Abstract
Synthesis of Indazoles
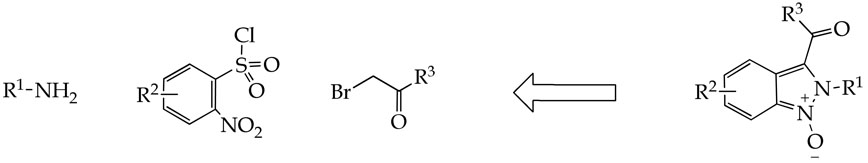
Base-catalyzed tandem carbon–carbon followed by nitrogen–nitrogen bond formations quantitatively converted N-alkyl-2-nitro-N-(2-oxo-2-aryl-ethyl)-benzenesulfonamides to 2H-indazoles 1-oxides under mild conditions. Triphenylphosphine or mesyl chloride/triethylamine-mediated deoxygenation afforded 2H-indazoles.
Introduction
Synthetic compounds comprising indazole core have recently become an increasingly frequent subject of biological studies, indazole derivatives possessed significant potency in a wide range of biological targets. A recently published excellent review article by Cerecetto and colleagues1 portrayed the diversity of biological activities exhibited by indazoles. Since then, numerous new studies identified indazole-based compounds as potent agents with anti-inflammatory, anticancer,2,3 antimicrobial,4 antifungal,5,6 anti-angiogenic,7 and cytotoxic8 activities.
Indazoles were found to be potent inhibitors of nitric oxide synthetase,9,10 factor Xa,11 protein kinases,12,13 tubulin,14 and TRPV1.15,16 It was also shown that they were active as male contraceptives17,18 and 5-HT2C receptor agonists.19
The majority of chemical routes developed for the indazole syntheses were targeted for the preparation of 1,3-derivatized-1H-indazoles (reviewed e.g. in1), and the synthesis has also been carried out on solid phase.20 Recent reports of 1,3-derivatized-1H-indazole syntheses include [3+2] cycloaddition of diazocompounds with o-(trimethylsilyl)aryl triflates,21 copper(I)-catalyzed intramolecular amination,22 and from 2-aminobenzoximes via mesyl chloride activation.23
Reported synthetic procedures for 2,3-derivatized-2H-indazoles often required preparation of dedicated intermediates and/or harsh reaction conditions. Substituted-2H-indazoles were prepared by thermal decomposition of 2-azidobenzylideneamines,24 by TiCl4-mediated cyclization of 2-arylazophenylacetic acid derived ketene acetal,25 by cyclization of 2-cyclopropylazobenzenes in concentrated sulfuric acid,26 from 2-nitrobenzylamines by electroreduction,27 by reaction of azobenzene with in situ prepared isopropylidenecarbene,28 and SnCl2-mediated cyclization of nitro arenes.29 2-Acylaminoindazoles were prepared via oxidative cyclization of N-acyl hydrazones of 2-aminoaryl ketones.30,31 Polyfused heterocycles containing indazole moiety were prepared by palladium-catalyzed cross-coupling reactions.32,33
Several other synthetic routes were also reported for preparation of 2H-Indazole 1-oxides. (2-Nitro-benzylidene)-aniline derivatives were reacted with potassium cyanide to produce 2-aryl-3-cyano-2H-indazole 1-oxides.34–40 Hungarian researches published several reports for the synthesis of indazole 1-oxides by 1,7-electrocyclization of azomethine ylides.41–44
2-Aryl-2H-Indazoles were prepared via SnCl2-mediated cyclization of 2-nitrobenzylamines.45 Indazole moiety has also been introduced as a side-chain of an amino acid.46
Our contribution describes a novel and very efficient tandem reaction that provided access to 2,3-substituted-2H-indazoles from 2-nitro-N-(2-oxo-2-aryl-ethyl)-benzenesulfonamides under mild conditions. These precursors are readily accessible using commercially available building blocks: amines, 2-nitrobenzenesulfonyl chlorides, and bromoketones.
Results
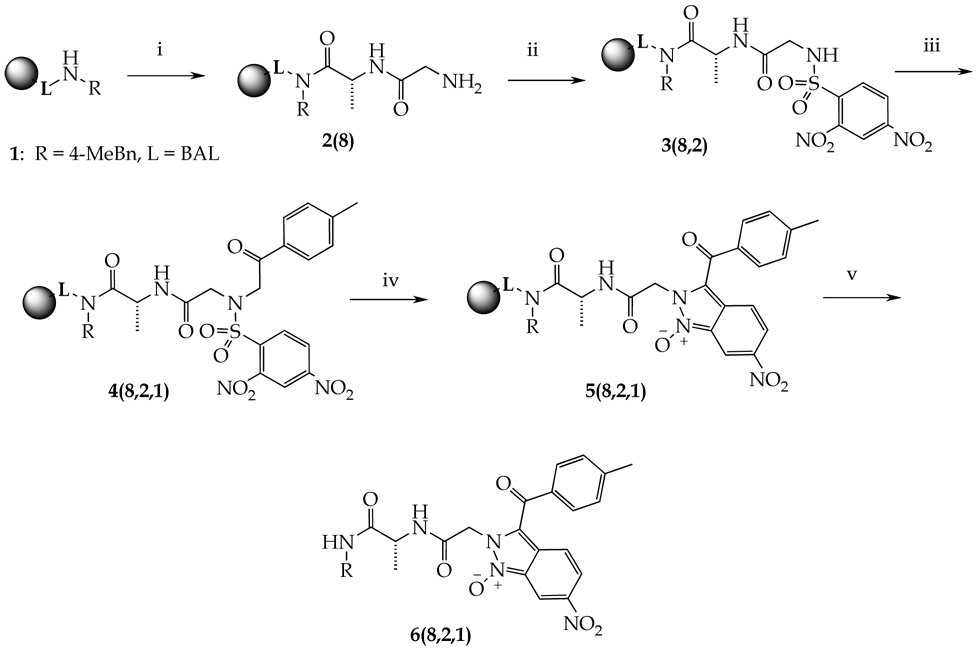
In our ongoing effort to develop new routes for solid-phase syntheses of combinatorial libraries of drug-like molecules of heterocyclic compounds, we were introducing an imidazole ring into a peptide chain. Our reaction sequence involved preparation of 2-nitro-N-(2-oxo-2-aryl-ethyl)-benzenesulfonamides 4 on the amino group of resin-bound peptides (Scheme 1). Resin-bound dipeptide Fmoc-Ala-Gly-NH-(4-Me)Bn 2(8) was synthesized on 4-(4-methylbenzyl)aminomethyl-3-methoxy-phenoxy)-butyric acid resin 1 (BAL resin). The Fmoc group was cleaved and resin-bound primary amines were reacted with 2,4-dinitrobenzenesulfonyl chloride to access sulfonamide 3. Alkylation with bromoketones afforded polymer-supported N-(2-oxo-2-aryl-ethyl)-2,4-dinitrobenzenesulfonamides 4(8,2,1) (for R-groups numbering refer to Table 1). Short exposure (30 min) to a base (DBU) in DMF led to transformation to 2H-indazoles 1-oxides 6(8,2,1).
