Abstract
Objective
To characterize foveal atrophy in a heterogeneous group of uveitis patients using clinical findings and high-definition optical coherence tomography (HD-OCT).
Design
Cross-sectional, retrospective case series.
Results
HD-OCT scans of 140 patients seen in a tertiary referral center were reviewed and 23 patients (33 eyes) with foveal atrophy were identified. All patients with foveal atrophy were diagnosed with intermediate uveitis, posterior uveitis, or panuveitis. The status of the photoreceptor layer as visualized with HD-OCT was associated with significant differences in mean visual acuity (p<0.0001). Clinical findings associated with foveal atrophy included atrophy of the retinal pigment epithelium (RPE) and/or choroid (91%), macular ischemia (39%), cystoid macular edema (15%), choroidal neovascularization (12%), retinal detachment involving the macula (6%), and serum antiretinal antibodies (6%).
Conclusions
Foveal atrophy can be a complication of intraocular inflammation in a variety of uveitic syndromes. The etiology of foveal atrophy is multi-factorial and may include dysfunction and atrophy of the RPE and/or choroid, cystoid macular edema, macular ischemia secondary to occlusive retinal vasculitis, choroidal neovascularization, retinal detachment, and possibly antibody-mediated damage directed against photoreceptors. Careful observation of the photoreceptor layer using HD-OCT may help to identify patients who are at risk for visual loss secondary to foveal atrophy.
INTRODUCTION
The sequelae of intraocular inflammation are responsible for a significant amount of visual morbidity associated with uveitis. Macular pathology secondary to intraocular inflammatory disease includes choroidal neovascularization (CNV), epiretinal membrane, macular hole, and cystoid macular edema (CME)1. Cross-sectional studies have demonstrated CME to be a significant cause of visual impairment in the uveitic population2, 3. Any type of uveitis can be complicated by CME; however, CME is more common with intermediate uveitis, posterior uveitis, and panuveitis3, 4. Macular edema has been described as a common cause of visual loss in specific uveitic syndromes including birdshot chorioretinopathy5, Behçet’s disease6, sarcoid uveitis7, and intermediate uveitis8. The pathogenic mechanisms underlying uveitic CME are multi-factorial and involve disruption of the inner blood-retina-barrier (BRB) secondary to inflammation and vitreous traction, choroidal inflammation, and retinal pigment epithelial (RPE) dysfunction9.
The clinical features and disease associations of uveitic CME have been extensively discussed in the literature. However, discussion of uveitic macular atrophy has been more limited. One retrospective series described macular atrophy as a predominant cause of compromised vision in patients with birdshot chorioretinopathy (BCR)5. A relationship between loss of the photoreceptor layer and decreased visual acuity has previously been shown in BCR using Stratus™ (Carl Zeiss Meditec, Inc., Dublin, CA) time domain optical coherence tomography (OCT)10. Although macular atrophy with photoreceptor degeneration has been described as a significant cause of vision loss in patients with BCR, the contribution of this entity to visual morbidity in other uveitic syndromes has not been well characterized.
OCT is an objective and reliable test for diagnosing maculopathy, and has been used to describe macular pathology in uveitis11, 12. The use of high-definition OCT (HD-OCT) using spectral (fourier) domain technology in the diagnosis and management of macular disease has become increasingly prevalent. A recent report has demonstrated that spectral domain imaging using the Cirrus™ HD-OCT (Carl Zeiss Meditec, Inc., Dublin, CA) provides superior imaging of macular pathology in uveitic eyes compared to the Stratus™ OCT13. The higher resolution of HD-OCT may be especially important in helping to define specific retinal structures such as the photoreceptor layer. Photoreceptor atrophy and disorganization of retinal layers has been described in a case of BCR using HD-OCT14. However, HD-OCT description of retinal morphology in patients with uveitic disease other than BCR has not been described to date.
The purpose of this study was to further characterize foveal atrophy in the setting of various uveitic syndromes seen at a tertiary referral center and to delineate the role of various pathological processes such as RPE damage, vascular disease, and photoreceptor degeneration in the development of uveitic foveal atrophy. To evaluate these questions, we retrospectively reviewed and categorized the clinical and Cirrus™ HD-OCT findings in a cohort of patients with uveitic foveal atrophy.
