Abstract
Toll-like receptors (TLR) play an important role in the recognition of microbes by host sentinel cells that leads to the subsequent innate and adaptive immune responses. In this study, we evaluated the patterns of TLR2-, TLR3- and TLR9-expressing antigen presenting cells (APCs) in spleen and blood of gnotobiotic (Gn) pigs after colonization with a mixture of two strains of lactic acid bacteria (LAB), Lactobacillus acidophilus and Lactobacillus reuteri or infection with the virulent human rotavirus (HRV) Wa strain. We also assessed the influence of LAB on TLR and serum innate cytokine responses induced by HRV. Distributions of subpopulations of APCs [CD14+/−SWC3+CD11R1− monocytes/macrophages and CD14+/−SWC3+CD11R1+ conventional dendritic cells (cDCs)] were described in our previous report (Zhang, W., Wen, K., Azevedo, M.S., Gonzalez, A.M., Saif, L.J., Li, G., Yousef, A.E., Yuan, L., 2008. Lactic acid bacterial colonization and human rotavirus infection influence distribution and frequencies of monocytes/macrophages and dendritic cells in neonatal gnotobiotic pigs. Vet. Immunol. Immunopathol. 121, pp. 222–231). We demonstrated that LAB induced strong TLR2-expressing APC responses in blood and spleen, HRV induced a TLR3 response in spleen, and TLR9 responses were induced by either HRV (in spleen) or LAB (in blood). LAB and HRV have an additive effect on TLR2- and TLR9-expressing APC responses, consistent with the adjuvant effect of LAB. Overall, the frequencies of TLR-expressing CD14+ APCs were higher than CD14− APCs. LAB enhanced the IFN-γ and IL-4 responses in serum, but it had a suppressive effect on the TLR3- and TLR9-expressing CD14− APC responses in spleen and the serum IFN-α response induced by HRV. These results elucidated the systemic TLR2-, TLR3-, and TLR9-expressing monocyte/macrophage and cDC responses after HRV infection, LAB colonization, and the two combined. Our findings facilitate the understanding of the mechanism of LAB’s adjuvant effect on rotavirus vaccines and the diverse innate and adaptive immune responses induced by commensal LAB colonization versus rotavirus infection and the interactions between them.
Keywords: Toll-like receptors Rotavirus, Lactobacilli, Dendritic cells, Monocytes/macrophages, Gnotobiotic pigs
1. Introduction
Toll-like receptors (TLRs), a type of pattern-recognition receptor (PRR), play an important role in viral antigen recognition, innate immunity and in bridging innate and adaptive immune responses (Kaisho and Akira, 2006). TLRs are expressed on many cell types, but mainly on professional antigen presenting cells (APCs), e.g. monocytes/macrophages and dendritic cells (DCs). TLR activation induces type I interferon production through several signaling pathways that lead to anti-viral and proinflammatory cytokine responses and induction of adaptive immune responses (Sandor and Buc, 2005; Seth et al., 2006). Different microbe-associated molecular patterns (MAMPs) are recognized by different TLRs (Akira and Takeda, 2004). TLR2, in association with TLR1 and/or TLR6, recognizes peptidoglycans, lipopeptides, and lipoteichoic acids from Gram-positive bacteria (Modlin, 2002). TLR3 recognizes double-stranded (ds) viral RNA, which is found in rotavirus genome and many other viruses during their replication cycles (e.g. negative-stranded RNA viruses), and synthetic polyinosine-polycytidylic acid (polyI:C). TLR9 recognizes unmethylated CpG DNA found abundantly in bacterial and viral genomes (Werling and Jungi, 2003). Both TLR3 and TLR9 play crucial role in defense against viral infection in vivo (Tabeta et al., 2004). Alterations in TLR expression levels in peripheral blood mononuclear cells (PBMCs) have been reported in various viral infections (Chen et al., 2008; de Kruif et al., 2008; Lester et al., 2008; Sato et al., 2007; Xu et al., 2007, 2008) and have been directly correlated with plasma viral load (Lester et al., 2008) or associated with the severity of disease outcomes (de Kruif et al., 2008; Xu et al., 2007).
Rotaviruses are the single most important etiologic agent of severe gastroenteritis in infants and young children worldwide (Parashar et al., 2006). Rotavirus virion has a non-enveloped, triple-layered capsid structure that surrounds the genome, which is composed of 11 segments of dsRNA. Rotavirus replicates mainly in the mature epithelial cells of the small intestinal villi and causes villous atrophy (Ward et al., 1996). However, recent studies confirmed that rotavirus infection is not restricted to the intestinal tract. Rotavirus infection has an acute phase of viremia (Azevedo et al., 2005; Blutt and Conner, 2007). Thus, TLR-expressing APC responses in systemic lymphoid tissues may contribute to the anti-viral immunity or pathogenesis in rotavirus infection. Patients with acute rotavirus infection had elevated mean levels of TLR2, TLR3, TLR4, TLR7, TLR8 mRNA expression in PBMCs within 3 days of onset of the disease (Xu et al., 2006).
Lactobacillus spp., Gram-positive rod-shaped bacteria, are normal components of the healthy human and pig intestinal microflora. Lactic acid bacteria (LAB), including lactobacilli are widely evaluated as probiotics in animals and humans (Vaughan et al., 2002) and have been shown to significantly stimulate gut epithelial cell proliferation (Ichikawa et al., 1999), enhance innate and acquired immunity in young lab animals (mice, rats) and children (Herias et al., 1999; Yasui et al., 1999) and suppress intestinal inflammation (Zocco et al., 2006). Several LAB strains have been shown to reduce the severity of acute rotavirus gastroenteritis in children (Majamaa et al., 1995; Shornikova et al., 1997). The mechanisms of LAB’s beneficial effects on human and animal health are the subject of many ongoing studies. Our studies are focused on LAB’s effect on innate and adaptive immune responses to rotavirus infection (Zhang et al., 2008a,c) and the adjuvant effect on rotavirus vaccines (Zhang et al., 2008b). The adjuvant effect of several LAB strains has been documented in humans and in pigs (de Vrese et al., 2005; Isolauri et al., 1995; Kaila et al., 1992; Link-Amster et al., 1994; Olivares et al., 2007; Zhang et al., 2008b); however, the mechanism is undefined. It is known that many LAB strains are full of CpG islands in the genome (Rachmilewitz et al., 2004); therefore, lactobacilli may exert an immunostimulating effect via activation of TLR9 on APCs.
The objectives of the present study were to (1) evaluate TLR2-, TLR3- and TLR9-expressing APC responses in rotavirus infection or LAB colonization in systemic lymphoid tissues of neonatal Gn pigs, and (2) to assess the influence of LAB on TLR2, TLR3 and TLR9, and innate cytokine responses to rotavirus infection. The Gn pig model of human rotavirus (HRV) infection and diarrhea has been well defined in our previous studies (Saif et al., 1996; Yuan and Saif, 2002). Early and late cytokine responses (e.g. IFN-γ, IL-4, IL-6, IL-10, IL-12, TGF-β1, and TNF-α) in serum of Gn pigs infected with the virulent Wa HRV have been reported (Azevedo et al., 2006). In this study, we evaluated the effect of LAB on early cytokine responses as they are indicators of activation of innate immune cells via PRR. Besides being a differentiation/maturation marker of monocytes/macrophages and DCs (Carrasco et al., 2001; Paillot et al., 2001; Summerfield et al., 2003), CD14 is also a PRR and plays a role in the innate immune response. It directly interacts with intracellular TLR3 and enhances dsRNA-mediated TLR3 activation by aiding uptake of dsRNA into cells (Lee et al., 2006). Because the monocytes (while in blood circulation) and macrophages (after entering spleen) are defined by the same cell markers, we refer to them as monocytes/macrophages in both locations (Zhang et al., 2008c). It was previously found that LAB significantly reduced the total, but not CD14+, frequencies of monocytes/macrophages and cDCs in spleen of LAB plus HRV pigs compared to HRV-only pigs (Zhang et al., 2008c). The influences of LAB on frequencies of TLR2-, TLR3- and TLR9-expressing CD14+ versus CD14− monocytes/macrophages and DCs in the spleen and blood of HRV-infected Gn pigs were evaluated in this study to further clarify the effect of LAB on the responses in different APC subpopulations in systemic lymphoid tissues.
2. Materials and methods
2.1. Bacteria
The Lactobacillus reuteri strain ATCC 23272 and Lactobacillus acidophilus strain NCFM™ (ATCC, Manassas, VA, USA) were used in this study. Both LAB strains were propagated in lactobacilli MRS broth (Weber, Hamilton, NJ, USA). LAB inoculums were prepared and titrated as previously described (Zhang et al., 2008c). The two LAB inoculums with known titers were diluted to the specified CFU/ml in 0.1% peptone water (BD Biosciences, Franklin Lakes, NJ, USA) and mixed in equal amounts on the day of feeding.