Scheme 1.
Formation of indazole oxides by tandem reaction
Reagents and conditions: (i) traditional Fmoc peptide synthesis; (ii) 2,4-dinitrobenzenesulfonyl chloride, lutidine, DCM, 16h; (iii) 2-bromo-4′-methyl-acetophenone, DIEA, DMF, 16h; (iv) DBU, DMF, 30 min; (v) 50% TFA in DCM, 30 min.
Table 1.
Synthesized indazole oxides 6 and indazoles 8
| Compound | –R1–H | R2 | R3 | MS ESI+ | Puritya (%) | Isolation | Yieldb (%) |
|---|---|---|---|---|---|---|---|
| 6(1,1,1) | –(CH2)2–CONH2 | H | 4-Me-Ph | 324 | 99 | B | 82 |
| 6(1,1,2) | –(CH2)2–CONH2 | H | 4-Cl-Ph | 344 | 98 | B | 63 |
| 6(1,1,3) | –(CH2)2–CONH2 | H | 4-NH2-3,5-diCl-Ph | 393 | 99 | B | 52 |
| 6(1,1,4) | –(CH2)2–CONH2 | H | 3-NO2-Ph | 355 | 77 | C | 50 |
| 6(1,1,5) | –(CH2)2–CONH2 | H | 4-OMe-Ph | 340 | 94 | A | 58 |
| 6(1,2,1) | –(CH2)2–CONH2 | 4-NO2 | 4-Me-Ph | 369 | 98 | B | 97 |
| 6(1,3,1) | –(CH2)2–CONH2 | 4-CF3 | 4-Me-Ph | 392 | 97 | B | 95 |
| 6(1,4,1) | –(CH2)2–CONH2 | 4-OMe | 4-Me-Ph | 354 | 89 | C | 59 |
| 6(2,1,1) | –CH(CH3)–CONH2 | H | 4-Me-Ph | 324 | 89 | C | 54 |
| 6(2,1,5) | –CH(CH3)–CONH2 | H | -OEt | 278 | 88 | C | 74 |
| 6(3,1,1) | –(CH2)2–CONH(4-Me-Bn) | H | 4-Me-Ph | 428 | 91 | C | 65 |
| 6(4,1,1) | –(CH2)2–COOH | H | 4-Me-Ph | 325 | 93 | C | 37 |
| 6(5,1,1) | –(CH2)2–OH | H | 4-Me-Ph | 297 | 93 | C | 80 |
| 6(5,1,5) | –(CH2)2–OH | H | -OEt | 251 | 72 | C | 23 |
| 6(6,2,1) | –(CH2)3–CONH2 | 4-NO2 | 4-Me-Ph | 383 | 95 | C | 39 |
| 6(6,2,2) | –(CH2)3–CONH2 | 4-NO2 | 4-Cl-Ph | 403 | 90 | C | 17 |
| 6(6,2,3) | –(CH2)3–CONH2 | 4-NO2 | 4-NH2-3,5-diCl-Ph | 452 | 91 | C | 23 |
| 6(6,2,5) | –(CH2)3–CONH2 | 4-NO2 | 4-OMe-Ph | 399 | 95 | C | 29 |
| 6(6,3,1) | –(CH2)3–CONH2 | 4-CF3 | 4-Me-Ph | 406 | 99 | A | 30 |
| 6(6,3,2) | –(CH2)3–CONH2 | 4-CF3 | 4-Cl-Ph | 426 | 84 | C | 28 |
| 6(6,3,3) | –(CH2)3–CONH2 | 4-CF3 | 4-NH2-3,5-diCl-Ph | 475 | 98 | B | 64 |
| 6(6,3,5) | –(CH2)3–CONH2 | 4-CF3 | 4-OMe-Ph | 422 | 99 | A | 69 |
| 6(6,4,1) | –(CH2)3–CONH2 | 4-OMe | 4-Me-Ph | 368 | 81 | C | 21 |
| 6(7,1,3) | –(CH2)3–NH2 | H | 4-NH2-3,5-diCl-Ph | 379 | 98 | A | 81 |
| 6(7,2,1) | –(CH2)3–NH2 | 4-NO2 | 4-Me-Ph | 355 | 92 | A | 93 |
| 6(7,2,3) | –(CH2)3–NH2 | 4-NO2 | 4-NH2-3,5-diCl-Ph | 424 | 97 | A | 99 |
| 6(7,2,5) | –(CH2)3–NH2 | 4-NO2 | 4-OMe-Ph | 371 | 90 | A | 92 |
| 6(7,3,1) | –(CH2)3–NH2 | 4-CF3 | 4-Me-Ph | 378 | 99 | A | 85 |
| 6(7,3,2) | –(CH2)3–NH2 | 4-CF3 | 4-Cl-Ph | 398 | 94 | A | 90 |
| 6(7,3,3) | –(CH2)3–NH2 | 4-CF3 | 4-NH2-3,5-diCl-Ph | 447 | 99 | A | 84 |
| 6(7,3,5) | –(CH2)3–NH2 | 4-CF3 | 4-OMe-Ph | 394) | 97 | B | 99 |
| 8(1,3,1) | –(CH2)2–CONH2 | 4-CF3 | 4-Me-Ph | 376 | 87 | C | 48 |
| 8(1,5,1) | –(CH2)2–CONH2 | 4-NH2 | 4-Me-Ph | 323 | 72 | C | 23 |
Purity of crude product before purification
yield after purification.
