Abstract
Background
Physician adherence to key recommendations of guidelines for community‐acquired pneumonia (CAP) is often not optimal. A better understanding of factors influencing optimal performance is needed to plan effective change.
Methods
The authors used semistructured interviews with care providers in three Dutch medium‐sized hospitals to qualitatively study and understand barriers to appropriate antibiotic use in patients with CAP. They discussed recommendations about the prescription of empirical antibiotic therapy that adheres to the guidelines, timely administration of antibiotics, adjusting antibiotic dosage to accommodate decreased renal function, switching and streamlining therapy, and blood and sputum culturing. The authors then classified the barriers each recommendation faced into categories using a conceptual framework (Cabana).
Results
Eighteen interviews were performed with residents and specialists in pulmonology and internal medicine, with medical microbiologists and a clinical pharmacist. Two additional multidisciplinary small group interviews which included nurses were performed. Each guideline recommendation elicited a different type of barrier. Regarding the choice of guideline‐adherent empirical therapy, treating physicians said that they worried about patient outcome when prescribing narrow‐spectrum antibiotic therapy. Regarding the timeliness of antibiotic administration, barriers such as conflicting guidelines and organisational factors (for example, delayed laboratory results, antibiotics not directly available, lack of time) were reported. Not streamlining therapy after culture results became available was thought to be due to the physicians' attitude of “never change a winning team”.
Conclusions
Efforts to improve the use of antibiotics for patients with CAP should consider the range of barriers that care providers face. Each recommendation meets its own barriers. Interventions to improve adherence should be tailored to these factors.
Community‐acquired pneumonia (CAP) is a common, potentially life‐threatening disease that is associated with much morbidity, mortality and use of healthcare resources. Recognition of the consequences of CAP and unexplained variation in quality of care has resulted in the development of clinical practice guidelines in various countries.1,2,3,4 Several papers have reported underperformance with respect to key recommendations of these guidelines and have shown that poor physician adherence may be associated with poorer patient outcome.5,6,7 However, implementation of such guidelines has not consistently resulted in improved antibiotic use in CAP.8,9,10
The limited ability of strategies to change physician prescribing behaviour may be due to a lack of understanding about specific factors impeding and facilitating optimal performance in CAP. Studies have shown that implementation strategies are more likely to be effective if they focus directly on problems in care provision and factors that influence change.11 Surveys of internists' attitudes toward clinical guidelines in general report barriers such as a lack of familiarity with or confidence in the guideline. Internists said they were worried about effects of guidelines on their clinical autonomy, on healthcare costs and on satisfaction with daily clinical practice.12,13,14,15 For CAP guidelines, a questionnaire has clarified that physicians' low awareness may account for poor compliance.16 In another study, professionals reported that a large variety of barriers inhibited successful implementation of a critical‐care pathway for CAP.17,18 These studies all focussed mainly on professional knowledge and attitudes.
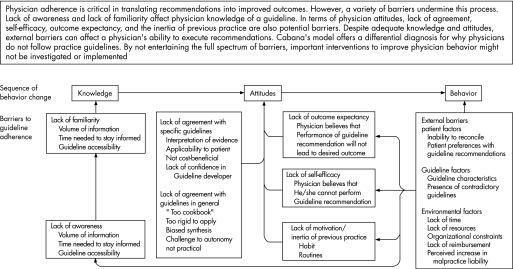
We used in‐depth interviews and small group sessions to qualitatively study the whole spectrum of patient, care‐provider, system and guideline barriers that impede judicious antibiotic treatment for CAP. We discussed six key recommendations from guidelines on antibiotic treatment for CAP and used a validated framework to standardise the reporting of barriers.19,20 This model suggests that physicians fail to adhere to guidelines in the presence of an internal barrier that has a cognitive (awareness or knowledge) or affective (attitude or motivation) component, or in the presence of an external barrier (patient, guideline and environmental factors) that restricts the professionals' ability.
Data obtained with these qualitative techniques will help us to better understand which barriers we should overcome and will enable us to generate hypotheses for potentially effective strategies to improve physician adherence.
Methods
Study design
We conducted semistructured interviews to understand the barriers to optimal performance with respect to six key recommendations of antibiotic treatment for CAP (table 1).
