Abstract
A highly convergent, enantioselective total synthesis of the potent antitumor agent apoptolidin A, has been completed. The key transformations include highly selective glycosylations to attach the C27 disaccharide and the C9 6′-deoxy-l-glucose, a cross metathesis to incorporate the C1-C10 trienoate unit, and a Yamaguchi macrolactonization to complete the macrocycle. Twelve stereocenters in the polypropionate segments and sugar units were established through diastereoselective chlorotitanium enolate aldol reactions.
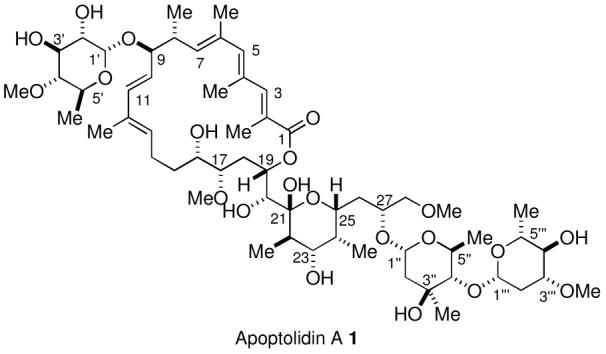
Apoptolidins A-D (Figure 1) are secondary metabolites of Nocardiopsis sp. which are potent, selective inducers of programmed cell death in rat glia cells transformed with the adenovirus E1A oncogene. Apoptolidin A was isolated by Hayakawa in 1997, and its structure was elucidated by a combination of NMR spectroscopy and molecular model techniques.1 The minor metabolites apoptolidins B, C, and D were recently isolated and identified by Wender.2,3 Importantly, apoptolidin A has no effect on normal cells at concentrations as high as 88 μM, while the E1A transformed glial cells undergo apoptotic cell death when exposed to apoptolidin A at concentrations of 10 nM.1 This selective activity of cancer therapeutics is a major factor in minimizing side effects of chemotherapeutic agents.
Figure 1.
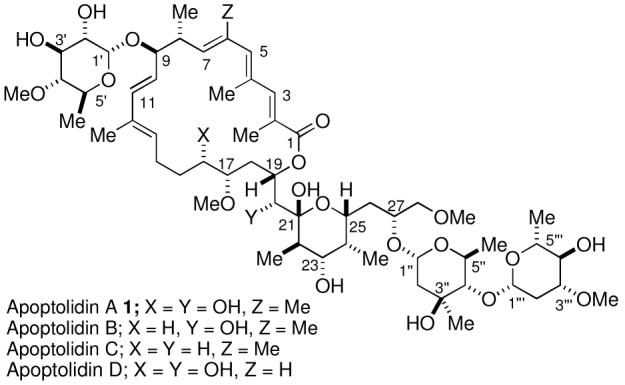
Structure of the Apoptolidins
The apoptolidins have been the subject of intense synthetic and biological4 investigations because of their potential as agents for the treatment of cancer.5 Two total syntheses of apoptolidin A,6 several syntheses of the aglycone, known as apoptolidinone,7 including one from our laboratory,8 a number of partial syntheses,9 as well as selective synthetic modifications10 have been reported.
Apoptolidin A (1, Scheme 1) is comprised of a macrocyclic lactone and two unusual carbohydrate units attached through glycosidic linkages at the C9 and C27 hydroxyl groups. Our highly convergent approach to the synthesis of apoptolidin A focused initially on the individual preparation of the aglycone, apoptolidinone,8 and the carbohydrate units,9e with the expectation that the approach to apoptolidinone could be adapted to a synthesis of apoptolidin A by a late-stage attachment of the sugar units to advanced intermediates in the apoptolidinone synthesis.
Scheme 1. Retrosynthesis for Apoptolidin A.
