Abstract
Because ascorbic acid (AA) is concentrated in synaptic vesicles containing glutamic acid, we hypothesized that AA might act as a neurotransmitter. Because AA is an antioxidant, it might therefore inhibit nitric oxidergic (NOergic) activation of luteinizing hormone-releasing hormone (LH-RH) release from medial basal hypothalamic explants by chemically reducing NO. Cell membrane depolarization induced by increased potassium concentration [K+] increased medium concentrations of both AA and LH-RH. An inhibitor of NO synthase (NOS), NG-monomethyl-l-arginine (NMMA), prevented the increase in medium concentrations of AA and LH-RH induced by high [K+], suggesting that NO mediates release of both AA and LH-RH. Calcium-free medium blocked not only the increase in AA in the medium but also the release of LH-RH. Sodium nitroprusside, which releases NO, stimulated LH-RH release and decreased the concentration of AA in the incubation medium, presumably because the NO released oxidized AA to dehydro-AA. AA (10−5 to 10−3 M) had no effect on basal LH-RH release but completely blocked high [K+]- and nitroprusside-induced LH-RH release. N-Methyl-d-aspartic acid (NMDA), which mimics the action of the excitatory amino acid neurotransmitter glutamic acid, releases LH-RH by releasing NO. AA (10−5 to 10−3 M) inhibited the LH-RH-releasing action of NMDA. AA may be an inhibitory neurotransmitter that blocks NOergic stimulation of LH-RH release by chemically reducing the NO released by the NOergic neurons.
Keywords: increased [K+] media, decreased [Ca2+] media, N-methyl-d-aspartate, nitroprusside, NG-monomethyl-l-arginine
Ascorbic acid (AA) is present in high concentrations in the hypothalamus (1–3) but its role in the modulation of hypothalamic hormone release is obscure. The brain accumulates AA from the blood and maintains a relatively high concentration (1.1–1.7 mM), independent of AA ingestion (4–7). Neuronal intracellular AA concentrations are 10 times greater than extracellular AA concentration (6, 7). Neuronal activity was shown to increase extracellular AA in vitro (6, 8–12) and in vivo as determined by voltametry (13). AA is further concentrated in isolated nerve terminals (14). Synaptic vesicles concentrate AA by active transport (15). AA is present in high concentrations in synaptic vesicles of glutamergic neurons (13) and in adrenal medullary catecholamine secretory granules (16, 17).
The presence of high concentrations of AA in synaptic vesicles and the evidence that it could be released into the extracellular space by neuronal activity suggested to us that AA might be a neurotransmitter. Since high concentrations of AA are already known to be present in the hypothalamus, we hypothesized that AA might control luteinizing hormone-releasing hormone (LH-RH) release, possibly by its antioxidant properties. LH-RH release is controlled by release of nitric oxide (NO) from NOergic interneurons that release the soluble gas in juxtaposition to the LH-RH terminals (18, 19). In the terminals, NO activates guanylyl cyclase, leading to an increase in cyclic guanosine monophosphate (cGMP), cyclooxygenase, resulting in increased concentrations of prostaglandin E2 (PGE2), and lipoxygenase, leading to increased concentrations of leukotrienes (20–23). All of these participate in causing the extrusion of the LH-RH secretory granules into the hypophyseal portal vessels for delivery of LH-RH to the pituitary gland, where it causes the release of luteinizing hormone. Glutamergic and noradrenergic terminals stimulate the NOergic neuron to release NO by action on their receptors to increase intracellular free calcium in the NOergic neurons, and the calcium combines with calmodulin that activates neural NO synthase (NOS), causing synthesis and release of NO from the neurons. Previous experiments have shown that LH-RH is released from medial basal hypothalamic explants by membrane depolarization by high potassium concentrations [K+] (24, 25), by the specific stimulant N-methyl-d-aspartate (NMDA) (26–32), and by a releaser of NO, nitroprusside (NP) (18, 26, 30, 33), whereas inhibition of NO synthesis with the competitive inhibitor of the enzyme NG-monomethyl-l-arginine (NMMA) blocked NMDA-induced stimulation of LH-RH release, indicating the crucial role of NO in the response.
Consequently, in these experiments, we evaluated the effect of AA on basal release of LH-RH and stimulated release produced by high-[K+] medium, NMDA, and NP and the consequences of blocking NO release by NMMA. Furthermore, we determined the effect of the three stimuli on the concentration of AA in the medium and its concentration in the medial basal hypothalamus (MBH). The results support the hypothesis that AA is a cotransmitter released with classical transmitters from synaptic vesicles that acts to reduce chemically the NO formed, thereby providing a negative feedforward inhibitory control over LH-RH release.
Materials and Methods
Animals.
tk;2Adult male rats of the Sprague–Dawley strain (Holtzman, Madison, WI; 200–250 g) were housed two per cage under controlled conditions of temperature (23–25°C) and lighting (on 05:00–17:00). The animals had free access to a pellet diet and tap water.
Chemicals.
Na+ ascorbate, NMMA, Na+ NP, and NMDA were purchased from Sigma.
In Vitro MBH Studies.