METHODS
This study was performed with informed patient consent and conducted under a protocol approved by the local institutional review board (IRB), in accordance with the ethical standards stated in the 1964 Declaration of Helsinki. All HD-OCT scans that were performed using the Cirrus™ HD-OCT during a six-month period (July 2007 to December 2007) at the National Eye Institute Uveitis Clinic were reviewed. Scans obtained using the 512x128 scan pattern from a total of 140 patients were analyzed. This protocol scans a 6×6mm area on the retina with 128 horizontal lines, each consisting of 512 A-scans per line (total of 65,536 sampled points) within a scan time of 2.4 seconds. Using the three dimensional topographical macular map output, the center of the fovea was determined using the cross-hair function of the Cirrus™ HD-OCT. Following this, the center point foveal thickness was determined using the caliper function of the software program included with the Cirrus™ HD-OCT. The measurement of center point foveal thickness using this technique is not dependent on patient fixation; hence the potential for erroneous measurements in eyes with eccentric fixation was avoided. As normal values for foveal thickness using Cirrus™ HD-OCT have not been established yet, we defined foveal atrophy based on our own experience with Cirrus™ HD-OCT scans in normal and diseased eyes. Foveal atrophy was defined as a center foveal point thickness of less than 150 μm. Normal foveal thickness was defined as 150–250 μm, and CME was defined as a macular thickness greater than 250 μm with the presence of intraretinal cysts. In order to determine the integrity of the photoreceptor layer, we examined the inner segment/outer segment junction (IS/OS junction), using the horizontal 5 line raster scan pattern, which performs 4096 A-scans per line. The photoreceptor layer was assessed by visualization of the IS/OS junction, which appears as a highly reflective line in the outer retina, adjacent and inner to the highly reflective line which demarcates the RPE layer. The status of the IS/OS junction was categorized as either intact, partially intact, or absent based on a previously described grading system10. Furthermore, the presence of the normal hyporeflective space between the IS/OS junction and the RPE line, which represents the photoreceptor outer segments, was noted.
The charts, fundus photographs, and fluorescein angiograms of all patients who were identified as having foveal atrophy based on Cirrus™ HD-OCT were reviewed. All patients had these ancillary tests performed. Macular findings seen on fundus photographs and angiographs, as well as corrected visual acuities obtained using Early Treatment of Diabetic Retinopathy Study (ETDRS) charts at the time of Cirrus™ HD-OCT scanning were documented. The status of the RPE and choroid was assessed based on color photographs and fluorescein angiograms. Severe disease of the RPE/choroid was defined as the presence of atrophy/fibrosis of the RPE/choroid with visualization of the underlying choroid/sclera. Mild to moderate pigmentary disease was defined as RPE mottling/hyperplasia/granularity. Angiographic macular ischemia was defined as closure of parafoveal capillaries identified by an enlarged and/or irregular foveal avascular zone (FAZ)15 or diffusely diminished macular perfusion. A history of prior CME, CNV, retinal vasculitis, and/or retinal detachment (RD) involving the macula was also noted. One patient had been screened for serum antiretinal antibodies using previously described methods16.
The relationship between center point foveal thickness and visual acuity in eyes with macular atrophy was determined using Pearson correlation. The mean visual acuity of all eyes with intact, partially intact, or absent IS/OS junction was calculated and then compared using analysis of variance (ANOVA) with Newman-Keuls post-test. All statistical analysis were two-tailed, and statistical significance was determined at an a value of 0.05. Analyses were performed using GraphPad Prism version 4.0 (GraphPad Software Inc., San Diego, California, USA).
RESULTS
We identified a total of 140 patients who had Cirrus™ HD-OCT scans performed during a 6 month period at the National Eye Institute Uveitis Clinic. A total of 23 patients were identified as having foveal atrophy based on our criteria. A summary of the uveitic diagnoses of our cohort of patients with uveitic foveal atrophy is shown in Table 1. Aside form uveitis, none of the patients had any other disease that could contribute to foveal atrophy such as diabetic retinopathy, age-related macular degeneration, or central serous chorioretinopathy. There were a total of 12 males and 11 females, with a median age of 43 years (range: 18–70). The median duration of follow-up was 52 months (range: 1–195). It should be noted that, as many of the patients in our cohort had the diagnosis of uveitis before being referred to our clinic, the duration of disease activity in many of these patients is longer than the duration of follow-up at our clinic. Among our cohort, 33 eyes had foveal atrophy, one eye had CME, 6 eyes had normal macular thickness, and 6 eyes could not be assessed due to the presence of significant media opacities or prostheses. All prostheses were for enucleated globes. A detailed description of the patient cohort with clinical findings is shown in Table 2. The mean visual acuity of eyes with foveal atrophy was 20/150 (range: 20/20–20/800). All patients with foveal atrophy were diagnosed with intermediate uveitis, posterior uveitis, or panuveitis according to established criteria17. We did not find a significant association between center point foveal thickness and visual acuity (p = 0.99) in eyes with foveal atrophy. However, the visual acuities of all eyes with intact, partially intact, or absent IS/OS junction as visualized in HD-OCT were significantly different from each other (p < 0.0001). All pairwise comparisons between these groups were also significantly different (p < 0.001, Figure 1). In all eyes with foveal atrophy, we observed a complete or partial loss of the normal hyporeflective space between the IS/OS junction and the RPE line suggestive of photoreceptor outer segment shortening. Figure 2 shows a representative HD-OCT scan of a normal macula, while Figure 3 shows representative HD-OCT scans from eyes with foveal atrophy and varying degrees of IS/OS junction atrophy. The prevalence of posterior segment sequelae of intraocular inflammation, either present or prior, in eyes with foveal atrophy is summarized in Table 3.
