Summary
Coronatine is an important virulence factor produced by several pathovars of the bacterial pathogen Pseudomonas syringae. The structure of coronatine is similar to a class of plant hormones called jasmonates (JAs). An important step in JA signaling is the SCFCOI1 E3 ubiquitin ligase-dependent degradation of JAZ repressor proteins. We recently showed that jasmonoyl-isoleucine (JA-Ile) could promote physical interaction between Arabidopsis JAZ1 and COI1 (the F-box component of SCFCOI1) proteins, and that the JA-Ile-dependent COI1-JAZ1 interaction could be reconstituted in yeast cells (i.e., in the absence of other plant proteins). Here, we show that coronatine, but not its two biosynthetic precursors, could also promote interaction between Arabidopsis COI1 and multiple JAZ proteins. The carboxyl terminal Jas motif, but not the N-terminal (NT) domain or central ZIM domain of JAZ proteins, is critical for JA-Ile/coronatine-dependent interaction with COI1. Two positively charged amino acid residues in the Jas domain were identified as being essential for coronatine-dependent COI1-JAZ interactions. These two mutations did not affect the ability of JAZ1 and JAZ9 to interact with the transcription factor AtMYC2. Importantly, transgenic Arabidopsis plants expressing JAZ1 carrying these two mutations exhibited JA-insensitive phenotypes, including male sterility and enhanced resistance to P. syringae infection. These results not only suggest that coronatine and JA-Ile target the physical interaction between COI1 and the Jas domain of JAZ repressors, but also illustrate a critical role of positively charged amino acids in the Jas domain in mediating JA-Ile/coronatine-dependent JAZ interaction with COI1.
Keywords: Coronatine, jasmonate, COI1, JAZ, plant immunity, Pseudomonas syringae
Introduction
Jasmonates regulate a wide range of biological processes in plants, from sexual reproduction to herbivore defense and pathogen responses (Browse, 2005; Howe and Jander, 2008; Browse and Howe, 2008). A critical component of JA signaling is COI1, which is the F-box protein subunit of SCFCOI1, a member of the Skip/Cullin/F-box (SCF) family of E3 ubiquitin ligases (Xie et al., 1998; Li et al., 2002). E3 ubiquitin ligases are involved in the ubiquitination of specific protein substrates, targeting them to the 26S proteasome for degradation. The cognate substrates of SCFCOI1 were identified recently; they are members of the JAZ (jasmonate ZIM-domain) protein family (Chini et al. 2007; Thines et al. 2007; Yan et al., 2007). Several JAZ proteins, including JAZ1, JAZ3, and JAZ6, have been shown to be degraded in Arabidopsis in response to JA treatments and this degradation is dependent on SCFCOI1 and the 26S proteasome (Chini et al. 2007; Thines et al. 2007).
All JAZ proteins contain two highly conserved sequence motifs: the TIF[F/Y]XG signature defines the so-called ZIM domain (Vanholme et al., 2007) in the central portion of the protein, and the C-terminal Jas motif having the consensus sequence SLX2FX2KRX2RX5PY (Yan et al., 2007). In addition, Thines and coworkers noticed a weakly conserved region at the N-terminus (referred to “NT” hereinafter). The JAZ C-terminal region, including the Jas domain, seems particularly important for controlling the SCFCOI1-dependent stability of JAZ proteins because JAZ1 and JAZ3 derivatives lacking this region are no longer degraded in response to JA treatment (Chini et al., 2007; Thines et al., 2007; Yan et al., 2007). Moreover, mutant or transgenic plants expressing JAZ1, JAZ3, and JAZ10 lacking the Jas domain exhibit constitutive repression of JA signaling and are insensitive to JA (Chini et al. 2007; Thines et al., 2007, Yan et al., 2007). Taken together, these results suggest that the Jas domain controls the stability of JAZ proteins in response to a JA signal in an SCFCOI1-dependent manner, whereas NT and ZIM domains are involved in repressing JA signaling. However, the specific mechanisms by which NT, ZIM, and Jas domains control JAZ stability and/or repressor function are not yet understood.
Coronatine is produced by several pathovars of the bacterial pathogen P. syringae. This toxin plays multiple functions in disease: suppressing plant defenses locally and systemically and inducing disease-associated tissue necrosis and chlorosis (Bender et al., 1999; Mittal and Davis, 1995; Block et al., 2005; Brooks et al., 2005; Cui et al., 2005; Melotto et al., 2006, 2008; Underwood et al. 2007). The overall structure of coronatine, consisting of coronafacic acid (CFA) and coronamic acid (CMA) (Bender et al., 1999), shows structural similarity to jasmonates (JAs), most notably JA-Ile (Staswick, 2008). Indeed, coronatine induces a large number of JA-responsive genes and other JA-related responses in plants (Bender et al., 1999; Feys et al., 1994; Lauchli and Boland, 2003; Uppalapati et al., 2005; Thilmony et al., 2006 Zhao et al., 2003). In addition, the virulence function of coronatine requires COI1 (Feys et al., 1994; Kloek et al., 2001; Zhao et al. 2003). Thus, it has been hypothesized that coronatine mimics jasmonates to exert its virulence function. However, the specific host targets of coronatine have not yet been identified.
Chini and colleagues (2007) recently showed that purified recombinant JAZ3 interacts with COI1 produced in wheat germ in vitro transcription/translation extract in the absence of exogenous jasmonates. In these experiments, the N-terminal/central regions (containing NT and ZIM domains) of JAZ3 were sufficient for interaction with COI1, whereas the C-terminus (containing the Jas domain) interacted with AtMYC2, a transcription factor known to be involved in JA signaling. On the other hand, Thines and co-workers (2007) showed that JAZ1 interaction with COI1 required JA-Ile. Other commonly used jasmonates, such as jasmonic acid (JA), methyl jasmonate (MeJA), or 12-oxo phytodienoic acid (OPDA), were not effective in promoting physical interaction between COI1 and JAZ1. Furthermore, the JA-Ile-dependent interaction between COI1 and JAZ1 was observed in protein pull down assays using total plant protein extracts, as well as in yeast cells expressing COI1 and JAZ1 (i.e., in the absence of any other plant proteins), strongly suggesting that the COI1-JAZ1 complex is a site for JA-Ile perception. However, it is currently unclear whether the JA-Ile dependence represents a unique situation for the COI1-JAZ1 interaction or a general mechanism applicable to COI1 interaction with other JAZ proteins. Similarly it is not known which domain in JAZ proteins mediates JA-Ile-dependent interaction with COI1.
In this study, we show that (i) coronatine mimics JA-Ile in its ability to promote the physical interaction between COI1 and JAZ proteins; (ii) the requirement of JA-Ile or coronatine is a general mechanism for COI1 interaction with multiple JAZ proteins, (iii) two positively charged amino acid residues in the Jas domain are essential for coronatine-dependent COI1-JAZ interactions; (iv) these two mutations do not affect the ability of JAZ1 and JAZ9 to interact with the transcription factor AtMYC2; and (v) transgenic Arabidopsis plants expressing JAZ1 carrying these two mutations exhibited JA-insensitive phenotypes, including enhanced resistance to P. syringae infection.
