Abstract
Purpose of review
This review reports recent findings on the multiple factors that regulate skeletal muscle growth in neonates.
Recent findings
Skeletal muscle is the fastest growing protein mass in neonates. The high rate of neonatal muscle growth is due to accelerated rates of protein synthesis accompanied by the rapid accumulation of muscle nuclei. Feeding profoundly stimulates muscle protein synthesis in neonates and the response decreases with age. The feeding-induced stimulation of muscle protein synthesis is modulated by enhanced sensitivity to the postprandial rise in insulin and amino acids. Insulin and amino acid signaling components have been identified that are involved in the feeding-induced stimulation of protein synthesis in neonatal muscle. The enhanced activation of these signaling components in skeletal muscle of the neonate contributes to the high rate of muscle protein synthesis and rapid gain in muscle protein mass in neonates.
Summary
Recent findings suggest that the immature muscle has a heightened capacity to activate signaling cascades that promote translation initiation in response to the postprandial rise in insulin and amino acids thereby enabling their efficient utilization for muscle growth. This capacity is further supported by enhanced satellite cell proliferation, but how these two processes are linked remains to be established.
Keywords: amino acids, insulin, mammalian target of rapamycin, protein synthesis, satellite cell
Introduction
In this review, we address our current understanding of the regulation of skeletal muscle growth in the neonatal period. The regulation of skeletal muscle growth is dependent on the stage of muscle development and birth, which defines the start of the neonatal period and occurs at different stages of development among species. It is important when evaluating processes that occur in the ‘neonatal’ period, therefore, that consideration is given to the species and developmental stage from which the data are derived. Humans present a special case because, with the advances in the clinical management of premature babies, the neonatal period can cover a wide range of developmental stages. We define the close of the ‘neonatal’ period as the stage when animals no longer depend on their caretakers for their nutrition, which for most species is by definition at weaning. This stage is coincident with the attainment of full biochemical and functional maturity of the skeletal muscle, which enables the organism to breathe, eat, and move, which are prerequisite functions for independent survival.
Given the aforementioned restrictions, we will address the period of development from when the skeletal muscle fibers are fully differentiated, fiber formation is established, and growth represents the hypertrophy of these fibers. Concurrently, there is maturation of the muscles' composition and structures that result in the attainment of functional maturity. The extent to which factors that influence fiber hypertrophy also affect maturation depends on an interaction between the muscles' stage of development and the underlying cause and severity of the growth deviation. Our early work [1] demonstrated that although muscle growth rate is highly sensitive to variations in overall nutrient intake in the neonatal rat, maturation is minimally altered. Thus, we will limit the review to the processes that drive hypertrophy, that is, protein accretion and myonuclear proliferation.
Regulation of neonatal muscle growth by protein synthesis
The rate of growth during the neonatal period is higher than at any other stage of postnatal life, and a majority of the mass increase is comprised of skeletal muscle. The more rapid accretion of muscle proteins than other tissue proteins results in a substantial increase in the proportion of the body protein pool that is muscle protein. For example, in the rodent, the amount of muscle protein relative to body protein increases from approximately 30% in the newborn to approximately 45% at weaning (Fig. 1a, [2,3]). The fractional rate of muscle growth, that is, the amount of muscle weight gained in relation to the existing muscle mass, however, decreases profoundly during the neonatal period (Fig. 1b).
Figure 1. Muscle protein accretion in early postnatal life.
(a) Relative changes in the proportion of whole-body protein mass attributable to skeletal muscle protein in the rat between birth and weaning (Fiorotto et al., unpublished observations). (b) Relationship between the postnatal change in the rate of muscle protein accretion and the fractional rates of protein synthesis and degradation in skeletal muscle proteins during the suckling period in the hind limb muscles of rats [2,3].  , Fractional synthesis rate;
, Fractional synthesis rate;  , fractional accretion rate;
, fractional accretion rate;  , fractional degradation rate.
, fractional degradation rate.
Growth occurs when the rate of protein synthesis is higher than that of protein degradation. The fractional synthesis rate of skeletal muscle proteins in newly formed muscles is substantially higher than their degradation rates resulting in the high accretion rates typical of the neonatal period. With maturation, synthesis rates decrease more than degradation rates until the two processes are in balance in adult nongrowing muscles (Fig. 1b). Thus, the high growth rate of neonatal muscle is attributable to the high rate of protein synthesis [2,3].
The maximal rate of protein synthesis is dictated by the abundance of ribosomes in a tissue and the efficiency with which they translate mRNA into protein. The elevated capacity for protein synthesis in immature muscle and its overall decline with development are in part driven by an elevated concentration of ribosomes at birth which decreases as the muscle matures [3,4].
Feeding stimulates protein synthesis in neonatal muscle
Because ethical considerations preclude the measurement of tissue protein synthesis in the human infant, the neonatal pig has been used as an animal model because of its similarity in anatomy, developmental physiology, and metabolism. Although rodents have been used extensively to study muscle growth mechanisms, their small size limits the types of nutritional studies that can be performed during the neonatal period. Thus, using the pig as an animal model, we determined that a heightened responsiveness of skeletal muscle protein synthesis to feeding during the neonatal period enables dietary amino acids to be used for growth with exceedingly high efficiency [5]. Although feeding stimulates protein synthesis in all tissues of the neonate, the magnitude of the increase is highest for skeletal muscle, a response that diminishes with development. These findings in the neonatal pig are consistent with the ability of feeding to stimulate protein synthesis in the whole body of newborn humans [6], but not in the whole body or skeletal muscle of adults [7].
