Abstract
Purpose
To determine whether pediatric patients treated with surgery only for low-grade tumors in the cerebral hemispheres, supratentorial midline, and exophytic brainstem evidence neurocognitive, academic, adaptive, or emotional/behavioral sequelae.
Patients and Methods
Ninety-three patients from a natural history study of low-grade astrocytomas were tested an average of 111 days after surgery. Rates of below average (≤ 25th percentile) scores in this sample were compared with test norms, and performances were compared across anatomic sites. Finally, the relationships of pre-, peri-, and postsurgical complications to outcome were investigated.
Results
For the entire sample, there was a significantly elevated rate of below average scores across intelligence quotient, achievement, and adaptive behavior, but not behavioral/emotional adjustment measures. Patients with hemispheric, midline, and brainstem tumors did not differ significantly. Patients with left hemisphere tumors generally performed worse than those with right hemisphere tumors. Finally, neurobehavioral outcome was unrelated to pre-, peri-, or postsurgery complications.
Conclusion
After surgery for low-grade brain tumors, a significant number of patients was found to function below average, by as much as 55% compared with 25% in the normative population. Moreover, these results suggest greater risk for patients with lesions situated in the left cerebral hemisphere. Routine neuropsychological follow-up of children after treatment for low-grade tumors is recommended.
INTRODUCTION
A substantial percentage of childhood tumors are classified as low grade (ie, nonmalignant) based on their histologic features. Because they are assumed to have a benign course, less is known about the outcomes of such patients compared with patients who have more malignant diseases and who receive more aggressive neurotoxic treatments. However, there is mounting evidence that children with low-grade tumors may suffer more long-term sequelae that are widely appreciated. For low-grade tumors of the cerebellum, we recently reported lower than expected intelligence quotients (IQs) and deficits in adaptive behavior in a large sample of patients treated with surgery only.1 These findings generally agree with several reports on similar patients documenting various forms of neurobehavioral morbidity,2-5 consistent with the growing literature implicating the cerebellum in circuits subserving several cognitive and emotional regulatory functions.6
Concerns about the outcomes of these understudied patients are compounded by their prevalence because these patients constitute approximately a quarter of the pediatric brain tumor patients diagnosed and presumably an even greater proportion of long-term survivors.7 From the perspective of net social burden, these children may be one of the most at-risk groups of children treated for brain tumors as a result of lifetime accrued costs associated with disability and underemployment.
Brain tumors in adults and children differ in a number of important respects.8 These differences notwithstanding, the best evidence to date indicates that reduced cognitive performance of adults with low-grade tumors, compared with other cancer patients and healthy controls, is mainly attributable to the effects of the tumor and, perhaps, antiepileptic medications. Neurocognitive sequelae attributable to conventional radiotherapy (45 to 54 Gy in 1.8- to 2-Gy fractions) are infrequent.9 When radiotherapy fractions exceed 2 Gy, however, radiation-related late effects can occur.10
Investigations into the outcomes of children with low-grade tumors are important to better define long-term risks and to provide appropriate interventions to mitigate these effects. This research can also inform the development of treatment approaches that reduce neurobehavioral sequelae. Extending the findings of our previously reported study of patients with cerebellar tumors,1 this article reports the neurobehavioral findings of the largest sample yet published of children with extracerebellar low-grade tumors.