Results
Coronatine promotes interaction between COI1 and JAZ1
To test the hypothesis that coronatine functionally mimics JA-Ile and facilitates the formation of the COI1-JAZ1 complex independent of other plant proteins, we examined COI1-JAZ1 interaction in yeast cells. As shown in Figure 1a, coronatine could effectively promoted COI1-JAZ1 interaction. Neither CFA nor CMA, the two nonfunctional precursors of coronatine biosynthesis (Brooks et al., 2005), had this activity, highlighting the importance of an intact coronatine structure for both the biological activity and promotion of the COI1-JAZ1 interaction in yeast cells (Figure 1a).
Figure 1. JA-Ile/coronatine-dependent interaction between COI1 and JAZ proteins in yeast.
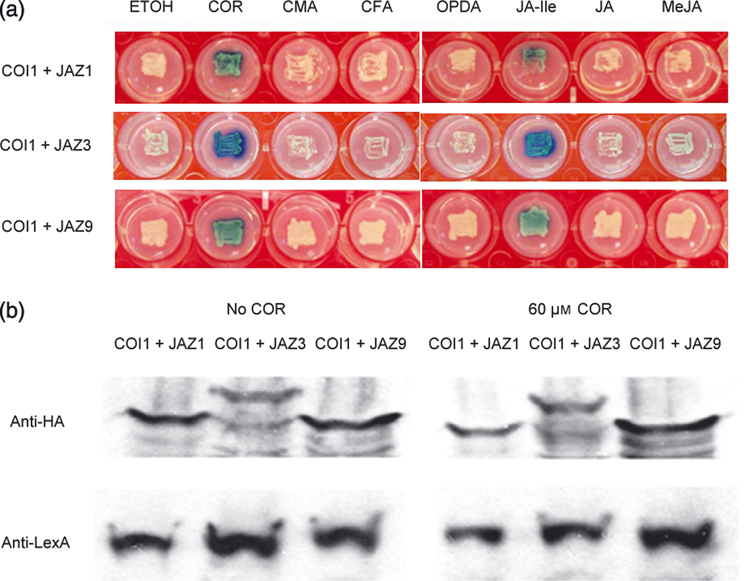
(a) Yeast strains carrying pGILDA-COI1 and pB42AD-JAZ were streaked on Y2H agar medium (placed in wells of a microtiter plate). Blue colony color indicates positive interaction, whereas white colony color indicates no interaction. The chemical coronatine (COR), JA, MeJA, JA-Ile, OPDA, CMA, or CFA were added to the medium at a concentration of 60 µM. Ethanol is the solvent for the chemicals used. Pictures were taken at day 4. (b) Western blotting analysis of the yeast strains used in (a) to detect protein expression. The COI1 and JAZ proteins expressed from pGILDA-COI1 and pB42AD-JAZ were detected as fusion proteins using anti-LexA and anti-HA antibodies, respectively. Each lane was loaded with total protein extract from one strain.
COI1 interaction with JAZ1 could result in degradation of JAZ1 in yeast cells if the Arabidopsis COI1 protein were able to form a functional E3 ubiquitin ligase complex through interaction with heterologous yeast SCF subunits. To examine this possibility, we analyzed expression of COI1 and/or JAZ1 proteins by western blot analysis. As shown in Figure 1b, co-expression of COI1 with JAZ1 did not lead to degradation of JAZ1 with or without coronatine, suggesting that COI1 does not form a functional E3 ubiquitin ligase in yeast cells or, if such a functional ligase is formed, the JAZ1 protein is not degraded by the yeast 26S proteasome.
JA-Ile/coronatine-dependent interaction is not unique to the COI1-JAZ1 interaction
Yeast two-hybrid (Y2H) screens of an Arabidopsis cDNA library (Holt et al., 2003) for coronatine-dependent COI1 interactors also yielded JAZ1 as well as several other JAZ proteins. Specifically, JAZ9 (formerly COI1-Interacting Protein 1; Melotto and He, 2007) clones were recovered in the Y2H screen in which the medium was supplemented with 1.5 µM coronatine; and JAZ1, JAZ2, and JAZ3 clones were isolated in the presence of 50 µM coronatine (data not shown). Furthermore, either coronatine or JA-Ile could promote these interactions, whereas JA, MeJA, OPDA, CFA or CMA did not (see Figure 1a for COI1-JAZ3 and COI1-JAZ9 interactions). Thus, the requirement of JA-Ile or coronatine is not unique to the COI1-JAZ1 interaction, but rather extends to several JAZ family proteins. Western blot analysis showed that co-expression of COI1 with JAZ3 or JAZ9 did not lead to degradation of JAZ3 or JAZ9 in yeast cells in the presence of coronatine (Figure 1b), again similar to what was observed for the COI1-JAZ1 interaction.
We next determined the concentrations of coronatine required for detecting various COI1-JAZ interactions. As low as 1.5 µM of coronatine in the medium was sufficient for detecting the COI1-JAZ9 interaction, whereas 30 µM coronatine was required for detecting the COI1-JAZ1 interaction (Figure 2). However, expression of JAZ1 appeared to be toxic to yeast cells, judging from slow yeast growth, which could have negatively affected detection of this interaction, especially at lower coronatine concentrations. We also compared the relative concentrations of coronatine and JA-Ile required for detecting COI1-JAZ9 interaction in yeast. Coronatine appears to be more effective than JA-Ile in these assays (Figure 2), suggesting that either coronatine is more potent than JA-Ile in promoting COI1-JAZ interactions, or yeast cells take up and/or metabolize coronatine and JA-Ile to varying extents.
Figure 2.
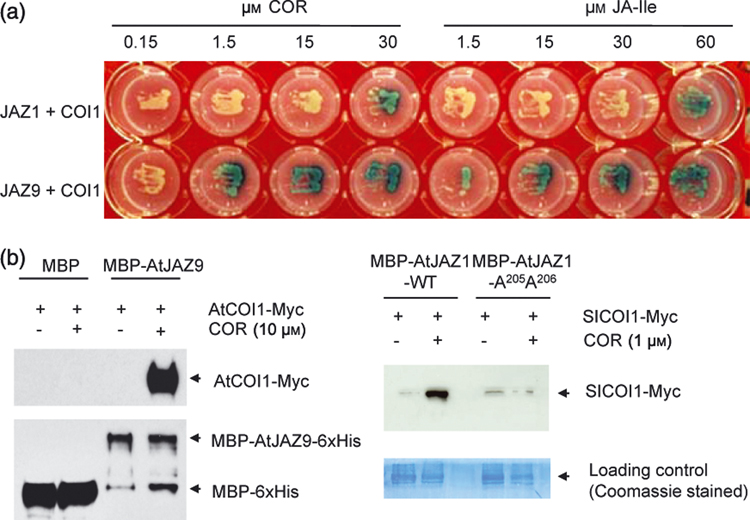
(a) The dose-response relationships for COI1 interaction with JAZ1 and JAZ9 in the presence of coronatine (COR) or JA-Ile. See Figure 1A for experimental setup. (b) Protein pull-down assays. Left, purified recombinant Arabidopsis JAZ1 protein (fused to MBP and 6xHis) was used to pull down COI1-Myc fusion protein from total Arabidopsis leaf extract in a coronatine (COR)-dependent manner. As control, purified recombinant MBP-6xHis protein did not pull down COI1-Myc. COI1-Myc and MBP-JAZ-6xHis were detected by western blotting using Myc epitope and MBP antibodies, respectively. Right, purified recombinant Arabidopsis JAZ1 protein (fused to MBP and 6xHis) was used to pull down SICOI1-Myc fusion protein from total tomato leaf extract in the presence of COR). In contrast, purified recombinant MBP-JAZ1-A205A206-6xHis protein did not pull down SICOI1-Myc. SICOI1-Myc was detected by western blotting using the Myc epitope antibody.