Insulin, amino acids, and glucose mediate the feeding-induced stimulation of muscle protein synthesis in neonates
Circulating concentrations of insulin, amino acids, and glucose rise after a meal and each play a role in mediating the postprandial stimulation of protein synthesis in skeletal muscle of the neonate. Studies performed in vitro [8] and in growing animals in vivo show that insulin stimulates protein synthesis in both skeletal muscle and whole body [9–11]. Although insulin infusion in extremely low-birth-weight infants did not stimulate whole-body protein synthesis [12], the response of skeletal muscle specifically was not determined, and it is possible that the insulin-stimulated reduction in circulating amino acid levels limited insulin-stimulated protein synthesis. By contrast, most [13,14], but not all [15], studies in adults have shown that insulin does not stimulate muscle protein synthesis, even when basal amino acids are maintained. This suggests that the ability of muscle protein synthesis to respond to insulin is age dependent. On the contrary, amino acids, either alone or concurrent with insulin, can upregulate protein synthesis in skeletal muscle throughout the lifespan [16,17].
Studies [4,18,19] using the pancreatic glucose–amino acid clamp technique in neonatal pigs have showed that increasing insulin from the fasting to the fed level stimulates protein synthesis in a dose–response manner in neonatal pigs, even when amino acids are at or below fasting levels. This response to insulin is specific to skeletal muscle [20•] and declines with development [21] in parallel with the decrease in the response of muscle protein synthesis to feeding [5]. Raising amino acids to fed levels, in the presence of fasting or below fasting insulin levels, will increase protein synthesis in most tissues; however, the highest response occurs in skeletal muscle [21,22]. The postprandial rise in glucose, independent of insulin, also contributes to the feeding-induced stimulation of protein synthesis, albeit modestly, and the response is unique to skeletal muscle [23•]. This ability of skeletal muscle in neonates to respond separately to the rise in insulin, amino acids, and glucose after a meal likely contributes to the more rapid gain in protein mass in skeletal muscle, as compared with other tissues of the body of neonates.
Insulin and amino acid signaling pathways are upregulated in neonatal muscle
The major steps of the insulin signaling cascade have been reviewed elsewhere [24–26] and are summarized in Fig. 2. Our studies [27–31] have shown that the high rate of protein synthesis in neonatal muscle is in part due to enhanced activation of the insulin signaling cascade, including the insulin receptor, insulin receptor substrate (IRS)-1/2, phosphatidylinositol 3-kinase (PI3K), phosphoinositide-dependent kinase 1 (PDK-1), and protein kinase B (PKB or Akt). Variations in insulin, but not amino acids, within the physiological range increase the activation of insulin signaling proteins in a dose-dependent manner [29]. Reduced activation of negative regulators of insulin signaling also contributes to the high rate of neonatal muscle protein synthesis [31,32]. These include protein tyrosine phosphatase 1B (PTP-1B), phosphatase and tensin homologue deleted on chromosome 10 (PTEN), and protein phosphatase 2A (PP2A). The action of AMP-activated protein kinase (AMPK) is unaffected.
Figure 2. Insulin and amino acid signaling pathways that lead to translation initiation.
4EBP1, 4E-binding protein 1; AMPK, AMP-activated protein kinase; mTOR, mammalian target of rapamycin; PDK1, phosphoinositide-dependent kinase 1; PI3-K, phosphatidylinositol 3-kinase; PKB, protein kinase B; PP2A, protein phosphatase 2A; PTEN, phosphatase and tensin homologue deleted on chromosome 10; PTP1B, protein tyrosine phosphatase 1B; TSC, tuberous sclerosis complex.
The amino acid signaling pathway that promotes translation initiation is less well understood than that of insulin. Intracellular amino acids may directly affect signaling components that stimulate protein synthesis [33,34]. Amino acid-induced signaling converts Rheb–guanosine diphosphate (GDP) to Rheb–guanosine tri-phosphate (GTP) [35••] and the association between Rheb and the mammalian target of rapamycin (mTOR) to promote mTOR activation [36]. Tuberous sclerosis complex 1 and 2 (TSC1/2) inhibit mTOR activation by promoting Rheb–GTP hydrolysis, converting it to an inactive state, but whether amino acid stimulation of mTOR involves TSC1/2 remains controversial [34,37].
Most studies that examined the regulation of amino acid signaling have been performed in cell culture, and there have been few studies in intact animals under physiologically relevant conditions. Recently, we demonstrated that the high rate of protein synthesis in neonatal muscle is associated with reduced TSC1/2 activation and more activation of mTOR [31]. We further showed that insulin, but not amino acids, increases PKB activation and decreased TSC2 activation. However, both insulin and amino acids increase mTOR phosphorylation [38••].
Translation initiation is enhanced in neonatal muscle
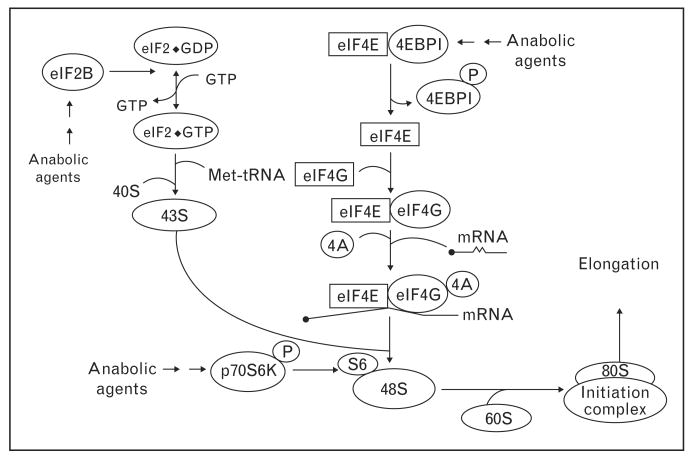
The activation of mTOR by insulin and amino acids promotes translation initiation by enhancing the phosphorylation of eukaryotic initiation factor (eIF), 4E-binding protein 1 (4EBP1), and ribosomal protein S6K1 (S6K1) [39,40••] (Figs. 2 and 3). The downstream series of events that result in translation initiation and elongation are summarized in Fig. 3 [41–45].
Figure 3. Regulation of translation initiation.
4EBP1, 4E-binding protein 1; GDP, guanosine diphosphate; GTP, guanosine triphosphate.