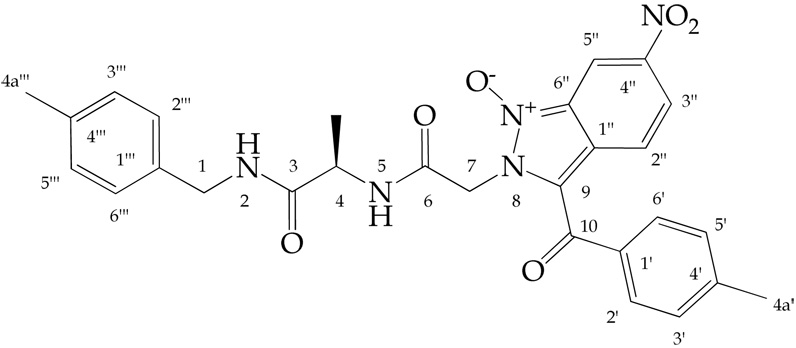
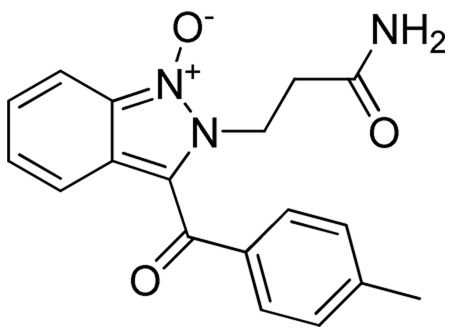
The structure of 6(8,2,1) was determined by analysis of 1D and 2D 1H and 13C NMR spectra. Proton connectivities were derived by examination of the DQF-COSY spectrum. Signals of all carbons with directly attached protons were assigned using the gHSQC spectrum. Finally, the gHMBC spectrum was used to assign quaternary carbons and to check the correctness of the connectivities established by the interpretation of the other spectra.
Two amide protons H-2 and H-5 (Figure 1) resonated at δ 8.39 and 8.84, respectively. Their corresponding signals showed as triplet and doublet. The amide H-5 signal exhibited a cross-peak with a signal (doublet of quartet) at δ 4.31 in the DQF-COSY spectrum. This signal simultaneously showed a cross-peak to the doublet at δ 1.27. These two resonances were, therefore, assigned to the methine CH-4 and methyl CH3-4a protons, respectively. The amide H-2 signal displayed a cross-peak to two protons doublet at δ 4.24 belonging to the methylene CH2-1 protons. The methylene CH2-7 protons resonated as two one proton doublets at δ 5.53 and 5.60, respectively. The doublets (2J=16.4 Hz) exhibited only a mutual cross-peak in the DQF-COSY spectrum. In the gHMBC spectrum, the resonance of the methylene CH2-1 protons showed a cross-peak with the carbon signal at δ 126.96 corresponding to the C-2”’ and C-6”’ carbons of the methyl benzyl moiety. Signals of the remaining carbons and protons of this moiety were then assigned by analyzing the gHSQC, DQF-COSY, and gHMBC spectra. The methylene CH2-7 proton signals displayed cross-peaks in the gHMBC spectrum only with the carbon signals at δ 163.64 and 118.23 corresponding to the carbonyl C-6 and olefinic C-9 carbons, respectively. Simultaneously, the C-9 carbon signal showed a cross-peak to the H-2” proton signal of the nitro aryl moiety and the carbonyl C-6 signal exhibited a cross-peak to the amide H-5 proton signal. Resonances of the other protons and carbons of this moiety as well as the 4-methyl phenyl moiety were finally allocated by examining the DQF-COSY, gHSQC, and gHMBC spectra. These facts unambiguously prove the structure of the 6(8,2,1).
Figure 1.
Atom numbering of compound 6(8,2,1)
Very efficient formation of indazole oxides from easily accessible precursors prompted us to evaluate the scope and limitations of this transformation. Three reaction components used in the synthesis included primary amines, 2-nitrobenzenesulfonyl chlorides, and bromoketones. For solid-phase synthesis, we have chosen to immobilize amines. To access target compounds with diversity of functional groups at R1 position of target indazoles, we prepared five types of resin-bound amines (Scheme 2). (i) Immobilization using carboxyl group of amino acids provided polymer-supported amines 2a by acylation of Rink amide resin.47 (ii) Secondary amides were prepared from backbone amide linker (BAL)48 via reductive amination with primary amines (resin 1b), followed by acylation with Fmoc-amino acids (2b). (iii) Free acids were accessed from resin 2c, obtained by esterification49 of Wang resin50 with Fmoc-amino acids. (iv) Diamines (unprotected) were attached to CDI-treated Wang resin51,52 to form resin 2d. (v) Fmoc-amino alcohols were linked to Wang resin via trichloroacetimidate intermediate53 and yielded resin 2e.
Scheme 2.
Preparation of five types of resin-bound amines
Reagents and conditions: (i) Fmoc-amino acid, DIC, HOBt, DMF/DCM, 16h; (ii) 50% piperidine, DMF, 15 min; (iii) amine, 10% AcOH, DMF, 16h, then NaBH(OAc)3, 5h; (iv) Fmoc-amino acid, PPh3, DIAD, 3h; (v) CDI, pyridine, DCM, 2h, then diamine, DCM, 2h; (vi) trichloroacetonitrile, DBU, DCM, 2h, then Fmoc-amino alcohol, BF3•Et2O, 1h.