Table 1.
Diagnoses of Patients with Uveitic Macular Atrophy
| Diagnosis | Number of Patients |
|---|---|
| Behçet’s Disease | 3 (13%) |
| Serpiginous Choroidopathy | 3 (13%) |
| Sympathetic Ophthalmia | 3 (13%) |
| Birdshot Chorioretinopathy | 2 (9%) |
| Idiopathic Granulomatous Panuveitis | 2 (9%) |
| Vogt-Koyanagi-Harada Syndrome | 2 (9%) |
| Lupus Vasculitis | 2 (9%) |
| Sarcoid Panuveitis | 1 (4%) |
| Acute Retinal Necrosis | 1 (4%) |
| Idiopathic Intermediate Uveitis | 1 (4%) |
| Idiopathic Retinal Vasculitis | 1 (4%) |
| Multifocal Choroiditis | 1 (4%) |
| Punctate Inner Choroidopathy | 1 (4%) |
Table 2.
Patient Characteristics and Clinical Findings
| Patient | Age/Gender | Eye | Diagnosis | Clinical/Angiographic Findings in Macula | Status of Photoreceptor Layer on HD-OCT | Foveal Thickness (μm) | ETDRS Acuity |
|---|---|---|---|---|---|---|---|
| 1 | 30/M | OD | Intermediate Uveitis (Idiopathic) | Unable to Assess (Cataract)† | Unable to Assess (Cataract) | N/A | 20/800 |
| OS | Intermediate Uveitis (Idiopathic) | RPE Mottling; Vasculitis, Macular Ischemia† | Partially Intact | 132 | 20/50 | ||
| 2 | 59/M | OD | Serpiginous Choroidopathy | Atrophy of RPE and Choroid | Partially Intact | 49 | 20/50 |
| OS | Serpiginous Choroidopathy | Atrophy of RPE and Choroid | Partially Intact | 29 | 20/63 | ||
| 3 | 43/F | OD | Serpiginous Choroidopathy | Atrophy of RPE and Choroid | Absent | 110 | 20/800 |
| OS | Serpiginous Choroidopathy | RPE Mottling | Partially Intact | 234 | 20/25 | ||
| 4 | 21/M | OD | Serpiginous Choroidopathy | Atrophy of RPE and Choroid | Absent | 41 | 20/200 |
| OS | Serpiginous Choroidopathy | Atrophy of RPE and Choroid | Absent | 137 | 20/100 | ||
| 5 | 70/M | OD | Birdshot Chorioretinopathy | RPE Mottling† | Absent | 142 | 20/400 |
| OS | Birdshot Chorioretinopathy | RPE Granularity | Partially Intact | 85 | 20/50 | ||
| 6 | 49/M | OD | Birdshot Chorioretinopathy | RPE Mottling | Partially Intact | 104 | 20/50 |
| OS | Birdshot Chorioretinopathy | Atrophy of RPE and Choroid* | Absent | 189 | 20/250 | ||
| 7 | 28/M | OD | Behçet’s Disease | Sclerotic Vessels, ERM, CME, RPE Mottling‡ | Partially Intact | 526 | 20/160 |
| OS | Behçet’s Disease | RPE Granularity; Macular Ischemia‡ | Partially Intact | 78 | 20/125 | ||
| 8 | 38/M | OD | Behçet’s Disease | Normal | Intact | 180 | 20/16 |
| OS | Behçet’s Disease | Sclerotic Vessels, RPE Mottling; Macular Ischemia†‡ | Absent | 121 | 20/800 | ||
| 9 | 40/M | OD | Behçet’s Disease | Sclerotic Vessels, RPE Mottling; Macular Ischemia†‡ | Partially Intact | 105 | 20/50 |
| OS | Behçet’s Disease | Sclerotic Vessels, RPE Mottling; Vasculitis, Macular Ischemia† | Partially Intact | 20 | 20/125 | ||
| 10 | 44/F | OD | Granulomatous Panuveitis (Idiopathic) | Subretinal Fibrosis and RPE Mottling; Macular Ischemia‡ | Absent | 70 | 20/200 |
| OS | Granulomatous Panuveitis (Idiopathic) | Unable to Assess (Band Keratopathy) | Unable to Asses (Band Keratopathy) | N/A | NLP | ||