We have examined the effects of nutrients on key components of these pathways. The ingestion of a meal does not alter the activation of eIF2B, a regulator of 43S preinitiation complex formation, in neonatal muscle although eIF2B activity decreases with development [46]. We further showed that feeding increases the activation of 4E-BP1, S6K1, and S6 and induces reciprocal changes in eIF4E binding to 4E-BP1 and eIF4G, which regulate mRNA binding to the 43S preinitiation complex [29,31,46,47]. These changes in translation initiation are profound in neonatal muscle, decrease with development, and are mTOR dependent [48]. Amino acids and insulin independently mediate the feeding-induced increase in phosphorylation of S6K1 and 4E-BP1 and formation of the active eIF4E–eIF4G complex [22,38••]. The activation of these translation initiation factors by insulin and amino acids is dose dependent. However, insulin, amino acids, and stage of maturity do not affect the phosphorylation of eukaryotic elongation factor 2, a regulator of the translocation of the ribosome along the mRNA. The modest increase in muscle protein synthesis by glucose stimulation is mTOR independent [23•].
Leucine and arginine as regulators of neonatal muscle growth
The amino acid, leucine, serves both as a precursor for protein synthesis and as a signal to activate protein synthesis [49]. Recently, we demonstrated that the postprandial rise in leucine, but not in isoleucine or valine alone, stimulates skeletal muscle protein synthesis in neonatal pigs, although this response to leucine is less than that to a complete amino acid mixture [50,51]. The leucine-induced stimulation of skeletal muscle protein synthesis involves the activation of mTOR and downstream signaling to translation initiation, that is, S6K1, 4EBP1, and eIF4G–eIF4E and can be completely blocked by rapamycin treatment [52••]. This process does not involve signaling proteins upstream of mTOR, that is, PKB, AMPK, or TSC2 or that regulate elongation, that is, eEF2. The acute leucine-induced stimulation of neonatal muscle protein synthesis is not sustained (despite continued activation of the mTOR signaling pathway) unless essential amino acids are also provided to prevent their decrease to less than fasting levels as they are utilized as substrates for protein synthesis [50,53••]. The stimulation of the mTOR signaling pathway and protein synthesis in muscle by leucine, similar to that which occurs with feeding, insulin, and a balanced amino acid mixture, decreases with age.
Arginine supplementation increases whole body and skeletal muscle growth of neonatal pigs, likely because arginine is limited in milk [54]. Chronic treatment with N-carbamylglutamate, which stimulates the synthesis of arginine thereby increasing circulating arginine, also increases muscle weight and protein synthesis [55]. Recently, it was demonstrated that the increase in muscle protein synthesis with arginine treatment in neonatal pigs involves activation of the mTOR signaling pathway [56•].
Myonuclear accretion is rapid in the neonatal period
The rapid accretion of protein in the neonatal period is accompanied by an increase in the fibers' myonuclear content, albeit at a proportionally slower rate. Because myonuclei are postmitotic, the addition of new myonuclei to the growing fibers is accomplished by the satellite cells, adult muscle progenitor cells, located in a niche between the sarcolemma and the basal lamina [57,58]. The satellite cell niche defines its physical location, the cell's axis of symmetry, and the nature of extracellular molecules to which it is exposed, and these factors all can influence the activity and fate of the cells [59••]. A number of excellent reviews [59••,60,61,62••,63••] on the biology of the satellite cell have been published recently and the readers are referred to these for greater detail. The origin of the satellite cells has been much debated. Recent studies that made use of a variety of ingenious genetically engineered animal models, however, generally conclude that the satellite cells in most muscles are characterized by the expression of Pax7 [64], and are derived from cells in the somites that express the transcription factors Pax3 and Pax7 [63••]. In newborns, satellite cells comprise a significant proportion of the total muscle nuclei, which decreases as the number of myonuclei per fiber increase. In rats, satellite cells comprise approximately 35% of muscle nuclei at birth and decrease to 10% at 4 weeks of age and less than 5% at sexual maturity when the cells are largely mitotically quiescent [65,66]. A similar pattern is seen in pigs [67,68]. The functional need for a higher number of satellite cells and proliferative activity to support neonatal muscle growth is further substantiated by the demonstration that during the suckling period, unweighting of the soleus muscle impairs satellite cell division and the normal developmental gain in total myonuclei resulting in a proportional impairment of muscle growth [69•]. The dependence of neonatal muscle hypertrophy on satellite cell proliferation is also demonstrated from the study of muscle growth in mice lacking Pax7 [70]. After birth, muscle growth is severely impaired in these mice in association with rapid loss of satellite cells, even though the total fiber number is not altered.
The rapid diminution in the proliferative activity of satellite cells and the decrease in their relative numbers during the normal process of muscle fiber maturation are attributable largely to the cells becoming quiescent. The mechanisms responsible for the induction of quiescence with muscle maturation are unclear but may involve alterations in the activities of the pathways that promote satellite cell activation and proliferation. For example, hepatocyte growth factor, which is essential for satellite cell activation, is high in neonatal rat muscle and decreases rapidly after 10 days of age [71]; the expression of myostatin increases in muscles as they mature [72] and myostatin has been shown to inhibit satellite cell activity [73]; the local expression of the insulin-like growth factors (IGFs) and the type 1 IGF receptor are down-regulated and this might be expected to mitigate cell cycle activity [74]. Recently, the cross-talk between the Notch and Wnt signaling pathways has been implicated in the regulation of satellite cell function and fate [59••,75,76••,77•], but their contribution to the postnatal transition to the quiescent state is uncertain. The importance of extracellular signals in regulating the activity of satellite cells is further underscored by results of parabiosis [78] or muscle cross-transplantation studies [79] between young adult and aged animals that uniformly demonstrate better responses in the younger host regardless of the donor's age.