PATIENTS AND METHODS
Sample
The sample was comprised of 93 children age 3 to 18 years who were enrolled onto Children's Cancer Group protocol 9891 or Pediatric Oncology Group protocol 9130 between 1991 and 1996 (Wisoff et al, manuscript under review). These protocols (which include the psychometrics reported here) were approved by the institutional review boards at all participating institutions, and all patients and/or their guardians gave informed consent. The sample included only children who had undergone surgical resection of their tumors but received no antineoplastic chemotherapies or radiotherapy before psychological testing. Because the psychological tests included in the collaborative protocols have poor technical qualities in very young children, only children age 3 years and older were considered. A previous article1 focused on children with cerebellar tumors, whereas this article focuses on the subgroup of children who had primary tumors outside the cerebellum. Of 256 such children who met these criteria, 93 underwent psychological testing within the first year after surgery (median, 90 days; range, 8 to 361 days); 10 patients were tested before surgery or greater than 1 year after surgery, 150 were unevaluated, and three were excluded as a result of problems with the testing. Reasons for failure to be tested were not consistently documented in this study. However, as is often the case in cooperative group protocols, the most frequently cited reasons were failure to refer for testing and the lack of availability of a psychologist to perform the testing. The psychological testing was a recommended but not required part of the parent study, and so this likely contributed to less than optimal compliance. The 93 patients tested after surgery were assigned by tumor location into one of the following three groups based on site data provided by the operating neurosurgeons and verified via central review: supratentorial-cerebral hemisphere (n = 58); supratentorial-midline (ie, chiasmatic, hypothalamic, thalamic, or third ventricle; n = 20); and exophytic brainstem (ie, midbrain, pons, or cerebellar peduncle; n = 15). The cerebral hemisphere patients were further divided into subgroups defined by location (right side, n = 23; left side, n = 31; and unknown/bilateral, n = 4; also, frontal, n = 9; extrafrontal, n = 43; and unknown, n = 6).
The median age when tested was 10.1 years, with a range of 3.3 to 18.6 years. Girls comprised 51% of the sample, which was largely white (77%), African American (12%), or Hispanic (3%). Of the 72 patients for whom parent educational information was available, the median parent education was 14 years (2 years of post–high school, college, or technical training). The three analyzed groups did not significantly differ in time since surgery, sex, or race (P > .15). However, there was a difference in age tested (P = .006); the hemispheric group had the oldest children, with a median age of 12.4 years compared with 9.4 years for the brainstem group and 7.4 years for the midline group. This reflects differences in the ages of diagnosis for the tumors comprising these groups.
Procedure
Eligibility for the parent study (Children's Cancer Group protocol 9891/Pediatric Oncology Group protocol 9130) was based on histopathologic evidence of low-grade astrocytoma, oligodendroglioma, mixed glioma, or ganglioglioma according to prevailing WHO standards confirmed by central review (see Wisoff et al, manuscript under review, for a more complete description of the parent study). Enrolled children underwent maximal tumor resection with follow-up supportive care. All children were judged to have no disease progression when psychologically tested. Data on medical complications were gathered at three time points, yielding presurgical, perisurgical, and postsurgical composite scores. The presurgical composite reflected the sum of the following factors, coded based on presurgical radiologic studies and physical examination just before the surgery (median of 1 day before the surgery): hydrocephalus (present = 1; absent = 0), seizures (present = 1; absent = 0), and level of consciousness (normal = 0; lethargic or somnolent = 1). The perisurgical composite reflected the sum of the following complications, coded by the neurosurgeon at the treating institution: CNS infection, aseptic meningitis, hematoma, new neurologic symptom, CSF leak, pseudomeningocele, or other specific complication (each present = 1; absent = 0). The postsurgical composite reflected the sum of the following ratings, made by the neurosurgeon 1 week after surgery compared with the presurgical baseline: level of consciousness and neurologic deficits (for each, better = −1; unchanged = 0; worse = +1). Higher composite scores reflected greater medical involvement or severity.
Psychological test data were gathered prospectively within the first year of surgery (mean, 111 days after surgery; standard deviation, 78 days after surgery). Intelligence was assessed with the age-appropriate Wechsler scale (Wechsler Preschool and Primary Scale of Intelligence–Revised, n = 17; Wechsler Intelligence Scale for Children [WISC] –Revised [WISC-R], n = 53; WISC-Third Edition, n = 8; Wechsler Adult Intelligence Scale–Revised, n = 8; no IQ data reported, n = 7), yielding a Verbal IQ score (reflecting language-based skills), a Performance IQ score (PIQ; reflecting spatial and visuomotor skills), and Full Scale IQ score (reflecting overall intelligence). In addition, on the basis of subtest scaled scores, measures of working memory (WM; average of digit span and arithmetic) and psychomotor speed (PS; coding or symbol substitution) were derived from the IQ tests. Visuomotor skills were further assessed by the Beery Test of Visual-Motor Integration. Academic skills were screened with the Wide Range Achievement Test, yielding scores in reading, spelling, and arithmetic. Parents were interviewed using the Vineland Adaptive Behavior Scales, which assessed the following functional domains: Communication, Daily Living Skills, and Socialization. Parents of children younger than 6 years also answered Vineland questions regarding the child's motor skills. Finally, parents were asked to complete the Achenbach Child Behavior Checklist (CBCL), which yields an index of internalizing symptoms (eg, depression, anxiety, somatic concern) and externalizing symptoms (eg, conduct problems).