Table 1 Key recommendations for antibiotic use in community‐acquired pneumonia.
| Initiation of treatment |
| Prescription of an empirical antibiotic regimen adherent to the guidelines |
| Timely initiation of antibiotic therapy |
| Re‐evaluation and change of treatment |
| Adjustment of antibiotic dosage and dosing interval to accommodate decreased renal function |
| Switching from IV to oral antibiotic therapy, according to existing criteria |
| Streamlining empirical therapy into pathogen‐directed therapy on the basis of culture results |
| Routine diagnostic procedures |
| Culturing blood samples, and culturing and Gram‐staining sputum samples |
Participants
We selected care providers with all levels of experience from various professional backgrounds and hospital settings (purposive sampling21). To do so, we asked medical directors of three secondary care hospitals in the South East of the Netherlands (two non‐university teaching and one non‐teaching hospital) to provide an exhaustive list of residents and specialists in internal medicine and pulmonology, medical microbiologists and clinical pharmacists. In the three hospitals a total of 42 residents, 20 specialists, 6 microbiologists and 5 clinical pharmacists were working. We then randomly selected professionals from this list. An invitational letter was sent to 12 residents, 6 specialists, 3 microbiologists and 3 clinical pharmacists.
Procedure
One trained interviewer performed the semistructured interviews. The interviewer (JS) was a resident in internal medicine, with no relationship to the hospitals or interviewees that were selected. Clinical doctors were asked to present a clinical case: we asked them to select their most recent patient with CAP who had been admitted in the four weeks preceding the interview. If no such patient could be found, the interviewer presented a previously prepared “dummy” patient before the interview. This was also done before the interviews of all non‐clinical care providers (medical microbiologists and clinical pharmacist). All sessions were audiotaped. New interviews took place until no new information was gleaned.
Questions
The questions were open‐ended and linked to the clinical case history as closely as possible. They focussed on perceived barriers to appropriate use of antibiotics in CAP as described in six key recommendations, and covered both diagnostic and therapeutic aspects of the process of care in a logical order (table 1). These recommendations had been systematically selected by an expert panel from national guidelines edited by the Dutch Working Party on Antibiotic Policy (SWAB) and the National Society for Respiratory Physicians (NVALT), international guidelines from the Infectious Diseases Society of America (IDSA), American Thoracic Society (ATS), British Thoracic Society (BTS) and the European Respiratory Society (ERS) and a systematic review of the literature.22 An interview guide for each key issue was developed, and then it was adapted after two pilot interviews with senior residents. The interview guide contained questions clarifying potential barriers to optimal antibiotic use at all possible levels (patient, doctor, system, and guideline).
Analysis of barriers
All audiotaped interviews were transcribed verbatim. Two researchers (JS and MH) independently reviewed the manuscripts and marked comments about barriers to adherence. Remarks of professionals were compared and classified into categories of potential barriers to physician adherence according to a conceptual model developped by Cabana et al.19,20 This model suggests that physicians fail to adhere to guidelines in the presence of an internal barrier that has a cognitive (awareness or knowledge) or affective (attitude or motivation) component, or in the presence of an external barrier that restricts the professionals' ability (see fig 1). The external barrier may contain factors relevant to the patient, the guideline or the environment. For example, the remark that “doctors often do not know whether they have to wait for collection of a sputum culture before starting antibiotic therapy” implies a knowledge‐based barrier (a lack of awareness or familiarity with the guideline recommendation). However, “Sometimes administration of antibiotics will have to wait until the doctor finds time to start an IV drip” would be classified as an external, organisational factor (lack of time). The two reviewers discussed all the remarks that they had individually highlighted and classified until consensus was reached. They consulted a third researcher (SN) to make a formal judgement about differences in classification. If controversy remained, the comment was considered ambiguous and was excluded. Our principal aim was to describe the whole spectrum of possible barriers rather than quantifying their relative importance, so all types of barriers that were mentioned are presented in table 2 with their most representative remark(s). However, the barriers that were mentioned most often by our interviewees are discussed more in detail in the results section.
Figure 1 Barriers to physician adherence to practice guidelines in relation to behaviour change. Reprinted with permission from Cabana MD, Rand CS, Powe NR, et al. Why don‘t physicians follow clinical practice guidelines? A framework for improvement. JAMA 1999;282:1458–1465.19
Table 2 Barriers to adherence to key recommendations for antibiotic use in CAP: an overview of comment transcripts.