Nutrition in the regulation of myonuclear accretion
The regulation of neonatal myonuclear accretion by the organism's nutrient intake has received scant attention, especially since the identification of markers that permit the detailed characterization of satellite cells. Quantitative studies have largely used total muscle DNA as a proxy for the number of myonuclei. This is not unreasonable because it has been demonstrated for skeletal muscle from normal healthy rats that the proportion of myonuclei to total nuclei present from birth to old age does not change [80]. These studies suggest that the relationship between protein and DNA accretion are closely regulated in neonatal muscle. As already discussed, the translational mechanisms in immature muscle are exquisitely sensitive to nutrient intake, and when differences in intake are sustained chronically, overall muscle growth is altered in parallel. Thus, when food intake is globally restricted or available in excess, the effects on total muscle DNA and protein are similar so that the ratio of protein to DNA in a given muscle is maintained within a fairly narrow range. This is demonstrated in Fig. 4 [81,82], which summarizes data from three older studies in which milk intake of suckling rats was suboptimal from birth to weaning. One study [83] has quantified by electron microscopy, the response of satellite cells in muscles of weanling rats that had been globally undernourished during gestation and the suckling period. The data suggest that there was a general impairment in satellite cell division with a proportional reduction in the growth of the cytoplasm and overall muscle growth, a result similar to those from the studies in Fig. 4. As recently debated, however, this may not necessarily be the case for adult muscle hypertrophy [84].
Figure 4. The relative consequence of suboptimal nutrition during the suckling period on total protein content and the ratio of protein to DNA in various hind limb muscles of weanling rats.

Data were compiled from three studies: (a) [81], (b) [82], (c) [3]. EDL, extensor digitorum longus; Gastroc, gastrocnemius muscle; SOL, soleus. ■, Total protein; ▨, protein : DNA.
A potential link between myonuclear accretion and the capacity for protein synthesis is through the regulation of ribosomal production and muscle ribosome concentration. The rate-limiting step for ribosome biogenesis is the rate of ribosomal RNA synthesis by ribosomal DNA (rDNA) transcription [85]. Upstream binding factor (UBF) is a key transactivator of rDNA genes and its availability is regulated by the phosphorylation state of the retinoblastoma gene product (pRb) [86]. Nader et al. [87] demonstrated that activation of mTOR in myotubes in culture by serum stimulation (but as may occur with feeding or exposure to growth factors in vivo) promote pRb phosphorylation, thereby releasing UBF for transactivation and increasing ribosomal production. Although there was no associated increase in total DNA, it would not be unreasonable to suggest that those factors responsible for the enhanced activation of mTOR in the immature myofibers of the neonate, that is, insulin, amino acids, and potentially other growth factors, also influence cell cycle activity and myonuclear accretion.
Conclusion
The high rate of muscle growth in the neonates is driven by the enhanced rate of protein synthesis when food is available and is sustained by a parallel capacity of the satellite cells to increase the myonuclear abundance of the muscle fibers. This feeding-induced stimulation of muscle protein synthesis is mediated by the enhanced sensitivity to the postprandial rise in insulin and amino acids. Recent studies, reviewed in this article, highlight some of the recent findings of the developmental changes in the activation of components of the insulin and amino acids signaling pathways and translation initiation and elongation in skeletal muscle of the neonate. The enhanced activation of these signaling components in neonatal muscle contributes to the high rate of protein synthesis and rapid gain in skeletal muscle mass in the neonate. There is unequivocal evidence that the maturational changes in protein synthesis and myonuclear accretion rates to promote muscle hypertrophy are coordinated; however, the underlying mechanism is still unclear.
Acknowledgments
This project has been funded in part by the National Institute of Arthritis and Musculoskeletal and Skin Disease Institute grant numbers AR-44474 and AR-46308 and the USDA/ARS under Cooperative Agreement no. 58-6250-6-001. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health or the US Department of Agriculture.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 103).
- 1.Fiorotto ML, Davis TA. Food intake alters muscle protein gain with little effect on Na(+)-K(+)-ATPase and myosin isoforms in suckled rats. Am J Physiol. 1997;272:R1461–R1471. doi: 10.1152/ajpregu.1997.272.5.R1461. [DOI] [PubMed] [Google Scholar]
- 2.Davis TA, Fiorotto ML, Nguyen HV, Reeds PJ. Protein turnover in skeletal muscle of suckling rats. Am J Physiol. 1989;257:R1141–R1146. doi: 10.1152/ajpregu.1989.257.5.R1141. [DOI] [PubMed] [Google Scholar]
- 3.Fiorotto ML, Davis TA, Reeds PJ. Regulation of myofibrillar protein turnover during maturation in normal and undernourished rat pups. Am J Physiol. 2000;278:845–854. doi: 10.1152/ajpregu.2000.278.4.R845. [DOI] [PubMed] [Google Scholar]