Animals were killed by decapitation and the brain was exposed by dorsal incision. MBH were dissected by vertical cuts along the lateral hypothalamic sulci, posterior edge of the optic chiasm, and the anterior edge of the mammillary bodies. A horizontal cut 1 mm from the base separated the island. The explants (8–12 mg) were incubated in vitro as previously reported (34). In brief, one MBH per tube was placed in 0.5 ml of Krebs–Ringer bicarbonate buffer (KRB, pH 7.4, 5.5 mM K+) supplemented with 20 μM bacitracin (Sigma) in an atmosphere of 95% O2 and 5% CO2 and incubated in a Dubnoff shaker (50 cycles per minute) for 60 min. After this preincubation, the tissues were incubated with 0.5 ml of KRB or KRB + K+ (28 or 56 mM) for 30 or 60 min. Constant ionic strength was maintained by equivalent reduction of sodium ion whenever KRB + K+ was used. At the end of the incubation the medium was aspirated and medium and tissues were stored at −80°C. A simultaneous incubation of MBH with high K+ + NMMA was also performed to evaluate the role of NO in depolarization-induced AA release. In another set of experiments MBH were incubated with KRB or KRB containing 10 or 50 mM NMDA or sodium NP (300 and 600 μM) or NMMA (300 and 600 μM) for 30 min after a preincubation period of 1 h. AA released into the incubation medium and its content were measured by HPLC. In addition, MBH were incubated with graded concentrations of AA (10−7 to 10−2 M) and incubated for 1 h. The medium was aspirated and the tissues were incubated with KRB or KRB + NMDA (10 mM) or KRB + NMDA + AA or KRB + NP (300 μM) or KRB + NP + AA for an additional 30 min. LH-RH released into the incubation medium was measured by RIA.
Chromatography.
Isocratic analyses were carried out with Beckman System Gold HPLC equipped with 126 module and diode array detector 168 operating at 254 nm at a sensitivity of 0.016 absorbance unit full scale. The separation was carried out on a μBondapak Beckman Ultrasphere C18 column (average particle size 5 μm, 25 cm × 4.6 mm). The mobile phase was a buffer consisting of 0.1 M NaH2PO4 and 0.2 mM Na2EDTA adjusted to pH 3.1 with orthophosphoric acid. The buffer was filtered through a 0.45-μm membrane filter (Gelman) and degassed before use. The column was maintained at room temperature and the mobile phase was used at a constant flow rate of 1.0 ml/min.
Preparation of Standard.
A sample buffer consisting of 5 mM each of metaphosphoric acid and Na2EDTA was prepared in HPLC grade water (VWR Scientific Products) and was used for preparing AA standards and MBH homogenates. This buffer was previously shown to be stable for 3–4 h, and all the estimations were completed within this time (35). A standard curve for AA was prepared from a stock solution of 1 mg/ml and was found to be linear from 487.5 to 7,800 ng. AA in standards, incubation medium, and homogenates was measured using a 507 ASE auto sampler (Beckman), and samples were used at a volume of 30 μl. Each sample (unknown) was passed through syringe filters (Gelman) before being placed in the vial for measurement. A standard calibration plot was obtained for AA concentrations (μg/ml) versus peak area (numerical units on the 126 module). Multiple plots for standard curve were constructed by using freshly prepared samples on different days.
MBH Homogenates.
MBH were weighed and placed in 600 μl of sample buffer containing 1 mg/ml of K+ metabisulfite and homogenized in a glass homogenizer. The homogenate was thoroughly mixed and centrifuged at 1000 × g for 10 min at 0°C. The supernatant was decanted and passed through syringe filters before placing in the vials for counting.
LH-RH Assay.
A highly specific antibody to LH-RH was provided by A. Barnea (University of Texas Southwestern Medical Center, Dallas). The minimal detectable LH-RH was 0.2 pg per tube and the curve was linear to 100 ng per tube.
Statistics.
Results were analyzed by one-way analysis of variance (ANOVA) or paired t tests wherever applicable, and P < 0.05 was considered significant.
Results
Effect of High [K+] on AA and LH-RH Release.
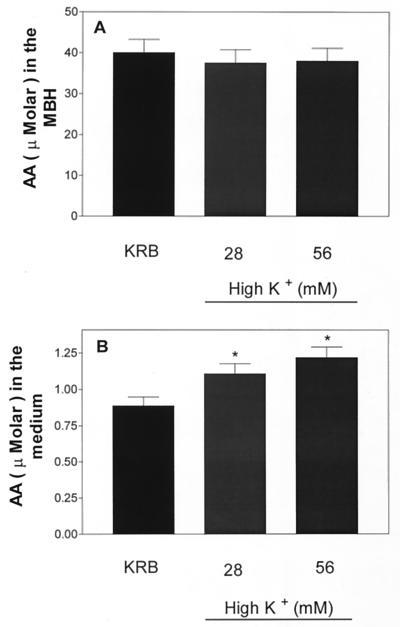
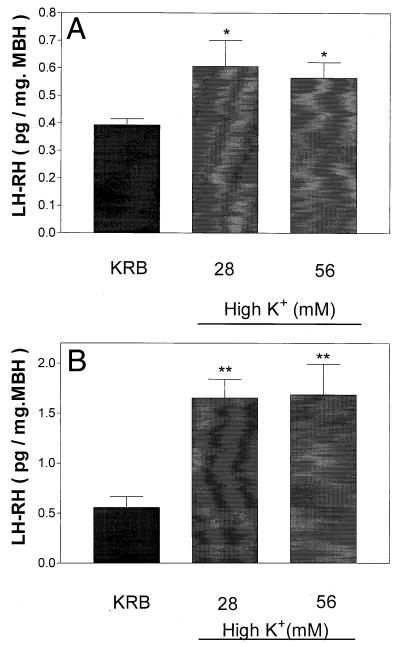
Incubation for 30 min had no significant effect on AA concentration in incubation medium (data not shown). When the incubation period was increased to 1 h, there was a significantly increased AA concentration in the medium with either 28 or 56 mM [K+], but the effect was not significantly dose related and tissue content was not altered (Fig. 1). As expected from prior research, both concentrations of K+ produced significant release of LH-RH after 30 and 60 min but again there was no dose-related relationship (Fig. 2).