| 11 | 49/M | OD | Granulomatous Panuveitis (Idiopathic) | Attenuated Vessels, RPE Mottling, Antiretinal Antibodies; Macular Ischemia | Absent | 119 | 20/400 |
| OS | Granulomatous Panuveitis (Idiopathic) | Attenuated Vessels, RPE Mottling, Antiretinal Antibodies; Macular Ischemia | Absent | 121 | 20/800 | ||
| 12 | 56/F | OD | VKH | Atrophy of the RPE and Choroid | Absent | 38 | 20/250 |
| OS | VKH | Atrophy of the RPE and Choroid | Absent | 71 | 20/100 | ||
| 13 | 62/F | OD | VKH | RPE Mottling | Partially Intact | 130 | 20/32 |
| OS | VKH | RPE Mottling | Partially Intact | 140 | 20/200 | ||
| 14 | 37/M | OD | SO | Subretinal Fibrosis and RPE Hyperplasia* | Absent | 100 | 20/160 |
| OS | SO | Unable to Assess (Prosthesis) | Unable to Assess (Prosthesis) | N/A | N/A | ||
| 15 | 62/F | OD | SO | Unable to Assess (Cataract) | Unable to Assess (Cataract) | N/A | NLP |
| OS | SO | Papillitis, RPE Granularity; Vasculitis, Macular Ischemia | Absent | 143 | 20/800 | ||
| 16 | 25/M | OD | SO | ERM+ | Partially Absent | 144 | 20/160 |
| OS | SO | Unable to Assess (Prosthesis) | Unable to Assess (Prosthesis) | N/A | N/A | ||
| 17 | 51/F | OD | Sarcoid Panuveitis | RPE Granularity | Partially Intact | 128 | 20/125 |
| OS | Sarcoid Panuveitis | RPE Granularity | Intact | 196 | 20/25 | ||
| 18 | 50/M | OD | Acute Retinal Necrosis | Attenuated Vessels, RPE Mottling+ | Absent | 85 | 20/63 |
| OS | Acute Retinal Necrosis | Unable to Assess (Silicone Oil) | Unable to Assess (Silicone Oil) | N/A | 20/800 | ||
| 19 | 17/F | OD | Lupus Vasculitis | Subretinal Fibrosis, RPE Hyperplasia; Macular Ischemia‡ | Absent | 100 | 20/320 |
| OS | Lupus Vasculitis | Subretinal Fibrosis, RPE Hyperplasia; Macular Ischemia‡ | Absent | 75 | 20/800 | ||
| 20 | 18/F | OD | Lupus Vasculitis | Irregular Foveal Reflex‡ | Intact | 140 | 20/20 |
| OS | Lupus Vasculitis | Irregular Foveal Reflex‡ | Intact | 146 | 20/20 | ||
| 21 | 58/F | OD | Retinal Vasculitis (Idiopathic) | Sclerotic Vessels, RPE Mottling; Macular Ischemia‡ | Absent | 64 | 20/250 |
| OS | Retinal Vasculitis (Idiopathic) | Sclerotic Vessels, RPE Mottling; Macular Ischemia‡ | Absent | 30 | 20/320 | ||
| 22 | 29/F | OD | Multifocal Choroiditis | Normal | Intact | 184 | 20/20 |
| OS | Multifocal Choroiditis | Atrophy of RPE and Choroid* | Absent | 120 | 20/400 | ||
| 23 | 36/F | OD | Punctate Inner Choroidopathy | RPE Mottling* | Preserved Foveal Photoreceptors | 188 | 20/16 |
| OS | Punctate Inner Choroidopathy | Subretinal Fibrosis, RPE Mottling* | Partial Foveal Photoreceptor Loss | 33 | 20/80 |
RPE = retinal pigment epithelium, ERM = epiretinal membrane, CME = cystoid macular edema, VKH = Vogt-Koyanagi-Harada disease, SO = sympathetic ophthalmia, ETDRS = Early Treatment of Diabetic Retinopathy Study
History of cystoid macular edema
History of choroidal neovascularization
History of retinal vasculitis
History of retinal detachment involving macula
Figure 1. Relationship Between Photoreceptor Layer Status and Visual Acuity.
