Abstract
Periodontitis is a periodontal tissue infectious disease and the most common cause for tooth loss in adults. It has been linked to many systemic disorders, such as coronary artery disease, stroke, and diabetes. At present, there is no ideal therapeutic approach to cure periodontitis and achieve optimal periodontal tissue regeneration. In this study, we explored the potential of using autologous periodontal ligament stem cells (PDLSCs) to treat periodontal defects in a porcine model of periodontitis. The periodontal lesion was generated in the first molars area of miniature pigs by the surgical removal of bone and subsequent silk ligament suture around the cervical portion of the tooth. Autologous PDLSCs were obtained from extracted teeth of the miniature pigs and then expanded ex vivo to enrich PDLSC numbers. When transplanted into the surgically created periodontal defect areas, PDLSCs were capable of regenerating periodontal tissues, leading to a favorable treatment for periodontitis. This study demonstrates the feasibility of using stem cell-mediated tissue engineering to treat periodontal diseases.
Keywords: Periodontal ligament stem cells, Periodontal disease, Tissue engineering, Regeneration
Introduction
Periodontitis is one of the most widespread infectious diseases; it is characterized by chronic bacterial infection of the supporting structures of the teeth, leading to tooth loss in adults. It is associated with a number of systemic diseases [1]. Systemic factors modify periodontitis principally through effects on the normal immune and inflammatory mechanisms [2–5]. Conversely, periodontitis may also trigger both local and systemic host inflammatory responses capable of exacerbating diabetes by decreasing glycemic control [6, 7] and conferring significantly elevated risk of cardiovascular disease and premature low birth weight [8]. Consequently, a new discipline, periodontal medicine, has emerged to further define interrelationships between periodontitis and systemic diseases through scientific inquiry. Ultimately, this new knowledge may prove useful in intervention strategies to reduce patient risks and prevent periodontitis-associated systemic diseases [9, 10]. Until now, the regenerative treatment of periodontal disease has been a major challenge in clinical periodontics. Conventional regeneration therapies, such as guided tissue regeneration, topical application of enamel matrix derivative, and use of various growth factors, can partially regenerate periodontal tissues [11–15]. However, the results in clinical applications vary greatly, depending on the individual anatomy of the defects or the amount of resident vital periodontal ligament [16]. The strategy of periodontal tissue regeneration therapies is to control inflammation and stimulate stem progenitors to regenerate new periodontal tissues. However, the residual periodontal ligament stem cells (PDLSCs) are limited in those patients with periodontitis because of long-term inflammation. Therefore, the current study examined whether functional periodontal tissue can be regenerated following transplantation of autologous PDLSCs. Human PDLSCs have previously been successfully isolated from extracted human teeth [17, 18]. Ex vivo-expanded PDLSCs are capable of regenerating a typical cementum/periodontal ligament-like structure when transplanted into immunocompromised mice using hydroxyapatite/tricalcium phosphate (HA/TCP) as a carrier. The current study investigated whether PDLSC-mediated tissue regeneration may provide an ideal approach for functional periodontal tissue regeneration. It has been speculated that transplantation of PDLSCs directly into periodontal defect areas caused by periodontal disease might be a viable therapeutic approach [19–21]. Because of the small size of the orofacial region in rodents, it is difficult to use rodent model to assess periodontal regeneration. Many studies have described the benefits of using miniature pigs as an ideal experimental animal model for many human diseases. The physiology, pathology, immunology, and anatomy structures of miniature pig are similar to those of humans [22]. Furthermore, spontaneous gingivitis and periodontitis can be observed in the miniature pig. In this study, we used miniature pig to generate an experimental animal model of periodontitis and investigated the utility of autologous PDLSC-mediated tissue engineering to treat periodontitis.
Materials and Methods
Animals
Fourteen inbred miniature pigs, 12 months old and weighing 30−40 kg, were obtained from the Institute of Animal Science of the Chinese Agriculture University (Beijing, China). Animals were housed under conventional conditions with free access to water and food. This study was reviewed and approved by the animal care and use committees of Capital Medical University.
Antibodies
The following antibodies were used: monoclonal mouse anti-human STRO-1 antibody (MAB1038; R&D Systems Inc., Minneapolis, http://www.rndsystems.com); monoclonal mouse anti-human Nestin antibody (MAB1259; R&D Systems); fluorescein-conjugated mouse anti-human Nestin monoclonal antibody (IC1259F; R&D Systems); alkaline phosphatase (ALP) detection kit (SCR004; Millipore, Billerica, MA, http://www.millipore.com); mouse monoclonal antibody to ALP (tissue nonspecific; ab17973; Abcam, Cambridge, MA, http://www.abcam.com); fluorescein-conjugated secondary goat anti-mouse polyclonal antibody (STAR 87F; AbD Serotec, Raleigh, NC, http://www.ab-direct.com); and Hoechst (14530 bis-benzimide Hoechst 33258; Fluka, Buchs, Switzerland, http://www.sigmaaldrich.com).
Generation of Periodontitis Model
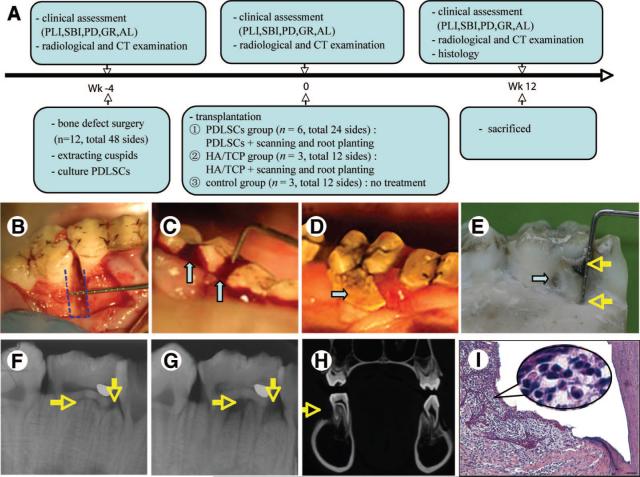
Twelve miniature pigs were used to generate periodontitis lesions. Two miniature pigs were sacrificed without any treatment at the end of the study, and the samples of the first molars were assessed by histology as representatives of normal periodontal tissues. Three separate cohorts of animals were studied (i.e., the experiments described were performed three separate times), using the same general experimental design (Fig. 1A). Animals were first anesthetized with a combination of ketamine chloride (6 mg/kg) and xylazine (0.6 mg/kg) injected intramuscularly. After clinical assessment (Fig. 1A), a mucoperiosteal flap was raised and alveolar bone was removed using surgical bur to create experimental periodontal defects in the mesial region of the maxilla and mandibular first molars. The created alveolar bone defect was 3 mm in width, 7 mm in length, and 5 mm in depth (Fig. 1B), and notch-shaped marks were made on the root surface at the level of the top of the alveolar crest and the floor of defect. In total, 48 defects were created in 12 miniature pigs. After the operation, 4−0 silk ligament was sutured around the cervical portion of the first molars. After the generation of periodontitis models, the 48 defects were randomly assigned to three different treatment groups (Fig. 1A).