Overview of Data Analysis
To assess for selection bias, we first used χ2 analyses to compare demographic and medical characteristics of the 93 children included in the analysis with those of the 163 children who were not included. We then focused on the present sample of 93 children, using single-sample t tests to compare mean scores on the psychological tests with the expected mean based on published norms (100 ± 15 for all tests, except the CBCL with a mean of 50 ± 10 and the WM and PS scores of 10 ± 3). As a complement to this group-average analysis, we also indexed the percentage of children in our sample who scored below average, which was defined as falling at or less than the 25th percentile based on norms (ie, standard score < 90 for IQ tests, Vineland, Wide Range Achievement Test, and Visual-Motor Integration; ≤ 8 for WM and PS; and ≥ 57 for the CBCL). This was compared with the population base rate of 25% via the normal approximation to the binomial test. This point of demarcation corresponds to conventional designations of average and subaverage performances on intelligence tests.11 Finally, we conducted group comparisons on the psychological variables (using analyses of variance) and neurologic composites (using χ2). Because of differences in sample sizes across measures, we ran univariate analyses rather than multivariate analyses that would require list-wise deletion of missing patients.
RESULTS
Comparing children included in the final analysis with those who were not included, there were no significant differences in age, sex, race, parental education, or pre-, peri-, or postsurgical neurologic composite indexes (all P > .10). Thus, there was no indication of selection bias.
Mean psychological test scores for the entire sample are listed in Table 1. Included are t tests versus published norms and percentages below average. The average to low average means reflect a shift to the left in the distributions of scores, resulting in higher rates of below average scores across all indexes except those from the CBCL (Fig 1). Given that multiple statistical tests were run, we used Holm's sequential procedure to guard against type I errors. This procedure has improved power over the Bonferroni correction without sacrificing control of type I error.12 Under this procedure, the most stringent Bonferroni α cutoff (0.05/17 in Table 1) is applied only to the most statistically significant finding in the family of analyses. If this threshold is crossed, then the next best significance level obtained in the family is compared with a slightly less stringent cutoff (0.05/16) and so on. Using this procedure, eight of 14 significant t test findings and nine of 14 binomial test findings remain after correction.
Table 1.
Total Sample Scores and Percentage of Patients Below Average
| Test | No. of Patients | Score
|
t Test | % of Patients Below Average | ||
|---|---|---|---|---|---|---|
| Median | Mean | SD | ||||
| Intelligence | ||||||
| VIQ | 83 | 95 | 95.6 | 17.9 | −2.3* | 37.4† |
| PIQ | 84 | 93 | 91.8 | 19.2 | −3.9‡§ | 46.4‡§ |
| FSIQ | 83 | 93 | 93.4 | 18.4 | −3.3† | 42.2‡§ |
| WM | 80 | 9 | 8.8 | 2.9 | −3.7‡§ | 40.0† |
| PS | 60 | 9 | 8.9 | 3.4 | −2.4* | 45.0‡§ |
| Beery VMI | ||||||
| Standard score | 70 | 91 | 89.8 | 14.3 | −6.0‡§ | 48.6‡§ |
| Achievement | ||||||
| Reading | 58 | 94.5 | 92.5 | 21.5 | −2.6* | 39.7† |
| Arithmetic | 59 | 93 | 89.6 | 20.4 | −3.9‡§ | 42.4† |
| Spelling | 56 | 91.5 | 89.8 | 20.3 | −3.8‡§ | 46.4‡§ |
| Adaptive behaviors | ||||||
| Communication | 57 | 93 | 88.2 | 24.0 | −3.7‡§ | 43.9‡§ |
| Daily Living Skills | 57 | 91 | 90.7 | 23.0 | −3.1† | 49.1‡§ |
| Socialization | 57 | 87 | 87.5 | 22.2 | −4.2‡§ | 54.4‡§ |
| Motor Skills | 22‖ | 81.5 | 80.8 | 27.3 | −3.3† | 54.6† |
| Composite | 54 | 88.5 | 84.3 | 23.8 | −4.8‡§ | 53.7‡§ |
| Achenbach CBCL | ||||||
| Internalizing | 55 | 52 | 52.2 | 11.5 | 1.4 | 27.3 |
| Externalizing | 55 | 52 | 51.5 | 11.0 | 1.1 | 29.1 |
| Sum | 53 | 51 | 51.3 | 12.3 | 0.8 | 32.1 |
Abbreviations: SD, standard deviation; VIQ, Verbal Intelligence Quotient; PIQ, Performance Intelligence Quotient; FSIQ, Full Scale Intelligence Quotient; WM, working memory; PS, psychomotor speed; VMI, Visual-Motor Integration; CBCL, Child Behavior Checklist.
