Abstract
Background
Rifampin is the cornerstone of antituberculosis therapy, but induction of hepatic cytochrome P450 (CYP) 3A by rifampin markedly lowers HIV protease inhibitor plasma concentrations.
Methods
This phase I, open-label, one-arm study was designed to assess pharmacokinetic interactions and safety of atazanavir, ritonavir, and rifampin among 14 evaluable HIV-seronegative volunteers. The study included three sequential periods of study drug dosing, with plasma sampling for pharmacokinetic analyses to occur on the last day of each period. During period 1, participants received rifampin 600 mg every 24 hours for 8 days. During period 2, participants continued rifampin 600 mg every 24 hours, and added atazanavir 300 mg and ritonavir 100 mg every 12 hours, to continue for at least 11 days. During period 3, atazanavir was to be increased to 400 mg every 12 hours.
Results
Upon adding atazanavir and ritonavir, the first three subjects developed vomiting and transaminase elevations resulting in study drug discontinuation. The study was therefore terminated.
Conclusions
Co-administration of rifampin with HIV protease inhibitors may not be a viable treatment option if rifampin administration precedes protease inhibitor initiation. Future studies which explore concomitant HIV protease inhibitors with rifampin must carefully consider the sequence in which drugs are initiated.
Keywords: atazanavir, ritonavir, rifampin, tuberculosis, hepatotoxicity
INTRODUCTION
Tuberculosis is a leading cause of mortality in human immunodeficiency virus (HIV)-infected individuals [1], and rifampin is the cornerstone of effective antituberculosis therapy. Unfortunately, induction of hepatic cytochrome P450 (CYP) 3A by rifampin markedly lowers plasma concentrations of HIV protease inhibitors [2]. The antituberculosis drug rifabutin induces CYP3A activity less than rifampin and may therefore be coadministered with many HIV protease inhibitors [2], but rifabutin is not available in most resource-limited countries. Furthermore, although rifampin may be co-administered with the non-nucleoside reverse transcriptase inhibitors efavirenz and nevirapine, the latter drugs are contraindicated in specific situations (e.g. when there is viral resistance to these drugs), underscoring the need for alternative safe and effective strategies to treat co-infection with HIV and Mycobacterium tuberculosis.
The HIV protease inhibitor atazanavir undergoes metabolism by CYP3A [3]. Ritonavir is a potent CYP3A inhibitor, and ritonavir coadministration increases atazanavir trough concentrations by over 600% [3]. Approved atazanavir dosages include 400 mg once-daily without ritonavir, and 300 mg once-daily when boosted with ritonavir 100 mg once-daily [2]. When administered with ritonavir 100 mg once-daily, atazanavir has shown similar efficacy to other ritonavir-enhanced protease inhibitor regimens in antiretroviral experienced patients [4].
In AIDS Clinical Trials Group (ACTG) protocol A5213 we previously showed that, among healthy HIV-seronegative volunteers, atazanavir 400 mg every 12 hours co-administered with rifampin 600 mg every 24 hours was safe and generally well tolerated but did not maintain adequate plasma atazanavir concentrations [5]. Similarly, in a healthy volunteer study by Burger et al, once-daily atazanavir (400 mg), ritonavir (200 mg), and rifampin (600 mg) did not maintain adequate plasma atazanavir concentrations toward the end of the 24 hour dosing interval [6]. We therefore revised protocol A5213 to test whether adequate atazanavir exposure could be safely achieved with twice-daily dosing of both atazanavir and ritonavir among healthy volunteers who first received rifampin to steady state.
METHODS
Study Participants and Design
This revised version of A5213 was designed to enroll 14 evaluable HIV-seronegative volunteers. Participants were at least 18, but no more than 55 years of age, with acceptable screening electrocardiogram, hematology, and chemistry studies including normal AST, ALT, and total bilirubin values. Exclusion criteria included use of medications known or predicted to interact with CYP3A. The study was approved by the Institutional Review Board at each site, and all participants provided written informed consent.