Figure 1.
Effect of high [K+] (28 and 56 mM) on AA content (A) and release into the medium (B) after 1 h of incubation. *, P < 0.05 vs. control group.
Figure 2.
Effect of high [K+] on LH-RH release after 30 (A) or 60 (B) min of incubation. *, P < 0.05; **, P < 0.01 vs. control group.
Effect of High K+ + NMMA on AA and LH-RH Release.
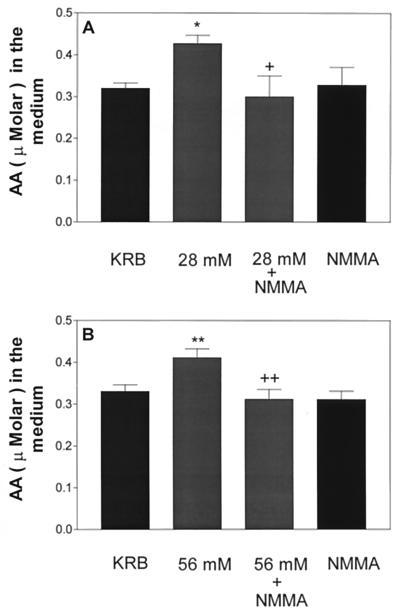
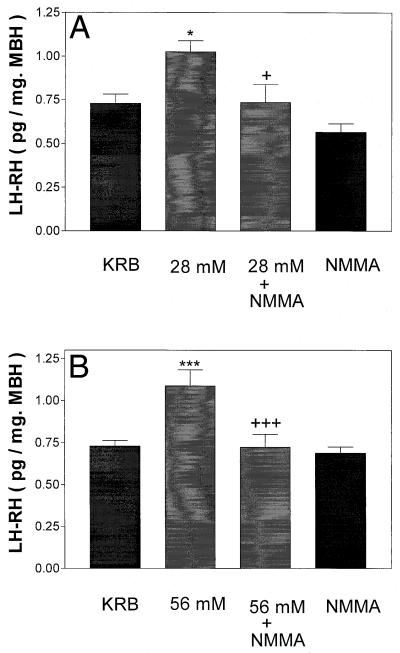
To determine whether NO was responsible for the K+-induced release of AA and also LH-RH, the tissue was incubated for 1 h with NMMA (300 μM) alone or together with 28 or 56 mM [K+]. NMMA completely blocked the release of AA into the medium induced by either concentration of K+ (Fig. 3) and also inhibited completely the release of LH-RH induced by both 28 and 56 mM [K+] (Fig. 4).
Figure 3.
Influence of high [K+] (28 mM in A and 56 mM in B) or a combination of high [K+] + NMMA on AA release. *, P < 0.05; **, P < 0.01 vs. control group. +, P < 0.05; ++, P < 0.01 vs. the group treated with high [K+].
Figure 4.
Effect of high [K+] (28 mM in A and 56 mM in B) or a combination of high [K+] + NMMA on LH-RH release. *, P < 0.05; ***, P < 0.001 vs. control group. +, P < 0.05; +++, P < 0.001 vs. the group treated with high [K+] (28 and 56 mM, respectively).
Effect of AA on High [K+]-Induced LH-RH Release.
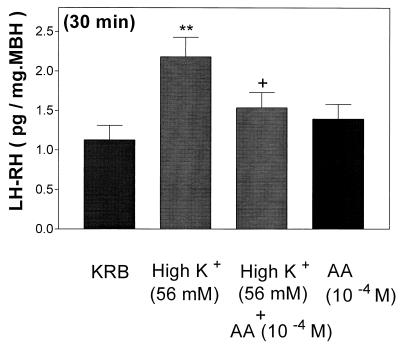
AA (10−4 M) had no effect on LH-RH release, but it completely blocked the LH-RH-releasing activity of high [K+] (Fig. 5).
Figure 5.
Effect of high [K+] (56 mM) or a combination of high [K+] (56 mM) + AA (10−4 M) on LH-RH release. **, P < 0.01 vs. control group. +, P < 0.05 vs. the group treated with high [K+].
Effect of Ca2+ Exclusion from the Medium on High [K+]-Induced AA and LH-RH Release.
