Abstract
OBJECTIVE
Stem cells from human exfoliated deciduous teeth (SHED) are a population of highly proliferative postnatal stem cells capable of differentiating into odontoblasts, adipocytes, neural cells, and osteo-inductive cells. To examine whether SHED-mediated bone regeneration can be utilized for therapeutic purposes, we used SHED to repair critical-size calvarial defects in immuno-compromised mice.
MATERIALS AND METHODS
We generated calvarial defects and transplanted SHED with hydroxyapatite/ tricalcium phosphate as a carrier into the defect areas.
RESULTS
SHED were able to repair the defects with substantial bone formation. Interestingly, SHED-mediated osteogenesis failed to recruit hematopoietic marrow elements that are commonly seen in bone marrow mesenchymal stem cell-generated bone. Furthermore, SHED were found to co-express mesenchymal stem cell marker, CC9/MUC18/CD146, with an array of growth factor receptors such as transforming growth factor β receptor I and II, fibroblast growth factor receptor I and III, and vascular endothelial growth factor receptor I, implying their comprehensive differentiation potential.
CONCLUSIONS
Our data indicate that SHED, derived from neural crest cells, may select unique mechanisms to exert osteogenesis. SHED might be a suitable resource for orofacial bone regeneration.
Keywords: stem cells from human exfoliated deciduous teeth (SHED), osteoblast, regeneration, bone
Introduction
Stem cells from human exfoliated deciduous teeth (SHED) were identified as a novel population of stem cells capable of differentiating into a variety of cell types including neural cells, odontogenic cells, and adipocytes (Miura et al, 2003). The most significant difference between SHED and adult dental pulp stem cells (DPSCs) is that SHED are able to induce bone formation when implanted into immunocompromised mice subcutaneously using hydroxyapatite/tricalcium phosphate as a carrier vehicle (Miura et al, 2003), while DPSCs generated a dentin/pulp-like structure (Gronthos et al, 2000). Importantly, SHED are derived from a readily accessible tissue source, human deciduous teeth that are expendable and routinely exfoliated in childhood with little or no morbidity to the patient. Although mesenchymal stem cells derived from bone marrow and adipocyte tissues have been used to treat critical-size bone defects in animal models (Krebsbach et al, 1998; Cowan et al, 2004), these cells originate from the mesoderm rather than from neural crest cells. Our hypothesis is that neural crest cell-derived SHED may offer optimal orofacial repairing with a matched neural crest origin.
Materials and methods
Subjects and cell culture
Stem cells from human exfoliated deciduous teeth were isolated and cultured as previously described (Miura et al, 2003). Briefly, normal exfoliated human deciduous upper and lower incisors were collected from 7- to 8-year-old children under approved guidelines set by the National Institutes of Health Office of Human Subjects Research. Collection of SHED for research has been approved by Institutional Review Board of University of Southern California. The pulp tissue was separated from a remnant crown and then digested in phosphate-buffered saline (PBS) containing 3 mg ml−1 collagenase type I (Worthington Biochemicals Corp., Freehold, NJ, USA) and 4 mg ml−1 dispase (Roche Diagnostic/Boehringer Mannheim Corp., Indianapolis, IN, USA) for 30 min at 37°C. Single cell suspensions were seeded into 6-well plates (Costar, Cambridge, MA, USA) with alpha Modification of Eagle's Medium (GIBCO/Invitrogen, Carlsbad, CA, USA) supplemented with 15% fetal calf serum (Equitech-Bio Inc., Kerrville, TX, USA), 100 μM l-ascorbic acid 2-phosphate (WAKO Pure Chemical Industries, Ltd, Osaka, Japan), 2 mM l-glutamine (Biosource/Invitrogen, Carlsbad, CA), 100 U ml−1 penicillin and 100 μg ml−1 streptomycin (Biosource/Invitrogen), then incubated at 37°C in 5% CO2. The above cell culture components were considered as regular cell culture medium. Human bone marrow cells were purchased from commercially available resources (AllCells LLC, Berkeley, CA, USA). To identify putative bone marrow mesenchymal stem cells, single cell suspension of 1 × 106 of bone marrow mononuclear cells were seeded into 15 cm culture dishes and non-adherent cells were removed after 3 h of incubation at 37°C. The adherent cells were cultured in regular cell culture medium mentioned above. To further confirm the bone marrow mesenchymal stem cells, STRO-1 expression, an identified mesenchymal stem cell marker, was used for verification. SHED and bone marrow mesenchymal stem cells used in this study were at one to three passages.