2.2. Virus
The virulent Wa strain (G1P1A[8]) HRV was passaged through Gn pigs and the pooled intestinal contents from the 23rd passage were used for inoculation at a dose of 1 × 105 fluorescent focus-forming units (FFU). The 50% infectious dose (ID50) of the Wa HRV in pigs was determined as approximately 1 FFU (Ward et al., 1996). Virus fecal shedding was detected by a cell-culture immunofluorescent (CCIF) assay and HRV antigen in serum was detected with an antigen capture enzyme-linked immunosorbent assay (ELISA) as previously described (Bohl et al., 1982; Hoblet et al., 1986).
2.3. Inoculation of Gn pigs
Near-term pigs were derived by surgery from two sows (Large White) and maintained in germ-free isolator units as described (Meyer et al., 1964). All pigs were confirmed as seronegative for rotavirus antibodies and germ-free prior to LAB and HRV exposure. Gn pigs (both males and females) were randomly assigned to four treatment groups with four pigs in each group as follows: (1) LAB colonization plus HRV infection (LAB+HRV+), (2) HRV only (LAB−HRV+), (3) LAB only (LAB+HRV−) or (4) mock control (LAB−HRV−). Pigs in LAB+ groups were orally dosed at 3, 5, 7 and 9 days of age with 103, 104, 105 and 106 CFU, respectively, of a 1:1 mixture of L. acidophilus and L. reuteri in 2 ml of 0.1% peptone water. The total dose of lactobacilli received by each pig was 1.1 × 106 CFU in four feedings. Non-LAB-fed pigs were given an equal volume of 0.1% peptone water. At 5 days of age, pigs in HRV+groups were orally inoculated with 105 FFU virulent Wa HRV in 5 ml of Dulbecco’s Modified Eagle’s Medium (DMEM). Non-infected pigs were given an equal volume of diluent. Pigs were given 5 ml of 100 mM sodium bicarbonate to reduce gastric acidity 20 min before HRV inoculation. Post-HRV-inoculation, pigs were examined daily for clinical signs, including % with diarrhea, duration of diarrhea and diarrhea scores as described (Ward et al., 1996; Yuan et al., 1996). Rectal swabs were collected daily for HRV and lactobacilli shedding. Serum samples were collected at post-HRV inoculation day (PID) 0, PID 2 and PID 5 for detection of cytokine responses. Pigs were euthanized at PID 5 (1 day after the last/7 days after the first LAB feeding) to isolate mononuclear cells (MNC) from spleen and peripheral blood. MNCs were isolated as previously described (Yuan et al., 1996) and stained with antibodies to porcine cell markers and TLR antibodies directly, without in vitro stimulation, on the same day of MNC isolation.
2.4. Enumeration of LAB
Each rectal swab was diluted in 4 ml of 0.1% peptone water (~1:10) and a 100 μl aliquot was diluted in 900 μl of peptone water and plated onto MRS agar. The plates were incubated in sealed BBL Gaspak jars (Fisher, Hanover Park, IL) containing Anaerogen packs (BD) for 24 h at 37 °C. The number of CFU on plates with 20–200 colonies were enumerated and recorded. LAB shedding was expressed as CFU/ml. Bacteremia was assessed by plating pig sera onto MRS agar plates and incubated in the same way as for LAB enumeration.
2.5. Staining cells for flow cytometry analysis
The MNCs (2 × 106 cells/tube) were first stained with antibodies to porcine monocyte/macrophage and cDC markers (SWC3, CD11R1 and CD14), followed by antibodies to TLR2, TLR3 and TLR9, respectively. Except when specifically noted, MNCs were washed once with a staining buffer (prepared according to BD Pharmingen™ BrdU Flow Kits Instruction Manual) and incubated for 15 min at room temperature (RT) at each step. Cells were first stained with mouse anti-porcine CD14 (IgG2b, Fitzgerald, clone MIL-2) and mouse anti-porcine CD11R1 (IgG1, Serotech: MCA1220, clone MIL4). After washing at 500 × g for 5 min at 4 °C, the secondary fluorescent-conjugated antibodies, rat anti-mouse IgG2b fluorescein isothiocyanate (FITC) (IgG2a, BD pharmingen, clone R12-3) and rat anti-mouse IgG1 allo-phycocyanin (APC) (IgG1, BD pharmingen, clone X56), were added. After washing the cells twice, mouse anti-porcine SWC3a-biotin (IgG1, Southern Biotech, clone 74-22-15) was added followed by streptavidin-conjugated with peridinine chlorophyll protein (PerCP). For staining of TLR2, which is expressed on the cell surface, mouse anti-human TLR2 phycoerythrin (PE) (mouse IgG1, eBioscience, clone T2.5) and the mouse IgG1 isotype control PE were added, respectively, to the TLR2 and its corresponding isotype-matched control tubes. For staining intracellular TLR3, TLR9 and their corresponding isotype controls, after staining with surface markers (SWC3, CD11R1 and CD14), cells were permeabilized with BD cytofix/cytoperm™ buffer (BD pharmingen) for 15 min at RT. In TLR3 and TLR9 tubes, mouse anti-human TLR3 PE (mouse IgG1, eBioscience, clone: TLR3.7) and rat anti-human TLR9 PE (rat IgG2a, eBioscience, clone: eB72-1665) antibodies were added, respectively. In the corresponding isotype-matched control tubes, mouse IgG1 isotype control PE and rat IgG2a isotype control PE were added, respectively. Cells were incubated, washed, fixed and resuspendedin staining buffer and kept in dark at 4 °C before flow cytometry analysis. All antibodies were titrated and used at optimal concentrations. Analysis of the stained cells was performed using a 4-color FACSCalibur flow cytometer (Becton Dickinson) and at least 20,000 cells were acquired. Data analysis was performed using CellQuest™ Pro (Becton Dickinson) or FlowJo 7.2.2 (Tree Star, Inc) software. Data are presented as mean frequencies of CD14+ or CD14− TLR-expressing monocytes/macrophages and cDCs. Any non-specific staining occurring in the isotype-matched control tubes was subtracted from the frequencies of TLR+ cells.
2.6. Detection of serum cytokine levels by ELISA
Blood samples were collected from pigs at PID 0, PID 2 and at euthanasia (PID 5). Sera were processed (without heat inactivation) and stored at −20 °C until tested. The ELISAs for detection of porcine IFN-γ, IL-4, IL-6, IL-10, IL-12, IFN-α, TGF-β, and TNF-α were conducted using anti-swine cytokine antibodies as previously described (Azevedo et al., 2006). Sensitivities of the ELISAs were 7.5 pg/ml for IFN-γ, IL-4, IL-10, and IL-12; 15 pg/ml for IL-6, TNF-α, TGF-β; and 75 pg/ml for IFN-α.
2.7. Statistical analysis
Non-parametric Kruskal–Wallis rank sum test was performed to compare frequencies of TLR-expressing cells in each cell subpopulation in spleen and blood among groups and the serum cytokine concentrations at PID 5 among groups. When differences among these groups were detected, the same test was used in a pairwise fashion to clarify the nature of the differences. Spearman’s rank correlation test was used for assessment of correlation between frequencies of TLR-expressing cDCs or monocytes/macrophages and cytokine concentrations in serum. Statistical significance was assessed at p < 0.05 throughout. All statistical analyses were performed using the SAS program (SAS Institute, NC, USA).
3. Results
3.1. LAB fecal counts, HRV infection and clinical signs
From post-HRV inoculation day 0–5, the average fecal LAB counts in LAB+ groups ranged between 5.9 × 106–8.1 × 107 CFU/ml, which were greater than the count of LAB in the original feeding inoculums (103–106 CFU/ml). The mean LAB count in the LAB+HRV+ group was significantly higher than the LAB+HRV− group on PID 5 (data not shown). The bacterial strains in the intestinal contents of the LAB-fed pigs were examined by plating serially diluted samples on selective MRS agar plates for Lactobacillus and culturing anaerobically at 37 °C over night. The bacterial colonies on MRS agar plates from the intestinal content samples had identical morphology as the colonies from the original LAB inoculum. The serially diluted intestinal contents and daily rectal swab samples were also plated on regular blood agar plates and cultured aerobically at 37 °C over night. No bacterial growth was detected on the blood agar plates from any of the pigs, confirming that no extraneous bacterial contamination occurred.
The kinetics and magnitude of virus fecal and nasal shedding and antigenemia after the virulent Wa HRV inoculation of Gn pigs have been characterized previously (Azevedo et al., 2005). HRV infection in this study was confirmed by detection of HRV titers in intestinal contents by CCIF and HRV antigen in serum by ELISA from all the pigs in the HRV+ groups (none in the HRV− groups) at PID 5. All the HRV+ pigs developed antigenemia, but none of the LAB+ pigs developed bacteremia (data not shown). There were no significant differences in HRV titers in intestinal contents (mean peak titer 6.4 × 104 FFU/ml vs. 3.3 × 104 FFU/ml) or antigen-ELISA OD values in serum (data not shown) between the two HRV+ groups, suggesting that LAB colonization did not reduce rotavirus intestinal replication or prevent viremia. The duration and severity of diarrhea between the LAB+HRV+ and LAB−HRV+ groups did not differ significantly (data not shown), thus intestinal colonization by the mixture of L. acidophilus and L. reuteri did not reduce rotavirus diarrhea.