In a previous study, recombinant tomato SlJAZ1 protein (fused to maltose-binding protein [MBP] and the 6xHis epitope tag) was used successfully to pull down the tomato SlCOI1 protein (fused to the Myc epitope tag) from total tomato leaf extract in the presence of JA-Ile (Thines et al., 2007). In this study, we performed similar protein pull-down assays using Arabidopsis COI1 and JAZ proteins. As shown in Figure 2b, the COI1 protein (fused to the Myc epitope tag) in Arabidopsis leaf extract could be pulled down using the recombinant JAZ9 protein (fused to MBP and the 6xHis epitope tag). Moreover, recombinant Arabidopsis JAZ1 protein (fused to MBP and the 6xHis tag) could even be used to pull down tomato SICOI1 (fused to the Myc tag), suggesting cross-species similarities in COI1-JAZ interactions (Figure 2b).
The C-terminal Jas domain, but not the NT or ZIM domain, is necessary and sufficient for interaction between COI1 and multiple JAZ proteins
Chini and colleagues (2007) have recently shown that the Arabidopsis JAZ3 protein could interact with COI1 without exogenous jasmonates and, furthermore, that COI1 interacts with the N-terminal and central portions (including domains NT and ZIM) of the JAZ3 protein. However, our sequence analysis of 32 COI1-interacting JAZ9 clones identified in the Y2H screening showed that the C-terminal polypeptides excluding the ZIM domain was sufficient for coronatine-dependent interaction with COI1 (Figure S1). To determine whether the C-terminus-dependent interaction was unique to JAZ9 or a general feature of COI1-JAZ interactions, we constructed several precise deletion derivatives of JAZ1, JAZ3, and JAZ9 and analyzed their abilities to interact with COI1. Deletion of the NT and/or ZIM domain in JAZ1 or JAZ9 did not affect the interaction with COI1 (Figure 3 B). Expression of COI1 and these truncated JAZ proteins were all detected in yeast (Figure 3c,d). Furthermore, we found that the C-terminus was sufficient for coronatine-dependent interaction between COI1 and JAZ1, JAZ3, and JAZ9 (Figure 3b,d). We conclude that it is the C-terminal Jas domain, not the NT or ZIM domain, that mediates JA-Ile- and coronatine-dependent JAZ interaction with COI1.
Figure 3. Requirement of the Jas domain for coronatine-dependent interaction between COI1 and JAZ1, 3, and 9.
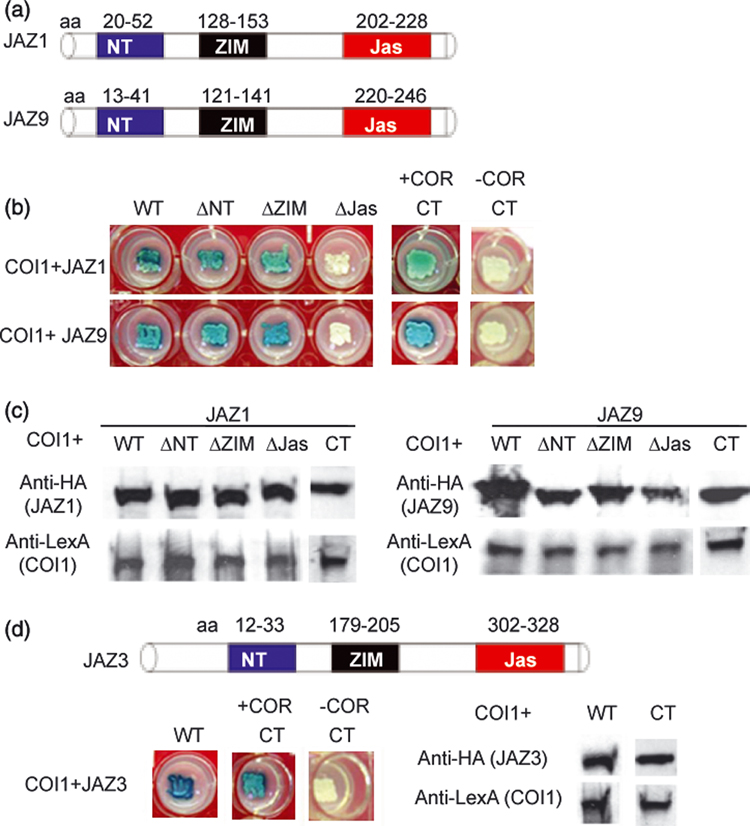
(a) A diagram showing domains (NT, ZIM, Jas) of the JAZ1 and JAZ9 proteins. Numbers indicate the amino acid positions in respective JAZ proteins. (b) Y2H assay of JAZ1 and JAZ9 interaction with COI1. Blue colony color indicates positive interaction, white colony color indicates no interaction. (c) Western blotting analysis of the yeast strains expressing COI1 in combination with various JAZ1 and JAZ9 constructs. Each lane was loaded with total protein extract from one strain and probed with anti-HA to detect the JAZ1 and JAZ9 proteins or anti-LexA to detect COI1 protein. (d) A diagram of JAZ3 and Y2H and western blot results for JAZ3. See panels (a) to (c) for experimental setup. Western blot results for WT, ΔNT, ΔZIM, and ΔJas of JAZ1 and JAZ9 were from the same experiment, whereas western blot results for Jas-containing C-terminus (CT) of JAZ, JAZ3, and JAZ9 were from a separate experiment.