Immobilization of amine via bifunctional building blocks does not provide a traceless solid-phase synthesis of indazoles. Five types of resin-bound amines allowed synthesis of target compounds with an amide, secondary amide, acid, amine, and alcohol in the R1 side-chain of the target indazoles.
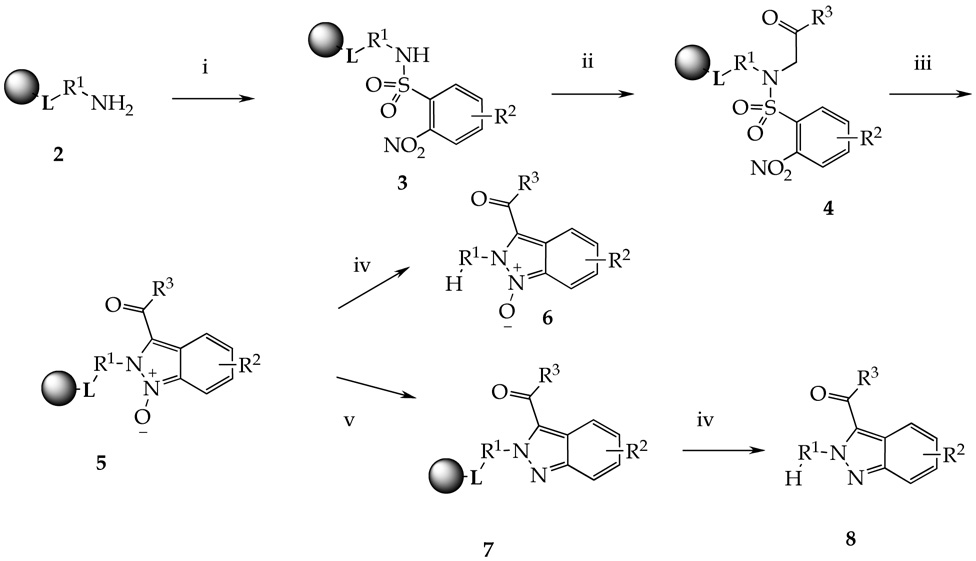
Synthesis of indazoles from five types of polymer-supported amines is portrayed in Scheme 3. Resin-bound primary amines 2 (L represents any linker described in Scheme 2), were reacted with 2-nitrobenzenesulfonyl chlorides to access sulfonamides 3. Alkylation with bromoketones cleanly afforded polymer-supported N-alkyl-2-nitro-N-(2-oxo-2-aryl-ethyl)-benzenesulfonamides 4. Short exposure to a base (DBU) mediated formation of indazole 1-oxides 5 by tandem of carbon–carbon followed by nitrogen–nitrogen bond-forming reactions. Cleavage of indazole oxides from the resin was accomplished by TFA solution in DCM to yield products 6. The TFA solution was evaporated and isolation and purification of indazole oxides 6 was carried out by three different methods depending on the purity of crude preparations: precipitation using diethyl ether (method A), passing through a C18 cartridge (method B) or HPLC purification (method C).
Scheme 3.
Indazoles synthesis
Reagents and conditions: (i) 2-nitrobenzenesulfonyl chloride, lutidine, DCM, 16h; (ii) bromoketone or bromoacetate, DIEA, DMF, 16h; (iii) DBU, DMF, 30 min; (iv) 50% TFA in DCM, 30 min; (v) mesyl chloride, TEA, DCM, 16h.
We synthesized a set of indazole oxides using four 2-nitrobenzenesulfonyl chlorides and four bromoketones with various electron-withdrawing as well as electron-donating substituents (Table 1). Five different linkers were used, which provided access to amides, N-alkyl amides, acids, amines, and alcohols, respectively.
The synthetic route is amenable to combinatorial synthesis. We prepared a combinatorial array of indazole oxides using two resin-bound amines prepared from β-Ala and 1,3-diaminopropane, two 2-nitrobenzenesulfonyl chlorides, and four bromoketones by using the split–split method.
To access the parent heterocycle, we evaluated several deoxygenation methods and reduced the resin-bound indazole oxides 5 to indazoles 7. Attempts to deoxygenate the resin-bound indazole oxides using InCl354,55 was not successful, overnight reaction at elevated temperature (100 °C) yielded indazoles only in single-digit percentage points. Reduction with SmI2 yielded a mixture of products. Deoxygenation using PPh340 provided clean indazoles, but required lengthy (2 days) exposure at elevated temperature (100 °C). Mesyl chloride in the presence of triethylamine56 at ambient temperature turned to be the reagent of choice for mild (ambient temperature) deoxygenation of indazole oxides to indazoles. We have not tested the “explosive” hexamethyldisilane method.57
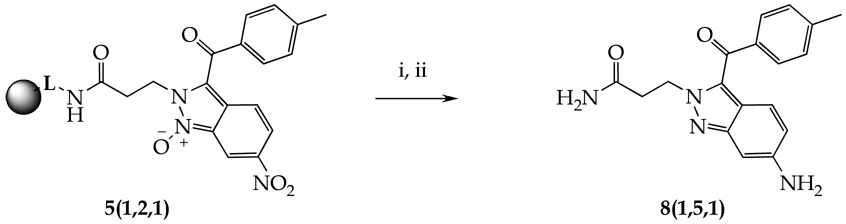
Interestingly, treatment of the resin-bound indazole oxide 5(1,2,1) with tin(II) chloride afforded not only reduction of the nitro group but deoxygenation of the product 8(1,5,1) as well (Scheme 4). Reduction of nitro group in compound 5(1,1,4) yielded mixture of products, nitro group reduced, but not deoxygenated, compound being the major component.
Scheme 4.