Figure 1.
Generation of periodontitis model in miniature pigs. (A): Schematic illustration of the timeline of the procedures conducted for this study. Three separate cohorts of miniature pigs were tested in this manner. (B): A 3-mm-wide, 7-mm-long, and 5-mm-deep bone defect was created in the mesial region of the maxilla or mandibular first molar (dotted line, arrow). (C): Four weeks after creating the bone defect by surgery and subsequent silk ligament, the inflammation of periodontal tissues was observed (arrows). (D, E): Typical periodontitis clinical findings presented in this model. Twelve weeks after the surgery, the gingival was red and swollen. A calculus could be seen around the margin of gingiva ([D], arrow), the inflammation was extended to the furcation area, and the whole mesial-buccal root of the first permanent molar was exposed ([E], arrows). (F–H): Imaging manifestations of this periodontitis model. X-ray image showed that the mineral density of the alveolar bone was decreased at the regions of furcation and the mesial side of the first permanent molar ([G], arrows) compared with control density of the alveolar bone ([F], arrows). CT coronal image showed obvious bone defect in the buccal alveolar region ([H], arrow). (I): Histological photomicrography showing typical periodontitis histopathological manifestations, including marked periodontal tissue reduction and inflammatory infiltration. Scale bar = 100 μm. Abbreviations: AL, attachment loss; CT, computed tomography; GR, gingival recession; HA/TCP, hydroxyapatite/tricalcium phosphate; PD, probing depth; PDLSC, periodontal ligament stem cell; SBI, sulcus bleeding index; Wk, week.
PDLSC Culture
The cuspids of the miniature pigs were extracted for the isolation of periodontal ligament cells 4 weeks before transplantation. The periodontal ligament from the middle third of the extracted cuspid root was gently separated from the surface of the root and then digested in a solution of 3 mg/ml collagenase type I (Worthington Biochemical, Freehold, NJ, http://www.worthington-biochem.com) and 4 mg/ml dispase (Roche Diagnostics, Basel, Switzerland, http://www.roche-applied-science.com) for 1 hour at 37°C [17]. Single-cell suspensions were obtained by passing the cells through a 70-μm strainer (Falcon; BD Labware, Franklin Lakes, NJ, http://www.bdbiosciences.com). To identify putative stem cells, single-cell suspensions (1 × 104∼1 × 105 cells) were seeded into 10-cm culture dishes (Corning Costar, Cambridge, MA, http://www.corning.com/lifesciences) with α-modification of Eagle's medium (Gibco, Carlsbad, CA, http://www.invitrogen.com) supplemented with 15% fetal calf serum (Equitech-Bio Inc., Kerrville, TX, http://www.equitechbio.com), 100 mol/l ascorbic acid 2-phosphate (Wako Chemical, Tokyo, http://www.wako-chem.co.jp/english), 2 mmol/l glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (Invitrogen, Carlsbad, CA, http://www.invitrogen.com) and then incubated at 37°C in 5% carbon dioxide. Colony-forming efficiency was assessed on day 14. Aggregates of 50 or more cells were scored as colonies.
To trace directly the distribution and differentiation of PDLSCs in vivo, the recombinant retroviral vector with green fluorescent protein (RV-GFP; Clontech, Palo Alto, CA, http://www.clontech.com) was used to label the third-passage PDLSCs. Recombinant RV-GFP expression vector was constructed and transfected into the packaging cell PT67. After G418 screening and amplification, cell clones producing high-level recombinant viruses were obtained and expanded in vitro. The virus supernatants from infected PT67 cell cultures were used to infect proliferating PDLSCs directly. Autologous GFP-labeled PDLSCs were seeded onto HA/TCP (Biomedical Materials and Engineering Center of Wuhan University of Technology, Wuhan, China, http://public.whut.edu.cn/shwzhx/english.htm) and transplanted into the periodontal bone defects.
Immunocytochemical Staining
Third-passage PDLSCs were subcultured in 24-chamber slides. Cells were fixed in 4% paraformaldehyde for 15 minutes and blocked with phosphate-buffered saline (PBS) containing 10% normal equine serum at room temperature for 45 minutes. Then, cells were incubated with diluted primary antibody (anti-STRO-1 or anti-Nestin) overnight at 4°C, washed with PBS, and then incubated with fluorescein-conjugated secondary antibody at room temperature in the dark for 45 minutes; Hoechst staining was then performed in the dark for 5 minutes. After being washed with PBS, the slides were mounted and then analyzed using a fluorescence microscope.
An alkaline phosphatase detection kit was used to test the expression of ALP according to the manufacturer's protocol. Third-passage PDLSCs were grown for 5 days in 24-well plates, fixed with 4% paraformaldehyde and 90% methanol/10% formaldehyde for 2 minutes, and washed with rinse buffer (20 mmol/l Tris-HCl, pH 7.4, 0.15 mmol/l NaCl, 0.05% Tween-20 [TBST]). The substrate solution was prepared by adding fast red violet (FRV) with naphthol AS-BI phosphate solution and water in a 2:1:1 ratio (FRV:naphthol:water). The substrate solution was added to each well and incubated in dark at room temperature for 15 minutes. Following this, the plates were washed with TBST and then stained with hematoxylin before being analyzed using a light microscope.
Flow Cytometric Analysis
Approximately 2.5 × 105 third-passage PDLSCs in each 1.5-ml Eppendorf tube were fixed with 4% paraformaldehyde for 15 minutes. Primary STRO-1 and ALP antibodies were added to the tubes and incubated at room temperature for 1 hour, followed by fluorescein-conjugated secondary antibody at room temperature in the dark for 45 minutes. The percentage of PDLSCs staining positive for STRO-1 and ALP was assessed using a FACSCalibur flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA, http://www.bd.com).
In parallel studies, the same numbers of PDLSCs were fixed as described above and resuspended in 2 ml of saponin buffer. Then, cells were incubated with diluted fluorescein-conjugated Nestin antibody at room temperature in the dark for 30 minutes. The percentage of PDLSCs staining positive to Nestin was assessed by flow cytometric analysis.
Scanning Electron Microscope
Ex vivo-expanded PDLSCs grown for 7 days were fixed using 2.5% glutaraldehyde in 0.1 mol/l sodium cacodylate buffer (pH 7.2) for 2 hours at 4°C. After being washed with sodium dimethylarsenate buffer, the cells were postfixed in 1% osmium tetroxide, dehydrated with gradient alcohol, and then incubated with isoamyl acetate. After gold coating, the samples were examined using a Hitachi S-520 scanning electron microscope (Hitachi, Tokyo, http://www.hitachi.com).