| Recommendation | Internal barriers: knowledge | Internal barriers: attitude | External barriers |
|---|---|---|---|
| Prescribing an empirical antibiotic regimen adherent to the guidelines | Lack of familiarity(R/S) “I do not know what the exact content of the guideline is.” Lack of insight in one's own behavior (R/S) “I realize now that I actually never follow our hospital guideline recommendations.” | Lack of outcome expectancy (R/M)“I think we are afraid of missing things, afraid to take risks with our own patients by prescribing narrow‐spectrum therapy even when the guidelines recommend it.”Lack of agreement with the guidelineInterpretation of evidence (R/S)“recent studies show that enterobacteriaceae should be covered by aspiration pneumonia … so penicillin is just not enough”Applicability to patient (R/S)“I will deliberately deviate from this guideline for a patient with comorbidities or one who is severely ill on admission.”Lack of confidence in guideline developer (S)“Microbiologists (who drew up the antibiotic guidelines) have a fundamentally different view than clinicians”Inertia of current practice, lack of motivation (S)“I have been treating patients with this non‐guideline‐adherent antibiotic since medical school and it is always successful” | Guideline factors (R/S) “The antibiotic booklet is unclear/confusing/poorly presented.” Social contextSocial pressure (R/S)“Everyone feels safe with cefuroxime (broad‐spectrum betalactam antibiotic) … colleagues will not quickly criticize you for this choice.”“Physicians and pulmonologists make many differing choices of antibiotics.” Organisational context (S) “You know, you don't see the patient yourself at night; it is often difficult to assess from your bed whether a patient needs broad‐spectrum antibiotic therapy” |
| Timely initiation of antibiotic therapy | Lack of awareness or insight (S/M) “I assume that antibiotics are always administered immediately, but I am not sure.” “Doctors and nurses do not realize how important timely administration of antibiotics is for outcome.” Lack of familiarity or experience (S/M/N) “Inexperienced doctors need all the results before they can establish the diagnosis of CAP and need to consult with the supervisor before starting therapy” | Lack of agreement with guideline Applicability to patient (R/S) “This rule only applies to a patient with CAP who is severely ill.” Lack of control of circumstances (R) “Once a patient is admitted to the ward, Ï am afraid I cannot control the schedule, I cannot guarantee timely administration.” Lack of a feeling of responsibility (R/S) “I believe the doctor should be responsible for seeing that his/her orders are carried out properly.” | Guideline factors Presence of conflicting guidelines (M/S/N) “Nurses take recommendations of getting blood and sputum cultures before first administration of antibiotics very literally, which may cause several hours of delay.” Guideline characteristics (R/S/M/N) “There is no clear recommendation on this subject in our guideline.” Social contextContrast between nurses in the ED and the ward (N)“Nurses in the ED are used to acute aspects of patient care, unlike those on the ward.”Social pressure on resident (N/S)“Both junior and senior residents will wait for all the results before conferring with their supervisor to discuss which antibiotic to choose.”Organisational contextCommunication between professionals (N)“Admission forms are not well structured and doctors' instructions are poorly written and unclear.”“Once a patient is admitted, there is no easy way to check whether antibiotics are administered.”Nursing protocols (N)“Nursing protocols do not necessarily put antibiotic administration ahead of less urgent aspects of care, such as dietary preferences.”Organisational constraints (eg, lack of time or resources, antibiotics not present, andlack of resident continuity on wards) (R/S/M/N)“Some antibiotics are just not present on the ward.”“If no IV drip has been started, this may cause delays.”“Nights are better: more attention is paid to the severely ill then.” |
| Adjusting antibiotic dosage and dosing interval for decreased renal function | Lack of awareness or insight (R/S/M)“Renal clearance of elderly patients with a moderately elevated serum creatinine concentration is often underestimated.”Lack of familiarity of experience (S)“Fifty percent of the residents do not know the content of the Cockroft‐Gault formula.” | Lack of agreement with guideline Applicability to patient (R/S) “No adjustment of dosage or dosing interval is required for treating patients with betalactam antibiotics.” | Guideline factors (R) “Calculating the creatinine clearance with the Cockroft‐Gault formula is time‐consuming and unpractical.” Social context (R) “With the exception of nephrologists, supervising specialists rarely pay attention this aspect of care.” Organisational context Lack of time (R/S) “That would be the first thing forgotten on a busy shift.” |