P < .05.
P < .01.
P < .001.
P value remained significant after correction for multiple comparisons.
The smaller number is attributable to the fact that the Motor Scale of the Vineland assesses only children < age 6 years.
Fig 1.
Percentage of scores below average (with 95% CI). VIQ, Verbal Intelligence Quotient; PIQ, Performance Intelligence Quotient; FSIQ, Full Scale Intelligence Quotient; WM, working memory; PS, psychomotor speed; VMI, Visual-Motor Integration; WRAT-R, Wide Range Achievement Test–Revised; Comm, Communication; DLS, Daily Living Skills; ABC, Adaptive Behavior Composite; Social, socialization; Internal, internalization; External, externalization.
No significant differences at the P = .05 level were found when comparing the three main subgroups (hemispheric, midline, and brainstem). Only two comparisons resulted in trends at the P = .10 level. One was based on PIQ (P = .08), with means of 95.4 for the hemispheric group, 85.4 for the midline group, and 86.5 for the brainstem group. The other was based on the PS (P = .09), with means of 9.6 for the hemispheric group, 8.3 for the midline group, and 7.3 for the brainstem group. It should be noted that PS and PIQ are not independent scores because coding and symbol search are on the Performance scale of the WISC.
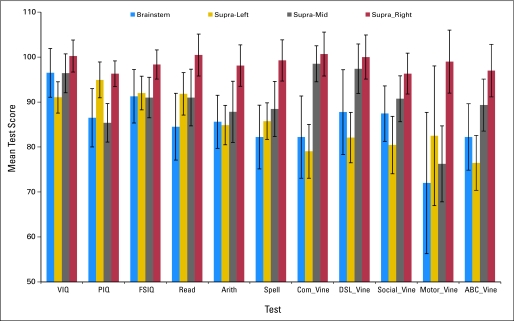
Table 2 lists the comparisons by hemispheric subgroup; several comparisons were significant. Patients with right hemisphere tumors generally had better scores. However, some of the sample sizes were rather small. Comparisons of frontal versus extrafrontal patients were precluded by the small number of patients with frontal lesions (n = 9), resulting in insufficient statistical power. Figure 2 presents the test score means for patients with brainstem, supratentorial left hemisphere, supratentorial midline, and supratentorial right hemisphere sites.
Table 2.