Transmission Electron Microscope
Ex vivo-expanded PDLSCs grown for 7 days were fixed using 2.5% glutaraldehyde in 0.1 mg/ml sodium cacodylate buffer (pH 7.2) for 2 hours at 4°C. After fixation, the samples were rinsed three times with 0.1 mol/l sodium cacodylate buffer (pH 7.2) for 0.5 hour. The samples were postfixed in 2% osmium tetroxide, washed for 1 hour, dehydrated in a graded ethanol series, and embedded in Epon 812 resin according to the manufacturer's instructions. Serial 0.5-μm sections were cut and examined using a light microscope (BHSRFK; Olympus, Tokyo, http://www.olympus-global.com) after staining with 2% toluidine blue for 5 minutes. For transmission electron microscopy analysis, 70-nm sections were cut, stained with 2% uranyl acetate for 30 minutes and 2% lead citrate for 5 minutes, and observed with a JEM1010 transmission electron microscope (JEOL, Tokyo, http://www.jeol.com).
Transplantation
One month after the generation of periodontitis lesions, clinical assessment was made for all 48 molars. The three treated groups were as follows: (a) control group (12 defects in three miniature pigs), no treatment; (b) HA/TCP group (12 defects in three miniature pigs), flap surgery, transplantation of HA/TCP scaffolds, and covering of the defects with gelatin membranes (Nanjing Jinling Medical Co., Nanjing, China, http://www.jlpharm.com/english.htm); and (c) PDLSC group (24 defects in six miniature pigs), flap surgery, transplantation of approximately 2.0 × 107 of the expanded third passage autologous PDLSCs combined with HA/TCP, and covering of the defects with gelatin membranes. During the treatment, the teeth were cleaned first, and then flap surgery, in which a mucoperiosteal flap was raised and granulation tissue was removed from the defect, was performed in all groups (except for the control group). All animals received 500 mg of amoxicillin and 200 mg of metronidazole three times daily for 7 days (mixed with food), and their mouths were washed daily with 0.2% chlorhexidine digluconate for 5 days.
Clinical and Radiological Evaluations
Clinical assessments, including plaque index (PLI) [23], sulcus bleeding index (SBI) [24], probing depth (PD), gingival recession (GR), and attachment loss (AL), were made on all experimental teeth pretransplantation and post-transplantation at weeks 4 and 12. The PD values were established with a Williams periodontal probe (Shanghai Kangqiao Dental Instruments Factory, Shanghai, China, http://www.kqdif.com). Bone regeneration was examined by x-ray (Sirona, Bensheim, Germany, http://www.sirona.com) and computed tomography (CT) (Siemens, Erlangen, Germany, http://www.medical.siemens.com) films at the indicated time points pretrans-plantation and 12 weeks after transplantation. The scanning length of CT was 0.75 mm.
Quantitative and Histological Assessments of Regenerated Periodontal Tissues
At 12 weeks after transplantation, all animals were sacrificed, and the samples from the experimental area were harvested and fixed with 4% formaldehyde. Following removal of the soft tissues from the experimental regions, the heights of new bone regeneration were measured by using a Williams periodontal probe. The distance from the top of newly formed bone to notch-shaped marks made during the operation was scaled. Each sample was measured at three different positions from the buccal to the lingual side. Mean values were recorded, and the heights of new bone regeneration were 7 mm minus mean values. Then, the harvested samples were assessed histologically. Parts of the samples were subsequently decalcified with buffered 10% edetic acid (pH 8.0) and embedded in paraffin. Sections were deparaffinized and stained with hematoxylin and eosin. For histopathological assessment, buccal-lingual-direction sections of experimental region were cut. Other parts of the samples were embedded in plastic and prepared as a nondecalcified series of slices [25]. The GFP-labeled PDLSCs were observed by fluorescence microscopy (Olympus BX/TF, U-LH100HG).
Statistical Analysis
Data were analyzed using one-way analysis of variance with pairwise comparisons using the Bonferroni method. Details about the statistical test performed are given in the legends to Tables 1 and 2.
Table 1.
Clinical assessment of periodontal situation in three groups
| −4 Weeksa |
0 Weeksb |
12 Weeksc |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | PD | GR | AL | PD | GR | AL | PD | GR | AL |
| PDLSC group | 2.6 ± 0.4 | 0 | 2.6 ± 0.4 | 10.0 ± 0.4 | 2.1 ± 1.8 | 12.1 ± 1.8 | 2.9 ± 0.3 | 0.4 ± 0.4 | 3.3 ± 0.6 |
| HA/TCP group | 2.6 ± 0.4 | 0 | 2.6 ± 0.4 | 10.2 ± 0.4 | 2.6 ± 1.6 | 12.8 ± 1.4 | 3.6 ± 0.8 | 1.8 ± 0.9 | 5.3 ± 0.3 |
| Control group | 2.3 ± 0.3 | 0 | 2.3 ± 0.3 | 10.0 ± 0.6 | 2.4 ± 1.5 | 12.4 ± 1.7 | 4.7 ± 0.4 | 1.6 ± 0.7 | 6.3 ± 0.5 |
Data are expressed as mean ± SD (mm). The differences of AL at each time point among three groups were analyzed using one-way analysis of variance. The pairwise comparisons were analyzed using Bonferroni method.
At the time point of −4 weeks, there was no significant difference among the three groups (F = 1.932; p = .161).
At the time point of 0 weeks, there was no significant difference among the three groups (F = 0.482; p = .622).
At the time point of 12 weeks, the difference among the three groups was statistically significant (F = 125.917; p = .000). The results of pairwise comparisons were as follows: PDLSC group and HA/TCP group, p = .000; PDLSC group and control group, p = .000; HA/TCP group and control group, p = .000.
Abbreviations: AL, attachment loss; GR, gingival recession; HA/TCP, hydroxyapatite/tricalcium phosphate; PD, probing depth; PDLSC, periodontal ligament stem cell.
Results
Generation of Periodontitis in Miniature Swine
To determine whether periodontitis-associated periodontal defects can be regenerated by PDLSC-mediated treatment, it is fundamental to generate a typical and stable periodontitis model in a large animal. In this study, we chose miniature pigs as the experimental animal to generate periodontitis. This animal model was chosen because it shares many of the characteristics with regard to physiology and pathophysiology of human periodontitis lesions [22, 26, 27]. We surgically removed alveolar bone and subsequently used silk suture of the ligament around the cervical portion of the first molars. General status of the periodontal tissues was assessed by commonly used clinical indexes, including PLI, SBI, PD, GR, and AL. In the early stages of periodontitis, GR and AL were limited, and PD could reflect the degree of inflammation in periodontal tissues. With the development of periodontitis, we needed to consider the three indexes comprehensively. Usually, AL is considered a better parameter to assess the periodontal situation more comprehensively. In addition, we used CT and histopathology to evaluate bone defects and inflammation conditions. Four weeks after the creation of the defects, the inflammation of periodontal tissues was obvious (Fig. 1C). PD was significantly increased from 2.6 to 10 mm, which was much deeper than what we had created in the periodontal tissue (Table 1). Plaque index and sulcus bleeding index were significantly increased compared with those in the preoperation group (data of PLI and SBI not shown). Twelve weeks after periodontitis formation, the inflammation of gingival tissue was still distinct in the untreated control group, which showed reddish and swelled gingival tissue along with calculus around the gingival margin (Fig. 1D). Although PD decreased, GR and AL were significantly increased (Table 1). This indicates that the periodontal tissues in the untreated group showed partial but limited restoration, below normal levels. Moreover, the inflammation was found to extend to the furcation area in some samples, and the whole mesialbuccal root of the first permanent molar was exposed (Fig. 1E). Radiographic analysis indicated a significant decrease in alveolar bone density in the regions of furcation and the mesial side of the first permanent molar compared with that in preoperation samples (Fig. 1F, 1G). Furthermore, the CT images of coronal scanning showed marked bone loss in buccal of the first molar (Fig. 1H). In addition, histological analysis showed marked erosion and ulcer development on the surface of sulcular epithelium, infiltration of inflammatory cells underlying connective tissue, and hyperemia and edema of blood vessels in the gingival tissues (Fig. 1I). These data indicate that a stable and typical periodontitis lesion could be successfully established in swine by surgical removal of alveolar bone in association with the placement of a silk suture ligature.