| Streamlining therapy (once culture results become available) | Lack of awareness (R) “Postgraduate education on the effect of antibiotic use on the development of resistance was a real eye‐opener for me.” Lack of familiarity (R/S/M) “Some doctors do not even recognize the name of the micro‐organism causing CAP.” “It may be difficult to differentiate between colonizing and pathogenic bacteria in a sputum culture.” | Lack of outcome expectancy (R/S/M)“Never change a winning team.”“You may still worry even if no other bacteria are present, you distrust the culture results, while you know that—on the basis of these results—you should actually tailor to narrow‐spectrum therapy .” Lack of agreement with guideline Applicability to patient (R/M) “Doctors often only look at culture results when a patient is deteriorating, not when he or she is recovering.” | Guideline factors Guideline characteristics (R/S) “There is no clear recommendation on this subject in our guideline.” Confounding guidelines (R/M) “Only when patients have recovered enough to switch from IV to oral therapy attention is paid to culture results.” Social context (R/S)“Clinicians do not accept the interference of clinical pharmacists and, to a lesser extent, medical microbiologists in clinical antibiotic management.”Organisational context (R/S/M/N)“Culture results sometimes arrive on day 4 or 5 of the empirical treatment, and it would be stupid to change the therapy for the remaining 2 or 3 days.”“At the discretion of the medical microbiologists, only a selection of the culture results are reported directly and personally to the clinicians.”“No effective control system exists to check what clinicians do with their antibiotics.”“One tends to postpone a decision about changing antibiotics until after the weekend or until the supervisor's ward round” |
| Switching therapy (from IV to oral) | Lack of familiarity (R/S/M)“It is sometimes difficult to choose an oral alternative with the same spectrum as the IV drug.”“Residents don't know the exact switch criteria, although they sort of feel what they mean in practice.” | Lack of outcome expectancy (R/S/M)“If a patient was severely ill on admission, I am worried that the infection will recur during oral therapy”“Some doctors still think that IV therapy works better than oral therapy.” Inertia (R/M) “One tends to postpone a decision on antibiotic change until after the weekend.” | Guideline factors Guideline characteristics (R/M) “Our guidelines make no clear recommendations about the exact formula, dose and dosage interval for switching therapies.” Social contextSocial pressure (R/S/M/N)“One tends to postpone a decision on antibiotic change until the supervisor's ward round” Organisational context (S/M/N) “Nurses play an important role in pressurizing doctors to consider the IV‐oral switch.” |
| Gram‐staining and culturing sputum; culturing blood | Lack of awareness and familiarity (R/M) “Nurses have no idea about the importance of careful and efficient handling of cultures, and doctors don't interfere with this process, they just give orders.” “It is still not clear to me how much time should be allowed between the culturing of two blood samples.” “Many doctors are not aware of the existence of guidelines on this subject.” | Lack of agreement with guidelineApplicability to patient (R/S)“We have always been taught that blood cultures are only useful for patients with a temperature >38.5°C.” Inertia of current practice (R/S) “It's sometimes easier to prescribe broad‐spectrum antibiotic therapy and not request any cultures at all; of course you should really think of resistance patterns” | Guideline factors Guideline characteristics (R/M) “Our guidelines make no clear recommendations.” Social contextSocial pressure (R/S/M)“You need prior approval from a medical microbiologist for some diagnostic tests, and this may influence patient management.”“There are culturing differences between physicians and pulmonologists.” Organisational context Communication between professionals (R/M)“Bacteriology forms are not clear, and clinical information is often omitted.” Organisational constraints (miscellaneous) (R/S/M/N) “Some diagnostic tests are more difficult to get during the weekend and at night.” “Sputum cultures can only be brought to the lab during certain hours, so at the weekend one tends to forget sputa in the fridge.” “Some samples coming from other hospitals are transported only once a day.” “I will give the patient a receptacle for sputum collection, but I will not wait until he/she produces it” |
ED, emergency department; M, barrier reported by medical microbiologists; N, barrier reported by nurses; R, barrier reported by residents; S, barrier reported by specialists.
Bolded areas indicate barriers that were mentioned more than seven times by different interviewees.
Results
Interviews took place in 2003. All invited professionals agreed to take part. Eighteen professionals (9 residents, 6 consultants, 2 microbiologists and 1 clinical pharmacist) were interviewed until no information that we hadn't received in preceding interviews was given. In our first interview sessions with residents, we discovered that for some recommended processes of care, barriers were mainly attributable to practical, organisational difficulties needing a multidisciplinary solution from those most closely involved. We therefore decided to add two interactive small group interviews with a nurse from the emergency department, a nurse from a pulmonology ward and a resident.