3.2. Detection of TLR expression in porcine APCs by flow cytometry using anti-human TLR antibodies
The cross reactivity and specificity of anti-human TLR2, TLR3 and TLR9 antibodies were evaluated for detection of porcine TLRs in MNCs by flow cytometry. Sufficient cross reactivity between human and porcine TLR antibodies was indicated by the strong fluorescent intensity and the specificity was indicated by the clear separation of the histograms of isotype-matched irrelevant control antibody-stained MNCs from the TLR antibody-stained MNC (data not shown). The cross reactivity of the antibodies can be explained by the high amino acid sequence homologies between human and porcine TLRs. There is a 78.1% for TLR2, 84.2% for TLR3, and 82.0% for TLR9 amino acid sequence identity, respectively, between humans and pigs based on comparing the predicted amino acid sequences from NCBI data base (TLR2 porcine NP_998926 vs. human AAH33756; TLR3 porcine ABB92547 vs. human NP_003256; and TLR9 porcine NP_999123 vs. human AAQ89443).
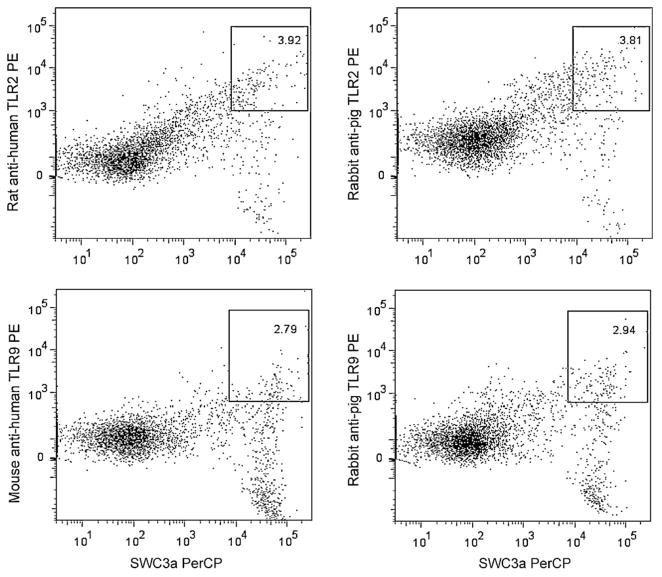
Rabbit anti-pig TLR2 and TLR9 polyclonal antibodies became available (Cosmo, Bio, Co.) later in the study. The sensitivity of the anti-human TLR antibodies were compared with the anti-pig TLR polyclonal antibodies using normal conventional pig blood MNCs. The MNCs were stimulated in vitro with CpG OND or peptidoglycan before staining. As shown in Fig. 1, similar frequencies of SWC3+TLR2+ and SWC3+TRL9+ monocytes were detected by anti-human and anti-pig TLR antibodies. We repeated the experiment in three pigs (Fig. 1 shows the representative frequencies). The differences in frequencies of SWC3+TLR2+ or SWC3+TRL9+ detected by anti-human and anti-pig antibodies were less than 0.5% and were not statistically significant. These results confirmed that the anti-human TLR antibodies are applicable for study of TLR responses in pigs. To date, anti-pig TLR3 is not commercially available. However, because TLR3 has a higher amino acid sequence similarity between humans and pigs than TLR2 and TLR9, similar results are expected when anti-pig TLR antibodies become available in the future.
Fig. 1.
Comparisons between anti-human and anti-pig TLR antibodies for detection of TLR2 and TLR9 expression in pig blood monocytes. The frequencies of SWC3+TLR2+ and SWC3+TLR9+ monocytes detected by the different TLR antibodies are labeled on each dot plot. Dot plots show the representative of results from three pigs. Blood MNCs isolated from three normal conventional pigs were stimulated in vitro with CpG OND D19 (1 μM) (Watarai et al., 2008) or Bacillus subtilis peptidoglycan (10 μg/ml) (Yamamoto et al., 2003) for 48 h before staining. The MNCs were stained with SWC3a-biotin and streptavidin-PerCP and followed by the PE conjugated anti-human TLR2 and TLR9, respectively (see Section 2) or followed by the rabbit anti-pig TLR2 and TLR9 polyclonal antibodies (Cosmo Bio Co., Japan) respectively, and then a PE conjugated goat anti-rabbit IgG (Open Biosystems, Huntsville, AL).
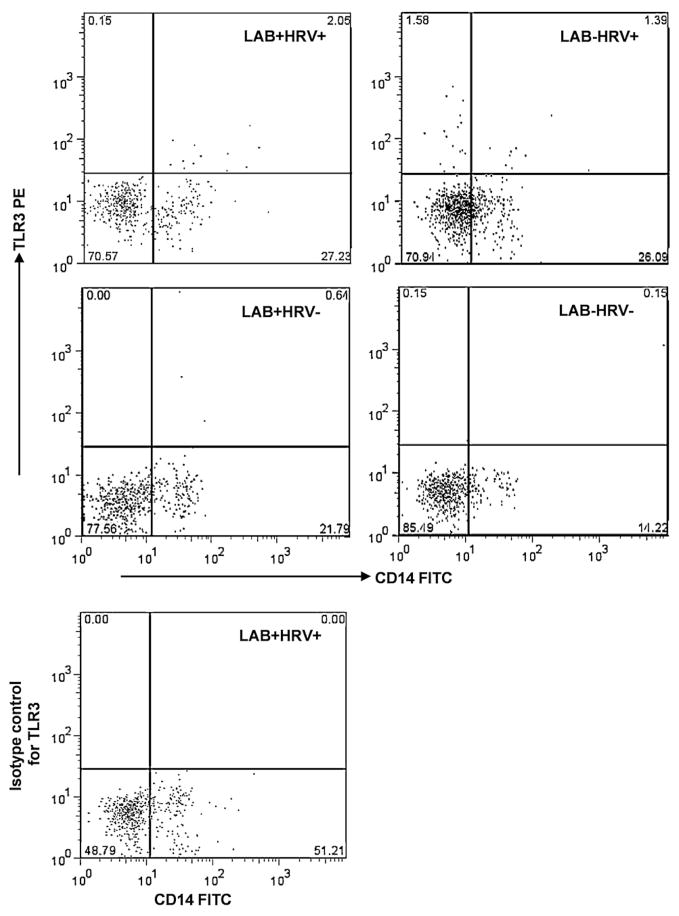
Detection of frequencies of TLR-expressing cDCs (SWC3+CD11R1+) and monocytes/macrophages (SWC3+CD11R1−) in the four treatment groups is depicted by the representative dot plots in Fig. 2 using TLR3 in spleen as an example. The frequencies and tissue distribution of porcine cDCs and monocytes/macrophages in the four groups have been described in a previous publication (Zhang et al., 2008c). As shown in Fig. 2 (dot plot at lower right corner), the MNCs stained with antibodies to cell markers and the isotype-matched irrelevant control antibody for TLR3 had zero percent TLR3+ cells, confirming the specificity of the antibody. The mean frequencies of TLR2-, TLR3- and TLR9-expressing CD14+ and CD14− monocytes/macrophages and cDCs are summarized in Fig. 3 (spleen; note the difference in y-axis scales between CD14+ and CD14− APCs) and Fig. 4 (blood; note the 10-fold difference in y-axis scales between TLR2-expressing CD14+ APCs and the others). Overall, higher frequencies of TLR-expressing CD14+ APCs than CD14− APCs were detected. High variability within treatment groups was observed for frequencies of TLR-expressing APCs in both spleen and blood. A matrix table was constructed based on both the frequencies and statistical comparison results among the four pig groups to depict the effect of treatment on frequencies of TLR-expressing APCs and serum cytokine levels (Table 1).
Fig. 2.
Example of frequencies of TLR3-expressing splenic monocytes/macrophages of Gn pigs from each treatment group. Treatment group is labeled on each dot plot: LAB+HRV+, pigs were inoculated with LAB and virulent Wa strain HRV; LAB−HRV+, pigs were inoculated with HRV only; LAB+HRV−, pigs were inoculated with LAB only; and LAB−HRV−, pigs were mock inoculated. Monocytes/macrophages were defined as CD14+/−SWC3+CD11R1− (Zhang et al., 2008c). CD14/TLR3 dot plots were performed within the SWC3+CD11R1− subpopulation. The dot plot at lower left corner shows a TLR3 isotype control staining which included antibodies to all the cell markers (SWC3, CD11R1 and CD14), except that the antibodies to TLR3 were replaced by the isotype-matched antigen-irrelevant control antibodies.