Characteristics of PDLSCs Derived from Miniature Pig
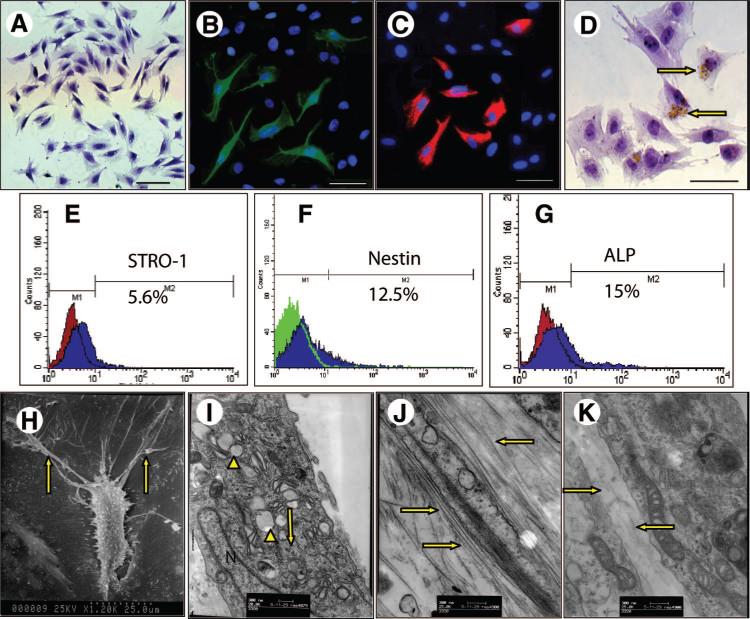
We then examined whether PDLSCs could be harvested from the periodontal ligament (PDL) tissue extracted from the cuspids of miniature pigs analogous to human PDL tissue containing condensed parallel collagen fibers and cellular components. To identify putative stem cells, single-cell suspensions were generated from miniature pig PDL and expanded ex vivo as previously described for human PDLSCs [17]. The ability of porcine PDL-derived cells to form adherent clonogenic cell clusters of fibroblast-like cells, similar to those recorded for human PDLSCs, was shown by the formation of approximately 23 single colonies generated per 105 single cells cultured at low density. The progeny of the colony-forming cells were fusiform in shape (Fig. 2A) and showed positive immunostaining for STRO-1, Nestin, and ALP (Fig. 2B–2D), where approximately 5.6% of these periodontal ligament cells stained positive for STRO-1, 12.5% for Nestin, and 15.0% for ALP, as assessed by flow cytometric analysis (Fig. 2E, 2F, 2G), suggesting that porcine PDLSCs are a heterogeneous cell population. Morphologically, PDLSCs displayed many short and long branching cytoplasmic processes under a scanning electron microscope, surrounded by secreted extracellular matrix (Fig. 2H). The cells contained abundant organelles, such as mitochondria, ribosome, and rough endoplasm, and secreted extracellular matrix, as assessed by transmission electron microscopy (Fig. 2I). The collagen fibrils produced by PDLSCs were larger and thicker than those produced by dental papilla stem cells under the same culture conditions (Fig. 2J, 2K).
Figure 2.
Characterization of miniature pig periodontal ligament stem cells (PDLSCs). (A): The cultured PDLSCs from single colonies showed typical fibroblast-like cells under a light microscope. (B–D): Immunocytochemical staining using STRO-1 (B), Nestin (C), and ALP (D) in PDLSCs showed positive staining. Approximately 5.6% of the third-passage PDLSCs stained positive for STRO-1 (E), 12.5% for Nestin (F), and 15.0% for ALP through flow cytometric analysis (G). (H–K): Ultrastructural observation of collagen produced by PDLSCs. Morphologically, PDLSC was fusiform-shaped, with many short and long ramifications under a scanning electron microscope, and the secreted extracellular matrix were around them (H). (I): The cells contained abundant organelles, such as mitochondria, ribosome (arrowheads) and rough endoplasm (arrow), as assessed by transmission electron microscopy. The miniature pig PDLSCs produced abundant collagen fibers ([J], arrows). The quantity of collagens produced by PDLSCs was much greater than that produced by dental papilla stem cells from the same animal ([K], arrows) under the same culture conditions. Scale bars = 100 μm. Abbreviations: ALP, alkaline phosphatase; N, nucleus.
PDLSC-Mediated Periodontal Tissue Regeneration
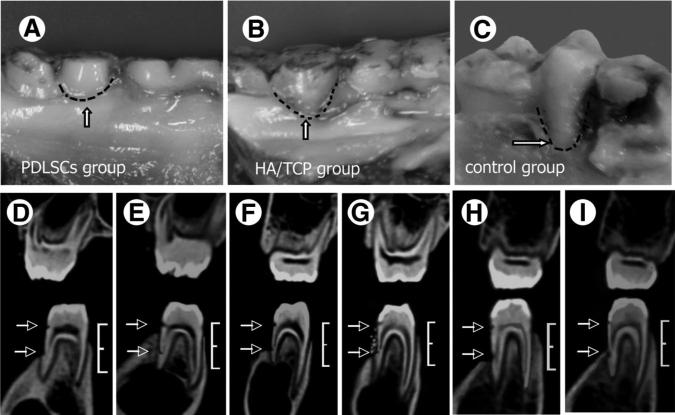
We hypothesized that PDLSC-mediated tissue regeneration may provide an ideal approach for functional periodontal tissue regeneration. To test this theory, we transplanted autologous PDLSCs combined with HA/TCP to the bone defects following experimentally induced periodontitis in miniature pigs. We have previously demonstrated that PDLSCs are capable of regenerating periodontal tissue when cotransplanted with root apical papilla stem cells for tooth root regeneration in a porcine model [26]. In this study, we used a common clinical assessment index, CT, and histopathology to evaluate the PDLSC-mediated periodontal tissue regeneration. At 12 weeks post-transplantation, the AL were 3.3 ± 0.6 mm in the PDLSC-mediated group, 5.3 ± 0.3 mm in the HA/TCP group, and 6.3 ± 0.5 mm in the untreated control group. Statistical analysis indicated that PDLSC treatment significantly improved periodontal tissue regeneration in comparison with the HA/TCP and control groups (Table 1; Fig. 3A–3C). CT scan analyses showed that the height of periodontal alveolar bone in the PDLSC-mediated group recovered to approximately the normal levels (Fig. 3D, 3E). In contrast, the HA/TCP group and the control group showed very limited or no bone regeneration (Fig. 3F–3I).