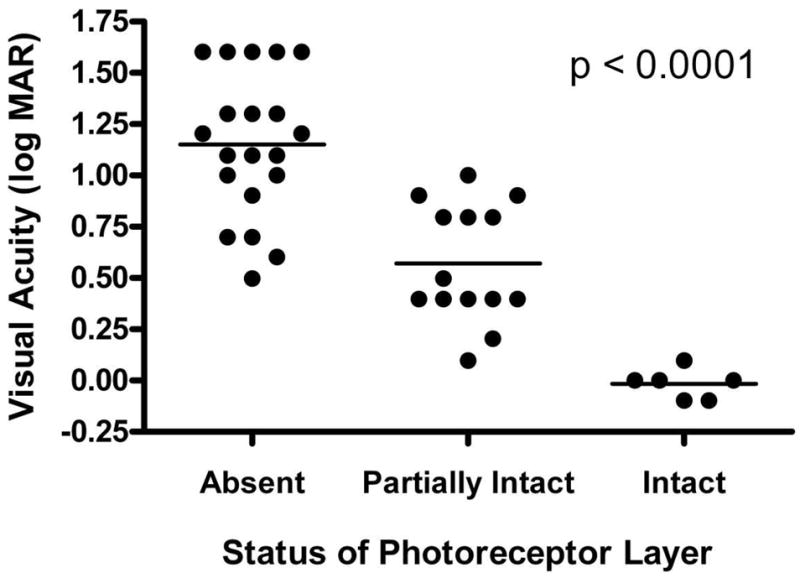
Visual acuities of eyes with intact, partially intact, or absent photoreceptor layers were significantly different from each other (p < 0.0001). All pairwise comparisons between these groups were also significantly different (p < 0.001). Horizontal lines indicate mean values.
Figure 2. Representative High-Definition Optical Coherence Tomography Image of a Normal Macula.
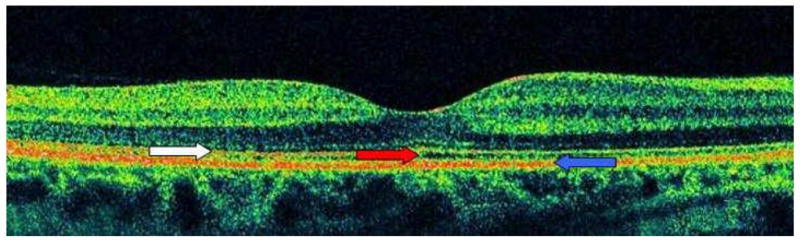
The right eye of patient 22 had normal macular thickness with a preserved photoreceptor layer. White arrow indicates hypereflective line corresponding to the photoreceptor layer, while the blue arrow indicates the hypereflective line corresponding to the RPE. The hyporeflective space in between these lines, denoted by the red arrow, corresponds to the photoreceptor outer segments. Image represents horizontal line scan through center of fovea.
Figure 3. Representative High-Definition Optical Coherence Tomography Images Demonstrating Varying Degrees of Macular Atrophy.
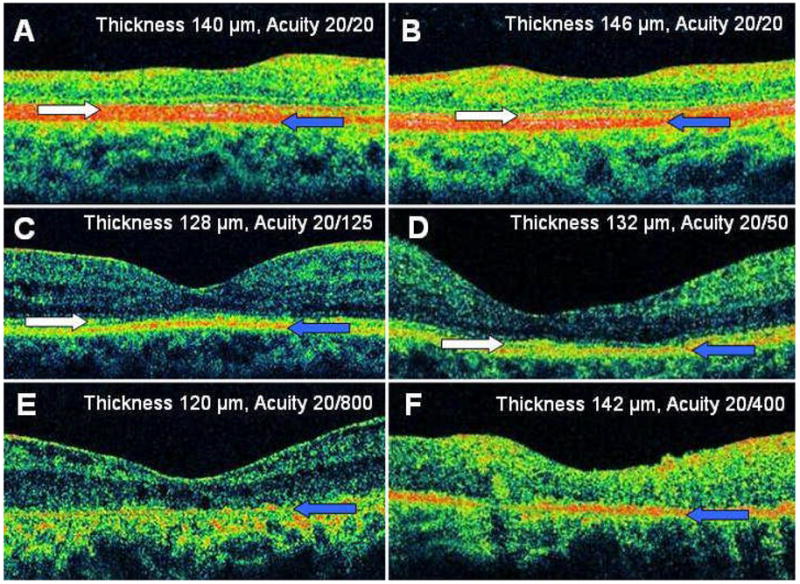
The right eye (A) and left eye (B) of patient 20 had an intact photoreceptor layer, while the photoreceptor outer segments appeared attenuated. The right eye of patient 17 (C) and the left eye of patient 1 (D) had a partially intact photoreceptor layer. The left eye of patient 11 (E) and the right eye of patient 5 (F) had an absent photoreceptor layer. White arrow indicates hypereflective line corresponding to the photoreceptor layer, while the blue arrow indicates the hypereflective line corresponding to the RPE. Images represent horizontal line scans through center of fovea.