Before performing our first interview with a clinical pharmacist, we already doubted whether he/she was a “care provider relevant to the process”. Due to the specific characteristics of our guideline, the pharmacist's contribution to our analysis of barriers was essentially limited to two recommendations: adjustment of antibiotic dose to renal function and switch therapy. In addition, a clinical pharmacist was reported to be seldomly present at ward meetings of general internal medicine and pulmonology in our recent survey at Dutch secondary care hospitals and thus may have little insight as to the reasons for non‐adherence to guideline recommendations.24 After interviewing the first clinical pharmacist, we decided not to recruite another one as our our doubts were confirmed.
The mean age of the 24 participants was 34.5 years (range 25–56); 10 participants were women. We encountered a wide spectrum of possible barriers to optimal antibiotic treatment for CAP. Table 2 presents transcripts of comments, grouped by theoretical barriers to adherence. All types of barriers, along with the most representative remarks, are presented. Barriers that were mentioned most often (⩾7 times) by different interviewees were considered frequent. In the table, these barriers are shown in bold text. Analysis of our data revealed that each single recommendation elicited its own specific pattern of barriers. We present the most important patterns for each of the recommendations.
Barriers to prescribing empirical antibiotic therapy adherent to the guidelines
Professional barriers to adherent prescribing included a doubt in outcome expectancy and predominantly reported by residents and specialists (“You never know … penicillin has a very narrow spectrum … I would not feel at ease treating my patient with only that” [specialist pulmonology S3]) and a lack of agreement with guidelines (“In the Netherlands, we always want to start with a very narrow spectrum, preferably with penicillin, and we add erythromycin only if a patient is really deteriorating … why not turn it around? Why not start with broad‐spectrum therapy and tailor it down to narrow‐spectrum therapy as soon as the culture results become available?” [resident internal medicine R6]). External barriers were mentioned to a lesser extent and were mainly related to the social context in which professionals operate: “Out of courtesy to colleagues, no criticism of the chosen antibiotic regimen is made at end‐of‐shift meetings” [resident internal medicine R4].
Barriers to timeliness of administration of antibiotics
Most interviewees (residents, specialists, microbiologists and nurses) mentioned external barriers related to organisational factors (for example, substantial delays in delivering laboratory results to the emergency department, antibiotics not present on the ward, IV drip not started). However, barriers were also created by the physician's lack of knowledge about the impact that timely antibiotic administration can have on patient outcome and a lack of agreement with the guideline (for example, several specialists and residents stated “this rule only applies to the patient with CAP who is severely ill”). Some remarks combine different barriers: “Ward nurses prioritize non‐medical issues (such as diet and social setting) during intake, leaving prescribed medication, including IV antibiotics, to the last or postponing administration until regular medication rounds” [awareness of importance and social‐organisational context, specialist internal medicine S2].
Barriers to adjusting the dosage and dosing interval to accommodate renal function
Reasons for omitting dosage adjustment to renal function were mainly attributable to lack of awareness—for example, that antibiotic dosage should be adjusted for patients with reduced renal function; that a moderately elevated serum creatinine concentration could conceal a significantly decreased renal clearance in elderly patients. Calculating the creatinine clearance with the Cockroft‐Gault formula for every patient was regarded as time consuming and unpractical by residents (external organisational barrier). According to medical specialists, only half of the residents were able to calculate creatinine clearance with this formula (lack of familiarity).
Barriers to streamlining therapy
Doctors said that they felt uncertain about tailoring empirical broad‐spectrum antibiotics to narrow‐spectrum antibiotic therapy (once culture results were known), especially when a patient had been very ill on admission. “Never change a winning team” was quoted by several residents and specialists and observed by medical microbiologists. Organisational barriers also apply: “The results only become available 3 to 5 days after culturing, and due to weekends and poorly computerized reporting systems, the time between availability and notification of the results becomes even greater”[specialist pulmonology S6] and “When patients do well with the initial treatment, streamlining therapy is postponed until the supervisory ward round, which is generally held only once or twice a week” [resident internal medicine R4]. Inexperienced residents, but not specialists, appreciated spontaneous interference with prescribed antibiotic therapy by non‐clinicians (medical microbiologists and clinical pharmacists).
Barriers to the intravenous‐oral switch
Treating a patient intravenously provided some clinicians with a subjective feeling of security, especially when a patient was severely ill on admission (attitude‐based barrier). Identical oral formulae are not available for some broad‐spectrum antibiotics (ceftriaxon and cefotaxim). This complicates switching to an oral antibiotic when no culture results are available: “I find it difficult to select an oral alternative with the same spectrum” was stated by several residents (knowledge‐based barrier). Social pressure may also postpone a timely switch: “Residents tend to wait until the supervisor's ward round before taking decisions” [nurse ward, N2]. Finally, clinicians said that nurses played a facilitating role in the IV‐to‐oral switch, asking for the IV drip to be discontinued at nearly every ward round (organisational facilitator).