Transplantation
Approximately 2.0 × 106 ex vivo expanded SHED or bone marrow mesenchymal stem cells were mixed with 40 mg of hydroxyapatite/tricalcium phosphate ceramic particle (Zimmer Inc., Warsaw, IN, USA) and then transplanted into the 2.7 mm diameter defect created by a trephine bur on the calvaria of immunocompromised mice (NIH-bg-nu-xid, Harlan Sprague–Dawley, Indianapolis, IN) as previously described (Krebsbach et al, 1997; Batouli et al, 2003). These immunocompromised mice were selected as a model for critical-size bone defects to avoid potential immunogenic and graft-rejection responses as the SHED are of human origin. These procedures were performed in accordance with the specifications of an approved small animal protocol (NIDCR No. 03−264). Totally 18 immunocompromised mice were used for three groups, including the SHED group (n = 6), bone marrow mesenchymal stem cell group (n = 6), and hydroxyapatite/tricalcium phosphate group (n = 6). Five mice from each group were harvested at 8 weeks posttransplantation and one mouse from each group was kept up to 6 months. The transplants were recovered either at 6−8 weeks or 6 months posttransplantation, fixed with 4% formalin, decalcified with buffered 10% EDTA (pH 8.0), and then embedded in paraffin. Sections were deparaffinized and stained with hematoxylin and eosin.
Immunohistochemistry
Deparaffinized sections were washed, and endogenous peroxidase activities were quenched by immersing in 3% H2O2/methanol for 15 min. Sections were then incubated with primary antibodies (1:200−1:300 dilution) for 1 h. Rabbit antibodies used for immunohistochemistry were anti: bone sialoprotein (BSP, LF21, 1:300 dilution), alkaline phosphatase (ALP, LF-47, 1:200 dilution), dentin sialoprotein (DSP, LF-151, 1:200 dilution), type I collagen (LF-67, 1:200 dilution) from Dr Larry Fisher (NIDCR/NIH, Bethesda, MD, USA). Mouse antibody was anti-human mitochondria (Chemicon, Temecula, CA, USA). Isotype-matched control antibodies were used under the same conditions. For enzymatic immunohistochemical staining, the Zymed SuperPicTure polymer detection kit (Zymed/Invitrogen, Carlsbad, CA, USA) was used according to the manufacturer's protocol. Subsequently, sections were counterstained with hematoxylin.
Immunocytochemistry
Primary SHED were sub-cultured into 8-chamber slides (2 × 104 cells/well) (NUNC Inc., Naperville, IL, USA). After 5 days in culture with 15 population doublings, the cells were fixed in freshly prepared 4% formalin for 15 min, then washed in PBS. The samples were subsequently blocked with 5% non-immune goat serum for 1 h at room temperature. Samples were incubated with primary antibodies in 5% non-immune goat serum for 1 h at room temperature. Rabbit antibodies used were anti: transforming growth factor β receptor (TGFβR)-I and II, fibroblast growth factor receptor (FGFR)-I and III, and vascular endothelial growth factor receptor (VEGFR)-I (1:200 dilution; Santa Cruz Biotechnology, Santa Cruz, CA); CC9/MUC18/CD146 from Dr. Stan Gronthos (Institute of Medical and Veterinary Science, Adelaide, Australia). After washing, the samples were incubated with goat anti-rabbit IgG-Rhodamine Red and IgG-FITC (Jackson ImmunoResearch, West Grove, PA, USA), for 45 min at room temperature, washed and mounted in VECTASHIELD mounting medium (Vector Laboratories Inc., Burlingame, CA, USA).
In situ hybridization
Human-specific alu and murine-specific pf1 sequences labeled with digoxigenin were used as probes for in situ hybridization as previously described (Batouli et al, 2003). Primers included: human alu (GenBank accession number: X53550), sense, 5′-TGGCTCACGCCTGTAATCC-3′, and antisense, 5′-TTTTTTGAGACGGAGTCTCGC-3′; and murine pf1 (GenBank accession number: X78319), sense, 5′-CCGGGCAGTGGTGGCGCATGCCTTTAAATCCC-3′, and antisense, 5′-GTTTGGTTTTTGAGCAGGGTTCTCTGTGTAGC-3′. The probes were prepared by PCR containing 1× PCR buffer (Perkin-Elmer, Foster City, CA, USA), 0.1 mM dATP, 0.1 mM dCTP, 0.1 mM dGTP, 0.065 mM dTTP, 0.035 mM digoxigenin-11-dUTP, 10 pmol of specific primers, and 100 ng of human genomic DNA as templates. Unstained sections were deparaffinized and hybridized with the digoxigenin-labeled alu probe using the mRNAlocator-Hyb Kit (Ambion, Inc., Austin, TX, USA). After hybridization, the presence of alu or pf1 in tissue sections was detected by immunoreactivity with an anti-digoxigenin ALP-conjugated Fab fragments (Roche Diagnostic/Boehringer Mannheim Corp.) (Gronthos et al, 2000, 2002).