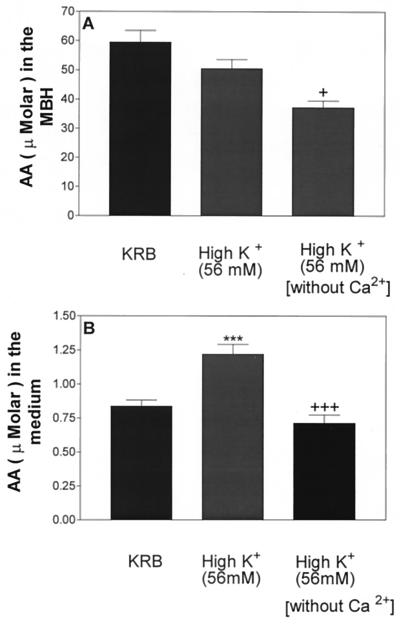
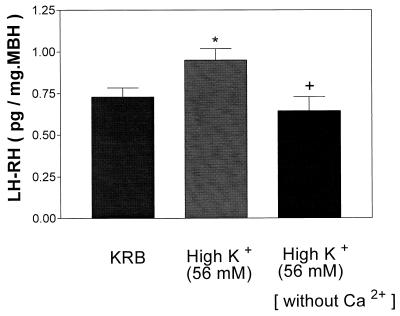
Incubation of MBH with high [K+] failed to alter AA content, but exclusion of Ca2+ from the medium containing high [K+] produced a significant decrease of AA content (Fig. 6A). High [K+] increased medium AA concentration as before. Increased medium [K+] in the absence of Ca2+ produced a significant decrease in AA concentration (Fig. 6B). As before (Fig. 7), incubation in the presence of high [K+] (56 mM) increased LH-RH release, whereas this increase was completely blocked in Ca2+-free medium.
Figure 6.
Effect of high [K+] (56 mM) in the presence or absence of Ca2+ on AA content (A) and release (B). ***, P < 0.001 vs. control group. +, P < 0.05; +++, P < 0.001 vs. the group treated with high [K+].
Figure 7.
Effect of high [K+] (56 mM) in the presence or absence of Ca2+ on LH-RH release. *, P < 0.05 vs. control group. +, P < 0.05 vs. the group treated with high [K+].
Effect of NP on AA Content in Incubation Medium and Tissue and LH-RH Release.
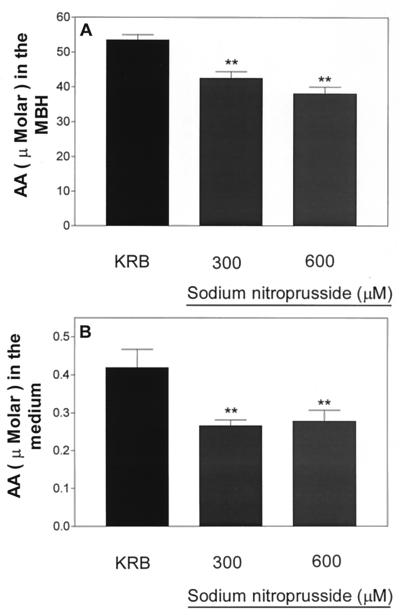
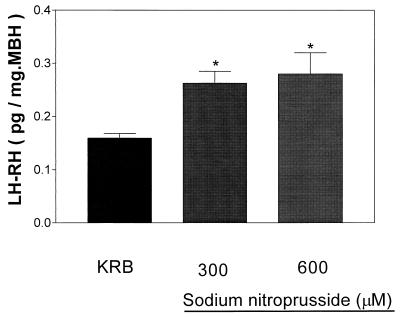
Incubation of MBH with two different concentrations of NP (300 and 600 μM) resulted in a significant decrease in tissue and medium concentrations of AA that was not dose dependent (Fig. 8 A and B, respectively). Incubation of MBH with these concentrations of NP stimulated LH-RH release but the effect was not dose dependent (Fig. 9).
Figure 8.
Effect of sodium NP (300 or 600 μM) on AA content (A) or release (B). **, P < 0.01 vs. control group.
Figure 9.
Effect of sodium NP on LH-RH release after 30 min of incubation. *, P < 0.05 vs. control group.
Effect of NMDA on LH-RH Release.
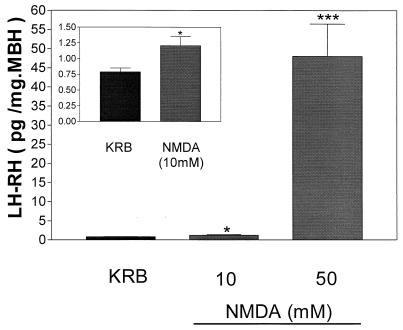
NMDA, an excitatory amino acid, produced a significant dose-dependent increase in LH-RH release as previously determined (Fig. 10). A 2-fold increase of LH-RH was observed in the group treated with the lower concentration (10 mM) (Fig. 10 Inset), whereas a 60-fold increase in LH-RH release was observed in the group treated with the higher concentration (50 mM) (Fig. 10).
Figure 10.
Effect of two different concentrations of NMDA (10 and 50 mM) on LH-RH release. Inset represents the stimulatory effect observed with 10 mM. *, P < 0.05; ***, P < 0.001 vs. control group.
Effect of NMDA on AA Medium and Tissue Concentration.