Identification of specific amino acid residues in the Jas domain that are important for COI1-JAZ1 interaction
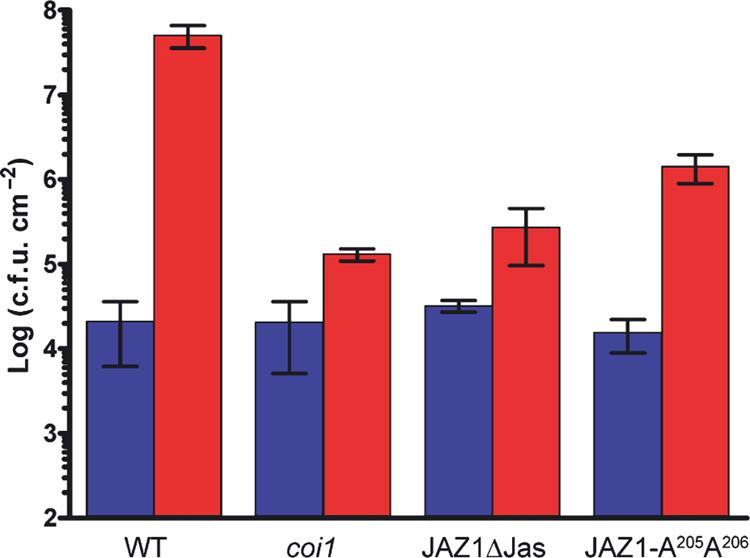
Because the Jas motif is the only conserved sequence in the C-terminal region of JAZ1, 3, 9, we conducted further mutagenesis experiments to identify specific amino acid residues in this motif important for COI1-JAZ interaction. We have previously shown that approximately 25% of T1 transgenic Arabidopsis plants overexpressing a truncated JAZ1 lacking the entire Jas domain (JAZ1Δ3A) exhibited JA-insensitivity, phenocopying the coi1 mutants (e.g., appearance of male-sterile phenotype and increased resistance to Pst DC3000 infection; Thines et al., 2007). To find out whether smaller changes in the Jas domain could also produce the same phenotype, we generated 10 variants of the JAZ1 cDNA encoding proteins with smaller deletions or alanine substitutions in the Jas domain. When expressed in wild-type plants under control of the 35S promoter, several of these resulted in T1 populations that contained significant proportions (4–16%) of male-sterile primary transgenics. In particular, a modified cDNA encoding a JAZ1 mutant protein in which R205 and R206 are both replaced by alanine residues (JAZ1-A205A206) produced 15 male-sterile plants within a tested T1 population of 93 plants (Table 1). As expected, there were no sterile plants among those transformed with the vector alone. Male-sterile JAZ1-A205A206 plants produced seed when pollinated with wild-type pollen. Among the T2 progeny, individuals inheriting the transgene were male-sterile while individuals lacking the transgene were fertile. Moreover, the transgenic T2 progeny were significantly more resistant to Pst DC3000 infection, compared with wild-type Col-0 plants, as reflected by reduced bacterial multiplication (Figure 4). Together, these results suggest that not only deletion of, but also specific mutations in the Jas domain can confer JA-insensitive phenotypes similar to that of the coi1 mutant plants.
Table 1.
Male-sterile phenotype of T1 transgenic plants expressing JAZ1 with deletion or amino acid mutations
| Transgene | No. of sterile plants | No. of total plants | % sterile plants | Y2H interaction | Jas domain sequences (relevant amino acid residues in red) |
|---|---|---|---|---|---|
| JAZ1 | + | ||||
| JAZ1Δ3 | 15 | 56 | 26.8 | − | |
| JAZ1-A205A206 | 15 | 93 | 16.1 | − | |
| Empty vector | 0 | 80 | 0 | N/A |
Figure 4. Susceptibility of Arabidopsis plants to Pst DC3000 infection.
Suspension (1×106 CFU.ml−1) of Pst DC3000 bacteria was vacuum-infiltrated into Col-0 (WT), coi1 mutant, and transgenic plants transformed with JAZ-ΔJas (JAZ1 with the entire Jas domain deleted) or JAZ1-A205A206 (JAZ1 with R205R206 replaced with alanine residues; see Figure 5a). Bacterial populations in leaves were determined three days after inoculation, with means and standard errors displayed (n=3). The bacterial growth experiment was repeated twice.
To investigate the possibility that the residues R205 and R206 are required for Jas domain-mediated interaction with COI1, we created A205, A206 and A205A206 double mutations in pB42AD-JAZ1 and conducted Y2H assays to detect interaction with COI1 in yeast. All three mutations disrupted the COI1-JAZ1 interaction (Figure 5b). Furthermore, unlike wild-type MBP-JAZ1-6xHis fusion protein, the MBP-JAZ1-A205A206-6xHis fusion protein failed to interact with SICOI1 in protein pull down assays (Figure 2b).
Figure 5. Role of Jas domain amino acid residues (R205R206 in JAZ1 and R223K224 in JAZ9) in JAZ interaction with COI1 and AtMYC2.
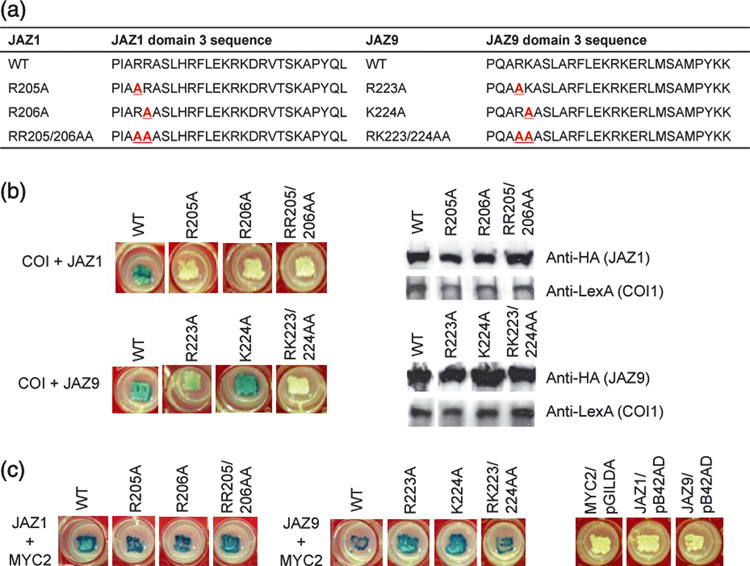
(a) The amino acid sequences of wild type (WT) and mutated domain 3 of JAZ1 and JAZ9. Mutated residues are in red and underlined. (b) Y2H (left panels) and western blot (right panels) assays of COI1 interaction with WT or mutant JAZ1 or JAZ9 protein. (c) Y2H assay of AtMYC2 interaction with WT or mutant JAZ1 protein or with empty Y2H vector (pGILDA or pB42AD). To detect COI1-JAZ interaction (b), we streaked yeast strains on Y2H agar medium containing 60 µM coronatine (COR). See Figure 1 for experimental setup. Interaction between JAZ and MYC2 does not require JA-Ile or coronatine; therefore, for (c) yeast strains were streaked on plain Y2H medium agar. Blue colony color indicates positive interaction, white colony indicates no interaction.
We next examined whether corresponding mutations in another JAZ protein have the same or different effects. The residues R223 and K224 (corresponding to R205 and R206 in JAZ1, respectively) in JAZ9 were substituted with alanine residues. Like the A205A206 mutations, the A223A224 double mutations abolished the COI1-JAZ9 interaction, although, in this case, single substitution mutations did not (Figure 5). Immunoblotting analysis showed that mutant JAZ1 and JAZ9 proteins were expressed (Figure 5b). Taken together, these results suggest that R205 and R206 in JAZ1 and R223 and K224 residues in JAZ9 play a critical role in mediating JA-Ile/coronatine-dependent COI1-JAZ interactions and that alanine-substitution mutations at these residues are sufficient to confer a dominant-negative effect on JAZ1 function in JA signaling (Table 1 and Figure 4).