Table 3.
Prevalence of Uveitic Sequelae (Present or Prior) in Eyes with Macular Atrophy
| Abnormal Findings | Number of Eyes |
|---|---|
| RPE/Choroid Defects | 30 (91%) |
| Macular Ischemia | 13 (39%) |
| Cystoid Macular Edema | 5 (15%) |
| Choroidal Neovascularization | 4 (12%) |
| Retinal Detachment | 2 (6%) |
| Antiretinal Antibodies | 2 (6%) |
Defects at the level at the RPE and/or choroid were observed in 30/33 (91%) eyes. The primary clinical and angiographic abnormalities seen in patients with serpiginous choroidopathy (patients 2, 3 and 4), Vogt-Koyanagi-Harada (VKH) disease (patient 12 and 13) and sarcoid granulomatous panuveitis (patient 17) were defects at the level of the RPE and/or choroid. These patients did not have current or prior evidence of CME, CNV, macular ischemia, or retinal detachment. Among these patients, all eyes with severe disease at the level of the RPE/choroid had an absent IS/OS junction on HD-OCT imaging. Eyes with mild to moderate RPE/choroid disease had a preserved or partially preserved IS/OS junction. Furthermore, in one case (patient 2) a clear delineation could be seen between an intact IS/OS junction overlying healthy appearing RPE/choroid and an absent IS/OS junction overlying atrophic RPE/choroid (Figure 4).
Figure 4. Status of Photoreceptor Layer in Relation to Atrophic Changes at the Level of the Retinal Pigment Epithelium and Choroid.
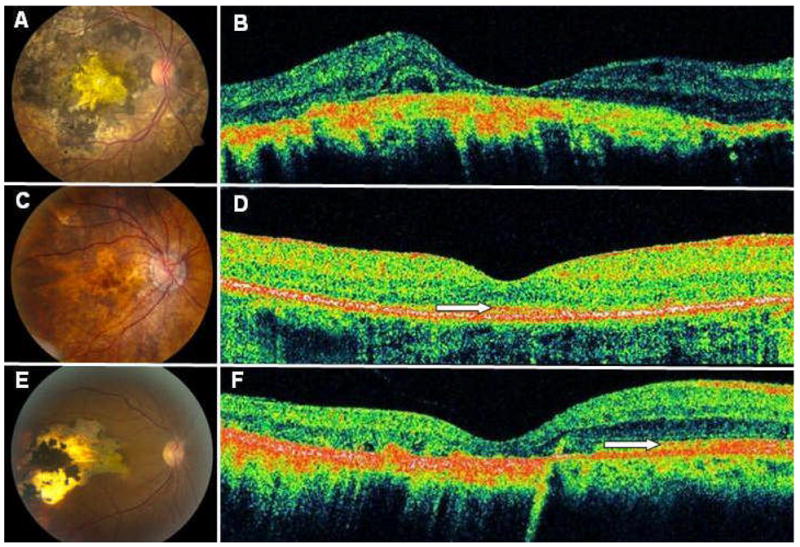
The right eye of patient 12 (A) with had severe RPE/choroid disease with absence of the overlying photoreceptors (B). The right eye of patient 13 (C) with had milder RPE disease, and the overlying photoreceptors were partially intact (D). The right eye of patient 2 (E) revealed a distinct boundary between intact photoreceptors overlying healthy appearing RPE/choroid and absent photoreceptors overlying atrophic RPE/choroid (F). White arrows denote the photoreceptor layer. OCT mages represent horizontal line scans through center of fovea.
Based on angiographic criteria, we identified a total of 13/33 (39%) eyes in our series with evidence of macular ischemia. The diagnoses in these cases were: idiopathic intermediate uveitis (1 eye, 8%), Behçet’s disease (4 eyes, 31%), idiopathic granulomatous panuveitis (3 eyes, 23%), sympathetic ophthalmia (1 eye, 8%), lupus chorioretinopathy (2 eyes, 15%), and idiopathic retinal vasculitis (2 eyes, 15%). The majority (11 eyes, 85%) of these eyes had active or resolved retinal vasculitis. In 2 eyes without a history of active or resolved retinal vasculitis, the presence of serum antiretinal antibodies had previously been demonstrated using immunohistochemical staining techniques (data not shown). In 2/4 (50%) of the eyes with active vasculitis, FA demonstrated macular ischemia with evidence of active vasculitis of the parafoveal vasculature (Figure 5). A review of charts demonstrated that 3/6 (50%) eyes with resolved vasculitis previously had a similar pattern of parafoveal vasculitis.