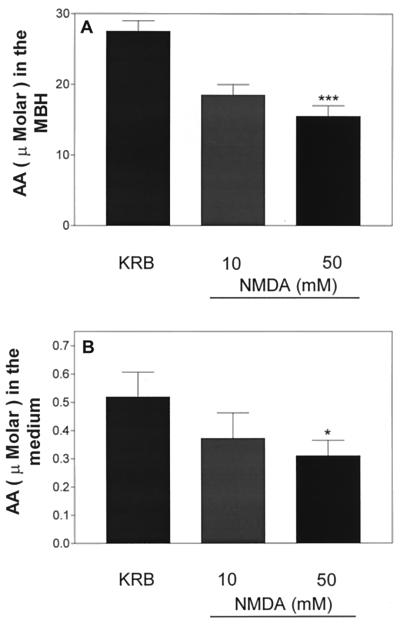
Incubation of MBH with NMDA (10 mM) for 30 min produced slight but not significant decreases in medium concentration and tissue content of AA, whereas the 5 times higher dose (50 mM) produced a significant reduction in both medium and tissue concentration of AA (Fig. 11).
Figure 11.
Effect of NMDA (10 or 50 mM) on AA content (A) or release (B) after 30 min of incubation. *, P < 0.05; ***, P < 0.001 vs. control group.
Effect of Graded Concentrations of AA on Basal and NMDA-Induced LH-RH Release.
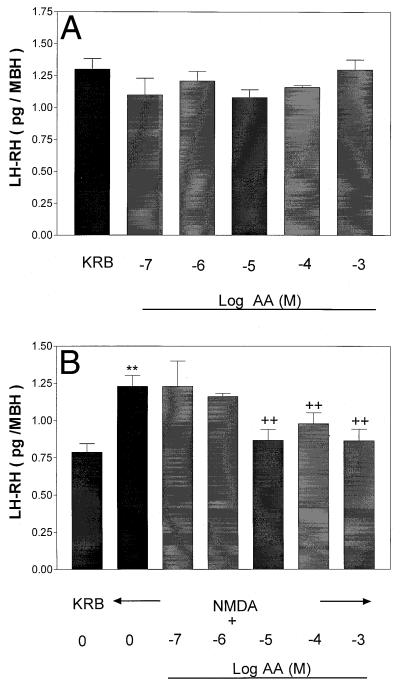
Because NMDA decreased medium AA concentrations, it was of interest to study the converse effect, namely the effect of graded concentrations of AA on LH-RH release and its influence on NMDA-induced LH-RH release. Incubation of MBH with AA (10−7 to 10−2 M) failed to alter basal LH-RH release (Fig. 12A). However, prior (60 min) and continued exposure to AA plus NMDA for 30 min totally blocked NMDA-induced LH-RH release. The minimal effective concentration of AA was 10−5 M, and the effect persisted at higher concentrations (10−4 and 10−3 M) (Fig. 12B).
Figure 12.
(A) Effect of graded concentrations of AA on basal LH-RH release after 1 h of incubation. (B) Effect of prior and continued exposure to AA or AA + NMDA on LH-RH release after 30 min of incubation. **, P < 0.01 vs. control group. ++, P < 0.01 vs. the group treated with NMDA alone.
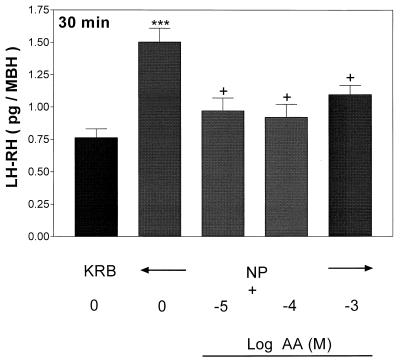
Effect of AA on NP-Induced LH-RH Release.
Incubation of MBH with graded concentrations of AA (10−5 to 10−3 M) for 1 h as expected failed to alter basal LH-RH release (data not presented). Prior and continued exposure to AA totally blocked NP-induced LH-RH release (Fig. 13). As above, the minimal effective dose was 10−5 M and the effect persisted at higher concentrations (10−4 and 10−3 M).
Figure 13.
Sodium NP-stimulated LH-RH release after 30 min of incubation. The tissues were subjected to prior and continued exposure to AA for 1 h (data not shown) followed by incubation with NP. ***, P < 0.001 vs. control group. +, P < 0.05 vs. the group treated with NP alone.
Discussion
Our results indicate that depolarization of cell membranes by means of high-[K+] medium, release of NO from NP, or the excitatory amino acid analog NMDA releases LH-RH from MBH explants. Because the release induced by membrane depolarization, NP, and NMDA is blocked by the NOS inhibitor NMMA, LH-RH release from all three stimuli appears to be mediated by NO. The release of LH-RH induced by all three stimuli was abolished by concentrations of AA as low as 10−5 M, although these concentrations of AA had no effect on basal release. Since the concentration of AA in plasma is normally 10−5 M or even greater and is 10 times lower than that intraneuronally and probably manyfold lower than inside synaptic vesicles of glutamergic neurons and probably others, such as catecholaminergic neurons, our hypothesis is that on excitation of neuronal activity by high [K+] or excitatory amino acids, such as NMDA, AA is coreleased along with the classical neurotransmitters, glutamic acid and norepinephrine. This release would provide high concentrations of AA in the synaptic cleft that may well be of physiologic significance, acting as a feedforward suppressant of the activation of LH-RH release from the LH-RH terminals. Interestingly, the concentration of AA required to inhibit stimulated LH-RH release (10−5 M) is much lower than that of glutamic acid or NMDA to activate LH-RH release (10−2 M) (30, 33). The concentration of norepinephrine required to stimulate LH-RH release in vitro (10−5 M) (18) is equivalent to the concentration of AA (10−5 M) required to inhibit stimulated LH-RH release. These comparisons lend credence to the concept that AA may be of physiological significance in control of LH-RH release.