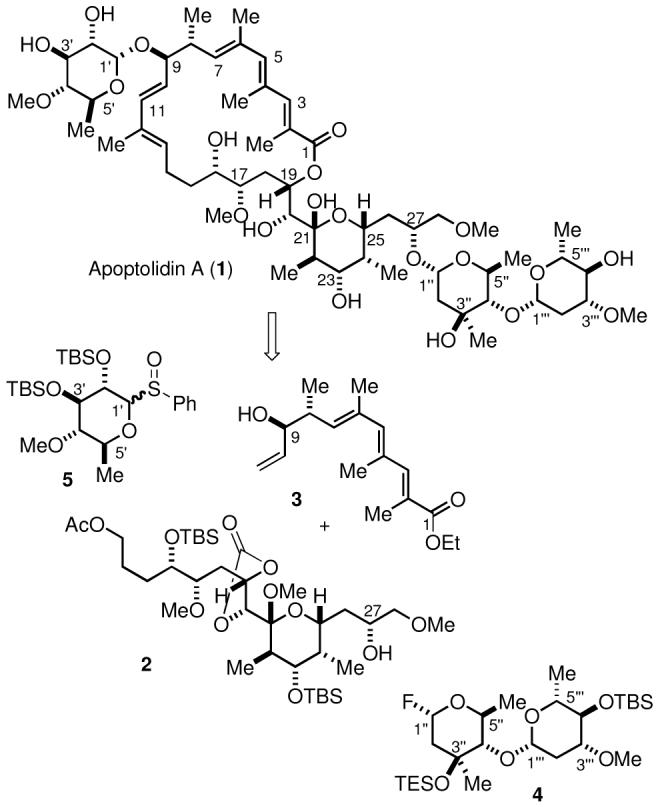
The glycosyl fluoride 4 derived from L-olivomycose and D-oleandrose would be utilized to attach the required disaccharide at C27 of mixed ketal 2 or a similar advanced intermediate prior to incorporation of the trienoate 3. Sulfoxide 5 would serve as the glycosylating agent for the incorporation of the 6′-deoxy-L-glucose unit at C9 either immediately before or after assembly of the C1-C10 and C10-C28 subunits via a stereoselective olefin cross-metathesis reaction. Mixed ketal 2 and trienoate 3 were advanced intermediates in our recently reported synthesis of apoptolidinone.8
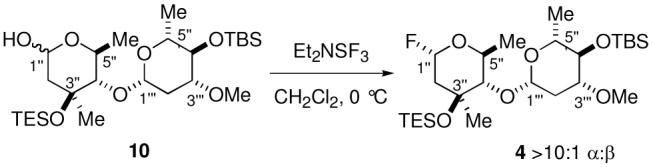
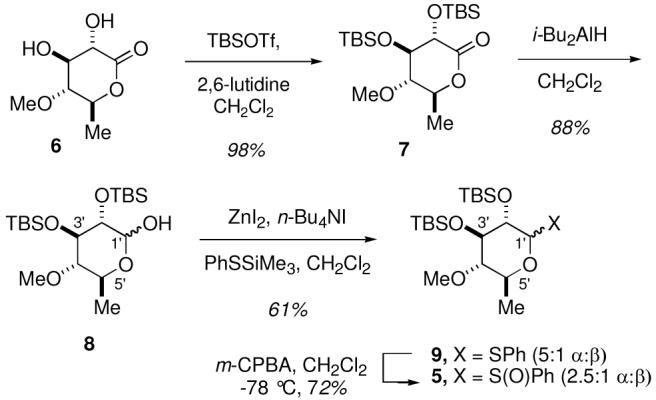
The required glycosyl donors 4 and 5 for the incorporation of the sugar units were obtained as illustrated in Schemes 2 and 3. Each of the individual monosaccharide units were obtained by de novo synthesis through the application of a chlorotitanium glycolate aldol approach to establish the C4 and C5 stereocenters of each of the monosaccharides.9e
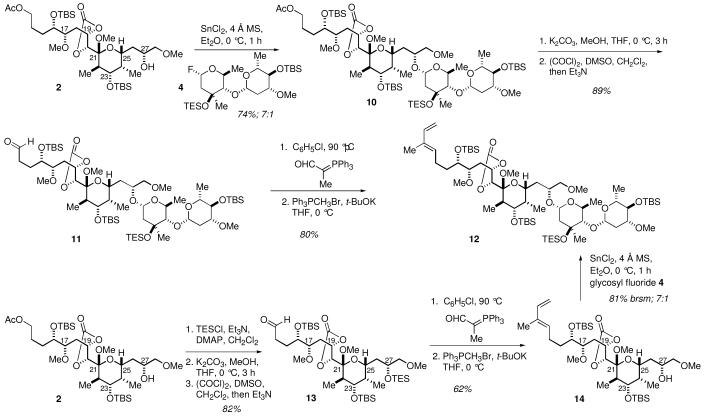
Scheme 2. Synthesis of the C27 Disaccharide.
Scheme 3. Synthesis of the C9 Sugar Unit.