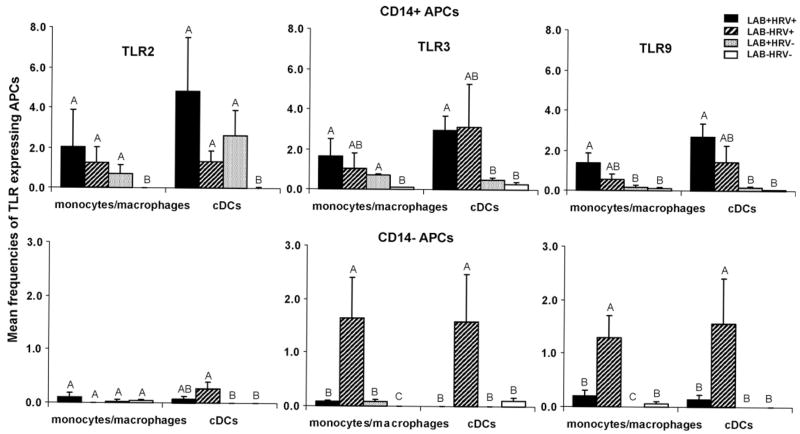
Fig. 3.
Frequencies of TLR2-, TLR3-, and TLR9-expressing CD14+ and CD14− APCs in spleen of Gn pigs at PID 5. Gn pigs were inoculated with LAB and virulent Wa strain HRV (LAB+HRV+), HRV only (LAB−HRV+), LAB only (LAB+HRV−) or mock (LAB−HRV−). The y-axis is the mean frequencies (%) of CD14+ or CD14− TLR-expressing cells among monocytes/macrophages or cDCs. Note the difference in y-axis scales between CD14+ and CD14− APCs. The error bars represent standard error of the mean (n = 4). The letters A, B, and C indicate the results of significance testing for difference between treatments. Unshared letters indicate significant difference between treatment groups on frequencies of the TLR-expressing APCs (Kruskal–Wallis rank sum test, p < 0.05), while shared letters indicate no significant difference.
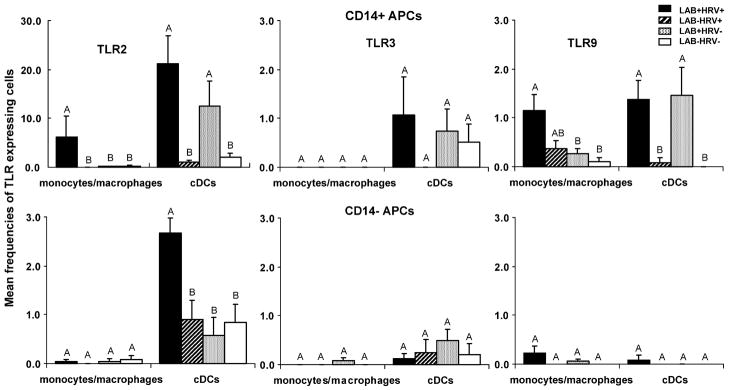
Fig. 4.
Frequencies of TLR2-, TLR3-, and TLR9-expressing CD14+ and CD14− APCs in blood of Gn pigs at PID 5. Gn pigs were inoculated with LAB and virulent Wa strain HRV (LAB+HRV+), HRV only (LAB−HRV+), LAB only (LAB+HRV−) or mock (LAB−HRV−). The y-axis is the mean frequencies (%) of CD14+ or CD14− TLR-expressing cells among monocytes/macrophages or cDCs. Note the difference in y-axis scales between CD14+ and CD14− TLR2-expressing APCs. The error bars represent standard error of the mean (n = 4). For letters A, B, C, see figure legend for Fig. 3.
Table 1.
Effect of treatment on frequencies of TLR-expressing APCs in spleen and blood and serum cytokine concentrations.
| Treatment | Spleen APCs | TLR2 | TLR3 | TLR9 | Blood APCs | TLR2 | TLR3 | TLR9 | Cytokines in serum | PID 2 | PID 5 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| IFN-α | +++ | + | |||||||||
| LAB plus HRV | CD14+ MPa | ++b,c | + | + | CD14+ MP | +++++ | − | + | IL-12 | +++ | + |
| CD14− MP | +/− | +/− | +/− | CD14− MP | − | − | +/− | IFN-γ | +++++ | ++ | |
| CD14+ cDCs | +++ | ++ | ++ | CD14+ cDCs | ++++++ | +/− | + | IL-4 | +++++ | ++ | |
| CD14− cDCs | +/− | − | +/− | CD14− cDCs | ++ | − | − | TGF-β | − − | − − | |
| IFN-α | +++ | ++ | |||||||||
| HRV-only | CD14+ MP | + | + | +/− | CD14+ MP | − | − | +/− | IL-12 | +++ | + |
| CD14− MP | − | + | + | CD14− MP | − | − | − | IFN-γ | ++ | +/− | |
| CD14+ cDCs | + | +++ | + | CD14+ cDCs | − | − | − | IL-4 | + | +/− | |
| CD14− cDCs | +/− | + | + | CD14− cDCs | − | − | − | TGF-β | − − | − − − − − | |
| IFN-α | − | − | |||||||||
| LAB-only | CD14+ MP | +/− | +/− | +/− | CD14+ MP | − | − | +/− | IL-12 | − | +++ |
| CD14− MP | − | +/− | − − | CD14− MP | − | − | − | IFN-γ | − | − | |
| CD14+ cDCs | ++ | +/− | +/− | CD14+ cDCs | +++++ | +/− | + | IL-4 | − | − | |
| CD14− cDCs | − | − | − | CD14− cDCs | − | +/− | − | TGF-β | − | − − − |
MP, monocytes/macrophages.
Plus and minus signs indicate various degree of effect of the treatment groups, from increased frequencies of APCs or cytokine concentrations (++) to equal (−) or lower than the baseline values (− −) of the mock control (LAB−HRV−) group.
Values differing significantly from the mock control group are in boldface (Kruskal–Wallis rank sum test; p < 0.05).
3.3. TLR-expressing APCs in spleen
3.3.1. TLR2-expressing CD14+ APCs were induced by either LAB or HRV and LAB plus HRV had an additive effect
As shown in Fig. 3, no or minimal frequencies of TLR-expressing monocytes/macrophages or cDCs were detected in spleen of mock control pigs (LAB−HRV−). LAB plus HRV (LAB+HRV+), HRV infection alone (LAB−HRV+), and LAB colonization alone (LAB+HRV−) induced significantly higher frequencies of CD14+ TLR2-expressing monocytes/macrophages and cDCs than the mock controls, indicating the involvement of TLR2 in recognition of LAB colonization and HRV infection. In contrast to the CD14+ APCs, CD14− monocytes/macrophages and cDCs had minimal or no TLR2 expression, except for the low TLR2 expression on cDCs in the HRV-only group.
3.3.2. TLR3-expressing CD14+ and CD14− APCs were induced by HRV whereas LAB had an antagonistic effect with HRV on frequencies of TLR3-expressing CD14− APCs
HRV alone induced 2- to 3-fold higher frequencies of TLR3-expressing CD14+ APCs compared to the mock control group. LAB plus HRV induced significantly higher frequencies of TLR3-expressing CD14+ APCs than mock controls, and they were similar to the HRV-only group. In contrast, frequencies of TLR3-expressing CD14− monocytes/macropahges and cDCs in the LAB plus HRV group were significantly lower than those in the HRV-only group, suggesting an antagonistic effect of LAB on the HRV-induced TLR3 response. LAB alone induced minimal frequencies of TLR3-expressing CD14+ and CD14− cDCs in spleen, coinciding with the low cytokine responses (except for IL-12) induced by LAB alone (Fig. 5). Still, frequencies of TLR3-expressing monocytes/macrophages in LAB-only group were, albeit low, significantly higher than mock controls. This is unexpected because LAB does not contain known TLR3 ligands.
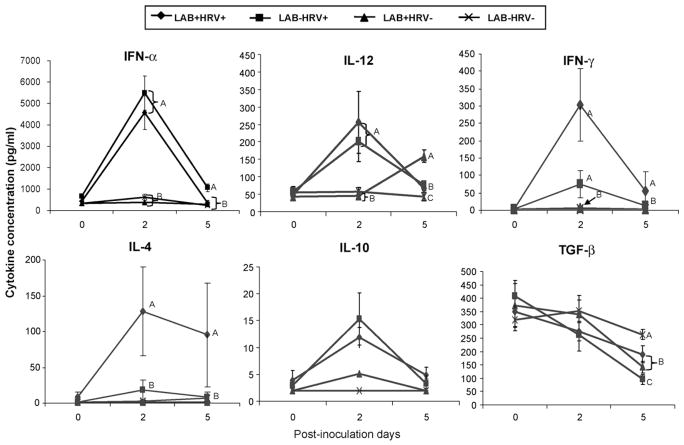
Fig. 5.
Cytokine levels in serum of Gn pigs. Gn pigs were inoculated with LAB and virulent Wa strain HRV (LAB+HRV+), HRV only (LAB−HRV+), LAB only (LAB+HRV−) or mock (LAB−HRV−). Cytokine concentrations were measured by ELISA on PID 0, PID 2, and PID 5. The error bars represent standard error of the mean. For letters A, B, C, see figure legend for Fig. 3.