Figure 3.
Gross and imaging assessment for PDLSC-mediated periodontal tissue regeneration. (A–C): Gross manifestations showed that 12 weeks after transplantation, PDLSC-mediated periodontal tissue regeneration was close to normal level ([A], dotted line, arrow). Only limited periodontal tissue was regenerated in HA/TCP group ([B], dotted line, arrow) and the control group ([C], dotted line). (D–I): Computed tomography imaging showed that the alveolar bone defect was obvious and at the same level prior to the PDLSCs transplantation (D, F, H). Height between the two arrows = 7 mm; bracket = 10 mm. Twelve weeks post-PDLSC transplantation, PDLSCs mediated a proximately complete periodontal tissue regeneration ([E], arrows), but limited regeneration and HA/TCP particles were noted in the HA/TCP group ([G], arrows). Very little alteration was found in the untreated control group in the same period ([I], arrows). Bracket = 10 mm. Abbreviations: HA/TCP, hydroxyapatite/ tricalcium phosphate; PDLSC, periodontal ligament stem cell.
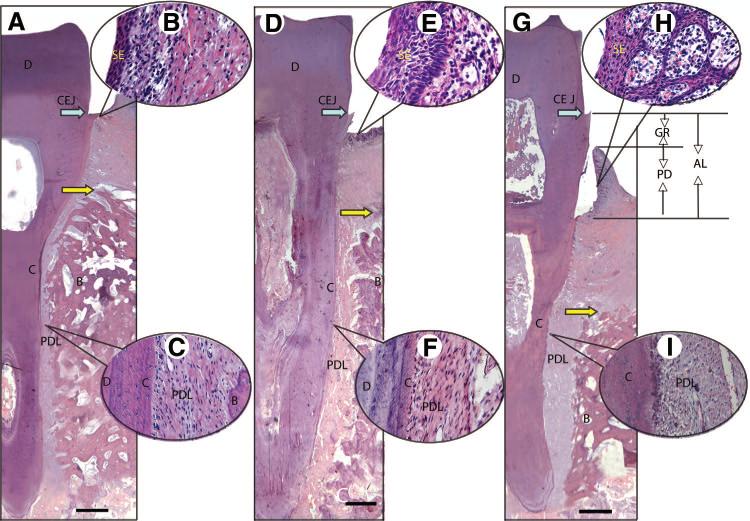
To evaluate the amount of periodontal tissue regeneration, a whole-view histopathological section of the experimental region in the buccal-lingual direction was made and stained by hematoxylin and eosin (Fig. 4). Histopathological photomicrographs showed that the position of junctional epithelium in normal periodontal tissues was on the cemento-enamel junction (Fig. 4A). The sulcular epithelium consisted of multilayered squamous epithelium in all three groups. It was thin and flat, and less infiltration of inflammatory cells could be observed in the connective tissues of the normal periodontal tissues (Fig. 4B). Sharpey's fibers were typical structures in the architecture of normal periodontal tissues (Fig. 4C). Although the position of junctional epithelium was below the cemento-enamel junction, the periodontal tissue regeneration in PDLSC-mediated group was much better than that in HA/TCP group (Fig. 4D). The sulcular epithelium in PDLSC-mediated group was thicker, and the epithelial pegs and dermal papillae were short and blunt, with fewer inflammatory cells in PDLSC-mediated group (Fig. 4E). New bone, cementum, and periodontal ligament were regenerated in the periodontal defect area in the PDLSC-mediated group (Fig. 4F), and the height of the new alveolar bone was much higher than that in HA/TCP group (Fig. 4G) but still lower than the normal level (Fig. 4A). Histopathological photomicrography showed that increased new bone and periodontal tissues, including cementum and periodontal ligament, were regenerated in the periodontal defect area, where newly formed Sharpey's fibers anchored into the newly regenerated cementum in the PDLSC-mediated group (Fig. 4F). Typical periodontitis, including marked periodontal tissue reduction (GR and AL), and infiltration of inflammatory cells were still found in the HA/TCP group (Fig. 4G). The epithelial pegs and dermal papillae were long and slender, with infiltration of inflammatory cells underlying connective tissue (Fig. 4H). Fibers lacking the typical structure of Sharpey's fibers filled in the periodontal defect in the HA/TCP group (Fig. 4I).
Figure 4.
Whole view of histopathological assessment for periodontal tissue regeneration by hematoxylin and eosin staining. A buccal-lingual direction histopathological section showed junctional epithelia attached on the cemento-enamel junction in normal periodontal tissues (A). The sulcular epithelium was thin and flat, and less infiltration of inflammatory cells could be observed in the connective tissues of the normal periodontal tissues (B). Sharpey's fibers were typically characteristic in the structure of normal periodontal tissues (C). Although the position of junctional epithelium was below the cemento-enamel junction, the periodontal tissue regeneration in periodontal ligament stem cell (PDLSC)-mediated group (D) was much better than that in HA/TCP group. The sulcular epithelium in PDLSC-mediated group was thicker, and the epithelial pegs and dermal papillae were short and blunt, with fewer inflammatory cells (E). New bone, cementum, and periodontal ligament were regenerated in the periodontal defect area in the PDLSC-mediated group (F), and the height of the new alveolar bone was much higher than that in HA/TCP group (G) but still lower than the normal level (A). Histopathological photomicrography showed that plenty of new bone and periodontal tissues, including cementum and periodontal ligament, were regenerated in the periodontal defective area, and the newly formed Sharpey's fibers plugged into the new regenerated cementum in the PDLSC-mediated group (F). Typical periodontitis, including marked periodontal tissue reduction (GR, AL), and infiltration of inflammatory cells were still found in HA/TCP group (G). The epithelial pegs and dermal papillae were long and slender, with infiltration of inflammatory cells underlying connective tissue (H). Fibers lacking the typical structure of Sharpey's fibers filled in the periodontal defect in the HA/TCP group (I). Scale bars = 1 mm. Yellow arrows show the top of the new bone. CEJ (blue arrows) is covered by junctional epithelium in normal periodontal tissue. GR is the height from CEJ to the margin of gingival; PD is the depth of periodontal pocket; AL is the length from CEJ to the bottom of the pocket (in this study, the bottom of the pocket was below CEJ, so AL is equal to the GR plus PD). Abbreviations: AL, attachment loss; B, bone; C, cementum; CEJ, cemento-enamel junction; D, dentin; GR, gingival recession; PD, probing depth; PDL, periodontal ligament; SE, sulcular epithelium.