The necessary glycosyl fluoride 4 was accessed from the protected disaccharide 10 as shown in Scheme 2. Direct exposure of the hemiacetal 10 to DAST provided the glycosyl fluoride 4 (>10:1 α:β).6c Since the glycosyl fluoride was somewhat unstable, it was utilized in the subsequent glycosylation of the C27 hydroxyl group without further purification.
The synthesis of 6′-deoxy-L-glucose (the C9 sugar unit) donor 5 was readily accomplished from lactone 69e (Scheme 3). Protection of the diol as the corresponding TBS ether 7 was followed by reduction of the lactone with i-Bu2AlH to deliver the protected 6′-deoxy-L-glucose 8. The hemiacetal 8 was converted to mixed acetal 9 ((5:1 α:β) by exposure to PhSSiMe3 in the presence of ZnI2. The required glycosyl donor 5 was obtained as a 2.5:1 mixture of isomers in 72% yield by oxidation of the thioacetal with m-CPBA.6c
The asssembly of the four key subunits for the completion of the synthesis of apoptolidin A began with the modification of mixed ketal 2. Mixed ketal 2 was an advanced intermediate from the recently completed synthesis of apoptolidinone, and was available in multigram quantities through 17 synthetic steps involving an interative aldol sequence. 8
The key C11-C28 diene 12 was accessible by either of two sequences from hemiketal 2 as illustrated in Scheme 4. Attachment of the C27 disaccharide could be accomplished in high yield (74%) with excellent stereoselectivity (7:1, α:β) by treatment of C27 alcohol 2 with stannous chloride in the presence of glycosyl fluoride 4. The C13 acetate was selectively cleaved in the presence of the C19,20 carbonate whereupon the resultant primary alcohol was oxidized under Swern11 conditions to produce aldehyde 11 in 89% yield. Two sequential Wittig olefinations completed the synthesis of diene 12 in 80% overall yield. Alternately, the diene unit could be incorporated prior to the attachment of the C27 discaccharide. The C27 alcohol 2 was protected as its triethylsilyl ether followed by removal of the C13 acetate and subsequent oxidation of the alcohol to the aldehyde 13. Stabilized Wittig olefination of the aldehyde in chlorobenzene at 90 °C was accompanied by cleavage of the C27 TES ether. Methylenation of the unsaturated aldehyde provided the desired diene alcohol 14. Stereoselective glycosylation of the C27 alcohol 14 as described previously for alcohol 2 delivered diene 12 in excellent yield.
Scheme 4. Attachment of the C27 Disaccharide.
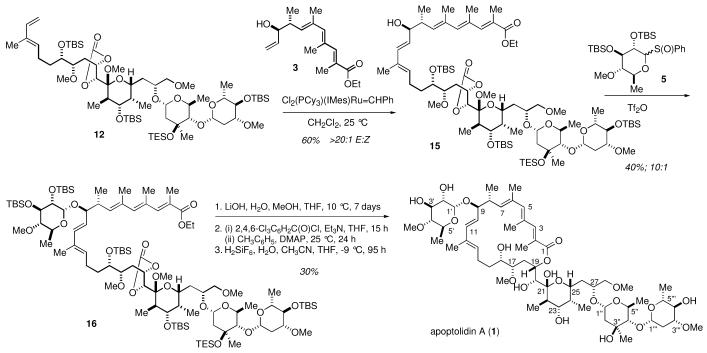
Completion of the synthesis of apoptolidin A is illustrated in Scheme 5. Exposure of the alkenes 38 and 12 to the Grubbs heterocyclic carbene catalyst [Cl2(Cy3P)(IMes)Ru=CHPh]12 provided the desired E isomer 15 through a critical complex cross-metathesis8,13 in good yield (>95:5 E:Z by 1H NMR analysis). An attempted cross metathesis reaction failed when the reaction was performed after the glycosidation of the C9 oxygen. To complete the synthesis of apoptolidin A, attachment of the sugar unit at C9 and macrolactonization were required. A mixture of alcohol 15 anhydride and sulfoxide 5 was exposure to triflic stereoselectively providing the glycoside 16. Treatment of the ester 16 with LiOH at room temperature rapidly cleaved the carbonate group and eventually the ester, to give a good yield of the desired seco acid. Regioselective macrolactonization at C19 proceeded smoothly under Yamaguchi’s conditions to deliver the macrolactone.14 Finally, cleavage of the silyl ethers and hydrolysis of the mixed methyl acetal were effected in one operation using H2SiF6 to furnish apoptolidinon A (1). Synthetic apoptolidin A was identical to an authentic sample.