- 4.Davis TA, Fiorotto ML, Beckett PR, et al. Differential effects of insulin on peripheral and visceral tissue protein synthesis in neonatal pigs. Am J Physiol Endocrinol Metab. 2001;280:E770–E779. doi: 10.1152/ajpendo.2001.280.5.E770. [DOI] [PubMed] [Google Scholar]
- 5.Davis TA, Burrin DG, Fiorotto ML, Nguyen HV. Protein synthesis in skeletal muscle and jejunum is more responsive to feeding in 7- than 26-day-old pigs. Am J Physiol. 1996;270:E802–E809. doi: 10.1152/ajpendo.1996.270.5.E802. [DOI] [PubMed] [Google Scholar]
- 6.Denne SC, Rossi EM, Kalhan SC. Leucine kinetics during feeding in normal newborns. Pediatr Res. 1991;30:23–27. doi: 10.1203/00006450-199107000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Tessari P, Zanetti M, Barazzoni R, et al. Mechanisms of postprandial protein accretion in human skeletal muscle. Insight from leucine and phenylalanine forearm kinetics. J Clin Invest. 1996;98:1361–1372. doi: 10.1172/JCI118923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimball SR, Horetsky RL, Jefferson LS. Signal transduction pathways involved in the regulation of protein synthesis by insulin in L6 myoblasts. Am J Physiol. 1998;274:C221–C228. doi: 10.1152/ajpcell.1998.274.1.C221. [DOI] [PubMed] [Google Scholar]
- 9.Wester TJ, Lobley GE, Birnie LM, Lomax MA. Insulin stimulates phenylalanine uptake across the hind limb in fed lambs. J Nutr. 2000;130:608–611. doi: 10.1093/jn/130.3.608. [DOI] [PubMed] [Google Scholar]
- 10.Liechty EA, Boyle DW, Moorehead H, et al. Effect of hyperinsulinemia on ovine fetal leucine kinetics during prolonged maternal fasting. Am J Physiol. 1992;263:E696–E702. doi: 10.1152/ajpendo.1992.263.4.E696. [DOI] [PubMed] [Google Scholar]
- 11.Brown LD, Hay WW., Jr Effect of hyperinsulinemia on amino acid utilization and oxidation independent of glucose metabolism in the ovine fetus. Am J Physiol Endocrinol Metab. 2006;291:E1333–E1340. doi: 10.1152/ajpendo.00028.2006. [DOI] [PubMed] [Google Scholar]
- 12.Poindexter BB, Karn CA, Denne SC. Exogenous insulin reduces proteolysis and protein synthesis in extremely low birth weight infants. J Pediatr. 1998;132:948–953. doi: 10.1016/s0022-3476(98)70389-0. [DOI] [PubMed] [Google Scholar]
- 13.Louard RJ, Fryburg DA, Gelfand RA, Barrett EJ. Insulin sensitivity of protein and glucose metabolism in human forearm skeletal muscle. J Clin Invest. 1992;90:2348–2354. doi: 10.1172/JCI116124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McNulty PH, Young LH, Barrett EJ. Response of rat heart and skeletal muscle protein in vivo to insulin and amino acid infusion. Am J Physiol. 1993;264:E958–E965. doi: 10.1152/ajpendo.1993.264.6.E958. [DOI] [PubMed] [Google Scholar]
- 15.Chevalier S, Marliss EB, Morais JA, et al. Whole-body protein anabolic response is resistant to the action of insulin in obese women. Am J Clin Nutr. 2005;82:355–365. doi: 10.1093/ajcn.82.2.355. [DOI] [PubMed] [Google Scholar]
- 16.Vary TC, Jefferson LS, Kimball SR. Amino acid-induced stimulation of translation initiation in rat skeletal muscle. Am J Physiol. 1999;277:E1077–E1086. doi: 10.1152/ajpendo.1999.277.6.E1077. [DOI] [PubMed] [Google Scholar]
- 17.Volpi E, Ferrando AA, Yeckel CW, et al. Exogenous amino acids stimulate net muscle protein synthesis in the elderly. J Clin Invest. 1998;101:2000–2007. doi: 10.1172/JCI939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wray-Cahen D, Nguyen HV, Burrin DG, et al. Response of skeletal muscle protein synthesis to insulin in suckling pigs decreases with development. Am J Physiol. 1998;275:E602–E609. doi: 10.1152/ajpendo.1998.275.4.E602. [DOI] [PubMed] [Google Scholar]
- 19.O'Connor PM, Bush JA, Suryawan A, et al. Insulin and amino acids independently stimulate skeletal muscle protein synthesis in neonatal pigs. Am J Physiol Endocrinol Metab. 2003;284:E110–E119. doi: 10.1152/ajpendo.00326.2002. [DOI] [PubMed] [Google Scholar]
- 20•.Suryawan A, O'Connor PM, Bush JA, et al. Differential regulation of protein synthesis by amino acids and insulin in peripheral and visceral tissues of neonatal pigs. Amino Acids. 2008 doi: 10.1007/s00726-008-0149-z. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study highlighted the uniqueness of skeletal muscle, compared with other tissues, in its ability to respond to stimulation by both insulin and amino acids to stimulate protein synthesis.
- 21.Davis TA, Fiorotto ML, Burrin DG, et al. Stimulation of protein synthesis by both insulin and amino acids is unique to skeletal muscle in neonatal pigs. Am J Physiol Endocrinol Metab. 2002;282:E880–E890. doi: 10.1152/ajpendo.00517.2001. [DOI] [PubMed] [Google Scholar]
- 22.O'Connor PMJ, Kimball SR, Suryawan A, et al. Regulation of translation initiation by insulin and amino acids in skeletal muscle of neonatal pigs. Am J Physiol Endocrinol Metab. 2003;285:E40–E53. doi: 10.1152/ajpendo.00563.2002. [DOI] [PubMed] [Google Scholar]
- 23•.Jeyapalan AS, Orellana RA, Suryawan A, et al. Glucose stimulates protein synthesis in skeletal muscle of neonatal pigs through an AMPK- and mTOR-independent process. Am J Physiol Endocrinol Metab. 2007;293:E595–E603. doi: 10.1152/ajpendo.00121.2007. [DOI] [PubMed] [Google Scholar]; The study emphasizes the importance of a balanced carbohydrate and protein-containing diet in the growth of skeletal muscle in the neonate.