This phase I, open-label, one-arm study was designed to include three sequential periods of study drug dosing, with serial plasma sampling for pharmacokinetic analyses to occur on the last day of each period. During period 1, participants received rifampin 600 mg every 24 hours for 8 days. During period 2, participants continued rifampin 600 mg every 24 hours, and added atazanavir 300 mg and ritonavir 100 mg every 12 hours, to continue for at least 11 days. During period 3, the atazanavir dose was to be increased to 400 mg every 12 hours. Safety assessments, including ALT and AST determinations, were to be performed during pharmacokinetic sampling visits, at the midpoint of each period, 2 days after adding atazanavir and ritonavir, and 14–21 days after the last dose of study drug.
Statistical Design
Sample size calculations for the primary objective assumed the use of a standard one-sided, two-sample t-test applied to natural log-transformed areas under the concentration-time curve (AUC) and fixing the alpha level at 5%. The sample size of 14 had 80% power to test if the estimated atazanavir AUC0–24h values were at least 70% of the historic mean AUC0–24h for atazanavir 400 mg every 12 h. The protocol-defined discontinuation criteria for participants included any confirmed grade 2 ALT elevation that did not decrease by at least 10% upon repeat determination, or any adverse event or toxicity of grade 3 or higher [7]. Toxicities were closely monitored.
RESULTS
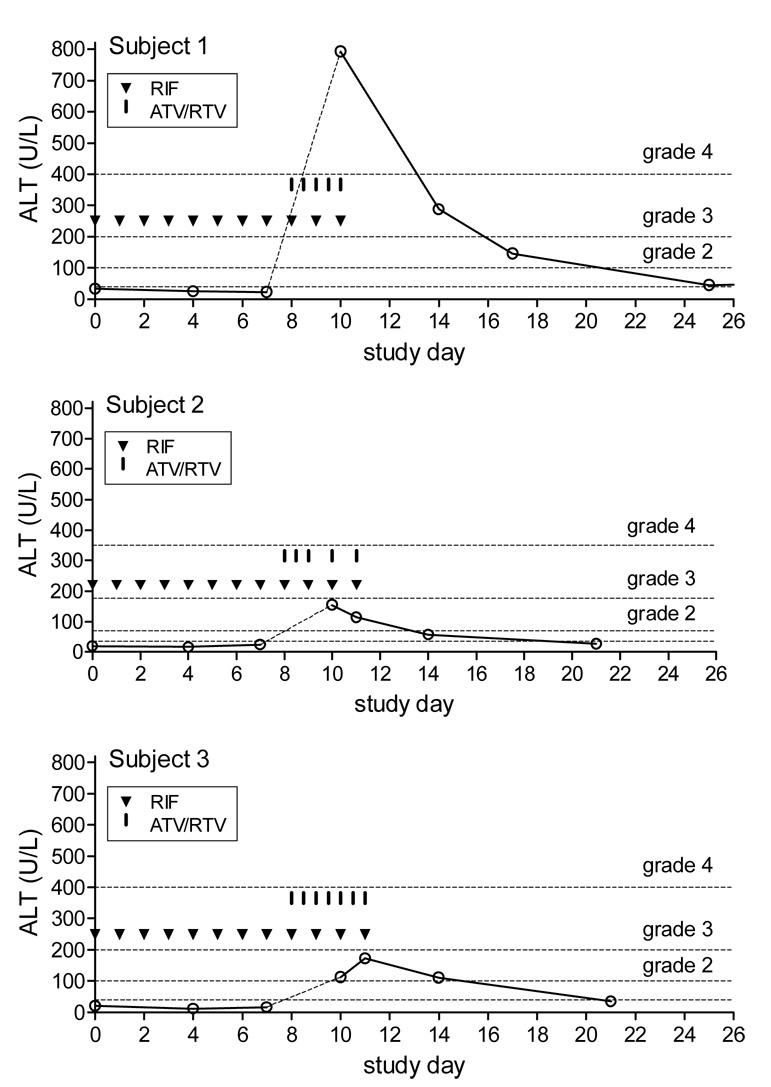
Three participants began study drug on the same calendar day. They ranged in age from 35 to 41 years, all were non-Hispanic white, 2 were male, and body mass index ranged from 26.3 to 35.3 kg/m2. Throughout period 1, all participants tolerated rifampin and had normal transaminase values. However, within 24 hours of adding atazanavir and ritonavir during period 2, all three experienced Grade 2 nausea and vomiting. Transaminase values were elevated in all participants two days after initiating atazanavir and ritonavir (Figure). The highest documented ALT value for each participant was 792 IU/L (grade 4), 173 IU/L and 154 IU/L (grade 2), with the highest ALT being in a male participant. All discontinued study drugs after no more than seven doses of atazanavir with ritonavir. Two participants were required to discontinue study drugs based on protocol-defined criteria. The protocol team instructed the third participant (who had missed two atazanavir/ritonavir doses because of nausea) to also discontinue study drugs. In all participants, nausea resolved within several days after stopping study drugs, and transaminase values returned to normal (Figure).