Scheme 5. Synthesis of Apoptolidin A.
The successful total synthesis utilized diastereoselective chlorotitanium aldol reactions to establish twelve (carbons 4′,5′; 4′′,5′′; 4′′′,5′′′, 8,9; 22, 23; and 24, 25) of the 24 stereocenters of apoptolidin A and exploited a complex cross metathesis to assemble the two major fragments and construct the C10-C13 diene unit.
Supplementary Material
Acknowledgment
Financial support from the National Cancer Institute (CA63572) is gratefully acknowledged. We thank Professor Paul A. Wender and Dr. Kate Longcore, Stanford University, for generously supplying an authentic sample of apoptolidin A.
References
- 1.(a) Kim JW, Adachi H, Shin-ya K, Hayakawa Y, Seto H. J. Antibiot. 1997;50:628. doi: 10.7164/antibiotics.50.628. [DOI] [PubMed] [Google Scholar]; (b) Hayakawa Y, Kim JW, Adachi H, Shin-ya K, Fujita K, Seto H. J. Am. Chem. Soc. 1998;120:3524. [Google Scholar]
- 2.Wender PA, Sukopp M, Longcore K. Org. Lett. 2005;7:3025. doi: 10.1021/ol051074o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wender PA, Longcore K. Org. Lett. 2007;9:691. doi: 10.1021/ol0630245. [DOI] [PubMed] [Google Scholar]
- 4.(a) Salomon AR, Voehringer DW, Herzenberg LA, Khosla C. Chem. and Biol. 2001;8:71. doi: 10.1016/s1074-5521(00)00057-0. [DOI] [PubMed] [Google Scholar]; (b) Salomon AR, Zhang YB, Seto H, Khosla C. Org. Lett. 2001;3:57. doi: 10.1021/ol006767d. [DOI] [PubMed] [Google Scholar]; (c) Salomon AR, Voehringer DW, Herzenberg LA, Khosla C. Proc. Nat. Acad. Sci. 2000;97:14766. doi: 10.1073/pnas.97.26.14766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daniel PT, Koert U, Schuppan J. Angew. Chem Int. Ed. 2006;45:872. doi: 10.1002/anie.200502698. [DOI] [PubMed] [Google Scholar]
- 6.(a) Wehlan H, Dauber M, Fernaud MTM, Schuppan J, Mahrwald R, Ziemer B, Garcia MEJ, Koert U. Angew. Chem., Int. Ed. 2004;43:4597. doi: 10.1002/anie.200460172. [DOI] [PubMed] [Google Scholar]; (b) Wehlan H, Dauber M, Fernaud MTM, Schuppan J, Kieper S, Mahrwald R, Garcia MEJ, Koert U. Chem. Eur. J. 2006;12:7378. doi: 10.1002/chem.200600462. [DOI] [PubMed] [Google Scholar]; (c) Nicolaou KC, Fylaktakidou KC, Monenschein H, Li YW, Weyershausen B, Mitchell HJ, Wei HX, Guntupalli P, Hepworth D, Sugita K. J. Am. Chem. Soc. 2003;125:15433. doi: 10.1021/ja0304953. [DOI] [PubMed] [Google Scholar]; (d) Nicolaou KC, Li YW, Sugita K, Monenschein H, Guntupalli P, Mitchell HJ, Fylaktakidou KC, Vourloumis D, Giannakakou P, O’Brate A. J. Am. Chem. Soc. 2003;125:15443. doi: 10.1021/ja030496v. [DOI] [PubMed] [Google Scholar]; (e) Nicolaou KC, Li Y, Fylaktakidou KC, Mitchell HJ, Sugita K. Angew. Chem., Int. Ed. 2001;40:3854. doi: 10.1002/1521-3773(20011015)40:20<3854::AID-ANIE3854>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]; (f) Nicolaou KC, Li Y, Fylaktakidou KC, Mitchell HJ, Wei HX, Weyershausen B. Angew. Chem., Int. Ed. 2001;40:3849. [PubMed] [Google Scholar]