- 24.Bevan P. Insulin signalling. J Cell Sci. 2001;114:1429–1430. doi: 10.1242/jcs.114.8.1429. [DOI] [PubMed] [Google Scholar]
- 25.Kwiatkowski DJ, Manning BD. Tuberous sclerosis: a GAP at the crossroads of multiple signaling pathways. Hum Mol Genet. 2005;14:R251–R258. doi: 10.1093/hmg/ddi260. [DOI] [PubMed] [Google Scholar]
- 26.Egawa K, Sharma PM, Nakashima N, et al. Membrane-targeted phosphati-dylinositol 3-kinase mimics insulin actions and induces a state of cellular insulin resistance. J Biol Chem. 1999;274:14306–14314. doi: 10.1074/jbc.274.20.14306. [DOI] [PubMed] [Google Scholar]
- 27.Suryawan A, Nguyen HV, Bush JA, Davis TA. Developmental changes in the feeding-induced activation of the insulin-signaling pathway in neonatal pigs. Am J Physiol Endocrinol Metab. 2001;281:E908–E915. doi: 10.1152/ajpendo.2001.281.5.E908. [DOI] [PubMed] [Google Scholar]
- 28.Suryawan A, Nguyen HV, Orellana RA, et al. Insulin/insulin-like growth factor-I hybrid receptor abundance decreases with development in suckling pigs. J Nutr. 2003;133:2783–2787. doi: 10.1093/jn/133.9.2783. [DOI] [PubMed] [Google Scholar]
- 29.Suryawan A, O'Connor PMJ, Kimball SR, et al. Amino acids do not alter the insulin-induced activation of the insulin signaling pathway in neonatal pigs. J Nutr. 2004;134:24–30. doi: 10.1093/jn/134.1.24. [DOI] [PubMed] [Google Scholar]
- 30.Suryawan A, Davis TA. Developmental regulation of protein kinase B activation is isoform specific in skeletal muscle of neonatal pigs. Pediatr Res. 2005;58:719–724. doi: 10.1203/01.PDR.0000180536.51032.AB. [DOI] [PubMed] [Google Scholar]
- 31.Suryawan A, Escobar J, Frank JW, et al. Developmental regulation of the activation of signaling components leading to translation initiation in skeletal muscle of neonatal pigs. Am J Physiol Endocrinol Metab. 2006;291:E849–E859. doi: 10.1152/ajpendo.00069.2006. [DOI] [PubMed] [Google Scholar]
- 32.Suryawan A, Davis TA. Protein-tyrosine-phosphatase 1B activation is regulated developmentally in muscle of neonatal pigs. Am J Physiol Endocrinol Metab. 2003;284:E47–E54. doi: 10.1152/ajpendo.00210.2002. [DOI] [PubMed] [Google Scholar]
- 33.Beugnet A, Wang X, Proud CG. Target of rapamycin (TOR)-signaling and RAIP motifs play distinct roles in the mammalian TOR-dependent phosphorylation of initiation factor 4E-binding protein 1. J Biol Chem. 2003;278:40717–40722. doi: 10.1074/jbc.M308573200. [DOI] [PubMed] [Google Scholar]
- 34.Kimball SR, Jefferson LS. New functions for amino acids: effects on gene transcription and translation. Am J Clin Nutr. 2006;83:500S–507S. doi: 10.1093/ajcn/83.2.500S. [DOI] [PubMed] [Google Scholar]
- 35••.Kimball SR. The role of nutrition in stimulating muscle protein accretion at the molecular level. Biochem Soc Trans. 2007;35:1298–1301. doi: 10.1042/BST0351298. [DOI] [PubMed] [Google Scholar]; An outstanding review article of current findings of the molecular mechanisms by which food consumption stimulates muscle protein synthesis.
- 36.Long X, Ortiz-Vega S, Lin Y, Avruch J. Rheb binding to mammalian target of rapamycin (mTOR) is regulated by amino acid sufficiency. J Biol Chem. 2005;280:23433–23436. doi: 10.1074/jbc.C500169200. [DOI] [PubMed] [Google Scholar]
- 37.Smith EM, Finn SG, Tee AR, et al. The tuberous sclerosis protein TSC2 is not required for the regulation of the mammalian target of rapamycin by amino acids and certain cellular stresses. J Biol Chem. 2005;280:18717–18727. doi: 10.1074/jbc.M414499200. [DOI] [PubMed] [Google Scholar]
- 38••.Suryawan A, Orellana RA, Nguyen HV, et al. Activation by insulin and amino acids of signaling components leading to translation initiation in skeletal muscle of neonatal pigs is developmentally regulated. Am J Physiol Endocrinol Metab. 2007;293:E1597–E1605. doi: 10.1152/ajpendo.00307.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrated that the postprandial rise in amino acids regulates mRNA translation through activation of the mTOR signaling pathway downstream of TSC2.
- 39.Corradetti MN, Guan KL. Upstream of the mammalian target of rapamycin: do all roads pass through mTOR? Oncogene. 2006;25:6347–6360. doi: 10.1038/sj.onc.1209885. [DOI] [PubMed] [Google Scholar]
- 40••.Vary TC, Lynch CJ. Nutrient signaling components controlling protein synthesis in striated muscle. J Nutr. 2007;137:1835–1843. doi: 10.1093/jn/137.8.1835. [DOI] [PubMed] [Google Scholar]; This outstanding review details the molecular mechanism by which feeding increases mRNA translation initiation in skeletal muscle.
- 41.Mamane Y, Petroulakis E, LeBacquer O, Sonenberg N. mTOR, translation initiation and cancer. Oncogene. 2006;25:6416–6422. doi: 10.1038/sj.onc.1209888. [DOI] [PubMed] [Google Scholar]
- 42.Stolovich M, Tang H, Hornstein E, et al. Transduction of growth or mitogenic signals into translational activation of TOP mRNAs is fully reliant on the phosphatidylinositol 3-kinase-mediated pathway but requires neither S6K1 nor rpS6 phosphorylation. Mol Cell Biol. 2002;22:8101–8113. doi: 10.1128/MCB.22.23.8101-8113.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Proud CG. Role of mTOR signalling in the control of translation initiation and elongation by nutrients. Curr Top Microbiol Immunol. 2004;279:215–244. doi: 10.1007/978-3-642-18930-2_13. [DOI] [PubMed] [Google Scholar]
- 44.Proud CG. Regulation of protein synthesis by insulin. Biochem Soc Trans. 2006;34:213–216. doi: 10.1042/BST20060213. [DOI] [PubMed] [Google Scholar]