Coronatine-independent interaction between JAZ and AtMYC2 proteins
The transcription factor AtMYC2 plays an important role in JA signaling and was previously shown to interact with JAZ3 in vitro and in Y2H assays, establishing an important connection from SCFCOI1-dependent degradation of JAZ3 to gene expression in JA signaling (Chini et al., 2007). Also, the C-terminus containing the Jas domain was identified as the AtMYC2-interacting domain (Chini et al., 2007). However, it is not known whether the AtMYC2-JAZ interaction is unique to JAZ3 or a general phenomenon that applies to other JAZ proteins. To address this question, we conducted Y2H assays with JAZ1 and JAZ9 proteins. As shown in Figure 4c, AtMYC2 interacted with both JAZ1 and JAZ9. Unlike the COI1-JAZ1/3/9 interactions, interaction between AtMYC2 and JAZ1 and JAZ9 proteins could be observed even in the absence of coronatine in the medium (Figure 5c); addition of coronatine or JA-Ile did not affect these interactions (data not shown). These results confirm that JAZ interaction with AtMYC is JA-Ile/coronatine-independent.
Because AtMYC2 interacts with the C-terminus (including the Jas domain) of JAZ3 (Chini et al., 2007), we investigated whether R205/R223 (JAZ1/JAZ9) and R206/K224 (JAZ1/JAZ9) in domain 3 are also involved in the AtMYC2-JAZ interactions. As shown in Figure 5C, these mutations did not affect AtMYC2 interaction with JAZ1 or JAZ9, demonstrating specificity of the effects of these mutations on COI1-JAZ interactions.
Discussion
In this study, we addressed several questions arising from the recent identification of the JAZ repressor proteins as the substrates of the SCFCOI1 E3 ubiquitin ligase in JA signaling. First, we show that the bacterial toxin coronatine could effectively promote physical interaction between Arabidopsis COI1 and JAZ proteins. Among biologically active jasmonates commonly used in the study of JA signaling (e.g., JA, MeJA, OPDA, and JA-Ile), only JA-Ile could promote COI1-JAZ interactions (Thines et al., 2007; Figure 1a, this study). Interestingly, the chemical structure of coronatine is most similar to that of JA-Ile and consists of CFA and CMA (an ethylcyclopropyl amino acid derived from isoleucine; Bender et al., 1999; Staswick, 2008). We found that neither CFA nor CMA promoted COI1-JAZ interactions (Figure 1a). Together, these results highlight the importance of the Ile (or modified Ile) moiety in JA signaling and coronatine action and suggest that coronatine is a potent microbial mimic of JA-Ile, targeting a key step of JA signaling, the formation of COI-JAZ complexes, as part of its virulence mechanism.
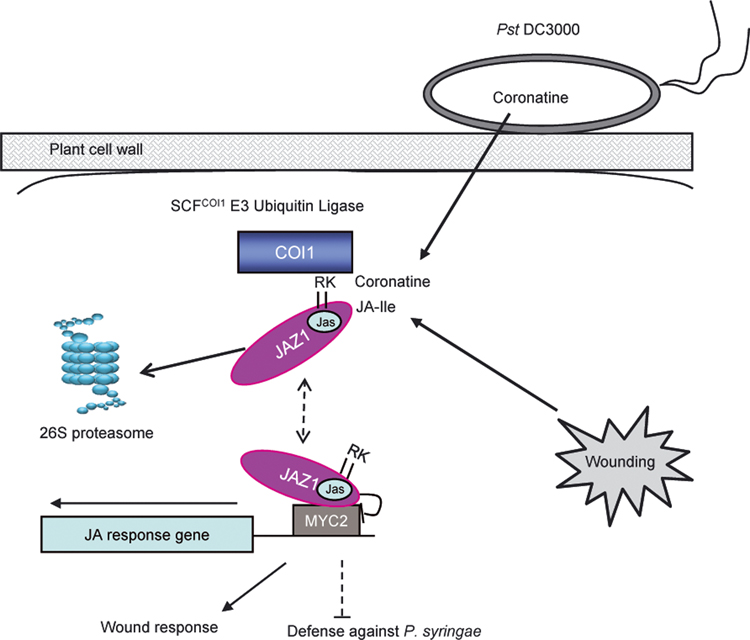
Another major conclusion from this study is that it is the C-terminus, not the N-terminus or central region, that is both necessary and sufficient for several Arabidopsis JAZ proteins to interact with Arabidopsis COI1 in a JA-Ile/coronatine-dependent manner (Figure 3). The C-terminal 157 aa of tomato SlJAZ3 (including the Jas motif) is also necessary and sufficient for hormone-dependent binding with tomato SlCOI1 (Katsir et al., 2008). However, it was not clear, from these results, whether the Jas motif itself or another sequence in the C-terminus is important for SlCOI1-SlJAZ3 interaction. In this study, we conducted further analyses with precise deletion of three individual intramolecular motifs of Arabidopsis JAZ1 and JAZ9 and show that neither the N-terminal motif nor the central ZIM motif is required, but the Jas motif is (Fig. 3). Furthermore, we performed site-directed mutagenesis in the Jas motif and have identified two basic amino acid residues (R205R206 in JAZ1 and R223K224 in JAZ9) in the Jas domain that are required for JAZ1 and JAZ9 to interact with COI1 (Figure 5). By transgenic expression we showed that these two positively charged residues are critical for JA signaling, as transgenic expression of JAZ1 carrying alanine substitutions of R205R206 conferred JA-insensitive phenotypes, including male sterility (Table 1) and enhanced resistance to Pst DC3000 infection (Figure 4). These results emphasize the importance of the Jas domain in coordinating molecular interactions of four key regulators of JA signaling: COI1 (a subunit of the SCFCOI1 E3 ubiquitin ligase), JAZs (repressors of JA signaling), AtMYC2 (transcription factor) and JA-Ile/coronatine (ligands) (Figure 6).
Figure 6. A model for the action of coronatine and JA-Ile in the plant cell.
In the absence of JA-Ile or coronatine, JAZ proteins bind to AtMYC2 and possibly other JA signaling transcription factors (not shown) via the C-terminal domain 3, repressing their transcription activity. When the concentration of JA-Ile or coronatine reaches a threshold during wounding or pathogen infection, COI1 binds to the Jas domain of JAZ proteins in a ligand-dependent manner. In this interaction, the positively charged residues of the Jas domain (R205R206 in JAZ1 and R223K224 in JAZ9) play a prominent role. The JAIle/coronatine-dependent interaction between COI1 and the C-terminus of JAZ proteins results in SCFCOI1-dependent ubiquitination and degradation of JAZ proteins through the 26S proteasome, thus derepressing JA signaling, which activates wound response and suppresses plant defense against Pseudomonas syringae bacteria.