3.3.3. TLR9-expressing CD14+ and CD14− APCs were induced by HRV whereas LAB had an antagonistic effect with HRV on frequencies of TLR9-expressing CD14− APCs
The patterns of TLR9-expressing CD14+ and CD14− APCs in the HRV+ groups were similar to those of TLR3 with slightly lower frequencies (Fig. 3, Table 1), suggesting that TLR9 is also involved in the HRV-induced innate immune responses. LAB alone induced minimal TLR9-expressing CD14+ and CD14− APCs in spleen.
In summary, similar frequencies of TLR3-expressing CD14+ APCs were detected in spleen of the HRV-infected Gn pigs with or without LAB colonization (Fig. 3 and Table 1), suggesting that HRV infection is mainly responsible for the increased frequencies of TLR3-expressing APCs. LAB plus HRV enhanced (not statistically significant) the frequencies of TLR2- and TLR9-expressing CD14+ APCs compared to LAB or HRV alone, suggesting an additive effect between LAB and HRV. The most striking observation in spleen is that the frequencies of TLR3- and TLR9-expressing CD14− APCs were significantly reduced in the LAB plus HRV group compared to the HRV-only group, thus LAB had a suppressive effect on the TLR responses to HRV in a subset of systemic APCs.
3.4. TLR-expressing APCs in blood
3.4.1. High frequencies of TLR2-expressing CD14+ APCs were induced by LAB (cDCs) and LAB plus HRV (cDCs and monocytes/macrophages)
In blood, minimal to no TLR-expressing CD14+ or CD14− monocytes/macrophages were detected in mock-inoculated pigs (Fig. 4). However, low frequencies of TLR2− and TLR3-expressing CD14+ and CD14− cDCs were detected, which likely reflect the baseline constitutive expression of the TLRs by blood cDCs. LAB plus HRV and LAB alone induced the highest mean frequencies of TLR2-expressing CD14+ cDCs in blood compared to all the others (note the y-axis scale difference) and they were significantly higher (6 to 21-fold) than the HRV-only and mock control groups.
LAB plus HRV, but not LAB alone, induced significantly higher frequencies of CD14+ TLR2-expressing monocytes/macrophages compared to the HRV-only and mock control groups. This additive effect was also observed for CD14− TLR2-expressing cDCs. HRV alone did not induce significant TLR-expressing APCs in blood. Frequencies of TLR2-expressing CD14− monocytes/macrophages and TLR3-expressing CD14+ and CD14− APCs were low to minimal and with high variability, and did not differ significantly among the four groups.
3.4.2. TLR9-expressing CD14+ APCs were induced by LAB (cDCs) and LAB plus HRV (cDCs and monocytes/macrophages)
The pattern of TLR9-expressing CD14+ APCs was similar to the pattern of TLR2, but at an approximately 10-fold lower magnitude, with the two LAB+ groups inducing significantly higher frequencies of TLR9-expressing CD14+ cDCs compared to the HRV-only and mock control groups. LAB plus HRV, but not HRV or LAB alone, induced significantly higher frequencies of TLR9-expressing CD14+ monocytes/macrophages compared to the mock controls. Frequencies of TLR9-expressing CD14− APCs were low and did not differ significantly among the groups.
In summary, high frequencies of TLR2-expressing CD14+ cDCs in blood were induced by LAB, or LAB plus HRV. Significant TLR9-expressing CD14+ cDCs were also detected in the two LAB+ groups, but not in the HRV-only group (Fig. 4 and Table 1). Overall, LAB plus HRV induced higher or significantly higher frequencies of TLR2- and TLR9-expressing-CD14+ monocytes/macrophages and cDCs and TLR2-expressing CD14− cDCs than HRV alone. These data indicate that TLR2- and TLR9-expressing cDCs in blood were mainly induced by LAB; however, LAB and HRV had an additive effect on frequencies of TLR2- and TLR9-expressing cDCs.
3.5. Cytokine levels in serum and the correlation with TLR responses
3.5.1. LAB plus HRV induced significantly higher levels of IFN-γ and IL-4 but significantly lower IFN-α than HRV alone at PID 5
The mean concentrations of anti-viral cytokine (IFN-α), proinflammatory cytokines (IL-12), Th1 cytokine (IFN-γ), Th2 cytokine (IL-4), and T regulatory cell (Treg) cytokines (IL-10 and TGF-β) at PID 2 and PID 5 in the sera of pigs are summarized in Fig. 5. The concentrations of IFN-γ and IL-4 in the LAB plus HRV group were higher or significantly higher than the HRV-only group, suggesting an adjuvant effect of LAB on both Th1 and Th2 type responses (Fig. 5). The mean IFN-γ and IL-4 concentrations were 4- to 11-fold higher in the LAB plus HRV group compared to the HRV-only group. On the other hand, the concentrations of IFN-α in the HRV-only group were higher or significantly higher than the LAB plus HRV group, indicating a down-regulating effect of LAB on the IFN-α response induced by HRV. The concentrations of IL-12 in the LAB plus HRV and HRV-only groups were significantly higher than the LAB-only and the mock control groups at PID 2. At PID 5, however, IL-12 concentrations in the LAB-only group were significantly higher than the LAB plus HRV and the HRV-only groups and they were all significantly higher than the mock control group. Thus, IL-12 responses were stimulated by both HRV infection and LAB colonization but with different kinetics. The concentrations of TGF-β in the HRV-only group were significantly lower than the LAB plus HRV and the LAB-only groups and they were all significantly lower than the mock control group at PID 5, thus LAB may play a role in maintaining the TGF-β levels in serum. The concentrations of IL-10 did not differ significantly among groups, but there was a clear trend for higher concentrations in the HRV+ groups at PID 2 and PID 5. The IL-6 and TNF-α levels in serum were low and did not differ among groups (data not shown).
3.5.2. Serum IFN-α concentrations significantly correlated with frequencies of TLR3- and TLR9-expressing CD14− APCs in spleen at PID 5
Spearman’s rank correlation analysis was performed to identify the correlations between frequencies of TLR2-, TLR3- and TLR9-expressing APCs in spleen or blood and the concentrations of serum cytokines at PID 5 among the four treatment groups. Significant positive correlations were found between IFN-α concentrations and frequencies of TLR3-expressing CD14− cDC (r = 0.6151, p = 0.0112) and monocytes/macrophages (r = 0.6372, p = 0.0079) and TLR9-expressing CD14− cDC (r = 0.6398, p = 0.0076) and monocytes/macrophages (r = 0.5417, p = 0.0302) in spleen. These correlations suggest that the CD14− APCs in spleen may contribute significantly to the levels of IFN-α in serum.
4. Discussion
This study is the continuation of our previous report (Zhang et al., 2008c) on the effects of probiotic LAB colonization and HRV infection on development of the innate immune responses in neonatal Gn pigs. In this study, we evaluated the frequencies of systemic TLR2-, TLR3- and TLR9-expressing CD14+ and CD14− APCs in Gn pigs colonized with LAB, infected with HRV, or both. Previously, the TLR expression in pigs has been studied only at the mRNA level in tissues by using real-time quantitative PCR because antibodies against porcine TLRs are not currently available (Burkey et al., 2007; Tohno et al., 2006; Willing and Van Kessel, 2007). Using cross reactive anti-human TLR antibodies enabled us to evaluate the expression pattern at the protein level for cell surface (TLR2) and intracellular (TLR3, TLR9) TLR expression in the defined porcine APC subpopulations.
LAB intestinal colonization alone had a profound stimulating effect on early postnatal development of the systemic innate immune cells, as evidenced by the significantly increased total frequencies of monocytes/macrophages and cDCs and the CD14+ monocyte/macrophage frequencies in spleen (Zhang et al., 2008c), the significantly increased TLR2- and TLR9-expressing CD14+ cDCs in blood, and the significantly elevated serum pro-Th1 cytokine (IL-12) level at PID 5. The induction of TLR2- and TLR9-expressingcDC andIL-12 responses can be explained, atleast partially, by the presence of TLR2 and TLR9 ligands (peptidoglycan in the cell wall and CpG motifs in the genome for TLR2 and TLR9, respectively) in the LAB. We identified 6405 CpG islands in the whole genome of L. acidophilus NCFM (Altermann et al., 2005) using EMBOSS CpGPlot (http://www.ebi.ac.uk/Tools/emboss/cpglot/index.html). Induction of TLR2- and TLR9-expressing cDC and enhancement of the IFN-γ and IL-4 responses may provide the mechanism for the adjuvanticity of LAB in enhancing cellular and humoral immune responses induced by influenza virus, poliovirus and rotavirus vaccines and rotavirus or Salmonella typhi Ty21a infections (de Vrese et al., 2005; Isolauri et al., 1995; Kaila et al., 1992; Link-Amster et al., 1994; Olivares et al., 2007; Zhang et al., 2008b). Our results are consistent with other studies, e.g. (1) feeding L. casei increased TLR2 expression in Peyer’s patches in mice (Galdeano and Perdigon, 2006); and (2) L. paracasei and L. salivarius significantly increased the production of IL-12 in PBMC of healthy adults (Castellazzi et al., 2007).