Left v Right Hemisphere Locations
| Test | Left Side (n = 31)
|
Right Side (n = 23)
|
ANOVA
|
|||||
|---|---|---|---|---|---|---|---|---|
| No. of Patients | Mean Score | SD of Score | No. of Patients | Mean Score | SD of Score | F | P | |
| Intelligence | ||||||||
| VIQ | 26 | 91.0 | 17.8 | 23 | 100.2 | 17.1 | 3.4 | .07 |
| PIQ | 26 | 94.9 | 20.3 | 23 | 96.3 | 13.7 | 0.1 | .78 |
| FSIQ | 26 | 92.0 | 19.1 | 23 | 98.3 | 15.5 | 1.6 | .21 |
| WM | 26 | 8.3 | 3.1 | 20 | 9.4 | 2.7 | 1.7 | .20 |
| PS | 18 | 9.6 | 3.9 | 18 | 10.0 | 2.6 | 0.2 | .69 |
| Beery VMI | ||||||||
| Standard score | 23 | 89.6 | 12.8 | 16 | 88.1 | 11.2 | 0.1 | .71 |
| Achievement | ||||||||
| Reading | 17 | 91.8 | 19.5 | 19 | 100.5 | 20.3 | 1.7 | .20 |
| Arithmetic | 17 | 84.9 | 18.1 | 19 | 98.1 | 20.0 | 4.3 | .05* |
| Spelling | 16 | 85.8 | 16.3 | 19 | 99.3 | 20.0 | 4.7 | .04* |
| Adaptive behaviors | ||||||||
| Communication | 20 | 79.1 | 26.8 | 9 | 100.7 | 14.6 | 5.1 | .03* |
| Daily Living Skills | 20 | 82.1 | 25.0 | 9 | 100.0 | 14.7 | 3.9 | .06 |
| Socialization | 20 | 80.5 | 28.6 | 9 | 96.3 | 13.7 | 2.5 | .13 |
| Motor Skills | 6 | 82.5 | 38.1 | 3 | 99.0 | 12.1 | 0.5 | .50 |
| Adaptive Behavior Composite | 19 | 76.5 | 26.6 | 8 | 97.0 | 16.5 | 4.0 | .06 |
| Achenbach CBCL | ||||||||
| Internalizing | 21 | 54.8 | 11.5 | 12 | 47.7 | 8.5 | 3.5 | .07 |
| Externalizing | 21 | 53.1 | 10.9 | 12 | 47.1 | 12.4 | 2.1 | .16 |
| Sum | 21 | 53.9 | 13.5 | 12 | 46.7 | 12.1 | 2.3 | .14 |
Abbreviations: ANOVA, analysis of variance; SD, standard deviation; VIQ, Verbal Intelligence Quotient; PIQ, Performance Intelligence Quotient; FSIQ, Full Scale Intelligence Quotient; WM, working memory; PS, psychomotor speed; VMI, Visual-Motor Integration; CBCL, Child Behavior Checklist.
P < .05.
Fig 2.
Means by tumor site (± 1 SE). VIQ, Verbal Intelligence Quotient; PIQ, Performance Intelligence Quotient; FSIQ, Full Scale Intelligence Quotient; Com_Vine, Vineland Communication; DLS_Vine, Vineland Daily Living Skills; ABC_Vine, Vineland Adaptive Behavior Composite.
The pre-, peri-, and postsurgical composites were not associated with cognitive or adaptive outcome. Of 51 correlations (three composites × 17 outcome indexes), none reached the P < .05 level. There was also no indication that age, sex, or time between surgery and testing correlated substantially with the test results; only two of 51 correlations reached the P < .05 level of significance (within expectations for chance events). Age was significantly correlated with motor skills on the Vineland test (P = .003).
DISCUSSION
It is concluded that, like children treated for low-grade brain tumors of the cerebellum,1 children with tumors in the cerebral hemispheres, supratentorial midline structures, and brainstem are at increased risk for compromised neurobehavioral functioning. Although the sample Full Scale IQ of 93 falls in the average range, it still represents approximately half a standard deviation decline compared with what would be expected based on the parent educational attainment of 14 years, which would portend, if anything, a somewhat above average IQ because the study sample is more highly educated than the normative sample for the test.13 Furthermore, the adverse impact of these tumors may be even more evident in the patients’ adaptive behavior, but not in their overall emotional and behavioral adjustment. Because adaptive behavior reflects, to some extent, the degree of success patients have in using their cognitive skills in a functional, goal-directed manner, this is a critical dimension of outcome that is often overlooked.
These results do not seem to be attributable to statistical artifact such as the Flynn Effect or confounds introduced by the different versions of the IQ tests used (WISC-R v WISC-Third Edition). Because by far the most frequently used test was the WISC-R (n = 53), which was standardized in 1973 but applied here in the 1990s, the Flynn Effect would be to artifactually increase the IQs of our participants, which would be null biasing. In addition, because the WISC-R was standardized earlier than all of the other versions of IQ tests used in this study (Wechsler Preschool and Primary Scale of Intelligence–Revised and Wechsler Adult Intelligence Scale–Revised), test version would have the effect of decreasing but not eliminating this conservative bias. So, taking into account the more highly educated parents in our sample and the Flynn Effect, ours would seem to be a conservative benchmark for comparison of the participants’ outcomes.