Reverse transcriptase polymerase chain reaction (RT-PCR) analysis
The PCR primers include: human BSP (GenBank accession number: L24759), sense, 5′-CTATGGAGAGGACGCCACGCCTGG-3′, and antisense, 5′-CATAGCCATCGTAGCCTTGTCCT-3′; human osteocalcin (OSC; GenBank accession number: X53698), sense, 5′-CATGAGAGCCCTCACA-3′, and antisense, 5′-AGAGCGACACCCTAGAC-3′; human GAPDH (GenBank accession number: M32599), sense, 5′-ACTTTGTCAAGCTCATTTCC-3′, and antisense, 5′-TGCAGCGAACTTTATTGATG-3′; mouse BSP (GenBank accession number: L20232), sense 5′-GAAACGGTTTCCAGTCCAG-3′, and antisense, 5′-TGAAACCCGTTCAGAAGG-3′; mouse OSC (GenBank accession number: X04142), sense, 5′-CATGAGAGCCCTCACA-3′, and antisense, 5′-AGAGCGACACCCTAGAC-3′. Total RNA isolation, first-strand cDNA synthesis and PCR processes were described previously (Gronthos et al, 2000, 2002).
Results
As shown in Figure 1, multi-colony-derived SHED were equivalent to bone marrow mesenchymal stem cells in restoring the parietal continuity with significant amounts of bone formation in all transplanted mice. Semi-quantitative analysis indicated that SHED and bone marrow mesenchymal stem cells consistently form robust amount of mineralized tissues to repair the defects (Figure 1g). However, hydroxyapatite/tricalcium phosphate control group lacked mineralized tissue (Figure 1g). After 6 months posttransplantation, SHED maintained bone continuity and complete calvarial repair (Figure 2). However, SHED-mediated bone lacked hematopoietic marrow elements, which were found routinely in bone marrow mesenchymal stem cell-generated bone (Krebsbach et al, 1997; Batouli et al, 2003). Further examination demonstrated that SHED were not only inducing recipient cells to differentiate into osteogenic cells to form bone as reported previously (Miura et al, 2003), but also actively contributing to bone formation (Figure 2), which was confirmed by human alu in situ hybridization, specific anti-human mitochondria antibody staining and RTPCR-amplified human-specific osteogenic cell markers, including BSP and OSC. These results suggest that SHED were capable of forming and inducing bone formation in vivo. Human and mouse bone can be differentiated by examining human or mouse-specific BSP and OSC RT-PCR products (Figure 2). In addition, the amount of human and mouse bone formation can be estimated by comparing expression levels of the respective BSP and OSC to the housekeeping gene GAPDH (Figure 2). The single-colony-derived transplanted SHED exhibited similar bone regeneration capabilities to the multi-colony-derived SHED. They also demonstrated robust bone formation without associated hematopoietic bone marrow (Figure 2). In addition, the amount of human and mouse bone formation can be estimated by comparing expression levels of the respective BSP and OSC to the housekeeping gene GAPDH (Figure 2). To further characterize SHED-mediated bone structure, we used immunohistochemical staining to demonstrate that osteogenic cells were positive to anti-ALP, BSP, and type I collagen antibody staining, but negative for anti-DSP antibody staining (Figure 3). Additionally, the cultured SHED were positive for a variety of osteogenic-associated growth factor receptors including TGFβ, FGF and VEGF receptors with co-localization with CC9/MUC18/CD146, an early mesenchymal stem cell marker (Figure 4). These data support that SHED possess osteogenic differentiation potential in vivo and in vitro, and are analogous to bone marrow mesenchymal stem cells in supporting bone regeneration.
Figure 1.

