Higher than normal plasma interleukin-6 concentrations in cancer patients with depression: Preliminary findings. Am J Psychiatry 158:1252-1257, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Jehn CF, Kuehnhardt D, Bartholomae A, et al: Biomarkers of depression in cancer patients. Cancer 107:2723-2729, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Rich T, Innominato PF, Boerner J, et al: Elevated serum cytokines correlated with altered behavior, serum cortisol rhythm, and dampened 24-hour rest-activity patterns in patients with metastic colorectal cancer. Clin Cancer Res 11:1757-1764, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Costanzo ES, Lutgendorf SK, Sood AK, et al: Psychosocial factors and interleukin-6 among women with advanced ovarian cancer. Cancer 104:305-313, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Raison CL, Miller AH: Depression in cancer: New developments regarding diagnosis and treatment. Biol Psychiatry 54:283-294, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Li Q: Inflammation-associated cancer: NF kappaB is the lynchpin. Trends Immunol 26:318-335, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Dantzer R, O'Connor JC, Freund GG, et al: From inflammation to sickness and depression: When the immune system subjugates the brain. Nat Rev Neurosci 9:46-57, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Snick J: Interleukin-6: An overview. Annu Rev Immunol 8:253-278, 1990 [DOI] [PubMed] [Google Scholar]
- 16.Obata NH, Tamakoshi K, Shibata K, et al: Effects of interleukin-6 on in vitro cell attachment, migration, and invasion of human ovarian carcinoma. Ann Rev Immunol 17:337-342, 1997 [PubMed] [Google Scholar]
- 17.Wu S, Rodabaugh K, Martinez-Maza O, et al: Stimulation of ovarian tumor cell proliferation with monocyte products including interleukin-1, interleukin-6, and tumor necrosis factor-alpha. Am J Obstet Gynecol 166:997-1007, 1992 [DOI] [PubMed] [Google Scholar]
- 18.Tempfer C, Zeisler H, Sliutz G, et al: Serum evaluation of interleukin 6 in ovarian cancer patients. Gynecol Oncol 66:27-30, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Scambia G, Testa U, Benedetti P, et al: Prognostic significance of IL-6 serum levels in patients with ovarian cancer. Br J Cancer 71:352-356, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berek JS, Chung C, Kaldi K, et al: Serum interleukin-6 levels correlate with disease status in patients with epithelial ovarian cancer. Am J Obstet Gynecol 164:1038-1043, 1991 [DOI] [PubMed] [Google Scholar]
- 21.Suarez EC, Lewis JG, Krishnan RR, et al: Enhanced expression of cytokines and chemokines by blood monocytes to in vitro lipopolysaccharide stimulation are associated with hostility and severity of depressive symptoms in healthy women. Psychoneuroendocrinology 29:1119-1128, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Dentino AN, Pieper CF, Rao MK, et al: Association of interleukin-6 and other biologic variables with depression in older people living in the community. J Am Geriat Soc 47:6-11, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Alesci S: Major depression is associated with significant diurnal elevations in plasma interleukin-6 levels, a shift of its circadian rhythm, and loss of physiological complexity in its secretion: Clinical implications. J Clin Endocrinol Metab 90:2522-2530, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Haack M: Plasma levels of cytokines and soluble cytokine receptors in psychiatric patients upon hospital admission: Effects of confounding factors and diagnosis. J Psychiatric Res 33:407-418, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Steptoe A: Lack of association between depressive symptoms and markers of immune and vascular inflammation in middle-aged men and women. Psychol Med 33:667-674, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Maier S, Watkins L: Cytokines for psychologists: Implications of bidirectional immune to brain communication for understanding behavior, mood, and cognition. Psychology Rev 105:83-107, 1998 [DOI] [PubMed] [Google Scholar]
- 27.Capuron L, Dantzer R: Cytokines and depression: The need for a new paradigm. Brain Behav Immun 17:S119-S124, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Raison CL, Miller AH: When not enough is too much: The role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry 160:1554-1565, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Sapolsky RM, Alberts SC, Altmann J: Hypercortisolism associated with social subordinance or social isolation among wild baboons. Arch Gen Psychiatry 54:1137-1143, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Deuschle M, Schweiger U, Weber B, et al: Diurnal activity and pulsatility of the hypothalamus-pituitary-adrenal system in male depressed patients and healthy controls. J Clin Endocrinol Metab 82:234-238, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Chrousos GP, Gold PW: A healthy body in a healthy mind—and vice versa—the damaging power of “uncontrollable” stress. J Clin Endocrinol Metab 83:1842-1845, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Abou-Saleh MT, Milln P, Coppen A: Dexamethasone suppression test in depression. Neuropharmacol 22:549-550, 1983 [DOI] [PubMed] [Google Scholar]
