Abstract
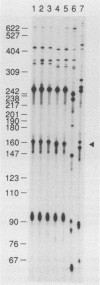
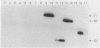
Intergenic tRNA spacers from strains of streptococcal groups A, B, and G were amplified by using the polymerase chain reaction (PCR) at low stringency with consensus tRNA gene primers. Cloning and sequencing showed that many of the homologous intergenic spacers differed in length between species. The sequences of the tRNA genes that flank these polymorphic spacers were determined and used to synthesize fully complementary primers. With these primers at high stringency, PCR products which varied in lengths from 53 to 71 bp, depending on the species or strain, were obtained from streptococcal DNAs, even in the presence of a 1,000-fold mass excess of human DNA. PCR products, the lengths of which could also be used for classification, were obtained at high stringency from a few genera closely related to Streptococcus. No products were obtained from genomic DNAs from more distantly related genera. Production of species- or strain-specific tRNA intergenic length polymorphisms with primers that generate characteristic products from a variety of species within the same genus should be applicable to many organisms, including those that would otherwise be difficult to culture or identify.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernet C., Garret M., de Barbeyrac B., Bebear C., Bonnet J. Detection of Mycoplasma pneumoniae by using the polymerase chain reaction. J Clin Microbiol. 1989 Nov;27(11):2492–2496. doi: 10.1128/jcm.27.11.2492-2496.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessesen M. T., Luo Q. A., Rotbart H. A., Blaser M. J., Ellison R. T., 3rd Detection of Listeria monocytogenes by using the polymerase chain reaction. Appl Environ Microbiol. 1990 Sep;56(9):2930–2932. doi: 10.1128/aem.56.9.2930-2932.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstain J. M., Grimprel E., Lukehart S. A., Norgard M. V., Radolf J. D. Sensitive detection of Treponema pallidum by using the polymerase chain reaction. J Clin Microbiol. 1991 Jan;29(1):62–69. doi: 10.1128/jcm.29.1.62-69.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger P., Siegrist C. A., Strautmann G., Belli D., Auckenthaler R. Evaluation of two ELISA tests for the rapid detection of group A streptococci. Eur J Pediatr. 1990 Jan;149(4):256–258. doi: 10.1007/BF02106286. [DOI] [PubMed] [Google Scholar]
- Hasin M., Furst A. Sore throat in family practice: comparison of blood agar throat culture with a rapid enzyme immunoassay test for diagnostic purposes. J R Coll Gen Pract. 1989 Aug;39(325):332–334. [PMC free article] [PubMed] [Google Scholar]
- Hoffmann S. Detection of group A streptococcal antigen from throat swabs with five diagnostic kits in general practice. Diagn Microbiol Infect Dis. 1990 May-Jun;13(3):209–215. doi: 10.1016/0732-8893(90)90061-y. [DOI] [PubMed] [Google Scholar]
- Kaplan E. L., Reid H. F., Johnson D. R., Kunde C. A. Rapid antigen detection in the diagnosis of group A streptococcal pyoderma: influence of a "learning curve effect" on sensitivity and specificity. Pediatr Infect Dis J. 1989 Sep;8(9):591–593. doi: 10.1097/00006454-198909000-00004. [DOI] [PubMed] [Google Scholar]
- Meier F. A., Howland J., Johnson J., Poisson R. Effects of a rapid antigen test for group A streptococcal pharyngitis on physician prescribing and antibiotic costs. Arch Intern Med. 1990 Aug;150(8):1696–1700. [PubMed] [Google Scholar]
- Muto A., Andachi Y., Yuzawa H., Yamao F., Osawa S. The organization and evolution of transfer RNA genes in Mycoplasma capricolum. Nucleic Acids Res. 1990 Sep 11;18(17):5037–5043. doi: 10.1093/nar/18.17.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa P. A., Schwan T. G. A specific and sensitive assay for the Lyme disease spirochete Borrelia burgdorferi using the polymerase chain reaction. J Infect Dis. 1989 Dec;160(6):1018–1029. doi: 10.1093/infdis/160.6.1018. [DOI] [PubMed] [Google Scholar]
- Tzianabos T., Anderson B. E., McDade J. E. Detection of Rickettsia rickettsii DNA in clinical specimens by using polymerase chain reaction technology. J Clin Microbiol. 1989 Dec;27(12):2866–2868. doi: 10.1128/jcm.27.12.2866-2868.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vold B. S. Structure and organization of genes for transfer ribonucleic acid in Bacillus subtilis. Microbiol Rev. 1985 Mar;49(1):71–80. doi: 10.1128/mr.49.1.71-80.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh J., McClelland M. Fingerprinting genomes using PCR with arbitrary primers. Nucleic Acids Res. 1990 Dec 25;18(24):7213–7218. doi: 10.1093/nar/18.24.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh J., McClelland M. Genomic fingerprints produced by PCR with consensus tRNA gene primers. Nucleic Acids Res. 1991 Feb 25;19(4):861–866. doi: 10.1093/nar/19.4.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh J., Petersen C., McClelland M. Polymorphisms generated by arbitrarily primed PCR in the mouse: application to strain identification and genetic mapping. Nucleic Acids Res. 1991 Jan 25;19(2):303–306. doi: 10.1093/nar/19.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. G., Kubelik A. R., Livak K. J., Rafalski J. A., Tingey S. V. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990 Nov 25;18(22):6531–6535. doi: 10.1093/nar/18.22.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson K. H., Blitchington R., Shah P., McDonald G., Gilmore R. D., Mallavia L. P. Probe directed at a segment of Rickettsia rickettsii rRNA amplified with polymerase chain reaction. J Clin Microbiol. 1989 Dec;27(12):2692–2696. doi: 10.1128/jcm.27.12.2692-2696.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]