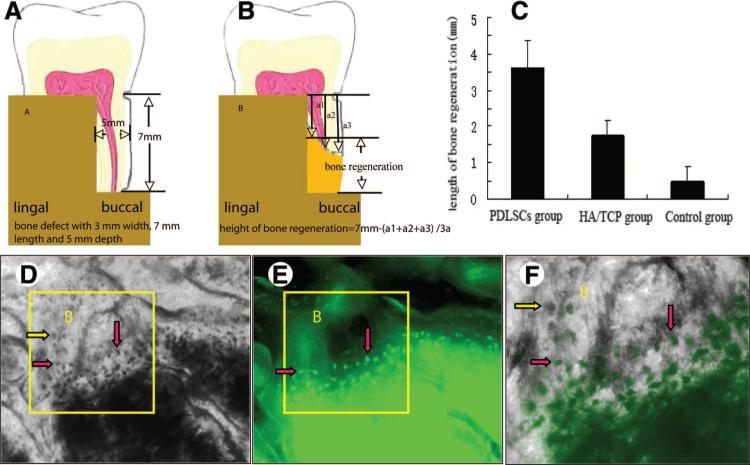
Furthermore, the quantity of regenerated alveolar bone was assessed. The original bone defect was created with dimensions of 3 mm width, 7 mm length, and 5 mm depth (Fig. 5A). At 12 weeks after transplantation, the distances from the top of newly formed bone to the notch-shaped mark made during the operation were scaled (Fig. 5B). The results showed that the height of regenerated alveolar bone was significantly higher in the PDLSC-mediated group than in the HA/TCP and control groups (F = 125.917; p = .000) (Fig. 5C).
Figure 5.
New bone regeneration assessment of PDLSC-mediated treatment for periodontitis in miniature pigs. (A–C): Quantitative assessment of regenerated alveolar bone. Originally, a bone defect with 3 mm wide, 7 mm long, and 5 mm deep was created (A). At 12 weeks after transplantation, the samples were harvested and fixed with 4% formaldehyde. Then, the soft tissues were removed from experimental regions, and the heights of new bone regeneration were measured by using Williams periodontal probe. The distances from the top of newly formed bone to the notch-shaped mark made during the operation were scaled. Each sample was measured in three different positions from buccal to lingual side. Mean values were recorded, and the heights of new bone regeneration were 7 mm minus mean values (B). The results showed that the height of regenerated alveolar bone was significantly higher in the PDLSC-mediated group than in the HA/TCP and control groups (F = 125.917; p = .000) (C). To identify whether the green fluorescent protein (GFP)-labeled PDLSCs implanted into the bone defects had differentiated to osteoblasts, nondecalcified slices were observed under the light microscope first, and osteoblasts and bone lacunas could clearly be seen in the new bone ([D], yellow and pink arrows). At the same visual field, the GFP+ PDLSCs were observed using a fluorescence microscope (E). When (D) and (E) were overlapped, the positions of some GFP+ cells were superposed on the osteoblasts and bone lacunas ([F], yellow arrow showing GFP− osteoblasts, red arrows showing GFP+ osteoblasts), which indicated that the GFP+ cells derived from PDLSCs had differentiated to osteoblasts. Abbreviations: B, bone; HA/TCP, hydroxyapatite/tricalcium phosphate; PDLSC, periodontal ligament stem cell.
To assess whether the GFP-labeled PDLSCs implanted into the bone defects had differentiated into osteoblasts, nondecalcified slices were first observed using light microscopy to identify osteoblasts and bone lacunas in the newly formed bone (Fig. 5D). In the same visual field, the GFP+ PDLSCs were then observed by fluorescence microscopy (Fig. 5E). When the images shown in Figure 5D and 5E were overlapped, the positions of some GFP+ cells were superposed with the osteoblasts and bone lacunas (Fig. 5F), which indicated that the GFP+ cells derived from autologous ex vivo-expanded PDLSCs had differentiated into osteoblasts in vivo.
Discussion
In this study, we established a preclinical model of periodontitis by creating alveolar bone defects in association with silk suture in miniature pigs. The results showed that typical periodontitis could be generated in miniature pig and that the gross and histological manifestations had features similar to those described for human cases of periodontitis. Clinical periodontitis caused by systemic diseases or other processes share similar manifestations, such as probing bleed, marked bone loss, deep periodontal pocket or clinical attachment loss, loose teeth, and histological inflammation. The purpose of various methods to establish the periodontal disease model, such as suture ligatures, bacteria, bone defect, and orthodontic elastics, was to mimic the clinical and histological manifestations regardless of the methodology used. In our model, periodontitis was induced by the surgical removal of the bone and subsequent silk ligament suture. The method could generate marked bone loss and cause significant inflammation in a relatively short period of time while displaying typical periodontitis manifestations, as described above. This approach was found to be a viable alternative to the establishment of a systemically induced disorder as observed in humans, which usually fails to show in animal models because of the short experimental time taken to induce periodontitis.
Large animal models are superior to models using small animals, such as rodents, for acquiring preclinical therapeutic data for the study of human diseases and assessing the safety and efficacy of different treatment regimes [22]. The oral maxillofacial region of miniature pigs is similar to that of humans in anatomy, development, physiology, pathophysiology, and disease occurrence [28, 29]. The miniature pig has both deciduous and permanent dentition. The morphology of the deciduous and permanent teeth and the anatomy structure of periodontal tissues are similar to that of humans [22]. In our previous study, we had demonstrated that transplantation of mesenchymal stem cells was a viable approach for oral and maxillofacial plastic surgery using a miniature pig model [27]. Gingivitis often presents in the miniature pig after the age of 6 months, and serious periodontitis can be found in the miniature pig after the age of 16 months. Histologically, the inflammatory process is similar to that seen in human periodontal diseases [30, 31]. Various methods have been used to establish the periodontal disease model, such as suture ligatures, bacteria [32, 33], bone defect [34], and orthodontic elastics [35]. The purposes of these methods were to generate clinical manifestations similar to those seen in human periodontitis, including probing bleed, marked bone loss, deep periodontal pocket, attachment loss, and loose tooth. In the present study, we demonstrated that the miniature pig is a suitable experimental model for the research and treatment of periodontal diseases, as a prelude to future human clinical trials.
Conventional treatment for periodontitis, such as chemical agents, mechanical methods, and periodontal surgery, are to alleviate the inflammation in periodontal tissues and improve the regeneration of periodontium (PDL, cementum, and alveolar bone). However, at present the clinical results are generally unsatisfactory, because the periodontium has a limited capacity for regeneration. The poor regenerative capacity may be attributed to inflammatory mediators produced during the long-term chronic inflammation in periodontal tissues that may change the characteristics and numbers of resident PDLSCs and therefore the potential of these stem cells to populate the bone and root surfaces. Several studies have indicated that transplanted cells can regenerate periodontal tissues, including cementum, periodontal ligament, and alveolar bone [36, 37], in animal defect models of periodontal disease. However, the capacity for tissue regeneration of different cell types is variable [38]. In our studies, we examined whether transplantation of autologous PDLSCs directly into the periodontal defects contributed to periodontal tissue repair in experimental periodontitis in miniature pigs. In our preliminary experiments, we found that gingival fibroblast transplant using HA/TCP as the carrier vehicle showed little tissue regeneration capacity, as seen in the HA/TCP group. Therefore, in this study, we used HA/TCP only as a control group.