Histology of SHED-mediated bone regeneration for repairing parietal defects in immunocompromised mice. Cross sections of hydroxyapatite/tricalcium phosphate carrier transplant (a, d) as negative control, SHED transplant (b, e), and bone marrow mesenchymal stem cell transplant (c, f) as positive control, at 8 weeks posttransplantation stained with hematoxylin and eosin. There was no mineralized tissue regeneration found in the negative control group as shown in low (a) and selected area with high (d) magnification. Only connective tissue (CT) was found around hydroxyapatite/tricalcium phosphate (HA) carrier. However, bone (B) formation presented on the surface of hydroxyapatite/tricalcium phosphate in SHED and bone marrow mesenchymal stem cell transplants as seen in low (b, c) and selected areas with high (e, f) magnification. Clearly, bone marrow elements (BM) were generated in bone marrow mesenchymal stem cell transplants (f), but absent in SHED transplants (e). Bar: 500 μm in (a)–(c), 100 μm in (d)–(f). Semi-quantitative analysis showed that bone regeneration capacity of SHED was similar to that of bone marrow mesenchymal stem cells when transplanted into immunocompromised mice (g) using Scion Image analysis (Scion Image, Rockville, MD, USA). Error bars represent the mean ± s.d. However, control hydroxyapatite/tricalcium phosphate transplant lacked bone formation (g)
Figure 2.

Characterization of SHED-mediated bone formation. After 6 months of transplantation, SHED were capable of maintaining bone structure (B) on the surfaces of hydroxyapatite/tricalcium phosphate (HA) along with connective tissue (CT, a). Same field of polarized picture showed dense collagen fibers (b). In contrast, bone marrow mesenchymal stem cells maintained both bone (B) and bone marrow elements (BM) after 6 months posttransplantation (c). Same field of polarized microscopy view showed dense collagen fibers (d). In situ hybridization studies showed the murine-specific pf1 DNA probe reacting with recipient osteoblasts and osteocytes associated with the new bone formation (B, black arrows in e). Mouse tissue (MT) reacted positive for pf1 probe in SHED transplant (open arrows in e). Human-specific alu in situ hybridization showed that SHED (black arrows in f) were associated with bone formation (B). Mouse tissue (MT) was negative for alu in situ hybridization. Immunohistochemical staining showed that SHED generated bone (B) and differentiated into osteocytes that were positive for anti-human-specific mitochondria antibody staining (open arrows in g). Mouse tissue (MT in g) and preimmunoserum control (h) were negative for anti-human-specific mitochondria antibody staining. Single colony-derived SHED were also capable of forming bone (B) on the surface of hydroxyapatite/tricalcium phosphate (HA) to repair critical size of calvarial defects (black line in i) in immunocompromised mice (i, j) same as seen in mixed population of SHED. CB indicates preexisting calvarial bone. The mRNA isolated from two different SHED transplants (T1 and T2) was applied for RT-PCR analysis (k). Both human (h) and mouse (m) BSP and OSC were positively detected on both transplants, suggesting that both human and mouse osteogenic cells involved in the bone formation in the SHED transplants. The mRNA extracted from human (H) and mouse (M) intact bones were used as positive and negative controls for RT-PCR amplification. GAPDH was used for internal control
Figure 3.
Characterization of SHED in vivo. After 8 weeks transplantation in immunocompromised mice, SHED were able to form bone (B) on the surface of hydroxyapatite/tricalcium phosphate (HA). Osteogenic cells were positive for anti-ALP (open arrows in a), BSP (open arrows in b) and type I collagen (open arrows in c) antibody staining. BSP and type I collagen showed a positive staining on the cells in connective tissue (CT) compartment. (d) Negative control of immunohistochemical staining on SHED transplant with preimmuno serum. The expressions of DSP as odontogenic marker were not detected in both bone marrow mesenchymal stem cells (e) and SHED (f). On the other hand, the expressions of BSP were positive in both bone marrow mesenchymal stem cell-mediated bone (g) and SHED-mediated bone (h). BM: bone marrow, HA: HA/TCP
Figure 4.