Barriers to blood and sputum culturing
Most residents and specialists mentioned that blood culturing was easily forgotten for elderly patients with CAP (who are often afebrile) or for severely ill, hypothermic patients: “We only request blood cultures if a patient with CAP has a fever” [lack of guideline applicability to patient, specialist pulmonology S1]. One nurse remarked: “I will give the patient a receptacle for sputum, but I will not wait until he/she produces it … it may then remain at a bedside table (or in the ward's fridge) for days” [nurse ward N1]. This reveals a variety of barriers: lack of awareness of the importance of careful and efficient handling of sputum; lack of motivation; lack of communication between doctors, nurses, and patients; and organisational constraints due to limited opening hours of microbiology labs. Influence of non‐clinical professionals (for example, medical microbiologists) regarding the availability of diagnostic tests (for example, urine antigen testing for Legionella spp) is considered undesirable by most clinical specialists (barrier of social interference).
Discussion
A large variety of barriers to key recommendations on antibiotic usefor CAP was reported by our interviewees. Each recommendation elicited its own pattern of barriers that should be overcome. Non‐adherence to guidelines for empirical antibiotic therapy was mainly attributable to physicians' negative attitudes towards the guideline. For another recommendation (“timely administration of antibiotics”), logistical and organisational factors were reported to be the most important barriers. Improving performance on either recommendation would obviously require a different approach.
Previous studies have reported barriers to CAP guideline (or critical‐care pathway) adherence as a whole,16,17,25 rather than to its various recommendations or they focus on only one recommendation.26 In addition, these studies do not consider the views of care providers other than clinical specialists. In our study, some very important barriers were, however, suggested by other care providers: nurses in the emergency department mentioned that some of the antibiotics recommended by the local guideline were not directly available at the emergency department (for example, due to a shortage of space to stock medication). This barrier was not perceived by physicians. In addition, our choice to interview physicians of various educational levels resulted in a variety of perceptions, which may have been overlooked when only medical specialists were interviewed. This clarified which professional levels our improvement strategy should target for some recommendations.
Since there is a general pattern in the type of barriers for each recommendation, improvement strategies should focus on this pattern. While many studies describe barriers to guideline implementation, there is only little practical advice on how they should be translated into practice changing strategies.27 It seems logical to provide an educational intervention in a situation where lack of knowledge is an important barrier, but this remains far less clear for other perceived barriers: for example, how can a social structure in a hospital or a department be changed? How can attitude towards a guideline be changed? Tailoring of an intervention can be difficult because there is no one‐to‐one relationship between the objectives and interventions, and empirical evidence on links between specific interventions and specific objectives is limited.
In our guideline implemantation study (submitted), we tried to develop interventions in a structured manner, using an exploraratory method.27,28 For each factor impeding adherence, the best possible intervention was chosen, using evidence of effectiveness when available, as well as experience, common sense and creativity.
In our case, improving guideline adherence for the prescription of empirical antibiotic therapy should aim at changing a physician's attitude rather than improving knowledge about the guideline. An intervention should be directed towards both specialists and residents, because—in line with a previous study18—our results suggest that residents do not decide independently about antibiotic policies in Dutch hospitals. There is a perceived lack of evidence justifying recommendations and a lack of confidence in the guideline developers, which leads to a lack of agreement with current guideline recommendations. This, along with the reported social pressure among professionals at end‐of‐shift meetings might reflect the ongoing discussion about benefits and doubts concerning empirical regimens of broad‐spectrum antibiotics covering both atypical and classic pathogens.29,30 A tailored intervention to remove these barriers would seem logical: involve clinical specialists in actively developing local guidelines based on the available evidence, organise small group discussions on appropriate prescribing and produce a clearly written and unequivocal critical‐care pathway.
In contrast, for other recommendations, such as “timely administration of antibiotics”, guideline adherence was predominantly impeded by external, organisational barriers. Some of these barriers may be dealt with easily: make the antibiotic available in the emergency department and integrate conflicting ward protocols. Other external barriers, such as either doctors' or nurses' lack of time, are often more difficult to address as they need interventions on a higher organisational or political level.