As in the case of JA-Ile, promotion of COI1-JAZ interactions by coronatine was found to occur in yeast cells without requiring any other plant proteins. The ability of coronatine and JA-Ile to reconstitute COI1-JAZ interactions in heterologous yeast cells strongly implicates the CO11-JAZ complexes as receptors for these ligands. In support of this notion, ligand-binding experiments show a critical role of SlCOI1 as part of a receptor for coronatine and JA-Ile (Katsir et al., 2008). It is therefore likely that the initial step of JA signaling may mirror that of auxin signaling in which auxin serves as molecular glue for the physical interaction between the TIR1 F-box protein and the substrates AUX/IAA repressor proteins (Tan et al. 2007). However, the requirement of the positively charged residues in the Jas domain (e.g., R205R206 in JAZ1 and R223K224 in JAZ9) for interaction with COI1 suggests that the molecular nature of the COI1-JAZ interaction in JA signaling may be different from that of the TIR1-IAA interaction in auxin signaling. For the latter interaction, hydrophobic residues seem to play a predominant role (Tan et al. 2007). For example, the IAA7 degron peptide, with the central hydrophobic consensus motif GWPPV for TIR1 binding, has a predominantly hydrophobic sequence and binds to the auxin-bound TIR1 pocket through extensive hydrophobic interactions (Tan et al. 2007). In contrast, the requirement of the positively charged residues for COI1-JAZ interactions suggests a critical role of electrostatic interactions.
Based on other findings (Chini et al., 2007; Thines et al., 2007; Katsir et al., 2008) and results obtained in this study we can propose a revised model for JA signaling (Figure 6). In the absence of JA-Ile or coronatine, the C-terminus of JAZ proteins binds to AtMYC2 and likely other JA signaling transcription factors, repressing their transcription activity. When the concentration of JA-Ile or coronatine reaches a threshold during wounding or pathogen infection (Chung et al., 2008), COI1 begins to bind to the Jas domain of JAZ proteins, presumably in a ligand-dependent manner. Interestingly, whereas R205R206 in JAZ1 and R223K224 in JAZ9 are important for JAZ1-COI1 or JAZ9-COI1 interactions, they are not important in mediating JAZ1-AtMYC2 or JAZ9-AtMYC2 interactions (Fig. 5), suggesting that the interacting surfaces for JAZ-COI1 and JAZ-AtMYC2 interactions are not identical. The JA-Ile/coronatine-dependent interaction between COI1 and the C-terminus of JAZ proteins would result in SCFCOI1-dependent ubiquitination and degradation of JAZ proteins through the 26S proteasome, thus derepressing JA signaling. This Jas domain-based model is different from an earlier model proposed by Chini and coworkers (2007), which is based on observations made on JAZ3 interaction with COI1 and AtMYC2 proteins. In these experiments, purified recombinant JAZ3 protein was found to interact with the COI1 protein produced in a wheat germ in vitro transcription/translation extract and the COI1-JAZ3 interaction did not require exogenous jasmonates. Moreover, the N-terminal/central regions (containing NT and ZIM domains), but not the C-terminus (containing the Jas domain), were sufficient for this interaction. Our revised model could in principle accommodate the observations made by Chini and coworkers on the COI1-JAZ3 interaction. For example, the JA-Ile-independent NT/ZIM-domain-mediated interaction could represent a basal-level interaction detectable only when COI1 is produced in the wheat germ in vitro transcription and translation extract (Chini et al. 2007)), but not by Y2H or protein pull-down assays (Thines et al. 2007; Katsir et al., 2008; this study). As proposed by Chini et al. (2007), this ligand-independent interaction between COI1 and the N-terminus of JAZ proteins may provide an explanation for the dominant negative effect of JAZ protein derivatives that lack a functional Jas motif. Future crystal structure analyses of COI1-JAZ complexes in the presence or absence of JA-Ile/coronatine would help to distinguish these possibilities.
Experimental procedures
Plant material and growth conditions
Arabidopsis thaliana plants were grown on soil under continuous illumination (100 µE m−2 s−1) at 22°C. In all experiments, wild type (WT) refers to the Columbia (Col-0) ecotype. To generate Arabidopsis Col-0 plants expressing the COI1-Myc fusion protein, the COI1 cDNA was amplified by reverse transcriptase-polymerase chain reaction (RT-PCR) using primers COI1up (5’-TTTTGTCGACCCGATGGAGGATC-3’; SalI recognition site underlined) and COI1dn1 (5’-GGGTGGTACCATATTGGCTCCTT-3’; KpnI recognition site underlined) and cloned into a pCambia1300 derivative, via SalI and KpnI, behind the CaMV 35S promoter and in front of a 9xMyc tag (398 bp) fragment. The DNA insert was fully sequenced. The resulting plasmid pCambia1300-COI1-9Myc was introduced into Agrobacterium tumefaciens strain GV3101 (pMP90) for transformation into Arabidopsis Col-0 (COI1/COI1) and heterozygous COI1/coi1-1 plants using the floral dip method (Clough and Bent, 1998). The correct functioning of the COI1-Myc fusion was confirmed by observing restoration of root sensitivity 50µM JA when the coi1/coi1/COI1-Myc progeny were germinated on MS agar plates and male fertility in the later stage of development (data not shown). Expression of COI1-9Myc in multiple T1 plants was confirmed by immunoblotting using the Myc epitope antibody. Homozygous T4 Col-0 plants expressing COI1-Myc were used for the experiments.
Transgenic plants expressing a modified JAZ1 cDNA encoding JAZ1-A205A206 were produced using techniques and vectors described in Thines et al. (2007). Tomato plants expressing the functional SICOI1-Myc fusion and growth conditions were described previously (Thines et al., 2007).
Protein expression constructs
The JAZ1 cDNA was amplified by PCR using the primers JAZ1-Not1 (5 ′ - GCGCGGCCGCCATGTCGAGTTCTATGGAATGTTCT - 3′) and JAZ1-Xho1 (5′ - CCCTCGGTATTTCAGCTGCTAAACCGAG - 3′). The PCR product was digested with NotI and XhoI and cloned into the corresponding sites of pRMG-nMAL (Thines et al., 2007). For making the MBP-JAZ9-6xHis fusion construct, the MBP-coding sequence was released from pMAL-c4x (NEB Laboratories) by NdeI and SalI digestion and sub-cloned into the same sites of pET42b (Novagen). The JAZ9 cDNA were amplified by polymerase chain reaction (PCR) using the primers JAZ9-F1 (5’- CACCGAATTCCCCATGGAAAGAGATTTTCTGGGTTTG; EcoRI recognition site underlined) and JAZ9-R1 (5’- TTACTCGAGGGCGCGCCCTGTAGGAGAAGTAGAAGAGTAA; XhoI recognition site underlined) and cloned into the EcoRI and XhoI sites of pET-MAL to create the MBP-JAZ9-6xHis fusion construct. Fusion proteins were expressed in E. coli strain Rosetta 2(DE3)pLysS. The recombinant proteins were purified by using amylase resin and eluted by 10 mM maltose in column buffer (20 mM Tris-HCl pH7.4, 200 mM NaCl, 10 mM β-mercaptoethanol).