An additive effect between LAB and HRV in activating TLR2- and TLR9-expressing CD14+ APCs in spleen and CD14− cDCs in blood was observed. On the other hand, the increased frequencies of TLR2- and TLR9-expressing APCs in LAB plus HRV pigs may simply be due to the significantly higher counts of LAB, which may translate to an increased magnitude of TLR agonists available to stimulate the host immune system. However, because the frequencies of total APCs or CD14+ APCs in the LAB plus HRV pigs did not differ from the LAB-only pigs in blood, and the total APCs in spleen of the LAB plus HRV pigs were significantly lower than the LAB-only group (Zhang et al., 2008c), it is not likely that the higher LAB count in the LAB plus HRV group played a more pertinent role in the significant increases of TLR2- and TLR9-expressing APC frequencies than did the co-infection of LAB and HRV.
HRV infection of the Gn pigs, with or without LAB colonization, induced similar frequencies of TLR3- and TLR9-expressing CD14+ APCs in spleen, indicating that HRV is chiefly responsible for the increases of TLR3- and TLR9-expressing CD14+ APCs. Notably, in LAB plus HRV pigs, the frequencies of TLR3- and TLR9-expressing CD14− APCs in spleen were significantly reduced compared to the HRV-only pigs. The reduced TLR3- and TLR9-expressing CD14− APC responses correlated significantly with the reduced serum IFN-α level in this pig group. Our previous study also showed that the LAB plus HRV pigs had significantly reduced total frequencies of APCs in spleen, reduced total frequencies of monocytes/macrophages in ileum, and significantly reduced frequencies of CD14+ monocytes/macrophages in ileum than the HRV-only pigs (Zhang et al., 2008c). Because the viremia level and fecal virus shedding titers did not differ significantly among the HRV-infected pigs with or without LAB, the reductions are not likely due to reduced HRV replication, although some studies have indicated that probiotic feeding can reduce rotavirus shedding (Mao et al., 2008; Saavedra et al., 1994; Yasui et al., 1999). Thus, the results may suggest that LAB colonization has a down-regulatory effect on subpopulations of APCs in HRV-infected pigs. Mechanisms and implications of such an effect of LAB on innate immune responses to HRV infection require further investigation.
Both CD14+ and CD14− APCs expressing TLR2, TLR3 and TLR9 were induced in spleen by HRV infection; however, the TLR-expressing APC responses in blood were very different from that of spleen. Frequencies of TLR2-, TLR3- and TLR9-expressing APCs in blood of the HRV-only pigs were similar to the control (LAB−HRV−) pigs. The lack of TLR responses in blood in the HRV-only pigs is consistent with our previous findings showing that HRV infection did not increase frequencies of total APCs or CD14+ cDCs, and significantly reduced the total frequencies of CD14+ monocytes/macrophages at PID 5 in blood (Zhang et al., 2008c). The potential reasons for the considerable differences between the APC response in spleen and blood may be because (1) MNCs were collected at only one time point (PID 5), TLR-expressing APC responses occurring in the blood at an earlier time point might have been missed; or (2) splenic and circulating APCs are diverse APC populations and they react to MAMP stimulation from LAB and HRV differently. To date, the only two previous studies of TLR responses induced by rotavirus infection were conducted by measuring TLR mRNA levels in intestinal tissues (Aich et al., 2007) or PBMC (Xu et al., 2006). Bovine rotavirus induced strong TLR3 mRNA expression in the intestinal tissue of newborn calves at 18 h post-inoculation (Aich et al., 2007). Rotavirus infected children had significantly elevated expression of TLR2, TLR3, TLR8 mRNA levels in PBMCs within 3 days of diarrhea onset and the elevated TLR mRNA expression lasted for 7–14 days (Xu et al., 2006). The time frame for sample collection in the children overlapped with that in Gn pigs. Thus, the lack of TLR responses in blood APCs of the HRV-only pigs may not be solely due to the single sample collection time point; however, it is important to investigate the TLR responses to HRV at an earlier time point (PID 2–3) in the future study.
Induction of TLR2-expressing APCs in spleen of Gn pigs by HRV infection is an interesting finding because the ligands for TLR2 have not been identified previously in rotavirus. Likewise were the TLR2, TLR4, TLR7 and TLR8 mRNA responses in rotavirus infected children (Xu et al., 2006). Further studies are needed to understand these observations. Rotavirus dsRNA are known to be recognized only by TLR3 (Matsumoto et al., 2003; Sato et al., 2006; Zhou et al., 2007). A recent study by Zhou et al. (2007) demonstrated that recognition of rotavirus dsRNA by TLR3 on intestinal epithelial cells triggers the secretion of IL-15, which functions to increase the percentage of intestinal intra epithelial lymphocytes and enhances their cytotoxicity in mice. Another study of rats (Sato et al., 2006) also showed that rotavirus dsRNA induced severe apoptosis and diminished wound repair in intestinal epithelial cells through TLR3 activation. Hence, TLR3 may be involved in the pathogenesis of rotavirus gastroenteritis. Study of the TLR3 response induced by HRV and LAB in the intestinal epithelial and lymphoid tissues is the objective of our ongoing investigation.
Whether TLR9 is involved in rotavirus infection and immunity has not been reported before. The patterns of TLR9-expressing CD14+ APCs were similar to those of TLR3 in spleen and to those of TLR2 in blood. These data provide incidental evidence that TLR9 is involved in the innate immune responses induced by both LAB and HRV. RNA viruses are found to have a low presence of CpG dinucleotides in their genomes (Greenbaum et al., 2008). We identified 52 CpG islands in the whole genome of the virulent Wa HRV (Yuan, unpublished data) using EMBOSS CpGPlot. The presence of CpG motifs may explain the HRV-induced TLR9-expressing APC responses. TLR9-expressing APC responses were induced by either LAB or HRV depending on the cell type and location. LAB alone induced a significant TLR9-expressing CD14+ cDC response in blood, whereas HRV alone induced significant TLR9-expressing CD14− (and some CD14+) monocyte/macrophage and cDC responses in spleen. LAB plus HRV induced the highest frequencies of TLR9-expressing CD14+ monocytes/macrophages in spleen and blood, which is consistent with the adjuvant effect of LAB on HRV vaccine-induced B and T cell immune responses (Zhang et al., 2008b).
The reduced IFN-α levels significantly correlated with the reduced frequencies of TLR3- and TLR9-expressing CD14− APCs in spleen and blood, which may suggest that the activity of CD14− APCs contributes significantly to the IFN-α response. LAB had significant influence on IFN-γ, IL-4, IFN-α and TGF-β serum responses induced by HRV infection. LAB plus HRV induced significantly lower levels of serum IFN-α and higher levels of TGF-β than HRV alone, suggesting the regulatory effect of LAB. However, LAB plus HRV pigs had significantly higher (4- to 11-fold) serum IFN-γ and IL-4 concentrations compared to the HRV-only pigs, which is consistent with the adjuvant effect of LAB on HRV-induced immune responses. In the previous study of cytokine responses to HRV in Gn pigs (Azevedo et al., 2006), serum TNF-α, IFN-γ, IL-4, IL-10, IL-6 and IL-12 levels after HRV infection peaked between PID 1–5, whereas TGF-β peaked later at PID 14. IFN-α levels were not measured previously. We selected PID 2 and PID 5 in this study to correspond with the peaks of most cytokines after HRV infection in Gn pigs. AlthoughserumIL-6 and TNF-α responses peaked onPID3in the early study (Azevedo et al., 2006), the responses in this study were low and no significant differences among groups were observed. Future studies should include more time points for detection of TLR and innate cytokine responses to better understand the kinetics of innate immune responses induced by LAB, HRV and the two combined.
In summary, this study elucidated the systemic TLR2-, TLR3-, and TLR9-expressing monocyte/macrophage and cDC responses after LAB colonization, HRV infection and the two combined. Our findings facilitate the understanding of the mechanism for LAB’s adjuvant effect on rotavirus vaccines and the diverse innate and adaptive immune responses induced by commensal LAB colonization versus rotavirus infection and the interactions between them.
Acknowledgments
We thank Dr. Juliette Hanson and Mr. Rich McCormick for animal care. This work was supported by grants from the National Institutes of Health (1R21AT002524 to LY and R01AI033561 to LJS) and Ohio Agricultural Research and Development Center, The Ohio State University (OHOA1208) to LY. Salaries and research support were provided by state and federal funds appropriated to the Ohio Agricultural Research and Development Center of The Ohio State University.