- 7.(a) Schuppan J, Wehlan H, Keiper S, Koert U. Angew. Chem., Int. Ed. 2001;40:2063. doi: 10.1002/1521-3773(20010601)40:11<2063::AID-ANIE2063>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]; (b) Wu B, Liu QS, Sulikowski GA. Angew. Chem., Int. Ed. 2004;43:6673. doi: 10.1002/anie.200461469. [DOI] [PubMed] [Google Scholar]; (c) Schuppan J, Wehlan H, Keiper S, Koert U. Chem. Eur. J. 2006;12:7364. doi: 10.1002/chem.200600461. [DOI] [PubMed] [Google Scholar]; (d) Ghidu VP, Wang JQ, Wu B, liu QS, Jacobs A, Marnett LJ, Sulikowski GA. J. Org. Chem. 2008;73:4949. doi: 10.1021/jo800545r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crimmins MT, Christie HS, Chaudhary K, Long A. J. Am. Chem. Soc. 2005;127:13810. doi: 10.1021/ja0549289. [DOI] [PubMed] [Google Scholar]
- 9.(a) Abe K, Kato K, Arai T, Rahim MA, Sultana I, Matsumura S, Toshima K. Tetrahedron Lett. 2004;45:8849. [Google Scholar]; (b) Bouchez LC, Vogel P. Chem. Eur. J. 2005;11:4609. doi: 10.1002/chem.200500165. [DOI] [PubMed] [Google Scholar]; (c) Chang SS, Xu J, Loh TP. Tetrahedron Lett. 2003;44:4997. [Google Scholar]; (d) Craita C, Didier C, Vogel P. J. Chem. Soc., Chem. Commun. 2007:2411. doi: 10.1039/b701293d. [DOI] [PubMed] [Google Scholar]; (e) Crimmins MT, Long A. Org. Lett. 2005;7:4157. doi: 10.1021/ol0515107. [DOI] [PubMed] [Google Scholar]; (f) Daniel PT, Koert U, Schuppan J. Angew. Chem., Int. Ed. 2006;45:872. doi: 10.1002/anie.200502698. [DOI] [PubMed] [Google Scholar]; (g) Jin BH, Liu QS, Sulikowski GA. Tetrahedron. 2005;61:401. [Google Scholar]; (h) Nicolaou KC, Li YW, Weyershausen B, Wei HX. J. Chem. Soc., Chem. Commun. 2000:307. [Google Scholar]; (i) Paquette WD, Taylor RE. Org. Lett. 2004;6:103. doi: 10.1021/ol0361397. [DOI] [PubMed] [Google Scholar]; (j) Schuppan J, Ziemer B, Koert U. Tetrahedron Lett. 2000;41:621. [Google Scholar]; (k) Sulikowski GA, Lee WM, Jin B, Wu B. Org. Lett. 2000;2:1439. doi: 10.1021/ol005769v. [DOI] [PubMed] [Google Scholar]; (l) Toshima K, Arita T, Kato K, Tanaka D, Matsumura S. Tetrahedron Lett. 2001;42:8873. [Google Scholar]; (m) Handa M, Scheidt KA, Bossart M, Zheng N, Roush WRJ. Org. Chem. 2008;73:1031. doi: 10.1021/jo702250z. [DOI] [PubMed] [Google Scholar]; (n) Handa M, Smith III, W J, Roush WR. J. Org. Chem. 2008;73:1036. doi: 10.1021/jo7022526. [DOI] [PubMed] [Google Scholar]
- 10.(a) Wender PA, Jankowski OD, Tabet EA, Seto H. Org. Lett. 2003;5:2299. doi: 10.1021/ol0346335. [DOI] [PubMed] [Google Scholar]; (b) Wender PA, Jankowski OD, Tabet EA, Seto H. Org. Lett. 2003;5:487. doi: 10.1021/ol027366w. [DOI] [PubMed] [Google Scholar]; (c) Wender PA, Gulledge AV, Jankowski OD, Seto H. Org. Lett. 2002;4:3819. doi: 10.1021/ol0266222. [DOI] [PubMed] [Google Scholar]
- 11.Swern D, Mancuso AJ, Huang S. J. Org. Chem. 1978;43:2480. [Google Scholar]
- 12.Scholl M, Ding S, Lee CW, Grubbs RH. Org. Lett. 1999;1:953. doi: 10.1021/ol990909q. [DOI] [PubMed] [Google Scholar]
- 13.Chatterjee AK, Choi TL, Sanders DP, Grubbs RH. J. Am. Chem. Soc. 2003;125:11360. doi: 10.1021/ja0214882. [DOI] [PubMed] [Google Scholar]
- 14.Inanaga J, Hirata K, Saeki H, Katsuki T, Yamaguchi M. Bull. Chem. Soc. Jpn. 1979;52:1989. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.