- 45.Browne GJ, Proud CG. Regulation of peptide-chain elongation in mammalian cells. Eur J Biochem. 2002;269:5360–5368. doi: 10.1046/j.1432-1033.2002.03290.x. [DOI] [PubMed] [Google Scholar]
- 46.Davis TA, Nguyen HV, Suryawan A, et al. Developmental changes in the feeding-induced stimulation of translation initiation in muscle of neonatal pigs. Am J Physiol Endocrinol Metab. 2000;279:E1226–E1234. doi: 10.1152/ajpendo.2000.279.6.E1226. [DOI] [PubMed] [Google Scholar]
- 47.Kimball SR, Farrell PA, Nguyen HV, et al. Developmental decline in components of signal transduction pathways regulating protein synthesis in pig muscle. Am J Physiol Endocrinol Metab. 2002;282:E585–E592. doi: 10.1152/ajpendo.00269.2001. [DOI] [PubMed] [Google Scholar]
- 48.Kimball SR, Jefferson LS, Nguyen HV, et al. Feeding stimulates protein synthesis in muscle and liver of neonatal pigs through an mTOR-dependent process. Am J Physiol Endocrinol Metab. 2000;279:E1080–E1087. doi: 10.1152/ajpendo.2000.279.5.E1080. [DOI] [PubMed] [Google Scholar]
- 49.Kimball SR, Jefferson LS. Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. J Nutr. 2006;136:227S–231S. doi: 10.1093/jn/136.1.227S. [DOI] [PubMed] [Google Scholar]
- 50.Escobar J, Frank JW, Suryawan A, et al. Physiological rise in plasma leucine stimulates muscle protein synthesis in neonatal pigs by enhancing translation initiation factor activation. Am J Physiol Endocrinol Metab. 2005;288:E914–E921. doi: 10.1152/ajpendo.00510.2004. [DOI] [PubMed] [Google Scholar]
- 51.Escobar J, Frank JW, Suryawan A, et al. Regulation of cardiac and skeletal muscle protein synthesis by individual branched-chain amino acids in neonatal pigs. Am J Physiol Endocrinol Metab. 2006;290:E612–E621. doi: 10.1152/ajpendo.00402.2005. [DOI] [PubMed] [Google Scholar]
- 52••.Suryawan A, Jeyapalan AS, Orellana RA, et al. Leucine stimulates protein synthesis in skeletal muscle of neonatal pigs by enhancing mTORC1 activation. Am J Physiol Endocrinol Metab. 2008;295:E868–E875. doi: 10.1152/ajpendo.90314.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]; The ability of rapamycin to completely block muscle protein synthesis in the current study, but only partially block the feeding-induced stimulation of muscle protein synthesis observed in previous studies, suggests that insulin, nonleucine amino acids, or both regulate muscle protein synthesis by mTOR-dependent and mTOR-independent mechanisms.
- 53••.Escobar J, Frank JW, Suryawan A, et al. Amino acid availability and age affect the leucine stimulation of protein synthesis and eIF4F formation in muscle. Am J Physiol Endocrinol Metab. 2007;293:E1615–E1621. doi: 10.1152/ajpendo.00302.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]; The importance of the work is that it clearly demonstrated that the ability of leucine to stimulate protein synthesis is dependent upon the availability of other amino acids for protein synthesis.
- 54.Wu G, Knabe DA, Kim SW. Arginine nutrition in neonatal pigs. J Nutr. 2004;134:2783S–2790S. doi: 10.1093/jn/134.10.2783S. [DOI] [PubMed] [Google Scholar]
- 55.Frank JW, Escobar J, Nguyen HV, et al. Oral N-carbamylglutamate supplementation increases protein synthesis in skeletal muscle of piglets. J Nutr. 2007;137:315–319. doi: 10.1093/jn/137.2.315. [DOI] [PubMed] [Google Scholar]
- 56•.Yao K, Yin YL, Chu W, et al. Dietary arginine supplementation increases mTOR signaling activity in skeletal muscle of neonatal pigs. J Nutr. 2008;138:867–872. doi: 10.1093/jn/138.5.867. [DOI] [PubMed] [Google Scholar]; The mechanism by which arginine increases muscle growth in neonatal pigs is detailed in this study.
- 57.Mauro A. Satellite cell of skeletal muscle fibers. J Cell Biol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moss FP, Leblond CP. Nature of dividing nuclei in skeletal muscle of growing rats. J Cell Biol. 1970;44:459–462. doi: 10.1083/jcb.44.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59••.Kuang S, Gillespie MA, Rudnicki MA. Niche regulation of muscle satellite cell self-renewal and differentiation. Cell Stem Cell. 2008;2:22–31. doi: 10.1016/j.stem.2007.12.012. [DOI] [PubMed] [Google Scholar]; This review summarizes succinctly how the satellite cell niche is an integral determinant of satellite cell behavior.
- 60.Dhawan J, Rando TA. Stem cells in postnatal myogenesis: molecular mechanisms of satellite cell quiescence, activation and replenishment. Trends Cell Biol. 2005;15:666–673. doi: 10.1016/j.tcb.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 61.Zammit PS, Partridge TA, Yablonka-Reuveni Z. The skeletal muscle satellite cell: the stem cell that came in from the cold. J Histochem Cytochem. 2006;54:1177–1191. doi: 10.1369/jhc.6R6995.2006. [DOI] [PubMed] [Google Scholar]
- 62••.Peault B, Rudnicki M, Torrente Y. Stem and progenitor cells in skeletal muscle development, maintenance, and therapy. Mol Ther. 2007;15:867–877. doi: 10.1038/mt.sj.6300145. [DOI] [PubMed] [Google Scholar]; A review of the current knowledge of the heterogeneous population of stem cells in skeletal muscle, including satellite cells, from the perspective of their potential therapeutic uses.
- 63••.Sambasivan R, Tajbakhsh S. Skeletal muscle stem cell birth and properties. Semin Cell Dev Biol. 2007;18:870–882. doi: 10.1016/j.semcdb.2007.09.013. [DOI] [PubMed] [Google Scholar]; A detailed review of the origins of muscle stem cells throughout the body and the signaling molecules, growth factors and molecular pathways responsible for orchestrating muscle development.