In this paper we have aimed to describe the whole range of barriers one could potentially identify for pneumonia guideline recommendations. We realise that barriers may be quite variable throughout different hospital settings. It is clear that several of the external, organisational barriers (such as the timing of a “supervisory ward round”) may only apply to a local hospital setting, but another Dutch study reported similar results and this suggests that our findings are—at least—not confined to these three hospitals but represent a Dutch hospital setting.18 This paper, however, also describes the patterns of barriers that surface when analysing the outcomes of all interviewees. One might think that these patterns identified were unique to the institutions studied. Our findings, however, correspond with findings from other qualitative research where similar antibiotic practice beliefs were found.17,26
A qualitative approach is the best method for exploring the reasons and hidden motives for non‐adherence to clinical guidelines. However, this study may have some limitations. First, there is a chance that we selected mainly cooperative care providers who underreported potential barriers, which may have limited generalisability. As our interviews were conducted in a non‐confrontational setting, we believe that care providers were less likely to give “professionally acceptable” or “socially desirable” responses. We deliberately choose to let interviews be performed by a resident in internal medicine (JS). Due to the (sometimes complex) clinical case study that was discussed, someone with a medical background was considered necessary. The interviewees were aware of the fact that the interviewer was medically trained, but they did not know the interviewer in a personal or professional context. They did not know his clinical specialty, but they knew that the interviewer was a researcher. As far as we know there has been no research performed to evaluate whether interviews performed by different types of professionals lead to different results. More methodological research on this topic is required.
Secondly, we realise that barriers that are reported by care providers may be different from those observed in real practice. However, results from a study on determinants of adherence to guidelines on antibiotic use for CAP in a sample of 498 patients confirm many of the barriers presented in this paper.31
In summary, we find that each key recommendation for the optimal antibiotic treatment of CAP meets its own pattern of barriers that must be overcome. This finding suggests that future improvement strategies should focus on different types of interventions for different aspects of the guideline.
Acknowledgements
Janine Trap, for administrative support. Grant support from Zon/Mw, Dutch department of Health.
Abbreviations
CAP - community‐acquired pneumonia
Footnotes
Competing interests: None.
The ethics board of the Radboud University Nijmegen Medical Centre approved the study protocol and all interviewees gave their written consent.
References
- 1.BTS Guidelines for the Management of Community Acquired Pneumonia in Adults Thorax. 2001;56 (Suppl 4):iv1–NaN64. doi: 10.1136/thorax.56.suppl_4.iv1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartlett J G, Dowell S F, Mandell L A.et al Practice guidelines for the management of community‐acquired pneumonia in adults. Infectious Diseases Society of America. Clin Infect Dis 200031347–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niederman M S, Mandell L A, Anzueto A.et al Guidelines for the management of adults with community‐acquired pneumonia. Diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am J Respir Crit Care Med 20011631730–1754. [DOI] [PubMed] [Google Scholar]
- 4.van Kasteren M E, Wijnands W J, Stobberingh E E.et al [Optimization of the antibiotics policy in the Netherlands. II. SWAB guidelines for the antimicrobial therapy of pneumonia in patients at home and as nosocomial infections. The Netherlands Antibiotic Policy Foundation]. Ned Tijdschr Geneeskd 1998142952–956. [PubMed] [Google Scholar]
- 5.Menendez R, Ferrando D, Valles J M.et al Influence of deviation from guidelines on the outcome of community‐acquired pneumonia. Chest 2002122612–617. [DOI] [PubMed] [Google Scholar]
- 6.Malone D C, Shaban H M. Adherence to ATS guidelines for hospitalized patients with community‐acquired pneumonia. Ann Pharmacother 2001351180–1185. [DOI] [PubMed] [Google Scholar]
- 7.Houck P M, Bratzler D W, Nsa W.et al Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community‐acquired pneumonia. Arch Intern Med 2004164637–644. [DOI] [PubMed] [Google Scholar]