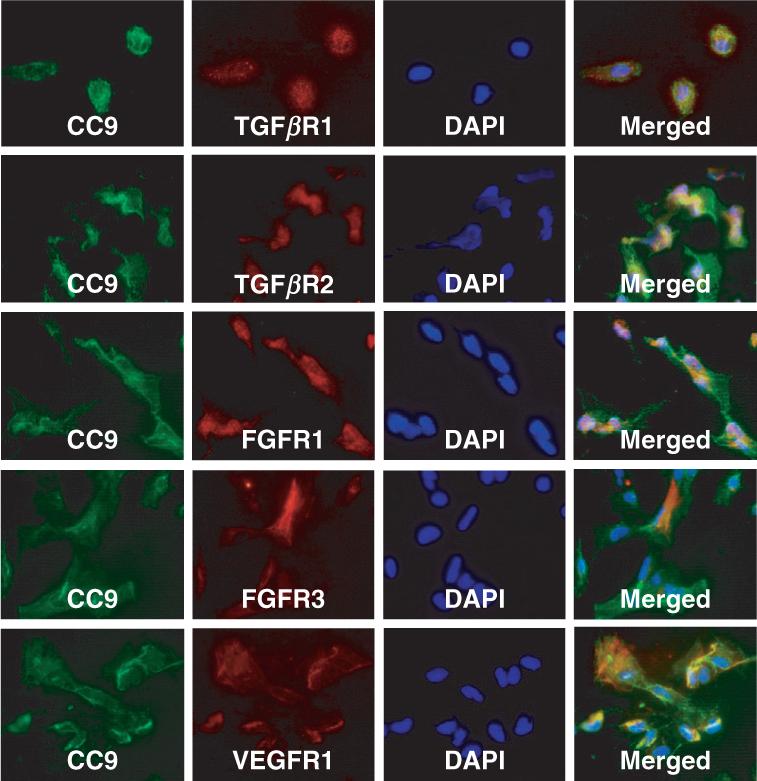
Immunocytochemical characterization of SHED. Osteogenic associated growth factor receptors including TGFβR-I/-II, FGFR-I/-III and VEGFR-I may co-express with CC9/MUC18/CD146, an early marker of mesenchymal stem cells. All those growth factor receptors may also not co-express with CC9/MUC18/CD146, implying a heterogenic property of SHED. Bars in merged images: 50 μm
Discussion
One of the most unique characteristics of SHED is their bone regeneration capacity when transplanted into immunocompromised mice using hydroxyapatite/tricalcium phosphate as a carrier (Miura et al, 2003). In this study we discovered that SHED are capable of repairing critical-size parietal defects in immunocompromised mice. However, SHED-mediated bone lacks hematopoietic marrow elements, unlike the bone/marrow organ-like structure generated by bone marrow mesenchymal stem cells. Clinically, autologous grafts from long bones to orofacial bone defects often result in an unfavorable outcome (Akintoye et al, 2006), which may be due, in part, to the fact that orofacial and long bones originate from neural crest cells and the mesoderm, respectively (Chai et al, 2000; Helms and Schneider, 2003), and mesenchymal stem cells derived from orofacial and long bones show distinctive differentiation traits (Matsubara et al, 2005; Wang et al, 2005; Akintoye et al, 2006). In addition, orofacial bone is not an optimal donor source, due to the limited size. Therefore, SHED, originating from neural crest cells and expressing many neural cell markers, could be a practical resource for repair of orofacial bone defects. There is a great demand for regeneration of orofacial defects caused by trauma, tumor, genetic malformation, and periodontal diseases. From a practical perspective, SHED would be a superior accessible tissue resource for autologous transplantation. Furthermore, SHED are an excellent cell population that maintain a higher proliferation rate than bone marrow mesenchymal stem cells and have the capacity to provide sufficient numbers of cells for clinical therapies (Miura et al, 2003). The novel discovery of SHED-mediated bone formation provided a promising stem cell resource for orofacial bone regeneration.
We have previously reported that in vivo transplanted SHED are capable of generating tiny amount of dentin and organizing significant amount of bones (Miura et al, 2003). This characteristic is distinct to human DPSCs that are able to generate a dentin/pulp-like structure in vivo when hydroxyapatite/tricalcium phosphate was used as a carrier vehicle (Gronthos et al, 2002). When SHED were transplanted into critical-size calvarial defect area, they generate bony tissue to repair the defect but without detectable amount of dentin formation as assessed by DSP immunohistochemical staining. Newly formed dentin is immunopositive for DSP antibody, which distinguishes it from other mineralized tissues such as bone, in which DSP is found below the immunohistochemical detectable level under our experimental condition (Batouli et al, 2003). Although DSP was expressed in bone (Qin et al, 2002), the level of expression may be too low to be detected by immunohistochemical staining (Batouli et al, 2003). Interestingly, although SHED were found to induce recipient cells to form bone structure as indicated in our previous report (Miura et al, 2003) and in this study, here we show that SHED are also capable of differentiating into osteoblast-like cells responding for active new bone formation.