Yeast-two hybrid (Y2H) methods
The Arabidopsis gene COI1 (At2g39940) was cloned into the Y2H bait vector pGILDA (Clontech) resulting in a LexA-COI1 protein fusion. This gene construct was transformed into yeast (Saccharomyces cerevisae) strain EGY48 (p8opLacZ) using the frozen-EZ yeast transformation II kit (Zymo Research). Tranformants were selected on SD-glucose medium (BD Biosciences) supplemented with —Ura/—His drop out solution (BD Biosciences). An Arabidopsis cDNA library (Holt et al., 2003) was screened twice for coronatine-dependent COI1-interactors using the Matchmaker LexA Two Hybrid System (Clontech Laboratories Inc.) following the manual provided by the manufacturer, except that coronatine (1.5 µM for one screen and 50 µM for the other) was added to the inducing medium [SD-galactose/rafinose inducing medium (BD Biosciences) containing —Ura/—His/—Trp drop out supplement and 80 µg/ml X-Gal].
To detect the interaction between JAZ protein and COI1 or MYC2, the JAZ genes were amplified by RT-PCR from Arabidopsis leaves collected 12 h after inoculation with the pathogen P. syringae pv. tomato (Pst) DC3000. JAZ genes were cloned into the Y2H prey vector pB42AD to obtain a B42-HA-JAZ fusion protein and co-transformed with pGILDA-COI1 into EGY48 (p8opLacZ) using the frozen-EZ yeast transformation II kit (Zymo Research). For Y2H assay with AtMYC2 and JAZ proteins, JAZ genes were cloned into the Y2H bait vector pGILDA to obtain a LexA-JAZ fusion protein and co-transformed with pB42AD-AtMYC2 into EGY48 (p8opLacZ) using the frozen-EZ yeast transformation II kit (Zymo Research). Tranformants were selected on SD-glucose medium supplemented with —Ura/—His/—Trp drop out solution. The expression of the LexA-COI1 and B42-HA-JAZ protein fusions was detected by western blotting using epitope-specific antibodies. To assess the JA-Ile or coronatine-dependent interaction between COI1 and JAZ proteins, transformed yeast strains were plated on SD-galactose/rafinose inducing medium containing —Ura/—His/—Trp drop out supplement, 80 µg/ml X-Gal. In some experiments, one of the following chemicals was also added: 60µM JA-Ile, 60µM jasmonic acid (Sigma), 60µM methyl jasmonate (Sigma), 60µM OPDA (Cayman Chemical Company), 60µM coronatine (Oklahoma State University or Sigma) or 10% ethanol (the solvent for all the chemicals) unless indicated otherwise. Plates were incubated for up to 6 days at 30°C. The positive control strain containing the pLexA-53 and pB42AD-T plasmids (Clontech) were also plated on inducing medium for comparison of the colony color.
Protein pull-down assay
Four-week-old Arabidopsis or 3-week-old tomato plants that stably express the COI1-Myc fusion protein were ground in liquid nitrogen to fine powder. The soluble proteins were extracted using binding buffer (50 mM Tris-HCl pH6.8, 150 mM NaCl, 10% glycerol, 0.1% Tween-20, 20 mM imidazole, 20 mM β-mercaptoethanol, 1 % Sigma protease inhibitor cocktail, and 10 mM MG-132) and clarified by centrifugation at 15,000g at 4 °C for 20 min. Protein concentrations were determined using a BioRad RC DC assay kit. All protein samples were aliquotted and stored in −80 °C. For each pull-down assay, 1 mg of soluble protein extracts from Arabidopsis or tomato plants were incubated with 25 µg purified MBP-6xHis or MBP-JAZ-6xHis fusion protein in the final volume of 0.5 ml. Procedure for protein pull-down experiments was the same as described previously (Thines et al., 2007).
Site-directed mutagenesis
Individual amino acid residues in the Jas domain of JAZ1 and JAZ9 proteins were mutated to alanine in pB42AD using the QuickChange II site directed mutagenesis kit (Stratagene). Mutant proteins were co-expressed with COI1 (expressed from pGILDA::COI1) in yeast to detect protein-protein interaction, as described above. For studying the effect of Jas domain mutations on JAZ-AtMYC2 interaction, we moved the mutated JAZ1 and JAZ9 inserts from pB42AD into pGILDA. Mutant JAZ1 and JAZ9 proteins were co-expressed with AtMYC2 (expressed from pB42AD::AtMYC2) in yeast to detect protein-protein interaction, as described above.
Bacterial infection assay
Pst DC3000 was cultured at 30°C in Luria-Bertani (LB) medium supplemented with appropriate antibiotics until an OD600 of 0.8 was reached. Bacteria were collected by centrifugation and resuspended in water to the final concentration of 106 CFU.ml−1. Four-week old Arabidopsis plants were infiltrated with bacterial suspension and kept under high humidity until disease symptoms developed. The bacterial population in the plant apoplast was determined as previously described (Katagiri et al., 2002).
Supplementary Material
The following supplemental material is available for this article online:
Figure S1. Alignment of JAZ9 cDNA clones isolated from a Y2H screening using Arabidopsis COI1 as bait. A total of 32 clones was recovered with sizes varying from 88 to 147 amino acid residues (C-terminus). Clones of 20 different lengths were aligned here representing deletions of every two amino acids between amino acids 120 and 179.
Acknowledgements
This work was supported by funding from the National Institutes of Health (S.Y.H. and G.A.H.) and the Department of Energy (S.Y.H., JB, and G.A.H.). We thank Jeff Dangl for providing us with an Arabidopsis cDNA library.