Figure 1. Study drug doses and ALT values in each participant.
Each graph represents a study participant. Open circles represent ALT values. The horizontal dashed lines represent ALT cut-offs for toxicity grades [7]. Triangles (▼) indicate each 600 mg rifampin dose taken. Vertical hash marks ( ▌) indicate each atazanavir (300 mg)/ritonavir (100 mg) dose taken.
DISCUSSION
Treatment of co-infection with M. tuberculosis and HIV is a major challenge, largely because rifampin enhances clearance of HIV protease inhibitors [2]. The present study demonstrated that, among healthy, HIV-negative volunteers who first took rifampin for eight days, the addition of twice-daily atazanavir 300 mg and ritonavir 100 mg caused considerable gastrointestinal intolerance and transaminase elevations. This required that we prematurely terminate the study. All participants fully recovered.
Two previous studies examined potential ways to maintain adequate atazanavir exposure with co-administered rifampin, neither of which succeeded [5,6]. Using an approach that did not involve ritonavir, we previously reported results from 10 HIV-negative subjects who first took atazanavir 300 mg every 12 h for at least 8 days, then added rifampin 600 mg every 24 hours for at least 11 days, and subsequently increased atazanavir to 400 mg every 12 h for at least 8 days [5]. Although study drugs were generally well tolerated and transaminases remained normal, the mean atazanavir C12 h value with 400 mg every 12 h was only 113 ng/ml. This was well below what has been reported for atazanavir 400 mg once-daily without ritonavir (geometric mean 159 ng/mL) [3]. Burger et al tested a ritonavir-boosted approach in which HIV-negative subjects received various once-daily combinations of atazanavir, ritonavir, and rifampin [6]. Among 14 volunteers who received the highest doses of atazanavir (400 mg) and ritonavir (200 mg), concomitant rifampin (600 mg) lowered the mean plasma atazanavir C24h to 86 ng/mL. These studies suggested that twice-daily dosing with both atazanavir and ritonavir may be necessary to maintain adequate plasma atazanavir exposure throughout the dosing interval among individuals taking concomitant rifampin.
The hepatotoxicity and gastrointestinal intolerance in the present study were similar to what was seen in two previous healthy volunteer studies involving HIV protease inhibitors other than atazanavir. In a study of twice-daily saquinavir (1000 mg) and ritonavir (100 mg) given with once-daily rifampin (600 mg), the study was prematurely terminated due to profound hepatic transaminase elevations, particularly among participants who received rifampin for 14 days before starting saquinavir and ritonavir [8]. Similarly, a study of concomitant lopinavir/ritonavir with rifampin was terminated due to nausea, vomiting, and transaminase elevations [9]. In that study, participants took rifampin 600 mg once daily for five days and then added twice-daily lopinavir/ritonavir. The total daily dose of lopinavir/ritonavir was either 1200mg/300mg or 1600mg/400mg. On the third day after adding lopinavir/ritonavir, all participants had transaminase elevations, which in 9 of 11 ranged from 764 to 1657 IU/L. Much less toxicity was seen in the study of Burger et al, in which only 1 of 14 participants had transaminase elevations greater than 5-times the upper limit of normal with once-daily atazanavir (400 mg), ritonavir (200 mg) and rifampin (600 mg) [6].