Our previous study demonstrated that DPSCs and periodontal ligament stem cells also express TGFβ, FGF and VEGF receptors (Gronthos et al, 2000; Seo et al, 2004), which may be involved in the recruitment of host cellular components into the transplants (Batouli et al, 2003). Although our studies indicate that SHED, like endothelial cells, express FGF and VEGF receptor, there is no evidence indicating that SHED are able to participate in blood vessel formation in vivo. FGF and VEGF are also considered to be potent mitogens of endothelial cells required for tissue vascularization and organogenesis (Bouma-ter Steege et al, 2001; Traver and Zon, 2002). The signaling cascade triggered by TGFβ, FGF and VEGF may govern proliferation and differentiation of SHED and their participation in the generation of the bone and dentin tissues. This study suggests that SHED are a distinctive population of postnatal stem cells. Further studies are required to elucidate the nature of SHED.
Acknowledgements
We thank Dr Larry Fisher for providing ALP, BSP and collagen type I antibodies, and Dr Stan Gronthos for providing CC9/MUC18/CD146 antibody. This work was supported by the University of Southern California School of Dentistry and the Division of Intramural Research, the National Institute of Dental and Craniofacial Research, the National Institutes of Health, Department of Health and Human Service.
References
- Akintoye SO, Lam T, Shi S, Brahim J, Collins MT, Robey PG. Skeletal site-specific characterization of orofacial and iliac crest human bone marrow stromal cells in same individuals. Bone. 2006;38:758–768. doi: 10.1016/j.bone.2005.10.027. [DOI] [PubMed] [Google Scholar]
- Batouli S, Miura M, Brahim J, et al. Comparison of stem-cell-mediated osteogenesis and dentinogenesis. J Dent Res. 2003;82:976–981. doi: 10.1177/154405910308201208. [DOI] [PubMed] [Google Scholar]
- Bouma-ter Steege JC, Mayo KH, Griffioen AW. Angiostatic proteins and peptides. Crit Rev Eukaryot Gene Expr. 2001;11:319–334. [PubMed] [Google Scholar]
- Chai Y, Jiang X, Ito Y, et al. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–1679. doi: 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- Cowan CM, Shi YY, Aalami OO, et al. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol. 2004;22:560–567. doi: 10.1038/nbt958. [DOI] [PubMed] [Google Scholar]
- Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronthos S, Brahim J, Li W, et al. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531–535. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- Helms JA, Schneider RA. Cranial skeletal biology. Nature. 2003;423:326–331. doi: 10.1038/nature01656. [DOI] [PubMed] [Google Scholar]
- Krebsbach PH, Kuznetsov SA, Satomura K, Emmons RV, Rowe DW, Robey PG. Bone formation in vivo: comparison of osteogenesis by transplanted mouse and human marrow stromal fibroblasts. Transplantation. 1997;63:1059–1069. doi: 10.1097/00007890-199704270-00003. [DOI] [PubMed] [Google Scholar]
- Krebsbach PH, Mankani MH, Satomura K, Kuznetsov SA, Robey PG. Repair of craniotomy defects using bone marrow stromal cells. Transplantation. 1998;66:1272–1278. doi: 10.1097/00007890-199811270-00002. [DOI] [PubMed] [Google Scholar]
- Matsubara T, Suardita K, Ishii M, et al. Alveolar bone marrow as a cell source for regenerative medicine: differences between alveolar and iliac bone marrow stromal cells. J Bone Miner Res. 2005;20:399–409. doi: 10.1359/JBMR.041117. [DOI] [PubMed] [Google Scholar]
- Miura M, Gronthos S, Zhao M, et al. SHED: Stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100:5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C, Brunn JC, Cadena E, et al. The expression of dentin sialophosphoprotein gene in bone. J Dent Res. 2002;81:392–394. doi: 10.1177/154405910208100607. [DOI] [PubMed] [Google Scholar]
- Seo BM, Miura M, Gronthos S, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- Traver D, Zon LI. Walking the walk: migration and other common themes in blood and vascular development. Cell. 2002;108:731–734. doi: 10.1016/s0092-8674(02)00686-4. [DOI] [PubMed] [Google Scholar]
- Wang L, Liu Y, Kalajzic Z, Jiang X, Rowe DW. Heterogeneity of engrafted bone-lining cells after systemic and local transplantation. Blood. 2005;106:3650–3657. doi: 10.1182/blood-2005-02-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]