REFERENCE
- Bender CL, Alarcon-Chaidez F, Gross DC. Pseudomonas syringae phytotoxins: mode of action, regulation, and biosynthesis by peptide and polyketide synthetases. Microbiol. Mol. Biol. Rev. 1999;63:266–292. doi: 10.1128/mmbr.63.2.266-292.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block A, Schmelz E, Jones JB, Klee HJ. Coronatine and salicylic acid: the battle between Arabidopsis and Pseudomonas for phytohormone control. Mol. Plant Pathol. 2005;6:79–83. doi: 10.1111/j.1364-3703.2004.00265.x. [DOI] [PubMed] [Google Scholar]
- Brooks DM, Bender CL, Kunkel BN. The Pseudomonas syringae phytotoxin coronatine promotes virulence by overcoming salicylic acid-dependent defences in Arabidopsis thaliana. Mol. Plant Pathol. 2005;6:629–639. doi: 10.1111/j.1364-3703.2005.00311.x. [DOI] [PubMed] [Google Scholar]
- Browse J. Jasmonate: an oxylipin signal with many roles in plants. In: Litwack G, editor. Vitamins and Hormones. New York: AP-Elsevier; 2005. pp. 431–456. [DOI] [PubMed] [Google Scholar]
- Browse J, Howe GA. Update on jasmonate signaling: New weapons and a rapid response against insect attack. Plant Physiol. 2008 doi: 10.1104/pp.107.115683. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini A, Fonseca S, Fernandez G, Adie B, Chico JM, Lorenzo O, Garcia-Casado G, Lopez-Vidriero I, Lozano FM, Ponce MR, Micol JL, Solano R. The JAZ family of repressors is the missing link in jasmonate siganlling. Nature. 2007;448:666–671. doi: 10.1038/nature06006. [DOI] [PubMed] [Google Scholar]
- Chung HS, Koo AJK, Gao X, Jayany S, Thines B, Jones AD, Howe GA. Regulation and function of Arabidopsis JAZ genes in response to wounding and herbivory. Plant Physiol. 2008 doi: 10.1104/pp.107.115691. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Cui J, Bahrami AK, Pringle EG, Hernandez-Guzman G, Bender CL, Pierce NE, Ausubel FM. Pseudomonas syringae manipulates systemic plant defenses against pathogens and herbivores. Proc. Natl. Acad. Sci. USA. 2005;102:1791–1796. doi: 10.1073/pnas.0409450102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feys B, Benedetti CE, Penfold CN, Turner JG. Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. Plant Cell. 1994;6:751–759. doi: 10.1105/tpc.6.5.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt BF, Boyes DC, Ellerstrom M, Siefers N, Wiig A, Kauffman S, Grant MR, Dangl JL. An evolutionarily conserved mediator of plant disease resistance gene function is required for normal Arabidopsis development. Dev. Cell. 2002;2:807–817. doi: 10.1016/s1534-5807(02)00174-0. [DOI] [PubMed] [Google Scholar]
- Howe GA, Jander G. Plant Immunity to herbivores. Ann. Rev. Plant Biol. 2008;59:41–66. doi: 10.1146/annurev.arplant.59.032607.092825. [DOI] [PubMed] [Google Scholar]
- Katagiri F, Thilmony R, He SY. The Arabidopsis thaliana-Pseudomonas syringae interaction. In: Somerville CR, Meyerowitz EM, editors. The Arabidopsis Book. Rockville, MD: American Society of Plant Biologists; 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsir L, Schilmiller AL, Staswick PE, He SY, Howe GA. COI1 is a critical component of a receptor for jasmonoyl-isoleucine and the bacterial virulence factor coronatine. Proc Natl Acad Sci USA. 2008;105:7100–7105. doi: 10.1073/pnas.0802332105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloek AP, Verbsky ML, Sharma SB, Schoelz JE, Vogel J, Klessig DF, Kunkel BN. Resistance to Pseudomonas syringae conferred by an Arabidopsis thaliana coronatine-insensitive (coi1) mutation occurs through two distinct mechanisms. Plant J. 2001;26:509–522. doi: 10.1046/j.1365-313x.2001.01050.x. [DOI] [PubMed] [Google Scholar]
- Lauchli R, Boland W. Indanoyl amino acid conjugates: tunable elicitors of plant secondary metabolism. Chem. Record. 2003;3:12–21. doi: 10.1002/tcr.10043. [DOI] [PubMed] [Google Scholar]
- Li L, Li CY, Lee GI, Howe GA. Distinct roles for jasmonate synthesis and action in the systemic wound response of tomato. Proc. Natl Acad. Sci. USA. 2002;99:6416–6421. doi: 10.1073/pnas.072072599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melotto M, Underwood W, Koczan J, Nomura K, He SY. Plant stomata function in innate immunity against bacterial invasion. Cell. 2006;126:969–980. doi: 10.1016/j.cell.2006.06.054. [DOI] [PubMed] [Google Scholar]
- Melotto M, He SY. Book of Abstracts, XIII International Congress on Molecular Plant-Microbe Interactions, p403. Italy: Sorrento; 2007. [July 21–27, 2007]. AtCOI1, an E3 ubiquitin ligase subunit, interacts with putative target proteins in a coronatine/jasmonate-dependent manner. [Google Scholar]
- Melotto M, Underwood W, He SY. Role of stomata in plant innate immunity and foliar bacterial diseases. Annu. Rev. Phytopathol. 2008;46 doi: 10.1146/annurev.phyto.121107.104959. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal SM, Davis KR. Role of the phytotoxin coronatine in the infection of Arabidopsis thaliana by Pseudomonas syringae pv tomato. Mol. Plant-Microbe Interact. 1995;8:165–171. doi: 10.1094/mpmi-8-0165. [DOI] [PubMed] [Google Scholar]
- Staswick PE. JAZing up jasmonate signaling. Trends Plant Sci. 2008;13:66–71. doi: 10.1016/j.tplants.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Tan X, Calderon-Villalobos LIA, Sharon M, Zheng C, Robinson CV, Estelle M, Zheng N. Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature. 2007;446:640–645. doi: 10.1038/nature05731. [DOI] [PubMed] [Google Scholar]
- Thilmony R, Underwood W, He SY. Genome-wide transcriptional analysis of the Arabidopsis thaliana interaction with the plant pathogen Pseudomonas syringae pv. tomato DC3000 and the human pathogen Escherichia coli O157:H7. Plant J. 2006;46:34–53. doi: 10.1111/j.1365-313X.2006.02725.x. [DOI] [PubMed] [Google Scholar]
- Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, Liu G, Nomura K, He SY, Howe GA, Browse J. JAZ repressor proteins are targets of the SCFCOI1 complex during jasmonate signaling. Nature. 2007;448:661–665. doi: 10.1038/nature05960. [DOI] [PubMed] [Google Scholar]
- Underwood W, Melotto M, He SY. Role of plant stomata in bacterial invasion. Cellular Microb. 2007;9:1621–1629. doi: 10.1111/j.1462-5822.2007.00938.x. [DOI] [PubMed] [Google Scholar]
- Uppalapati SR, Ayoubi P, Weng H, Palmer DA, Mitchell RE, Jones W, Bender CL. The phytotoxin coronatine and methyl jasmonate impact multiple phytohormone pathways in tomato. Plant J. 2005;42:201–217. doi: 10.1111/j.1365-313X.2005.02366.x. [DOI] [PubMed] [Google Scholar]
- Vanholme B, Grunewald W, Bateman A, Kohchi T, Gheysen G. The tify family previously known as ZIM. Trends Plant Sci. 2007;12:239–244. doi: 10.1016/j.tplants.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Xie DX, Feys BF, James S, Nieto-Rostro M, Turner JG. COI1: an Arabidopsis gene required for jasmonate-regulated defense and fertility. Science. 1998;280:1091–1094. doi: 10.1126/science.280.5366.1091. [DOI] [PubMed] [Google Scholar]
- Yan Y, Stolz S, Chetelat A, Reymong P, Pagni M, Dubugnon L, Farmer EE. A downstream mediator in the growth repression limb of the jasmonate pathway. Plant Cell. 2007;19:2470–2483. doi: 10.1105/tpc.107.050708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Thilmony R, Bender CL, Schaller A, He SY, Howe GA. Virulence systems of Pseudomonas syringae pv. tomato promote bacterial speck disease in tomato by targeting the jasmonate signaling pathway. Plant J. 2003;36:485–499. doi: 10.1046/j.1365-313x.2003.01895.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The following supplemental material is available for this article online:
Figure S1. Alignment of JAZ9 cDNA clones isolated from a Y2H screening using Arabidopsis COI1 as bait. A total of 32 clones was recovered with sizes varying from 88 to 147 amino acid residues (C-terminus). Clones of 20 different lengths were aligned here representing deletions of every two amino acids between amino acids 120 and 179.