The mechanism underlying the transaminase elevations in the present study is not known. However, its rapid onset in this and previous healthy volunteer studies [8,9] strongly suggests that the rifampin lead-in created a condition which favored toxicity when the HIV protease inhibitors were added. This is supported by the observation that toxicity was substantially less in other healthy volunteer studies that examined the combined use of rifampin with ritonavir-boosted HIV protease inhibitors, but without a rifampin lead-in period [6,8,10]. World Health Organization guidelines, however, state that among patients with concomitant active tuberculosis and HIV infection, priority is given to treating the tuberculosis, with at least two weeks of antituberculosis therapy before starting antiretroviral therapy [11]. Avoiding a rifampin lead-in in clinical practice is thus problematic.
Rifampin undergoes enterohepatic circulation, during which the drug is progressively metabolized to 25-O-desacetyl rifampin. It is possible that inhibition of CYP3A4 and/or a drug transporter by ritonavir and/or atazanavir blocks clearance of a rifampin metabolite which mediates toxicity. It is not known whether rifampin hepatotoxicity is mediated by the 25-O-desacetyl metabolite or other minor metabolites. Alternatively pre-induction of CYP3A4 by rifampin may generate a protease inhibitor metabolite. Since ultimate steady-state concentration-time profiles of parent drugs and metabolites would be independent of the sequence of drug initiation, toxicity following the rifampin lead-in likely reflects the time course of metabolite exposure. The rifampin lead-in may cause toxic metabolite(s) to very rapidly achieve eventual steady-state concentrations. We speculate that slower accumulation of metabolites without the rifampin lead-in may allow tachyphylaxis to ameliorate toxicity.
The effect of drug sequence could be addressed by studying individuals who first receive atazanavir and ritonavir, then subsequently initiate rifampin. Because no study participant reached the second pharmacokinetic sampling day, specimens during period 2 were not available to quantify study drugs or their metabolites. Furthermore, we cannot exclude the possibility that toxicity will be less among HIV-infected individuals than has been seen in healthy volunteer studies. In this regard, among 15 children (ages 7 months to 3.9 years) co-infected with HIV and M. tuberculosis, and who received rifampin-based antituberculosis therapy plus twice-daily lopinavir/ritonavir, none had treatment interrupted because of ALT elevations [12]. In that study most children initiated antituberculosis therapy before initiating lopinavir/ritonavir (Y. Ren, personal communication).
Based on available data, co-administration of rifampin with an HIV protease inhibitor may not be a viable treatment option if rifampin administration precedes protease inhibitor initiation. If future studies are to further explore concomitant HIV protease inhibitors with rifampin, the sequence in which drugs are initiated must be carefully considered.
ACKNOWLEDGMENTS
The authors acknowledge Robin DiFrancesco, MT, MBA for her outstanding contributions to this work. The authors are also grateful to the individuals who volunteered for this study. This study was supported in part by NIH grants AI068636 (ACTG Leadership Grant), AI69452 (EA), AI32775 (JG), AI068634 (MAK), AI694742 (SLK and LL), AI069556 (ARZ), RR024975 and AI069439 (DWH). Study drugs were provided by Bristol-Myers Squibb, Co.
Potential conflicts of interest:
DWH has received research grants from Bavarian Nordic, Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Merck, Tanox, and Tibotec. He is on scientific advisory boards for Glaxo Smith Kline and Tibotec.
EPA has consulted for or served on scientific advisory boards for Boehringer-Ingelheim, Bristol-Myers Squibb and Merck.
JGG has been a consultant for Bristol-Myers Squibb, and Merck and Tibotec.