Previous experiments showed that freshly isolated and cryopreserved human periodontal ligaments contain stem cells that are capable of differentiating into cementoblastic cells in vitro and forming cementum/PDL-like tissues in vivo [17, 18]. In this study, miniature pig PDLSCs were successfully isolated from PDL and shared similar characteristics to human PDLSCs [39]. A subset of these cells retained expression of early-stage markers of stem cells, such as STRO-1 (5.6%), Nestin (12.5%), and ALP (15.0%). In this study, 23 single colonies were generated from 105 single cells cultured at low density, and they demonstrated the capacity to produce a collagen matrix in vitro. When autologous porcine PDLSCs were transplanted into surgically created periodontal defects, they appeared to have an excellent capacity to form bone, cementum, and periodontal ligament. Twelve weeks after transplantation, the GFP-labeled cells were present in newly formed periodontal bones and had differentiated to osteo-blasts, suggesting that transplanted PDLSCs had contributed to periodontal tissue regeneration in vivo. In contrast, defects were largely replaced by fibrous tissue and epithelia, and limited new attachment was observed in the control and HA/TCP groups. The evidence of GFP-positive osteoblasts indicated that these new bone tissues formed from proliferating and differentiating GFP-labeled PDLSCs in surrounding periodontitis legions. In the visual fields observed, more than 50% of osteoblasts appeared as GFP-positive cells. This therapeutic approach appears superior to clinical conventional treatment methods for periodontitis, which produce only limited bone regeneration, presumably via residual PDLSCs and osteoblast precursors in patients with periodontitis. Collectively, these studies support the concept of using PDLSC as a potential stem cell technology to treat periodontal diseases.
Our observations indicated that a multilevel cellular/bio-material treatment strategy may offer an optimal therapeutic approach for periodontal tissue regeneration. It is likely that the transplanted PDLSCs generated some of the new tissues and helped remodel the local microenvironment, which prevented epithelial down-growth to optimize recovery of the periodontal defects. Furthermore, we speculate that the interactions among the transplanted stem cells, the residual precursor cells, and the local periodontal microenvironment may play an important role. The periodontal microenvironment could stabilize and induce the transplanted PDLSCs to growth and differentiation into periodontium, whereas the transplanted PDLSCs themselves could also secrete various cytokines that may stimulate the function of residual progenitor cells [17, 18]. Furthermore, the assessment of different bioscaffold materials could help identify suitable carriers to help induce transplanted precursor cells and better integrate them into the surrounding environment to improve PDLSC-mediated periodontal tissue regeneration.
Conclusion
The present study demonstrates the utility of using an autologous PDLSC therapeutic approach to treat periodontitis in a miniature pig preclinical model.
Acknowledgments
This work was supported by grants from Beijing Major Scientific Program (D0906007000091; to S.W.), the National Natural Science Foundation of China (Grants 30430690, 30428009, and 30700941; to S.W., S.S., and Y.L.), the University of Southern California School of Dentistry Start Fund (to S.S.), the National Institute of Dental and Craniofacial Research, NIH (R01DE17449; to S.S.), and the Beijing New Star Program (2007B067; to Y.L.).
Footnotes
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
EFERENCES
- 1.Kinane DF, Marshall GJ. Periodontal manifestations of systemic disease. Aust Dent J. 2001;46:2–12. doi: 10.1111/j.1834-7819.2001.tb00267.x. [DOI] [PubMed] [Google Scholar]
- 2.Behle JH, Papapanou PN. Periodontal infections and atherosclerotic vascular disease: An update. Int Dent J. 2006;56:256–262. doi: 10.1111/j.1875-595x.2006.tb00110.x. [DOI] [PubMed] [Google Scholar]
- 3.Geismar K, Stoltze K, Sigurd B, et al. Periodontal disease and coronary heart disease. J Periodontol. 2006;77:1547–1554. doi: 10.1902/jop.2006.050405. [DOI] [PubMed] [Google Scholar]
- 4.Andriankaja OM, Genco RJ, Dorn J, et al. The use of different measurements and definitions of periodontal disease in the study of the association between periodontal disease and risk of myocardial infarction. J Periodontol. 2006;77:1067–1073. doi: 10.1902/jop.2006.050276. [DOI] [PubMed] [Google Scholar]
- 5.Tan WC, Tay FB, Lim LP. Diabetes as a risk factor for periodontal disease: Current status and future considerations. Ann Acad Med Singapore. 2006;35:571–581. [PubMed] [Google Scholar]
- 6.Heitz-Mayfield LJ. Disease progression: Identification of high-risk groups and individuals for periodontitis. J Clin Periodontol. 2005;32:196–209. doi: 10.1111/j.1600-051X.2005.00803.x. [DOI] [PubMed] [Google Scholar]
- 7.Kiran M, Arpak N, Unsal E, et al. The effect of improved periodontal health on metabolic control in type 2 diabetes mellitus. J Clin Periodontol. 2005;32:266–272. doi: 10.1111/j.1600-051X.2005.00658.x. [DOI] [PubMed] [Google Scholar]
- 8.Holmlund A, Holm G, Lind L. Severity of periodontal disease and number of remaining teeth are related to the prevalence of myocardial infarction and hypertension in a study based on 4,254 subjects. J Periodontol. 2006;77:1173–1178. doi: 10.1902/jop.2006.050233. [DOI] [PubMed] [Google Scholar]
- 9.Paquette DW, Madianos P, Offenbacher S, et al. The concept of “risk” and the emerging discipline of periodontal medicine. J Contemp Dent Pract. 1999;1:1–8. [PubMed] [Google Scholar]
- 10.Williams RC, Offenbacher S. Periodontal medicine: The emergence of a new branch of periodontology. Periodontol 2000. 2000;23:9–12. doi: 10.1034/j.1600-0757.2000.2230101.x. [DOI] [PubMed] [Google Scholar]
- 11.Gonçalves PF, Gurgel BC, Pimentel SP, et al. Effect of two different approaches for root decontamination on new cementum formation following guided tissue regeneration: A histomorphometric study in dogs. J Periodontal Res. 2006;41:535–540. doi: 10.1111/j.1600-0765.2006.00902.x. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann T, Richter S, Meyle J, et al. A randomized clinical multi-centre trial comparing enamel matrix derivative and membrane treatment of buccal class II furcation involvement in mandibular molars. Part III: Patient factors and treatment outcome. J Clin Periodontol. 2006;33:575–583. doi: 10.1111/j.1600-051X.2006.00947.x. [DOI] [PubMed] [Google Scholar]