Figure 5. Macular Ischemia Associated with Macular Atrophy.
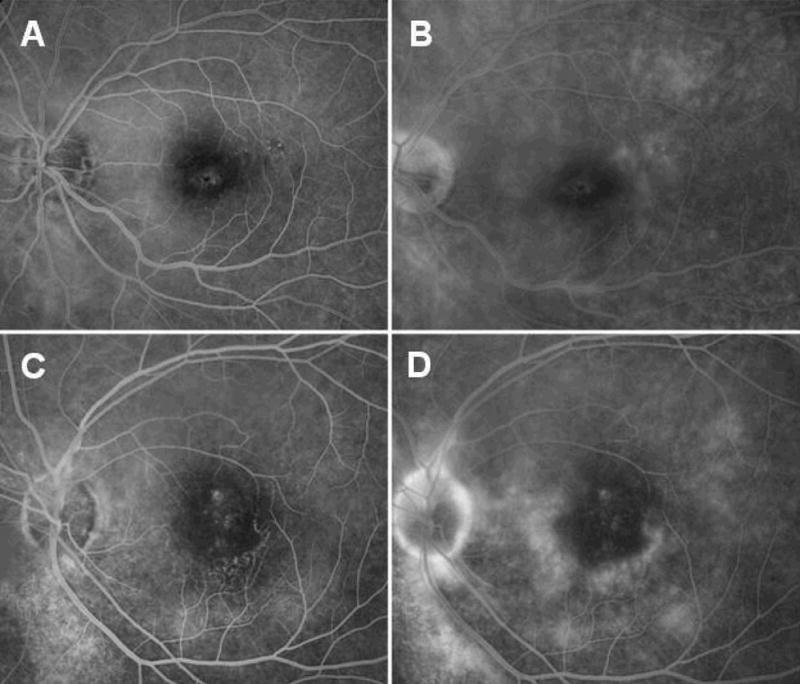
Arteriovenous (A, C) and late venous phase (B, D) fluorescein angiogram of the left eye of patient 1 (A, B) and the left eye of patient 9 (C, D) demonstrated active vasculitis at the border of the fovea with an irregular foveal avascular zone.
A total of 5 of 33 (15%) eyes with foveal atrophy had a history of prior CME, while 5/33 (15%) and 2/33 (6%) eyes had a history of CNV or RD involving the macula, respectively. CME was seen in eyes with idiopathic intermediate uveitis, BCR, and Behçet’s disease. CNV was a complication in eyes with BCR, sympathetic ophthalmia, multifocal choroiditis, and punctate inner choroidopathy. A history of RD involving the macula was noted in eyes with acute retinal necrosis and sympathetic ophthalmia.
DISCUSSION
In this report, we describe clinical features and associations of foveal atrophy in a heterogeneous group of patients with uveitis. Patients in our cohort were diagnosed with a variety of uveitic syndromes which could be classified as intermediate uveitis, posterior uveitis, or panuveitis. We found that visual acuity was related to the status of the IS/OS junction as seen on HD-OCT imaging. The visual outcome of uveitic CME has been shown to depend on anatomic location, with uveitic entities affecting the posterior segment (intermediate uveitis, posterior uveitis, and panuveitis) having a poorer visual prognsosis3. This suggests that posterior segment inflammation may be associated with other sequelae, such as foveal atrophy, which portend a poor visual outcome. This theory is supported by our observation of foveal atrophy in patients with intermediate uveitis, posterior uveitis, and panuveitis. Numerous manifestations of posterior segment inflammation including dysfunction and atrophy of the RPE/choroid, CME, CNV, macular ischemia secondary to vasculitis, RD involving the macula, and retinal autoimmunity could conceivably be involved in the development of foveal atrophy.
We observed HD-OCT evidence of marked photoreceptor degeneration in eyes with severe atrophy of the RPE and choroid in which no other posterior segment sequelae of intraocular inflammation could be identified. In the remaining majority of cases of foveal atrophy, we observed RPE pigmentary change suggestive of damage to this cellular layer. The photoreceptor layer, along with other components of the outer retina, relies heavily on the RPE and choroid for nutrient and oxygen support. Dysfunction of the RPE and choroid, mediated by various inflammatory factors, likely represents a contributing factor in the development of uveitic foveal atrophy.