Our previous experiments indicate that NMDA stimulates noradrenergic terminals that synapse on NOergic neurons and activate α1-adrenergic receptors. This activation causes an increase in intracellular free Ca2+ in the NOergic neuron, and the Ca2+ combines with calmodulin to activate NOS within these neurons. In the presence of cofactors and equimolar quantities of oxygen, arginine is converted into NO and equimolar quantities of citrulline. The NO diffuses to the LH-RH terminal, where it activates guanylyl cyclase that converts GTP into cGMP. We postulate that cGMP would increase intracellular free [Ca2+] (18, 33). Indeed, increased Ca2+ has been demonstrated in pancreatic acinar cells after NO activation of guanylyl cyclase (36, 37). The increased intracellular free Ca2+ would then participate in exocytosis of LH-RH secretory granules. NO also activates cyclooxygenase and lipoxygenase, leading to increased prostaglandin and leukotriene release. Because blockers of cyclooxygenase and lipoxygenase also block LH-RH release (38), all three of these mechanisms appear to act in concert to produce exocytosis of LH-RH secretory granules into the hypophyseal portal vessels, which transport it to the pituitary where it releases luteinizing hormone.
Our results also indicate that substantial amounts of AA are released by high-[K+] depolarization of cell membranes that could be accounted for by the exocytosis of synaptic vesicles containing the vitamin. We had not previously suspected a role for NO in the release of LH-RH, long known to be evoked by high-[K+] medium, but quite clearly this LH-RH release was also blocked by the inhibitor of NOS, NMMA. NMMA also blocked the release of AA, presumably by blocking the exocytosis of AA-rich synaptic vesicles. As expected, because exocytosis is Ca2+ dependent, the release of both AA and LH-RH induced by high-[K+] medium was abolished in Ca2+-free medium.
We do not know the mechanism by which NO evokes depolarization-induced release of AA and LH-RH; however, membrane depolarization is known to activate Ca2+ influx through Ca2+ channels. Therefore, it would activate NOS, leading to production of NO in NOergic neurons. As suggested above, NO by activation of guanylyl cyclase would further increase intracellular free [Ca2+] required for exocytosis of AA and LH-RH. Because NMMA blocked not only AA but also LH-RH release from MBH explants, NO appears to be essential for these exocytotic events, either by producing a further increase in intracellular [Ca2+] through activation of guanylyl cyclase as suggested above, or because of its activation of cyclooxygenase and lipoxygenase as well, because activation of all three enzymes by NO appears to be essential for release of the neuropeptides corticotropin-releasing hormone and LH-RH (23, 32, 39–42).
Although depolarization by K+ increased medium AA concentrations, activation of LH-RH release either by NP, a releaser of NO, or by the higher dose of NMDA, also a releaser of NO, was associated with a decreased medium concentration and tissue content of AA. We hypothesize that this occurs because of the oxidation of AA to dehydro-AA by NO released from NOergic neurons. Dehydro-AA, which cannot be measured by our method, is rapidly destroyed or reenters the tissue and is reduced to AA. In the case of K+ depolarization, AA would be released from all neurons containing AA as opposed to NMDA, which would affect only neurons with NMDA receptors. This higher dose of NMDA released massive amounts of LH-RH, presumably by stimulating the release of massive amounts of NO that would oxidize much of the AA released, accounting for the decrease of AA in the medium only with the higher dose of NMDA. Changes in medium concentration are hard to interpret because they are the balance of the secretion of AA into the medium and the degradation of AA to dehydro-AA that was not measurable. AA presumably blocks the release of LH-RH evoked by all three stimuli by chemically reducing NO as it is oxidized to dehydro-AA.
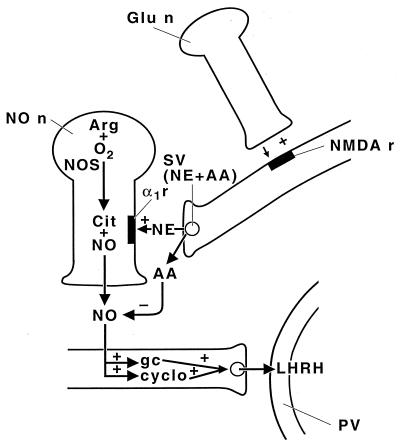
Our hypothesis of the mechanism of action of AA to inhibit the release of LH-RH produced by high-[K+] media, NP, and NMDA is presented in Fig. 14. According to this concept, the axons of glutamergic neurons synapse on the axons of noradrenergic neurons that in turn synapse on the NOergic neurons. Therefore, NMDA would cause the release of norepinephrine that by α1-noradrenergic receptors would increase intracellular [Ca2+] in the NOergic neuron. This increase would activate NOS, causing generation of NO that would diffuse to the LH-RH neuron and activate release by activating guanylyl cyclase, cyclooxygenase, and lipoxygenase. NO is thought to be responsible also for exocytosis of synaptic vesicles in neurons containing AA by a calcium-dependent mechanism, because NNMA also blocked AA release into the medium induced by high-[K+] medium. Again, this is by a Ca+2-dependent mechanism, presumably related to the activation of guanylyl cyclase and production of cGMP by NO because AA release, as well as LH-RH release, was blocked in the absence of Ca2+. We hypothesize that AA is the first known vitaminergic neurotransmitter.