- 13.Needleman IG, Worthington HV, Giedrys-Leeper E, et al. Guided tissue regeneration for periodontal infra-bony defects. Cochrane Database Syst Rev. 2006:CD001724. doi: 10.1002/14651858.CD001724.pub2. [DOI] [PubMed] [Google Scholar]
- 14.Venezia E, Goldstein M, Boyan BD, et al. The use of enamel matrix derivative in the treatment of periodontal defects: A literature review and meta-analysis. Crit Rev Oral Biol Med. 2004;15:382–402. doi: 10.1177/154411130401500605. [DOI] [PubMed] [Google Scholar]
- 15.Kaigler D, Cirelli JA, Giannobile WV. Growth factor delivery for oral and periodontal tissue engineering. Expert Opin Drug Deliv. 2006;3:647–662. doi: 10.1517/17425247.3.5.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blumenthal NM. A clinical comparison of collagen membranes with e-PTFE membranes in the treatment of human mandibular buccal class II furcation defects. J Periodontol. 1993;64:925–933. doi: 10.1902/jop.1993.64.10.925. [DOI] [PubMed] [Google Scholar]
- 17.Seo BM, Miura M, Gronthos S, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 18.Seo BM, Miura M, Sonoyama W, et al. Recovery of stem cells from cryopreserved periodontal ligament. J Dent Res. 2005;84:907–912. doi: 10.1177/154405910508401007. [DOI] [PubMed] [Google Scholar]
- 19.Thesleff I, Tummers M. Stem cells and tissue engineering: Prospects for regenerating tissues in dental practice. Med Princ Pract. 2003;12:43–50. doi: 10.1159/000069840. [DOI] [PubMed] [Google Scholar]
- 20.Young CS, Abukawa H, Asrican R, et al. Tissue-engineered hybrid tooth and bone. Tissue Eng. 2005;11:1599–1610. doi: 10.1089/ten.2005.11.1599. [DOI] [PubMed] [Google Scholar]
- 21.Bartold PM, Shi S, Gronthos S. Stem cells and periodontal regeneration. Periodontol 2000. 2006;40:164–172. doi: 10.1111/j.1600-0757.2005.00139.x. [DOI] [PubMed] [Google Scholar]
- 22.Wang SL, Liu Y, Fang DJ, et al. The miniature pig: A useful large animal model for dental and orofacial research. Oral Dis. 2007;13:530–537. doi: 10.1111/j.1601-0825.2006.01337.x. [DOI] [PubMed] [Google Scholar]
- 23.Quigley GA, Hein JW. Comparative cleansing efficiency of manual and power brushing. J Am Dent Assoc. 1962;65:26–29. doi: 10.14219/jada.archive.1962.0184. [DOI] [PubMed] [Google Scholar]
- 24.Mazza JE, Newman MG, Sims TN. Clinical and antimicrobial effect of stannous fluoride on periodontitis. J Clin Periodontol. 1981;8:203–212. doi: 10.1111/j.1600-051x.1981.tb02031.x. [DOI] [PubMed] [Google Scholar]
- 25.Donath K, Breuner G. A method for the study of undecalcified bones and teeth with attached soft tissues. The Sage-Schliff (sawing and grinding) technique. J Oral Pathol. 1982;11:318–326. doi: 10.1111/j.1600-0714.1982.tb00172.x. [DOI] [PubMed] [Google Scholar]
- 26.Sonoyama W, Liu Y, Fang D, et al. Postnatal stem cell-mediated functional tooth regeneration. PLoS ONE. 2006;1:e79–92. doi: 10.1371/journal.pone.0000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fang D, Seo BM, Liu Y, et al. Transplantation of mesenchymal stem cells is an optimal approach for plastic surgery. STEM CELLS. 2007;25:1021–1028. doi: 10.1634/stemcells.2006-0576. [DOI] [PubMed] [Google Scholar]
- 28.Weaver ME, Jump EB, McKean CF. The eruption pattern of deciduous teeth in miniature swine. Anat Rec. 1966;154:81–86. doi: 10.1002/ar.1091540107. [DOI] [PubMed] [Google Scholar]
- 29.Weaver ME, Jump EB, McKean CF. The eruption pattern of permanent teeth in miniature swine. Arch Oral Biol. 1969;14:323–331. doi: 10.1016/0003-9969(69)90235-0. [DOI] [PubMed] [Google Scholar]
- 30.Kalkwarf KL, Krejci RF. Effect of inflammation on periodontal attachment levels in miniature swine with mucogingival defects. J Periodontol. 1983;54:361–364. doi: 10.1902/jop.1983.54.6.361. [DOI] [PubMed] [Google Scholar]
- 31.Singh G, O'Neal RB, Brennan WA, et al. Surgical treatment of induced peri-implantitis in the micro pig: Clinical and histological analysis. J Periodontol. 1993;64:984–989. doi: 10.1902/jop.1993.64.10.984. [DOI] [PubMed] [Google Scholar]
- 32.Hickey JS, O'Neal RB, Scheidt MJ, et al. Microbiologic characterization of ligature-induced peri-implantitis in the microswine model. J Periodontol. 1991;62:548–553. doi: 10.1902/jop.1991.62.9.548. [DOI] [PubMed] [Google Scholar]
- 33.Sigusch BW, Pfitzner A, Albrecht V, et al. Efficacy of photodynamic therapy on inflammatory signs and two selected periodontopathogenic species in a beagle dog model. J Periodontol. 2005;76:1100–1105. doi: 10.1902/jop.2005.76.7.1100. [DOI] [PubMed] [Google Scholar]
- 34.Kawaguchi H, Hirachi A, Hasegawa N, et al. Enhancement of periodontal tissue regeneration by transplantation of bone marrow mesenchymal stem cells. J Periodontol. 2004;75:1281–1287. doi: 10.1902/jop.2004.75.9.1281. [DOI] [PubMed] [Google Scholar]
- 35.Lang H, Schiuler N, Nolden R. Attachment formation following replantation of cultured cells into periodontal defects-a study in minipigs. J Dent Res. 1998;77:393–405. doi: 10.1177/00220345980770020801. [DOI] [PubMed] [Google Scholar]
- 36.Yamada Y, Ueda M, Hibi H, et al. A novel approach to periodontal tissue regeneration with mesenchymal stem cells and platelet-rich plasma using tissue engineering technology: A clinical case report. Int J Periodontics Restorative Dent. 2006;26:363–369. [PubMed] [Google Scholar]
- 37.Dogğan A, Ozdemir A, Kubar A, et al. Healing of artificial fenestration defects by seeding of fibroblast-like cells derived from regenerated periodontal ligament in a dog: A preliminary study. Tissue Eng. 2003;9:1189–1196. doi: 10.1089/10763270360728099. [DOI] [PubMed] [Google Scholar]
- 38.Shi S, Bartold PM, Miura M, et al. The efficacy of mesenchymal stem cells to regenerate and repair dental structures. Orthod Craniofac Res. 2005;8:191–199. doi: 10.1111/j.1601-6343.2005.00331.x. [DOI] [PubMed] [Google Scholar]
- 39.Park JC, Kim YB, Kim HJ, et al. Isolation and characterization of cultured human periodental ligament fibroblast-specific cDNAs. Biochem Biophys Res Commun. 2001;282:1145–1153. doi: 10.1006/bbrc.2001.4694. [DOI] [PubMed] [Google Scholar]