Tin(II) chloride reduction of compound 5(1,2,1)
Reagents and conditions: (i) SnCl2•2H2O, DIEA, DMF, 2h; (ii) 50% TFA, DCM, 1h.
Discussion
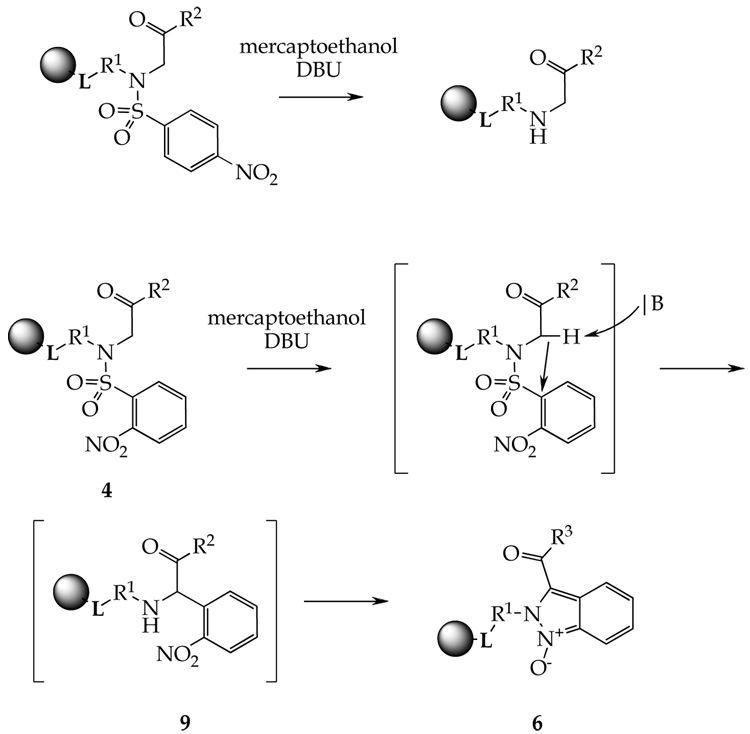
Tandem reaction of 2-nitrobenzenesulfonylated α–aminoketones 4 leading to indazole oxides 6 involved two consecutive transformations, C–C bond formation followed by N–N bond formation. Unlike 4-nitrobenzenesulfonyl derivatives, treatment of the 2-nitroderivatives 4 with mercaptoethanol and DBU does not cleave the 2-Nos group by the thiol (Scheme 5). Instead, the 2-Nos group is cleaved by an internal C-nucleophile generated by DBU from the α-ketosulfonamide. This transformation can be regarded as a variation of S–C Smiles rearrangement involving an internal C-nucleophile. The precedent for analogous carbon–carbon bond formation can be found in base-catalyzed formation of methyl-[9-(4-nitro-phenyl)-9H-fluoren-9-yl]-amine from N-(9H-fluoren-9-yl)-N-methyl-4-nitro-benzenesulfonamide.58
Scheme 5.
Different reactivities of 2- and 4-nitrobenzenesulfonyl derivatives.
The 2-nitrobenzylamine intermediate 9 underwent spontaneous cyclative dehydratation to form indazole oxides 6. This second step in the tandem reaction, formation of nitrogen–nitrogen bond, has been known for years and it was employed e.g., in the synthesis of 3,4-fused cinnolines59,60 and indazole oxides.39,40,42,44
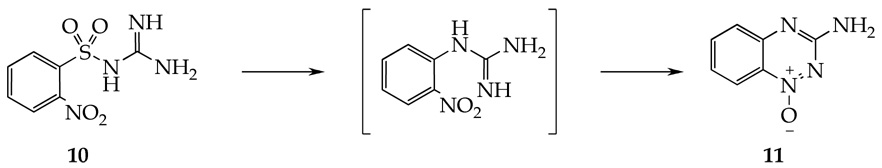
A distant analogy to the tandem synthesis of indazole oxides can be also found in the synthesis of 1-oxy-benzo[1,2,4]triazin-3-ylamine 11 (Scheme 6), reported more than 60 years ago.61 2-Nitrobenzenesulfonyl guanidine 10 was converted to 3-aminobenzo[e][1,2,4]triazine 1-oxide via intramolecular cleavage of the Nos group by an internal N-nucleophile followed by cyclative dehydratation involving N–N bond formation.
Scheme 6.
Synthesis of 1-oxy-benzo[1,2,4]triazin-3-ylamine and 3-phenyindazole
Conclusion
Tandem reaction involving C–C and N–N bond formations quantitatively converted polymersupported N-alkyl-2-nitro-N-(2-oxo-2-aryl-ethyl)-benzenesulfonamides to 2H-indazoles 1-oxides under mild conditions without any noticeable side-reaction. The transformation tolerated a range of substituents and was insensitive to electronic properties of R groups. Synthesis did not require preparation of dedicated building blocks and it was carried out using commercially available compounds. Triphenylphosphine or mesyl chloride/TEA-mediated deoxygenation yielded 2H-indazoles.
Experimental Section
Reaction with 2-Nos-Cl (resins 3)
Resins 2, ~1 g, were washed with DCM, a solution of 2-Nos-Cl (3 mmol) and lutidine (3.3 mmol, 382 µL) in 10 mL DCM was added to the resin and slurry was shaken overnight at ambient temperature. The resin was washed 3 × with DMF and 3 × DCM.
Reaction with bromoketones (resins 4)
Resins 3, ~250 mg, were washed 3 × with DCM and 5 × DMF. A solution of 0.5 M bromoketone (1.5 mmol) and 1 M DIEA (3 mmol, 522 µL) in 3 mL DMF was added. The slurry was shaken overnight at ambient temperature. The resin was washed 3 × with DMF and 5 × DCM.
Cyclization to indazole oxides (resins 5)
Resins 4, ~250 mg, were washed 5 × with DMF. A solution of 0.2 M DBU (1 mmol, 150 µL) in 5 mL DMF was added and resin slurry was shaken at ambient temperature for 30 min. Resins were washed 3 × with DMF, 5 × DCM and 3 × MeOH, and dried.