Across the three major groupings by location (hemispheric, midline, and brainstem), there was no significant difference, although there was a noteworthy trend toward better outcomes in patients with cerebral hemisphere tumors. Whether this trend suggests location effects or age at diagnosis effects cannot be determined because these two variables were confounded in this sample. Consistent with the literature on adult low-grade gliomas,10 within the hemispheric group, patients with left hemisphere tumors were at significantly greater risk than patients with right hemisphere tumors. The impact of left hemisphere tumors was especially evident on language functions (Verbal IQ and Communication), which is consistent with what is known about the functional organization of the brain. This also lends credence to the interpretation that these are tumor/treatment-related effects, not just normal variation. Two other trends that were found also make sense vis-à-vis functional neuroanatomy—that PIQ and PS are more affected in patients with midline and brainstem lesions. This is because of the likely encroachment on neural systems involved in vision (eg, chiasmatic tumors) and pyramidal tracts (eg, brainstem tumors) in these patients; tasks comprising both PIQ and PS place heavy demands on perceptual-motor speed. The significant motor skills × age correlation is accounted for by the fact that only young children receive a score in this area on the Vineland and that patients with midline and brainstem tumors were over-represented in this group.
The absence of a relationship between outcome and time since surgery would argue against these results being attributable to resolving postsurgical effects or, conversely, as representing the emergence of late effects commonly seen in patients treated with radiation therapy. With these cross-sectional data, it is not possible to disentangle the multitude of disease and treatment factors that could affect neurobehavioral outcome, although the correlational analyses showing no relationship to pre-, peri-, and postsurgical complications would argue against these having a significant impact.
There are several ways in which further late effects research on low-grade tumors in children can be of benefit. First, from a limited resource allocation standpoint, it is important in providing effective follow-up treatment and surveillance to know which children are at increased risk for which type of neurobehavioral complication. For example, this study would suggest that patients with tumors of the left cerebral hemisphere are at particular risk for problems in language and communication, whereas emotional/behavioral problems are not common. Second, smaller effect sizes notwithstanding, interventions to ameliorate brain tumor sequelae may be more effective when applied to these less damaged children, moving more of them into the normal range, which is an important consideration for clinically significant change.13 Third, this research offers a more sensitive metric for ascertaining costs and benefits associated with more and less aggressive surgery, as has been demonstrated in the case of craniopharyngioma where less aggressive surgery results in decreased morbidity.14 There is also the matter of the repeated treatments these patients often undergo. For patients with subtotal resections, half or more will have recurrences requiring further treatment.15 Presently, little is known about the neurobehavioral outcome after a single treatment, and almost nothing is known about morbidity associated with recurrences and subsequent therapies.
Although this study of an unusually large sample of patients diagnosed and treated in a highly uniform way yielded important findings about the outcomes of children with low-grade tumors, the conclusions to be drawn are limited by several factors. First, although there was no evidence of sampling bias, this cannot be totally ruled out. However, our experience with many cooperative group studies argues that the failure to complete psychological testing is most often a consequence of availability of testing resources at the treating institution, rather than patient characteristics. Second, the mean length of follow-up was approximately 3.5 months after surgery, and thus, the longer term risks associated with these tumors cannot be determined. However, correlational analyses and other research on smaller samples2-4 would argue that these effects do not resolve over time. Third, although the overall sample was large, contrasts for individual tests/scores and those comparing subgroups were sometimes limited in power, thereby increasing the chance of spurious null findings. Fourth, although an extensive battery of diverse neurobehavioral functions was used in this study, it would be important to study such patients using more precise neuropsychological instruments to better understand the extent and nature of their long-term vulnerabilities.16 Finally, the design used in this study is limited in that it can only suggest what causal factors are at play in producing these outcomes. Therefore, the apportionment of variance to host (including pre-existing), contextual, disease, and treatment factors remains to be determined. However, the results of this study argue that neuropsychological surveillance of children treated for low-grade brain tumors should be routine and would allow early and, perhaps, preemptive intervention to optimize their outcomes.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: M. Douglas Ris, Dean W. Beebe, F. Daniel Armstrong, John Fontanesi, Robert A. Sanford, Jeffrey H. Wisoff
Administrative support: F. Daniel Armstrong
Collection and assembly of data: M. Douglas Ris, F. Daniel Armstrong, John Fontanesi, Robert A. Sanford, Jeffrey H. Wisoff
Data analysis and interpretation: M. Douglas Ris, Dean W. Beebe, Emi Holmes
Manuscript writing: M. Douglas Ris, Dean W. Beebe, F. Daniel Armstrong, John Fontanesi
Final approval of manuscript: M. Douglas Ris, Dean W. Beebe, F. Daniel Armstrong, Robert A. Sanford, Jeffrey H. Wisoff
Acknowledgments
We acknowledge the contribution of the late Raymond Mulhern, PhD, of St Jude Children's Research Hospital who was involved in the earlier phases of this study.