ARZ has received research grants from Gilead Sciences, Bristol-Myers Squibb and Merck. He is on scientific advisory boards for Bristol-Myers Squibb, Tibotec, Gilead Sciences, Schering, VIRxSYS and Monogram Biosciences.
RB and MJC are employees of Bristol-Myers Squibb.
SLK, LL, MAK, CS, LH and BAS have no potential conflicts.
Footnotes
These data were presented in part at the 15th Conference on Retroviruses and Opportunistic Infections, February 2008, Boston, MA.
References
- 1.World Health Organization. [updated March 2007];Geneva, Switzerland: World Health Organization; Tuberculosis fact sheet no. 104. 2006 Available at: http://www.who.int/en/
- 2.Panel on Clinical Practices for Treatment of HIV Infection. Washington, DC: U.S. Department of Health and Human Services; Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2007 Available at: http://www.hivatis.org/
- 3.Bristol-Myers Squibb Company. Reyataz (atazanavir sulfate) prescribing information. Princeton, NJ: Bristol-Myers Squibb Company; 2008. [Google Scholar]
- 4.Johnson M, Grinsztejn B, Rodriguez C, et al. Atazanavir plus ritonavir or saquinavir, and lopinavir/ritonavir in patients experiencing multiple virological failures. AIDS. 2005;19:685–694. doi: 10.1097/01.aids.0000166091.39317.99. [DOI] [PubMed] [Google Scholar]
- 5.Acosta EP, Kendall MA, Gerber JG, et al. Effect of concomitantly administered rifampin on the pharmacokinetics and safety of atazanavir administered twice daily. Antimicrob Agents Chemother. 2007;51:3104–3110. doi: 10.1128/AAC.00341-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burger DM, Agarwala S, Child M, Been-Tiktak A, Wang Y, Bertz R. Effect of rifampin on steady-state pharmacokinetics of atazanavir with ritonavir in healthy volunteers. Antimicrob Agents Chemother. 2006;50:3336–3342. doi: 10.1128/AAC.00461-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. [Accessibility verified February 12, 2008];National Institutes of Health; Division of AIDS table for grading severity of adult adverse experiences. 1992 http://rcc.techres-intl.com/tox_tables.htm.
- 8.Grange S, Schutz M, Schmitt C, et al. Unexpected hepatotoxicity observed in a healthy volunteer study on the effects of multiple dose rifampicin on the steady-state pharmacokinetics of ritonavir-boosted saquinavir and vice versa [Abstract 35]; Presented at: Sixth Int. Workshop Clin. Pharmacol. HIV Ther; 28 to 30 April 2005; Montreal, Quebec, Canada. 2005. [Google Scholar]
- 9.Nijland HM, L'homme RF, Rongen GA, et al. High incidence of adverse events in healthy volunteers receiving rifampicin and adjusted doses of lopinavir/ritonavir tablets. AIDS. 2008;22:931–935. doi: 10.1097/QAD.0b013e3282faa71e. [DOI] [PubMed] [Google Scholar]
- 10.la Porte CJ, Colbers EP, Bertz R, et al. Pharmacokinetics of adjusted-dose lopinavir-ritonavir combined with rifampin in healthy volunteers. Antimicrob Agents Chemother. 2004;48:1553–1560. doi: 10.1128/AAC.48.5.1553-1560.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Geneva, Switzerland: World Health Organization; Antiretroviral Therapy for HIV Infection in Adults and Adolescents: Recommendations for a Public Health Approach (2006 revision) Available at : http://www.who.int/hiv/pub/guidelines/adult/en/ [PubMed]
- 12.Ren Y, Nuttall JJ, Egbers C, et al. Effect of Rifampicin on Lopinavir Pharmacokinetics in HIV-Infected Children With Tuberculosis. J Acquir Immune Defic Syndr. 2008;47:566–569. doi: 10.1097/QAI.0b013e3181642257. [DOI] [PubMed] [Google Scholar]