- 64.Seale P, Sabourin LA, Girgis-Gabardo A, et al. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 65.Allbrook DB, Han MF, Hellmuth AE. Population of muscle satellite cells in relation to age and mitotic activity. Pathology. 1971;3:233–243. doi: 10.3109/00313027109073739. [DOI] [PubMed] [Google Scholar]
- 66.Schultz E. Satellite cell proliferative compartments in growing skeletal muscles. Dev Biol. 1996;175:84–94. doi: 10.1006/dbio.1996.0097. [DOI] [PubMed] [Google Scholar]
- 67.Campion DR, Richardson RL, Reagan JO, Kraeling RR. Changes in the satellite cell population during postnatal growth of pig skeletal muscle. J Anim Sci. 1981;52:1014–1018. doi: 10.2527/jas1981.5251014x. [DOI] [PubMed] [Google Scholar]
- 68.Mesires NT, Doumit ME. Satellite cell proliferation and differentiation during postnatal growth of porcine skeletal muscle. Am J Physiol Cell Physiol. 2002;282:C899–C906. doi: 10.1152/ajpcell.00341.2001. [DOI] [PubMed] [Google Scholar]
- 69•.Kawano F, Takeno Y, Nakai N. Essential role of satellite cells in the growth of rat soleus muscle fibers. Am J Physiol Cell Physiol. 2008;295:C458–C467. doi: 10.1152/ajpcell.00497.2007. [DOI] [PubMed] [Google Scholar]; An unusual experimental paradigm highlights the significance of satellite cell proliferation for skeletal muscle growth during the suckling period in rats.
- 70.Kuang S, Charge SB, Seale P, et al. Distinct roles for Pax7 and Pax3 in adult regenerative myogenesis. J Cell Biol. 2006;172:103–113. doi: 10.1083/jcb.200508001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jennische E, Ekberg S, Matejka GL. Expression of hepatocyte growth factor in growing and regenerating rat skeletal muscle. Am J Physiol. 1993;265:C122–C128. doi: 10.1152/ajpcell.1993.265.1.C122. [DOI] [PubMed] [Google Scholar]
- 72.Suryawan A, Frank JA, Nguyen HV, et al. Expression of the TGF-B family of ligands is developmentally regulated in skeletal muscle of neonatal rats. Pediatr Res. 2006;59:175–179. doi: 10.1203/01.pdr.0000196718.47935.6e. [DOI] [PubMed] [Google Scholar]
- 73.McCroskery S, Thomas M, Maxwell L, et al. Myostatin negatively regulates satellite cell activation and self-renewal. J Cell Biol. 2003;162:1135–1147. doi: 10.1083/jcb.200207056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alexandrides T, Moses AC, Smith RJ. Developmental expression of receptors for insulin, insulin-like growth factor I (IGF-I), and IGF-II in rat skeletal muscle. Endocrinology. 1989;124:1064–1076. doi: 10.1210/endo-124-2-1064. [DOI] [PubMed] [Google Scholar]
- 75.Luo D, Renault VM, Rando TA. The regulation of Notch signaling in muscle stem cell activation and postnatal myogenesis. Semin Cell Dev Biol. 2005;16:612–622. doi: 10.1016/j.semcdb.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 76••.Brack AS, Conboy IM, Conboy MJ, et al. A temporal switch from notch to Wnt signaling in muscle stem cells Is necessary for normal adult myogenesis. Cell Stem Cell. 2008;2:50–59. doi: 10.1016/j.stem.2007.10.006. [DOI] [PubMed] [Google Scholar]; An elegant set of experiments demonstrates how the balance between Notch and Wnt signaling pathways control the fate of satellite cells, that is, self-renewal or differentiation.
- 77•.Otto A, Schmidt C, Luke G, et al. Canonical Wnt signalling induces satellite-cell proliferation during adult skeletal muscle regeneration. J Cell Sci. 2008;121:2939–2950. doi: 10.1242/jcs.026534. [DOI] [PubMed] [Google Scholar]; … not all Wnts are created equal… a study that highlights the complexity of this family of signaling molecules in regulating satellite cell behavior.
- 78.Conboy IM, Conboy MJ, Wagers AJ, et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 79.Carlson BM, Faulkner JA. Muscle transplantation between young and old rats: age of host determines recovery. Am J Physiol Cell Physiol. 1989;256:C1262–C1266. doi: 10.1152/ajpcell.1989.256.6.C1262. [DOI] [PubMed] [Google Scholar]
- 80.Enesco M, Puddy D. Increase in the number of nuclei and weight in skeletal muscle of rats of various ages. Am J Anat. 1964;114:235–244. doi: 10.1002/aja.1001140204. [DOI] [PubMed] [Google Scholar]
- 81.Winick M, Noble A. Cellular Response in rats during malnutrition at various ages. J Nutr. 1966;89:300–306. doi: 10.1093/jn/89.3.300. [DOI] [PubMed] [Google Scholar]
- 82.Glore SR, Layman DK. Cellular development of skeletal muscle during early periods of nutritional restriction and subsequent rehabilitation. Pediatr Res. 1983;17:602–605. doi: 10.1203/00006450-198307000-00017. [DOI] [PubMed] [Google Scholar]
- 83.Beerman DH, Hood LF, Liboff M. Satellite cell and myonuclei populations in rat soleus and extensor digitorum longus muscles after maternal nutritional deprivation and realimentation. J Anim Sci. 1983;57:1618–1625. doi: 10.2527/jas1983.5761618x. [DOI] [PubMed] [Google Scholar]
- 84.O'Connor RS, Pavlath GK, McCarthy JJ, Esser KA. Last word on Point:Counterpoint: Satellite cell addition is/is not obligatory for skeletal muscle hypertrophy. J Appl Physiol. 2007;103:1107. doi: 10.1152/japplphysiol.00502.2007. [DOI] [PubMed] [Google Scholar]
- 85.Zahradka P, Larson DE, Sells BH. Regulation of ribosome biogenesis in differentiated rat myotubes. Mol Cell Biochem. 1991;104:189–194. [PubMed] [Google Scholar]
- 86.Hannan KM, Kennedy BK, Cavanaugh AH, et al. RNA polymerase I transcription in confluent cells: Rb downregulates rDNA transcription during confluence-induced cell cycle arrest. Oncogene. 2000;19:3487–3497. doi: 10.1038/sj.onc.1203690. [DOI] [PubMed] [Google Scholar]
- 87.Nader GA, McLoughlin TJ, Esser KA. mTOR function in skeletal muscle hypertrophy: increased ribosomal RNA via cell cycle regulators. Am J Physiol Cell Physiol. 2005;289:C1457–C1465. doi: 10.1152/ajpcell.00165.2005. [DOI] [PubMed] [Google Scholar]