Cleavage and isolation (6)
Indazole oxides 6 were obtained after cleavage with 50% TFA in DCM for 1h. TFA solution was collected, resin was washed 3 × with 50 % TFA, combined extracts were evaporated by a stream of nitrogen and oily product was triturated with ether. Three isolation and purification methods were used depending on the purity of crude product. Compounds that precipitated after being triturated with ether were collected and precipitate dried (method A). Compounds that did not precipitate after ether trituration were dissolved in minimum quantity of methanol (~ 0.5 mL), diluted with 20 mL 10 mM aqueous ammonium acetate buffer and passed through C18 cartridge. The cartridge was washed by ammonium acetate buffer and compounds eluted by 66% MeCN in ammonium acetate buffer. The solution was evaporated and residue lyophilized overnight (method B). Alternatively, compounds were purified by semi-preparative reverse phase HPLC (method C). Samples were analyzed by LCMS.
Deoxygenation (resins 7)
Resins 5, ~250 mg, were washed 5 × with DCM. A solution of 2.5 mL of 0.7 M TEA (245 µL) in DCM was added. The syringe was connected with the second syringe containing 2.5 mL 0.5 M mesyl chloride (96 µL). Connected syringes were left in a freezer for 30 min and the mesyl chloride solution was drawn into the syringe with the resin. The resin slurry was shaken at ambient temperature for 16h. Resins were washed 5 × DCM and 3 × MeOH, and dried. Cleavage from the resin and purification was carried out as described for compounds 6.
Deoxygenation and reduction of the nitro group (resin 7(1,5,1))
Resin 5(1,2,1), 250 mg, was washed 3 × DMF and 2 mL of a solution of 1 M tin chloride dihydrate and 1 M DIEA, prepared under nitrogen bubbling, was added to the resin and the resin slurry was shaken overnight. The resin was washed very thoroughly 7 × with DMF, and 3 × DCM. Cleavage from the resin and purification was carried out as described for compounds 6.
Analytical data of individual compounds
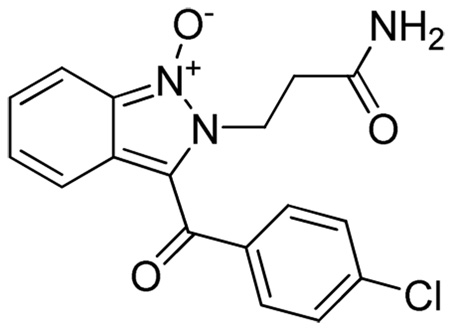
2-(3-amino-3-oxopropyl)-3-(4-methylbenzoyl)-2H-indazole 1-oxide 6(1,1,1)
Yield 32 mg (82%). ESI-MS m/z = 324, [M+H]+. 1H NMR (300 MHz, DMSO-d6) δ: 7.76 (d, J=8.56 Hz, 1 H) 7.68 (d, J=8.01 Hz, 2 H) 7.44 (br. s., 1 H) 7.40 (d, J=8.01 Hz, 2 H) 7.31 - 7.38 (m, 1 H) 7.19 - 7.29 (m, 1 H) 6.93 (br. s., 1 H) 6.85 (d, J=8.84 Hz, 1 H) 4.94 (t, J=7.18 Hz, 2 H) 2.73 (t, J=7.32 Hz, 2 H) 2.45 (s, 3 H). 13C NMR (75 MHz, DMSO-d6) δ: 181.6, 170.9, 143.1, 136.5, 129.3, 129.2, 128.5, 127.5, 126.3, 120.1, 119.6, 117.4, 113.3, 42.1, 32.6, 21.3. HRMS (FAB) m/z calcd for C18H18N3O3 [M + H]+ 324.1348, found 324.1353
2-(3-amino-3-oxopropyl)-3-(4-chlorobenzoyl)-2H-indazole 1-oxide 6(1,1,2)
Yield 26 mg (63%). ESI-MS m/z = 344, [M+H]+. 1H NMR (300 MHz, DMSO-d6) δ: 7.73 - 7.83 (m, 3 H) 7.68 (d, J=8.56 Hz, 2 H) 7.45 (br. s., 1 H) 7.33 - 7.42 (m, 1 H) 7.19 - 7.33 (m, 1 H) 6.94 (br. s., 1 H) 6.84 (d, J=8.56 Hz, 1 H) 4.95 (t, J=7.18 Hz, 2 H) 2.73 (t, J=7.32 Hz, 2 H). 13C NMR (75 MHz, DMSO-d6) δ: 180.5, 170.9, 137.9, 137.5, 131.0, 128.9, 128.6, 128.0, 126.4, 120.0, 119.9, 117.0, 113.4, 42.1, 32.4. HRMS (FAB) m/z calcd for C17H15N3O3Cl [M + H]+ 344.0802, found 344.0783
Supplementary Material
Supporting information contains details of experimental procedures, spectroscopic data for synthesized compounds, and copies of NMR spectra. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgment
This research was supported by the Department of Chemistry and Biochemistry University of Notre Dame and the NIH (GM079576). We gratefully appreciate the use of the NMR facility at the University of Notre Dame.