Figure 14.
Schematic diagram of the hypothesized mechanism of action of AA to inhibit NOergic stimulation of LH-RH release. For description see text. Glu n, glutamergic neuron; NMDA r, NMDA receptor; SV, synaptic vesicle; NE, norepinephrine; α1r, α1-adrenergic receptor; AA, ascorbic acid; NO n, NOergic neuron; Arg, arginine; NOS, NO synthase; Cit, citrulline; gc, guanylyl cyclase; cyclo, cyclooxygenase; PV, portal vesicle; +, stimulates; −, inactivates.
These results have been obtained in static incubations and must be confirmed in a dynamic perifusion system to further test their validity. Also, it would be very important to see whether the results would change in a situation in which AA has been depleted, such as in scorbutic guinea pigs. AA not only may alter physiological neurotransmission but also is very important in scavenging free radicals in cases of their excessive endogenous production, as in inflammation and severe infection with toxic shock (43).
Acknowledgments
We thank Judy Scott for her excellent secretarial assistance. This work was supported by National Institutes of Health Grant MH51853.
Abbreviations
- AA
ascorbic acid
- LH-RH
luteinizing hormone-releasing hormone
- NOS
NO synthase
- NMDA
N-methyl-d-aspartate
- NP
nitroprusside
- NMMA
NG-monomethyl-l-arginine
- MBH
medial basal hypothalamus (hypothalami)
References
- 1.Chatterjee I B. World Rev Nutr Diet. 1978;30:69–87. doi: 10.1159/000401236. [DOI] [PubMed] [Google Scholar]
- 2.Das P C, Das K P, Bagchi K, Dey C D. Life Sci. 1993;52:1493–1498. doi: 10.1016/0024-3205(93)90111-f. [DOI] [PubMed] [Google Scholar]
- 3.Schreiber M, Trojan S. Physiol Res. 1991;40:413–418. [PubMed] [Google Scholar]
- 4.Chatterjee I B, Majumdar A K, Nandi B K, Subramanian N. Ann NY Acad Sci. 1975;258:24–47. doi: 10.1111/j.1749-6632.1975.tb29266.x. [DOI] [PubMed] [Google Scholar]
- 5.Oke A F, May L, Adams R N. Ann NY Acad Sci. 1987;498:1–12. doi: 10.1111/j.1749-6632.1987.tb23747.x. [DOI] [PubMed] [Google Scholar]
- 6.Schenko J O, Miller E, Gaddis R, Adams R N. Brain Res. 1982;253:353–356. doi: 10.1016/0006-8993(82)90709-0. [DOI] [PubMed] [Google Scholar]
- 7.Spector R, Lorenzo A V. Am J Physiol. 1973;225:757–763. doi: 10.1152/ajplegacy.1973.225.4.757. [DOI] [PubMed] [Google Scholar]
- 8.Rice M E, Nicholson C. J Neurophysiol. 1991;65:264–272. doi: 10.1152/jn.1991.65.2.264. [DOI] [PubMed] [Google Scholar]
- 9.Rice M E, Cammack J. Neurosci Lett. 1991;132:141–145. doi: 10.1016/0304-3940(91)90287-4. [DOI] [PubMed] [Google Scholar]
- 10.Boutelle M G, Svensson L, Fillenz M. Neuroscience. 1989;30:11–17. doi: 10.1016/0306-4522(89)90349-7. [DOI] [PubMed] [Google Scholar]
- 11.Clemens J A, Phebus L A. Life Sci. 1984;35:671–677. doi: 10.1016/0024-3205(84)90262-5. [DOI] [PubMed] [Google Scholar]
- 12.Grunewald R A, O'Neill R D, Fillenz M, Albery W J. Neurochem Int. 1983;5:773–778. doi: 10.1016/0197-0186(83)90103-1. [DOI] [PubMed] [Google Scholar]
- 13.Grunewald R A. Brain Res Rev. 1993;18:123–133. doi: 10.1016/0165-0173(93)90010-w. [DOI] [PubMed] [Google Scholar]
- 14.Hadjiconstantinou M, Neff N H. Neuropharmacology. 1983;22:939–943. doi: 10.1016/0028-3908(83)90209-5. [DOI] [PubMed] [Google Scholar]
- 15.Thorn N A, Christensen B L, Jeppesen C, Nielsen F S. Acta Physiol Scand. 1985;124:87–92. doi: 10.1111/j.1748-1716.1985.tb07635.x. [DOI] [PubMed] [Google Scholar]
- 16.Daniels A J, Dean G, Viveros O H, Diliberto E J., Jr Science. 1982;216:737–739. doi: 10.1126/science.7079733. [DOI] [PubMed] [Google Scholar]
- 17.Diliberto E J, Jr, Menniti F S, Knoth J, Daniel A J, Kizer J S, Viveros O H. Ann NY Acad Sci. 1987;498:28–53. doi: 10.1111/j.1749-6632.1987.tb23749.x. [DOI] [PubMed] [Google Scholar]