References
- Aich P, Wilson HL, Kaushik RS, Potter AA, Babiuk LA, Griebel P. Comparative analysis of innate immune responses following infection of newborn calves with bovine rotavirus and bovine coronavirus. J Gen Virol. 2007;88:2749–2761. doi: 10.1099/vir.0.82861-0. [DOI] [PubMed] [Google Scholar]
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Altermann E, Russell WM, Azcarate-Peril MA, Barrangou R, Buck BL, McAuliffe O, Souther N, Dobson A, Duong T, Callanan M, Lick S, Hamrick A, Cano R, Klaenhammer TR. Complete genome sequence of the probiotic lactic acid bacterium Lactobacillus acidophilus NCFM. Proc Natl Acad Sci USA. 2005;102:3906–3912. doi: 10.1073/pnas.0409188102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo MS, Yuan L, Jeong KI, Gonzalez A, Nguyen TV, Pouly S, Gochnauer M, Zhang W, Azevedo A, Saif LJ. Viremia and nasal and rectal shedding of rotavirus in gnotobiotic pigs inoculated with Wa human rotavirus. J Virol. 2005;79:5428–5436. doi: 10.1128/JVI.79.9.5428-5436.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo MS, Yuan L, Pouly S, Gonzales AM, Jeong KI, Nguyen TV, Saif LJ. Cytokine responses in gnotobiotic pigs after infection with virulent or attenuated human rotavirus. J Virol. 2006;80:372–382. doi: 10.1128/JVI.80.1.372-382.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blutt SE, Conner ME. Rotavirus: to the gut and beyond! Curr. Opin Gastroenterol. 2007;23:39–43. doi: 10.1097/MOG.0b013e328011829d. [DOI] [PubMed] [Google Scholar]
- Bohl EH, Saif LJ, Theil KW, Agnes AG, Cross RF. Porcine pararotavirus: detection, differentiation from rotavirus, and pathogenesis in gnotobiotic pigs. J Clin Microbiol. 1982;15:312–319. doi: 10.1128/jcm.15.2.312-319.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkey TE, Skjolaas KA, Dritz SS, Minton JE. Expression of toll-like receptors, interleukin 8, macrophage migration inhibitory factor, and osteopontin in tissues from pigs challenged with Salmonella enterica serovar Typhimurium or serovar Choleraesuis. Vet Immunol Immunopathol. 2007;115:309–319. doi: 10.1016/j.vetimm.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Carrasco CP, Rigden RC, Schaffner R, Gerber H, Neuhaus V, Inumaru S, Takamatsu H, Bertoni G, McCullough KC, Summerfield A. Porcine dendritic cells generated in vitro: morphological, phenotypic and functional properties. Immunology. 2001;104:175–184. doi: 10.1046/j.0019-2805.2001.01299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellazzi AM, Valsecchi C, Montagna L, Malfa P, Ciprandi G, Avanzini MA, Marseglia GL. In vitro activation of mono-nuclear cells by two probiotics: Lactobacillus paracasei I 1688, Lactobacillus salivarius I 1794, and their mixture (PSMIX) Immunol Invest. 2007;36:413–421. doi: 10.1080/08820130701361160. [DOI] [PubMed] [Google Scholar]
- Chen Z, Cheng Y, Xu Y, Liao J, Zhang X, Hu Y, Zhang Q, Wang J, Zhang Z, Shen F, Yuan Z. Expression profiles and function of toll-like receptors 2 and 4 in peripheral blood mononuclear cells of chronic hepatitis B patients. Clin Immunol. 2008;128:400–408. doi: 10.1016/j.clim.2008.04.006. [DOI] [PubMed] [Google Scholar]
- de Kruif MD, Setiati TE, Mairuhu AT, Koraka P, Aberson HA, Spek CA, Osterhaus AD, Reitsma PH, Brandjes DP, Soemantri A, van Gorp EC. Differential gene expression changes in children with severe dengue virus infections. PLoS Negl Trop Dis. 2008;2:e215. doi: 10.1371/journal.pntd.0000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vrese M, Rautenberg P, Laue C, Koopmans M, Herremans T, Schrezenmeir J. Probiotic bacteria stimulate virus-specific neutralizing antibodies following a booster polio vaccination. Eur J Nutr. 2005;44:406–413. doi: 10.1007/s00394-004-0541-8. [DOI] [PubMed] [Google Scholar]
- Galdeano CM, Perdigon G. The probiotic bacterium Lactobacillus casei induces activation of the gut mucosal immune system through innate immunity. Clin Vac Immunol. 2006;13:219–226. doi: 10.1128/CVI.13.2.219-226.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum BD, Levine AJ, Bhanot G, Rabadan R. Patterns of evolution and host gene mimicry in influenza and other RNA viruses. PLoS Pathog. 2008;4:e1000079. doi: 10.1371/journal.ppat.1000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herias MV, Hessle C, Telemo E, Midtvedt T, Hanson LA, Wold AE. Immunomodulatory effects of Lactobacillus plantarum colonizing the intestine of gnotobiotic rats. Clin Exp Immunol. 1999;116:283–290. doi: 10.1046/j.1365-2249.1999.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoblet KH, Saif LJ, Kohler EM, Theil KW, Bech-Nielsen S, Stitzlein GA. Efficacy of an orally administered modified-live porcine-origin rotavirus vaccine against postweaning diarrhea in pigs. Am J Vet Res. 1986;47:1697–1703. [PubMed] [Google Scholar]
- Ichikawa H, Kuroiwa T, Inagaki A, Shineha R, Nishihira T, Satomi S, Sakata T. Probiotic bacteria stimulate gut epithelial cell proliferation in rat. Dig Dis Sci. 1999;44:2119–2123. doi: 10.1023/a:1026647024077. [DOI] [PubMed] [Google Scholar]
- Isolauri E, Joensuu J, Suomalainen H, Luomala M, Vesikari T. Improved immunogenicity of oral D × RRV reassortant rotavirus vaccine by Lactobacillus casei GG. Vaccine. 1995;13:310–312. doi: 10.1016/0264-410x(95)93319-5. [DOI] [PubMed] [Google Scholar]
- Kaila M, Isolauri E, Soppi E, Virtanen E, Laine S, Arvilommi H. Enhancement of the circulating antibody secreting cell response in human diarrhea by a human Lactobacillus strain. Pediatr Res. 1992;32:141–144. doi: 10.1203/00006450-199208000-00002. [DOI] [PubMed] [Google Scholar]
- Kaisho T, Akira S. Toll-like receptor function and signaling. J Allergy Clin Immunol. 2006;117:979–987. doi: 10.1016/j.jaci.2006.02.023. quiz 988. [DOI] [PubMed] [Google Scholar]
- Lee HK, Dunzendorfer S, Soldau K, Tobias PS. Double-stranded RNA-mediated TLR3 activation is enhanced by CD14. Immunity. 2006;24:153–163. doi: 10.1016/j.immuni.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Lester RT, Yao XD, Ball TB, McKinnon LR, Kaul R, Wachihi C, Jaoko W, Plummer FA, Rosenthal KL. Toll-like receptor expression and responsiveness are increased in viraemic HIV-1 infection. AIDS. 2008;22:685–694. doi: 10.1097/QAD.0b013e3282f4de35. [DOI] [PubMed] [Google Scholar]
- Link-Amster H, Rochat F, Saudan KY, Mignot O, Aeschlimann JM. Modulation of a specific humoral immune response and changes in intestinal flora mediated through fermented milk intake. FEMS Immunol Med Microbiol. 1994;10:55–63. doi: 10.1111/j.1574-695X.1994.tb00011.x. [DOI] [PubMed] [Google Scholar]
- Majamaa H, Isolauri E, Saxelin M, Vesikari T. Lactic acid bacteria in the treatment of acute rotavirus gastroenteritis. J Pediatr Gastroenterol Nutr. 1995;20:333–338. doi: 10.1097/00005176-199504000-00012. [DOI] [PubMed] [Google Scholar]
- Mao M, Yu T, Xiong Y, Wang Z, Liu H, Gotteland M, Brunser O. Effect of a lactose-free milk formula supplemented with bifidobacteria and streptococci on the recovery from acute diarrhoea. Asia Pac J Clin Nutr. 2008;17:30–34. [PubMed] [Google Scholar]
- Matsumoto M, Funami K, Tanabe M, Oshiumi H, Shingai M, Seto Y, Yamamoto A, Seya T. Subcellular localization of toll-like receptor 3 in human dendritic cells. J Immunol. 2003;171:3154–3162. doi: 10.4049/jimmunol.171.6.3154. [DOI] [PubMed] [Google Scholar]
- Meyer RC, Bohl EH, Kohler EM. Procurement and maintenance of germ-free seine for microbiological investigations. Appl Microbiol. 1964;12:295–300. doi: 10.1128/am.12.4.295-300.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modlin RL. Mammalian toll-like receptors. Ann Allergy Asthma Immunol. 2002;88:543–547. doi: 10.1016/S1081-1206(10)61883-2. quiz 548–550, 583. [DOI] [PubMed] [Google Scholar]