References
- 1.Cerecetto H, Gerpe A, Gonzalez M, Aran VJ, de Ocariz CO. Mini-Rev. Med. Chem. 2005;5:869–878. doi: 10.2174/138955705774329564. [DOI] [PubMed] [Google Scholar]
- 2.Chen CJ, Hsu MH, Huang LJ, Yamori T, Chung FG, Lee FY, Teng CM, Kuo SC. Biochem. Pharm. 2008;75:360–368. doi: 10.1016/j.bcp.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Yakaiah T, Lingaiah BPV, Narsaiah B, Shireesha B, Kumar BA, Gururaj S, Parthasarathy T, Sridhar B. Bioorg. Med. Chem. Lett. 2007;17:3445–3453. doi: 10.1016/j.bmcl.2007.03.087. [DOI] [PubMed] [Google Scholar]
- 4.Yakaiah T, Lingaiah BPV, Narsaiah B, Kumar KP, Murthy USN. Eur. J. Med. Chem. 2008;43:341–347. doi: 10.1016/j.ejmech.2007.03.031. [DOI] [PubMed] [Google Scholar]
- 5.Park JS, Yu KA, Yoon YS, Han MR, Kang TH, Kim S, Kim NJ, Yun H, Suh YG. Drugs of the Future. 2007;32:121. [Google Scholar]
- 6.Park JS, Yu KA, Kang TH, Kim SH, Suh YG. Bioorg. Med. Chem. Lett. 2007;17:3486–3490. doi: 10.1016/j.bmcl.2007.03.074. [DOI] [PubMed] [Google Scholar]
- 7.Huang LJ, Shih ML, Chen HS, Pan SL, Teng CM, Lee FY, Kuo SC. Bioorg. Med. Chem. 2006;14:528–536. doi: 10.1016/j.bmc.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 8.Rakib E, Oulemda B, Abouricha S, Bouissane L, Mouse HA, Zyad A. Lett. Drug Des. Discovery. 2007;4:467–470. [Google Scholar]
- 9.Matsumura N, Kikuchi-Utsumi K, Nakaki T. J. Pharmacol. Exp. Ther. 2008;325:357–362. doi: 10.1124/jpet.107.135160. [DOI] [PubMed] [Google Scholar]
- 10.Boulouard M, Schumann-Bard P, Butt-Gueulle S, Lohou E, Stiebing S, Collot V, Rault S. Bioorg. Med. Chem. Lett. 2007;17:3177–3180. doi: 10.1016/j.bmcl.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 11.Lee YK, Parks DJ, Lu T, Thieu TV, Markotan T, Pan W, Mccomsey DF, Milkiewicz KL, Crysler CS, Ninan N, Abad MC, Giardino EC, Maryanoff BE, Damiano BP, Player MR. J. Med. Chem. 2008;51:282–297. doi: 10.1021/jm701217r. [DOI] [PubMed] [Google Scholar]
- 12.Zhu GD, Gandhi VB, Gong JC, Thomas S, Woods KW, Song XH, Li TM, Diebold RB, Luo Y, Liu XS, Guan R, Klinghofer V, Johnson EF, Bouska J, Olson A, Marsh KC, Stoll VS, Mamo M, Polakowski J, Campbell TJ, Martin RL, Gintant GA, Penning TD, Li Q, Rosenberg SH, Giranda VL. J. Med. Chem. 2007;50:2990–3003. doi: 10.1021/jm0701019. [DOI] [PubMed] [Google Scholar]
- 13.Lee J, Choi H, Kim KH, Jeong S, Park JW, Baek CS, Lee SH. Bioorg. Med. Chem. Lett. 2008;18:2292–2295. doi: 10.1016/j.bmcl.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Meng FY, Cai XH, Duan JX, Matteucci MG, Hart CP. Cancer Chemother. Pharmacol. 2008;61:953–963. doi: 10.1007/s00280-007-0549-x. [DOI] [PubMed] [Google Scholar]
- 15.Gomtsyan A, Bayburt EK, Schmidt RG, Surowy CS, Honore P, Marsh KC, Hannick SM, McDonald HA, Wetter JM, Sullivan JP, Jarvis MF, Faltynek CR, Lee CH. J. Med. Chem. 2008;51:392–395. doi: 10.1021/jm701007g. [DOI] [PubMed] [Google Scholar]
- 16.Perner RJ, DiDomenico S, Koenig JR, Gomtsyan A, Bayburt EK, Schmidt RG, Drizin I, Zheng GZ, Turner SC, Jinkerson T, Brown BS, Keddy RG, Lukin K, McDonald HA, Honore P, Mikusa J, Marsh KC, Wetter JM, George KS, Jarvis MF, Faltynek CR, Lee CH. J. Med. Chem. 2007;50:3651–3660. doi: 10.1021/jm070276i. [DOI] [PubMed] [Google Scholar]
- 17.Tash JS, Attardi B, Hild SA, Chakrasali R, Jakkaraj SR, Georg GI. Biol. Reprod. 2008;78:1127–1138. doi: 10.1095/biolreprod.106.057810. [DOI] [PubMed] [Google Scholar]
- 18.Tash JS, Chakrasali R, Jakkaraj SR, Hughes J, Smith SK, Hornbaker K, Heckert LL, Ozturk SB, Hadden MK, Kinzy TG, Blagg BSJ, Georg GI. Biol. Reprod. 2008;78:1139–1152. doi: 10.1095/biolreprod.107.062679. [DOI] [PubMed] [Google Scholar]
- 19.Shimada I, Maeno K, Kazuta KI, Kubota H, Kimizuka T, Kimura Y, Hatanaka KI, Naitou Y, Wanibuchi F, Sakamoto S, Tsukamoto SI. Bioorg. Med. Chem. 2008;16:1966–1982. doi: 10.1016/j.bmc.2007.10.100. [DOI] [PubMed] [Google Scholar]
- 20.Yan B, Gstach H. Tetrahedron Lett. 1996;37:8325–8328. [Google Scholar]
- 21.Liu ZJ, Shi F, Martinez PDG, Raminelli C, Larock RC. J. Org. Chem. 2008;73:219–226. doi: 10.1021/jo702062n. [DOI] [PubMed] [Google Scholar]
- 22.Liu R, Zhu YM, Qin LN, Ji SJ. Synth. Commun. 2008;38:249–254. [Google Scholar]