- 33.McEwen BS: Mood disorders and allostatic load. Biol Psychiatry 54:200-207, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Mormont M, Levi F: Circadian system alterations during cancer processes: A review. Int J Cancer 70:241-247, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Cooper BC, Ritchie JM, Broghammer CLW, et al: Preoperative serum vascular endothelial growth factor (VEGF) levels: Significance in ovarian cancer. Clin Cancer Res 8:3193-3197, 2002 [PubMed] [Google Scholar]
- 36.McKenney JK, Balzer BL: Patterns of stromal invasion in ovarian serous tumors of low malignant potential (borderline tumors): A reevaluation of the concept of stromal microinvasion. Am J Surg Pathol 30:1209-1221, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Burger RA, Grosen EA, Loli GR, et al: Spontaneous release of interleukin-6 by primary cultures of lymphoid and tumor cell populations purified from human ovarian carcinoma. J Interfer Cyto Res 15:255-260, 1995 [DOI] [PubMed] [Google Scholar]
- 38.Kirschbaum C, Hellhammer D: Salivary cortisol in psychoneuroendocrine research: Recent developments and applications. Psychoneuroendocrinology 19:313-333, 1994 [DOI] [PubMed] [Google Scholar]
- 39.Kirschbaum C, Hellhammer DH: Salivary cortisol in psychobiological research: An overview. Neuropsychobiology 22:150-169, 1989 [DOI] [PubMed] [Google Scholar]
- 40.Kirschbaum C, Hellhammer DH. Salivary cortisol, in Fink G (ed): Encyclopedia of Stress (Vol 3) Burlington, MA, Academic Press, 2000
- 41.Kraemer HC, Giese-Davis J, Yutsis M, et al: Design decisions to optimize reliability of daytime cortisol slopes in an older population. Am J Geriatr Psychiatry 14:325-333, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Rohleder N, Beulen S, Chen E, et al: Stress on the dance floor: The cortisol response to social-evaluative threat in competitive ballroom dancers. Pers Soc Psychol Bull 33:69-84, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Radloff L: The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas 1:385-401, 1977 [Google Scholar]
- 44.Ensel WM: Measuring depression: The CES-D scale, in Adwe NL (ed): Social support, life events, and depression. New York, NY, Academic Press, 1986
- 45.Sheehan TJ, Fifield J, Reisine S, et al: The measurement structure of the Center for Epidemiologic Studies depression scale. J Pers Assess 64:507-521, 1995 [DOI] [PubMed] [Google Scholar]
- 46.Sephton S, Spiegel D: Circadian disruption in cancer: A neuroendocrine-immune pathway from stress to disease? Brain Behav Immun 17:321-328, 2003 [DOI] [PubMed] [Google Scholar]
- 47.Snijders TA, Bosker RJ: Multilevel analysis: An introduction to basic and advanced multilevel modeling. London, United Kingdom, Sage 1999
- 48.Harris TB, Ferrucci L, Tracy RP, et al: Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med 106:506-512, 1999 [DOI] [PubMed] [Google Scholar]
- 49.Swiergiel AH, Dunn AJ: Feeding, exploratory, anxiety- and depression-related behaviors are not altered in interleukin-6-deficient male mice. Behav Brain Res 171:94-108, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raison CL, Capuron L, Miller AH: Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends Immunol 27:24-31, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chrousos GP: Stressors, stress, and neuroendocrine integration of the adaptive response: The Hans Selye memorial lecture. Ann NY Acad Sci 851:311-335, 1998 [DOI] [PubMed] [Google Scholar]
- 52.Miller GE, Cohen S, Ritchey AK: Chronic psychological stress and the regulation of pro-inflammatory cytokines: A glucocorticoid-resistance model. Health Psychol 21:531-541, 2002 [DOI] [PubMed] [Google Scholar]
- 53.Pariante CM, Pearce BD, Pisell TL, et al: The proinflammatory cytokine, interleukin-1 alpha, reduces glucocorticoid receptor translocation and function. Endocrinology 140:4359-4366, 1999 [DOI] [PubMed] [Google Scholar]
- 54.Woenckhaus J, Franke FE, Hackethal A, et al: Glucocorticosteroid receptors in ovarian carcinomas. Oncol Rep 15:1137-1140, 2006 [PubMed] [Google Scholar]
- 55.Xu M, Song L, Wang Z: Effects of Dexamethasone on glucocorticoid receptor expression in a human ovarian carcinoma cell line 3AO. Chin Med J 116:392-395, 2003 [PubMed] [Google Scholar]
- 56.Hughes JW, Watkins L, Blumenthal JA, et al: Depression and anxiety symptoms are related to increased 24-hour urinary norepinephrine excretion among healthy middle-aged women. J Psychosom Res 57:353-358, 2004 [DOI] [PubMed] [Google Scholar]
- 57.Grossman F, Potter WZ: Catecholamines in depression: A cumulative study of urinary norepinephrine and its major metabolites in unipolar and bipolar depressed patients versus healthy volunteers at the NIMH. Psychiatry Res 87:21-27, 1999 [DOI] [PubMed] [Google Scholar]
- 58.Lake CR, Pickar D, Ziegler MG, et al: High plasma norepinephrine levels in patients with major affective disorder. Am J Psychiatry 139:1315-1318, 1982 [DOI] [PubMed] [Google Scholar]
- 59.Mausbach BT, Dimsdale JE, Ziegler MG, et al: Depressive symptoms predict norepinephrine response to a psychological stressor task in Alzheimer's caregivers. Psychosom Med 67:638-642, 2005 [DOI] [PubMed] [Google Scholar]
- 60.Nilsson MB, Takahashi R, Trevino J, et al: Stress hormones regulate IL-6 expression by human ovarian carcinoma cells through a SRC-dependent mechanism J Biol Chem 282:29919-29926, 2007 [DOI] [PubMed] [Google Scholar]
- 61.Illman J, Corringham R, Robinson DJ, et al: Are inflammatory cytokines the common link between cancer-associated cachexia and depression. J Support Oncol 3:37-50, 2005 [PubMed] [Google Scholar]