- Olivares M, Diaz-Ropero MP, Sierra S, Lara-Villoslada F, Fonolla J, Navas M, Rodriguez JM, Xaus J. Oral intake of Lactobacillus fermentum CECT5716 enhances the effects of influenza vaccination. Nutrition (Burbank, Los Angeles County, California) 2007;23:254–260. doi: 10.1016/j.nut.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Paillot R, Laval F, Audonnet JC, Andreoni C, Juillard V. Functional and phenotypic characterization of distinct porcine dendritic cells derived from peripheral blood monocytes. Immunology. 2001;102:396–404. doi: 10.1046/j.1365-2567.2001.01200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parashar UD, Gibson CJ, Bresse JS, Glass RI. Rotavirus and severe childhood diarrhea. Emerg Infect Dis. 2006;12:304–306. doi: 10.3201/eid1202.050006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachmilewitz D, Katakura K, Karmeli F, Hayashi T, Reinus C, Rudensky B, Akira S, Takeda K, Lee J, Takabayashi K, Raz E. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology. 2004;126:520–528. doi: 10.1053/j.gastro.2003.11.019. [DOI] [PubMed] [Google Scholar]
- Saavedra JM, Bauman NA, Oung I, Perman JA, Yolken RH. Feeding of Bifidobacterium bifidum and Streptococcus thermophilus to infants in hospital for prevention of diarrhoea and shedding of rotavirus. Lancet. 1994;344:1046–1049. doi: 10.1016/s0140-6736(94)91708-6. [DOI] [PubMed] [Google Scholar]
- Saif LJ, Ward LA, Yuan L, Rosen BI, To TL. The gnotobiotic piglet as a model for studies of disease pathogenesis and immunity to human rotaviruses. Arch Virol Suppl. 1996;12:153–161. doi: 10.1007/978-3-7091-6553-9_17. [DOI] [PubMed] [Google Scholar]
- Sandor F, Buc M. Toll-like receptors. II Distribution and pathways involved in TLR signalling. Folia Biol (Praha) 2005;51:188–197. [PubMed] [Google Scholar]
- Sato A, Iizuka M, Nakagomi O, Suzuki M, Horie Y, Konno S, Hirasawa F, Sasaki K, Shindo K, Watanabe S. Rotavirus double-stranded RNA induces apoptosis and diminishes wound repair in rat intestinal epithelial cells. J Gastroenterol Hepatol. 2006;21:521–530. doi: 10.1111/j.1440-1746.2005.03977.x. [DOI] [PubMed] [Google Scholar]
- Sato K, Ishikawa T, Okumura A, Yamauchi T, Sato S, Ayada M, Matsumoto E, Hotta N, Oohashi T, Fukuzawa Y, Kakumu S. Expression of toll-like receptors in chronic hepatitis C virus infection. J Gastroenterol Hepatol. 2007;22:1627–1632. doi: 10.1111/j.1440-1746.2006.04783.x. [DOI] [PubMed] [Google Scholar]
- Seth RB, Sun L, Chen ZJ. Antiviral innate immunity pathways. Cell Res. 2006;16:141–147. doi: 10.1038/sj.cr.7310019. [DOI] [PubMed] [Google Scholar]
- Shornikova AV, Casas IA, Mykkanen H, Salo E, Vesikari T. Bacteriotherapy with Lactobacillus reuteri in rotavirus gastroenteritis. Pediatr Infect Dis J. 1997;16:1103–1107. doi: 10.1097/00006454-199712000-00002. [DOI] [PubMed] [Google Scholar]
- Summerfield A, Guzylack-Piriou L, Schaub A, Carrasco CP, Tache V, Charley B, McCullough KC. Porcine peripheral blood dendritic cells and natural interferon-producing cells. Immunology. 2003;110:440–449. doi: 10.1111/j.1365-2567.2003.01755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabeta K, Georgel P, Janssen E, Du X, Hoebe K, Crozat K, Mudd S, Shamel L, Sovath S, Goode J, Alexopoulou L, Flavell RA, Beutler B. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc Natl Acad Sci USA. 2004;101:3516–3521. doi: 10.1073/pnas.0400525101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohno M, Shimosato T, Moue M, Aso H, Watanabe K, Kawai Y, Yamaguchi T, Saito T, Kitazawa H. Toll-like receptor 2 and 9 are expressed and functional in gut-associated lymphoid tissues of presuckling newborn swine. Vet Res. 2006;37:791–812. doi: 10.1051/vetres:2006036. [DOI] [PubMed] [Google Scholar]
- Vaughan EE, de Vries MC, Zoetendal EG, Ben-Amor K, Akkermans AD, de Vos WM. The intestinal LABs. Antonie Van Leeuwenhoek. 2002;82:341–352. [PubMed] [Google Scholar]
- Ward LA, Rosen BI, Yuan L, Saif LJ. Pathogenesis of an attenuated and a virulent strain of group A human rotavirus in neonatal gnotobiotic pigs. J Gen Virol. 1996;77(Pt 7):1431–1441. doi: 10.1099/0022-1317-77-7-1431. [DOI] [PubMed] [Google Scholar]
- Watarai H, Sekine E, Inoue S, Nakagawa R, Kaisho T, Taniguchi M. PDC-TREM, a plasmacytoid dendritic cell-specific receptor, is responsible for augmented production of type I interferon. Proc Natl Acad Sci USA. 2008;105:2993–2998. doi: 10.1073/pnas.0710351105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werling D, Jungi TW. Toll-like receptors linking innate and adaptive immune response. Vet Immunol Immunopathol. 2003;91:1–12. doi: 10.1016/s0165-2427(02)00228-3. [DOI] [PubMed] [Google Scholar]
- Willing BP, Van Kessel AG. Enterocyte proliferation and apoptosis in the caudal small intestine is influenced by the composition of colonizing commensal bacteria in the neonatal gnotobiotic pig. J Anim Sci. 2007;85:3256–3266. doi: 10.2527/jas.2007-0320. [DOI] [PubMed] [Google Scholar]
- Xu J, Yang Y, Sun J, Ding Y, Su L, Shao C, Jiang B. Expression of toll-like receptors and their association with cytokine responses in peripheral blood mononuclear cells of children with acute rotavirus diarrhoea. Clin Exp Immunol. 2006;144:376–381. doi: 10.1111/j.1365-2249.2006.03079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Yang Y, Sun JE, Ding YZ, Su LY, Shao CH. Expression of Toll-like receptors in mononuclear cells from children with acute rotavirus diarrhea. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2007;21:38–40. [PubMed] [Google Scholar]
- Xu N, Yao HP, Sun Z, Chen Z. Toll-like receptor 7 and 9 expression in peripheral blood mononuclear cells from patients with chronic hepatitis B and related hepatocellular carcinoma. Acta Pharmacol Sin. 2008;29:239–244. doi: 10.1111/j.1745-7254.2008.00711.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- Yasui H, Shida K, Matsuzaki T, Yokokura T. Immunomodulatory function of lactic acid bacteria. Antonie Van Leeuwenhoek. 1999;76:383–389. [PubMed] [Google Scholar]
- Yuan L, Saif LJ. Induction of mucosal immune responses and protection against enteric viruses: rotavirus infection of gnotobiotic pigs as a model. Vet Immunol Immunopathol. 2002;87:147–160. doi: 10.1016/S0165-2427(02)00046-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Ward LA, Rosen BI, To TL, Saif LJ. Systematic and intestinal antibody-secreting cell responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J Virol. 1996;70:3075–3083. doi: 10.1128/jvi.70.5.3075-3083.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Azevedo MS, Gonzalez AM, Saif LJ, Van Nguyen T, Wen K, Yousef AE, Yuan L. Influence of probiotic Lactobacilli colonization on neonatal B cell responses in a gnotobiotic pig model of human rotavirus infection and disease. Vet Immunol Immunopathol. 2008a;122:175–181. doi: 10.1016/j.vetimm.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Azevedo MSP, Wen K, Gonzalez AM, Saif LJ, Li G, Yousef AE, Yuan L. Probiotic Lactobacillus acidophilus enhances the immunogenicity of an oral rotavirus vaccine in gnotobiotic pigs. Vaccine. 2008b;26:3655–3661. doi: 10.1016/j.vaccine.2008.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Wen K, Azevedo MS, Gonzalez A, Saif LJ, Li G, Yousef AE, Yuan L. Lactic acid bacterial colonization and human rota-virus infection influence distribution and frequencies of monocytes/macrophages and dendritic cells in neonatal gnotobiotic pigs. Vet Immunol Immunopathol. 2008c;121:222–231. doi: 10.1016/j.vetimm.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Wei H, Sun R, Tian Z. Recognition of double-stranded RNA by TLR3 induces severe small intestinal injury in mice. J Immunol. 2007;178:4548–4556. doi: 10.4049/jimmunol.178.7.4548. [DOI] [PubMed] [Google Scholar]
- Zocco MA, dal Verme LZ, Cremonini F, Piscaglia AC, Nista EC, Candelli M, Novi M, Rigante D, Cazzato IA, Ojetti V, Armuzzi A, Gasbarrini G, Gasbarrini A. Efficacy of Lactobacillus GG in maintaining remission of ulcerative colitis. Aliment Pharmacol Ther. 2006;23:1567–1574. doi: 10.1111/j.1365-2036.2006.02927.x. [DOI] [PubMed] [Google Scholar]