- 18.Rettori V, Belova N, Dees W L, Nyberg C L, Gimeno M, McCann S M. Proc Natl Acad Sci USA. 1993;90:10130–10134. doi: 10.1073/pnas.90.21.10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhat G K, Mahesh V B, Lamar C A, Ping L, Aguan K, Brann D W. Neuroendocrinology. 1995;62:187–197. doi: 10.1159/000127004. [DOI] [PubMed] [Google Scholar]
- 20.Mayer B, Koesling D, Bohne E. Adv Second Messenger Phosphoprotein Res. 1993;28:111–119. [PubMed] [Google Scholar]
- 21.Vallebuona F, Raiteri M. Neuroscience. 1993;57:577–585. doi: 10.1016/0306-4522(93)90007-3. [DOI] [PubMed] [Google Scholar]
- 22.Uno H, Arakawa T, Fukuda T, Yu H, Fujiwara Y, Higuchi K, Inoue M, Kobayashi K. Prostaglandins. 1997;53:153–162. doi: 10.1016/s0090-6980(97)00013-0. [DOI] [PubMed] [Google Scholar]
- 23.Rettori V, Gimeno M, Lyson K, McCann S M. Proc Natl Acad Sci USA. 1992;89:11543–11546. doi: 10.1073/pnas.89.23.11543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramirez V D, Gallardo E, Hartter D. J Endocrinol Invest. 1980;3:29–37. doi: 10.1007/BF03348214. [DOI] [PubMed] [Google Scholar]
- 25.Bigdeli H, Snyder P J. Endocrinology. 1978;103:281–286. doi: 10.1210/endo-103-1-281. [DOI] [PubMed] [Google Scholar]
- 26.Bonavera J J, Sahu A, Kalra P S, Kalra S P. Endocrinology. 1993;133:2481–2487. doi: 10.1210/endo.133.6.8243268. [DOI] [PubMed] [Google Scholar]
- 27.Donoso A O, Lopez F J, Negro-Vilar A. Endocrinology. 1990;126:414–420. doi: 10.1210/endo-126-1-414. [DOI] [PubMed] [Google Scholar]
- 28.Bourguignon J P, Gerard A, Franchimont P. Neuroendocrinology. 1989;49:402–408. doi: 10.1159/000125145. [DOI] [PubMed] [Google Scholar]
- 29.Bourguignon J P, Gerard A, Mathieu J, Simons J, Franchimont P. Endocrinology. 1989;125:1090–1096. doi: 10.1210/endo-125-2-1090. [DOI] [PubMed] [Google Scholar]
- 30.Moretto M, Lopez F J, Negro-Vilar A. Endocrinology. 1993;133:2399–2402. doi: 10.1210/endo.133.5.8104781. [DOI] [PubMed] [Google Scholar]
- 31.Price M T, Olney J W, Cicero T J. Neuroendocrinology. 1978;26:352–358. doi: 10.1159/000122790. [DOI] [PubMed] [Google Scholar]
- 32.Mahachoklertwattana P, Black S M, Kaplan S L, Bristow J D, Grumbach M M. Endocrinology. 1994;135:1709–1712. doi: 10.1210/endo.135.4.7523101. [DOI] [PubMed] [Google Scholar]
- 33.Rettori V, Kamat A, McCann S M. Brain Res Bull. 1994;33:501–503. doi: 10.1016/0361-9230(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 34.Karanth S, Lyson K, Aguila M C, McCann S M. Neuroimmunomodulation. 1995;2:166–173. doi: 10.1159/000096888. [DOI] [PubMed] [Google Scholar]
- 35.Harapanhalli R S, Howell R W, Rao D V. J Chromatogr. 1993;614:233–243. doi: 10.1016/0378-4347(93)80314-t. [DOI] [PubMed] [Google Scholar]
- 36.Ahn S H, Seo D W, Ko Y K, Sung D S, Bae G U, Yoon J W, Hong S Y, Han J W, Lee H W. Arch Pharm Res. 1998;21:657–663. doi: 10.1007/BF02976753. [DOI] [PubMed] [Google Scholar]
- 37.Yoshida H, Tsunado Y, Owyang C. Eur J Physiol. 1997;434:25–37. doi: 10.1007/s004240050359. [DOI] [PubMed] [Google Scholar]
- 38.McCann S M, Rettori V. Proc Soc Exp Biol Med. 1996;211:7–15. doi: 10.3181/00379727-211-43950b. [DOI] [PubMed] [Google Scholar]
- 39.Salvemini D, Misko T P, Masferrer J L, Seibert K, Currie M C, Needleman P. Proc Natl Acad Sci USA. 1993;90:7240–7244. doi: 10.1073/pnas.90.15.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bugajski J, Gadek M A, Borycz J, Glod R. Brain Res. 1999;817:220–225. doi: 10.1016/s0006-8993(98)01209-8. [DOI] [PubMed] [Google Scholar]
- 41.Brunetti L, Preziosi P, Ragazzoni E, Vacca M. Life Sci. 1993;53:219–222. doi: 10.1016/0024-3205(93)90267-7. [DOI] [PubMed] [Google Scholar]
- 42.Karanth S, Lyson K, McCann S M. Proc Natl Acad Sci USA. 1993;90:3383–3387. doi: 10.1073/pnas.90.8.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Niki E. Am J Clin Nutr. 1991;54:11195–11245. doi: 10.1093/ajcn/54.6.1119s. [DOI] [PubMed] [Google Scholar]