- 23.Counceller CM, Eichman CC, Wray BC, Stambuli JP. Org. Lett. 2008;10:1021–1023. doi: 10.1021/ol800053f. [DOI] [PubMed] [Google Scholar]
- 24.Krbechek L, Takimoto H. J. Org. Chem. 1964;29:1150–1152. [Google Scholar]
- 25.Hutchings MG, Devonald DP. Tetrahedron Lett. 1989;30:3715–3718. [Google Scholar]
- 26.Fedotov AN, Shishkina IN, Kutateladze TG, Mochalov SS, Shabarov YuS. Khimiya Geterotsiklicheskikh Soedinenii. 1987:1063–1068. [Google Scholar]
- 27.Frontana-Uribe BA, Moinet C. Tetrahedron. 1998;54:3197–3206. [Google Scholar]
- 28.Krageloh K, Anderson GH, Stang PJ. J. Am. Chem. Soc. 1984;106:6015–6021. [Google Scholar]
- 29.Sawant D, Kumar R, Maulik PR, Kundu B. Org. Lett. 2006;8:1525–1528. doi: 10.1021/ol053033y. [DOI] [PubMed] [Google Scholar]
- 30.Kotali A, Harris PA. Heterocycles. 1994;37:1541–1548. [Google Scholar]
- 31.Kotali A, Harris PA. J. Heterocyclic Chem. 1996;33:605–606. [Google Scholar]
- 32.Timari G, Hajos G, Riedl Z, Beres M, Soos T, Messmer A, Gronowitz S. Molecules. 1996;1:236–241. [Google Scholar]
- 33.Hajos G, Riedl Z, Timari G, Matyus P, Maes BUW, Lemiere GLF. Molecules. 2003;8:480–487. [Google Scholar]
- 34.Johnston D, Smith DM. Tetrahedron Lett. 1975:1121–1124. [Google Scholar]
- 35.Heller G, Spielmeyer G. Chem. Ber. 1925;58:834–838. [Google Scholar]
- 36.Reibert A, Lemmer F. Chem. Ber. 1926;59:351–359. [Google Scholar]
- 37.Behr LC. J. Am. Chem. Soc. 1954;76:3672–3675. [Google Scholar]
- 38.Behr LC, Alley EG, Levand O. J. Org. Chem. 1962;27:65–67. [Google Scholar]
- 39.Johnston D, Smith DM, Shepherd T, Thompson D. J. Chem. Soc., Perkin Trans. I. 1987:495–500. [Google Scholar]
- 40.Gerpe A, Aguirre G, Boiani L, Cerecetto H, Gonzalez M, Olea-Azar C, Rigol C, Maya JD, Morello A, Piro OE, Aran VJ, Azqueta A, de Cerain AL, Monge A, Rojas MA, Yaluff G. Bioorg. Med. Chem. 2006;14:3467–3480. doi: 10.1016/j.bmc.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 41.Nyerges M, Fejes I, Viranyi A, Groundwater PW, Toke L. Tetrahedron Lett. 2001;42:5081–5083. [Google Scholar]
- 42.Nyerges M, Viranyi A, Zhang WM, Groundwater PW, Blasko G, Toke L. Tetrahedron. 2004;60:9937–9944. [Google Scholar]
- 43.Nyerges M, Somfai B, Toth J, Toke L, Dancso A, Blasko G. Synthesis-Stuttgart. 2005:2039–2045. [Google Scholar]
- 44.Nyerges M, Viranyi A, Toth J, Blasko G, Toke L. Synthesis-Stuttgart. 2006:1273–1278. [Google Scholar]
- 45.Shi DQ, Dou GL, Ni SN, Shi JW, Li XY, Wang XS, Wu H, Ji SJ. Synlett. 2007:2509–2512. [Google Scholar]
- 46.Abdel-Rahman AAH. Monatsh. Chem. 2008;139:289–297. [Google Scholar]
- 47.Rink H. Tetrahedron Lett. 1987;28:3787–3790. [Google Scholar]
- 48.Jensen KJ, Alsina J, Songster MF, Vagner J, Albericio F, Barany G. J. Am. Chem. Soc. 1998;120:5441–5452. [Google Scholar]
- 49.Krchnak V, Cabel D, Weichsel AS, Flegelova Z. Lett. Peptide Sci. 1995;2:277–282. [Google Scholar]
- 50.Wang S-S. J. Am. Chem. Soc. 1973;95:1328–1333. doi: 10.1021/ja00785a602. [DOI] [PubMed] [Google Scholar]
- 51.Hauske JR, Dorff P. Tetrahedron Lett. 1995;36:1589–1592. [Google Scholar]
- 52.Munson MC, Cook AW, Josey JA, Rao C. Tetrahedron Lett. 1998;39:7223–7226. [Google Scholar]
- 53.Yan LZ, Mayer JP. J. Org. Chem. 2003;68:1161–1162. doi: 10.1021/jo026405e. [DOI] [PubMed] [Google Scholar]
- 54.Jeevanandam A, Cartwright C, Ling YC. Synth. Commun. 2000;30:3153–3160. [Google Scholar]
- 55.Ilias M, Barman DC, Prajapati D, Sandhu JS. Tetrahedron Lett. 2002;43:1877–1879. [Google Scholar]
- 56.Morimoto Y, Kurihara H, Yokoe C, Kinoshita T. Chem. Lett. 1998:829–830. [Google Scholar]
- 57.Vorbruggen H, Krolikiewicz K. Tetrahedron Lett. 1983;24:5337–5338. [Google Scholar]
- 58.Meng Q, Thibblin A. J. Am. Chem. Soc. 1997;119:1224–1229. [Google Scholar]
- 59.Muth CW, Abraham N, Linfield ML, Wotring RB, Pacofsky EA. J. Org. Chem. 1960;25:736–740. [Google Scholar]
- 60.Slevin A, Koolmeister T, Scobie M. Chem. Commun. 2007:2506–2508. doi: 10.1039/b618318b. [DOI] [PubMed] [Google Scholar]
- 61.Backer HJ, Moed H. Recl. Trav. Chim. 2008;66:689–704. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information contains details of experimental procedures, spectroscopic data for synthesized compounds, and copies of NMR spectra. This material is available free of charge via the Internet at http://pubs.acs.org.