published online ahead of print at www.jco.org on September 8, 2008
Supported by Children's Oncology Group (COG) Grant No. CA 98543. A complete listing of grant support for research conducted by the Children's Cancer Group and Pediatric Oncology Group before initiation of the COG grant in 2003 is available online at http://www.childrensoncologygroup.org/admin/grantinfo.htm.
Presented in part at the 31st Annual Meeting of the International Neuropsychological Society, February 5-8, 2003, Honolulu, HI.
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
REFERENCES
- 1.Beebe DW, Ris MD, Armstrong FD, et al: Cognitive and adaptive outcome in low grade pediatric cerebellar astrocytomas: Evidence of increased risk in national collaborative research studies (CCG9891/POG9130). J Clin Oncol 23:5198-5204, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Chadderton RD, West CGH, Schulz S, et al: Radiotherapy in the treatment of low-grade astrocytomas: II. The physical and cognitive sequelae. Child Nerv Syst 11:443-448, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Levisohn L, Cronin-Golomb A, Schmahmann JD: Neuropsychological consequences of cerebellar tumor resection in children: Cerebellar cognitive affective syndrome in a pediatric population. Brain 123:1041-1050, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Berger A, Sadeh S, Tzur G, et al: Task switching after cerebellar damage. Neuropsychology 19:362-370, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Aarsen FK, Paquier PF, Reddingius RE, et al: Functional outcome after low-grade astrocytomas treatment in childhood. Cancer 106:396-402, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Karatekin C, Lazareff JA, Asarnow, RF: Relevance of the cerebellar hemispheres for executive functions. Pediatr Neurol 22:106-112, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Central Brain Tumor Registry of the United States: Statistical Report: Primary Brain Tumors in the United States, 1995-1999. Hinsdale, IL, Central Brain Tumor Registry of the United States, 2002
- 8.Armstrong CL, Gyato K, Awadalla AW, et al: A critical review of the clinical effects of therapeutic irradiation damage to the brain: The roots of controversy. Neuropsychol Rev 14:65-86, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Schiff D, Brown PD, Giannini C: Outcome in adult low-grade glioma. Neurology 69:1366-1373, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Klein M, Heimans JJ, Aaronson NK, et al: Effect of radiotherapy and other treatment-related factors on mid-term to long-term cognitive sequelae in low-grade gliomas: A comparison study. Lancet 360:1361-1368, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Wechsler D: Manual for the Wechsler Intelligence Scale for Children-Revised. San Antonio, TX, The Psychological Corporation, 1974
- 12.Aickin M, Gensler H: Adjusting for multiple testing when reporting research results: The Bonferroni vs Holm methods. Am J Public Health 86:726-728, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kendall PC, Marrs-Garcia A, Nath SR, et al: Normative comparisons for the evaluation of clinical significance. J Consult Clin Psychol 67:285-299, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Merchant TE, Kiehna EN, Sanford RA, et al: Craniopharyngioma: The St. Jude Children's Research Hospital experience 1984-2001. Int J Radiat Oncol 53:533-542, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Palma L, Guidetti B: Cystic pilocytic astrocytomas of the cerebral hemispheres: Surgical experience with 51 cases and long-term results. J Neurosurg 62:811-815, 1985 [DOI] [PubMed] [Google Scholar]
- 16.Ris MD: Lessons in pediatric neuropsycho-oncology: What we’ve learned since Johnny Gunther. J Pediatr Psychol 32:1029-1037, 2007 [DOI] [PubMed] [Google Scholar]