- 8.Chu L A, Bratzler D W, Lewis R J.et al Improving the quality of care for patients with pneumonia in very small hospitals. Arch Intern Med 2003163326–332. [DOI] [PubMed] [Google Scholar]
- 9.Halm E A, Horowitz C, Silver A.et al Limited impact of a multicenter intervention to improve the quality and efficiency of pneumonia care. Chest 2004126100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simpson S H, Marrie T J, Majumdar S R. Do guidelines guide pneumonia practice? A systematic review of interventions and barriers to best practice in the management of community‐acquired pneumonia. Respir Care Clin N Am 2005111–13. [DOI] [PubMed] [Google Scholar]
- 11.Grol R, Grimshaw J. From best evidence to best practice: effective implementation of change in patients' care. Lancet 20033621225–1230. [DOI] [PubMed] [Google Scholar]
- 12.Tunis S R, Hayward R S, Wilson M C.et al Internists' attitudes about clinical practice guidelines. Ann Intern Med 1994120956–963. [DOI] [PubMed] [Google Scholar]
- 13.Hayward R S, Wilson M C, Tunis S R.et al Practice guidelines. What are internists looking for? J Gen Intern Med 199611176–178. [DOI] [PubMed] [Google Scholar]
- 14.Hayward R S, Guyatt G H, Moore K A.et al Canadian physicians' attitudes about and preferences regarding clinical practice guidelines. CMAJ 19971561715–1723. [PMC free article] [PubMed] [Google Scholar]
- 15.Vogtlander N P, van Kasteren M E, Natsch S.et al Improving the process of antibiotic therapy in daily practice: interventions to optimize timing, dosage adjustment to renal function, and switch therapy. Arch Intern Med 20041641206–1212. [DOI] [PubMed] [Google Scholar]
- 16.Switzer G E, Halm E A, Chang C C.et al Physician awareness and self‐reported use of local and national guidelines for community‐acquired pneumonia. J Gen Intern Med 200318816–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Majumdar S R, Simpson S H, Marrie T J. Physician‐perceived barriers to adopting a critical pathway for unity‐acquired pneumonia. Jt Comm J Qual Saf 200430387–395. [DOI] [PubMed] [Google Scholar]
- 18.Mol P G, Rutten W J, Gans R O.et al Adherence barriers to antimicrobial treatment guidelines in teaching hospital, the Netherlands. Emerg Infect Dis 200410522–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cabana M D, Rand C S, Powe N R.et al Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA 19992821458–1465. [DOI] [PubMed] [Google Scholar]
- 20.Grol R, Wensing M. What drives change? Barriers to and incentives for achieving evidence‐based practice. Med J Aust 2004180(Suppl 6)S57–S60. [DOI] [PubMed] [Google Scholar]
- 21.Grol R, Baker R, Moss F.Quality improvement research: understanding the science of change in health care. London: BMJ Publishing Group, 2004 [DOI] [PMC free article] [PubMed]
- 22.Schouten J A, Hulscher M E, Wollersheim H.et al Quality of antibiotic use for lower respiratory tract infections at hospitals: (how) can we measure it? Clin Infect Dis 200541450–460. [DOI] [PubMed] [Google Scholar]
- 23.Verheij T J, Salome P L, Bindels P J.et al NHG‐standaard Acuut hoesten. Huisarts en Wetenschap 200346496–506. [Google Scholar]
- 24.Schouten J A, Hulscher M E, Natsch S.et al Antibiotic control measures in Dutch secondary care hospitals. Neth J Med 20056324–30. [PubMed] [Google Scholar]
- 25.Halm E A, Atlas S J, Borowsky L H.et al Change in physician knowledge and attitudes after implementation of a pneumonia practice guideline. J Gen Intern Med 199914688–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halm E A, Switzer G E, Mittman B S.et al What factors influence physicians' decisions to switch from intravenous to oral antibiotics for community‐acquired pneumonia? J Gen Intern Med 200116599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Bokhoven M A, Kok G, van der W T. Designing a quality improvement intervention: a systematic approach. Qual Saf Health Care 200312215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bartholomew L, Kok G, Parcel G.et alIntervention mapping: designing theory‐ and evidence‐based health promotion programs. New York: McGraw Hill, 2001
- 29.Oosterheert J J, Bonten M J, Hak E.et al How good is the evidence for the recommended empirical antimicrobial treatment of patients hospitalized because of community‐acquired pneumonia? A systematic review. J Antimicrob Chemother 200352555–563. [DOI] [PubMed] [Google Scholar]
- 30.Oosterheert J J, Bonten M J, Schneider M M.et al [Community acquired pneumonia; no reason to revise current Dutch antibiotic guidelines]. Ned Tijdschr Geneeskd 2003147381–386. [PubMed] [Google Scholar]
- 31.Schouten J A, Hulscher M E, Kullberg B J.et al Understanding variation in quality of antibiotic use for community‐acquired pneumonia: effect of patient, professional and hospital factors. J Antimicrob Chemother 200556575–582. [DOI] [PubMed] [Google Scholar]