Macular ischemia was observed in a large percentage of eyes with evidence of foveal atrophy and photoreceptor degeneration on HD-OCT. The majority of these eyes had evidence of active or prior retinal vasculitis, and in some of these cases we observed vasculitic changes within the parafoveal vasculature. Macular ischemia secondary to occlusive parafoveal vasculitis has previously been described in cases of Behçet’s disease15, 18, 19, sarcoidosis15, and idiopathic retinal vasculitis15. We observed macular ischemia in eyes with these syndromes, as well as in eyes with idiopathic intermediate uveitis, idiopathic granulomatous panuveitis, sympathetic ophthalmia, and lupus vasculitis. Furthermore, we observed angiographic evidence of active occlusive parafoveal vasculitis in eyes with idiopathic intermediate uveitis, Behçet’s disease, and lupus vasculitis. Retinal vasculitis in uveitis typically involves vessels of larger caliber but our observations, along with those of other investigators, suggests that the foveal vasculature is susceptible to vasculitis as well. Furthermore, our finding of macular thinning and photoreceptor degeneration in this group of patients demonstrates that macular ischemia secondary to parafoveal occlusive retinal vasculitis is an important cause of foveal atrophy in uveitis patients.
Cystoid macular edema may represent another pathogenic factor in uveitic foveal atrophy. A significant percentage of eyes in our series had a history of CME. The visual morbidity of uveitic CME has been well established2, 3. The potential relationship between CME and foveal atrophy has not been well described in the literature. Photoreceptor dysfunction has been observed in eyes with macular edema secondary to diabetes and uevitis20. Nitric oxide has been shown to contribute to breakdown of the blood-retina barrier in diabetic macular edema21, and aqueous levels of nitric oxide are elevated in patients with uveitis22, 23. Nitric oxide-mediated oxidative stress and apoptotic death of photoreceptors has been demonstrated in animal models of uveitis24–26. Although the role of uveitic CME in the development of foveal atrophy remains to be fully elucidated, it is possible that inflammatory mediators (e.g. nitric oxide) in uveitic CME may be involved in the induction of apoptotic photoreceptor death and are involved in the pathogenesis of foveal atrophy.
A small percentage of eyes with foveal atrophy in our series had a history of CNV, which is a known but rare complication of posterior uveitis and panuveitis. CNV has been reported in numerous uveitic entities including multifocal choroiditis, BCR, sympathetic ophthalmia, serpiginous choroidopathy, Behçet’s disease, and punctate inner choroiopathy27, 28. Choroidal neovascular membranes can be classified into three growth patterns: subretinal, sub-RPE, or combined29. The sequelae of CNV include exudation, hemorrhage, and fibrosis. Photoreceptor degeneration could conceivably occur directly as a result of growth of CNV in the subretinal space, or secondary to RPE dysfunction induced by a sub-RPE choroidal neovascular complex.
The presence of uveitis has been associated with RD in specific uveitis entities such as VKH and acute retinal necrosis30, 31. RD occurs more frequently in patients with panuveitis than in those with other anatomic locations of uveitis32. RD involving the macula was a rare complication in our cohort, and was observed in eyes with posterior uveitis or panuveitis. Photoreceptor apoptosis has been observed in patients following primary and recurrent RD33. Apoptotic death of photoreceptors following RD involving the macula may contribute to the development of foveal atrophy in some patients with uveitis.
The serum of one of our patients was found to contain antiretinal antibodies using immunohistochemical techniques. Previous experiments have demonstrated immunohistochemical labeling of photoreceptors by antiretinal antibodies from the serum of patients with VKH, Behçet’s disease, and sympathetic ophthalmia34. Interestingly, we observed cases of foveal atrophy in patients with all of these diagnoses. Antiretinal antibodies have been shown to induce apoptotic death of photoreceptors in vivo35. The pathogenic role of antiretinal antibodies in uveitis remains to be fully established, but our observations suggest that this form of humoral autoimmunity may play a role in the development of uveitic foveal atrophy.
In summary, uveitic foveal atrophy may be a complication of intermediate uveitis, posterior uveitis, or panuveitis. Macular thinning and, more importantly, photoreceptor degeneration are serious sequelae of this process. Several etiologic factors may contribute to the evolution of foveal atrophy in the setting of uveitis. Inflammatory damage to the RPE and choroid can cause dysfunction and atrophy of these tissues, leading to hypoxia and nutritional deprivation of the macula. Longstanding CME may also contribute to the development of foveal atrophy via toxic inflammatory mediators. Occlusive parafoveal retinal vasculitis can lead to foveal ischemia and infarction in some cases. Complications such as CNV and RD can also result in photoreceptor death. Finally, antiretinal antibodies may induce apoptotic death of retinal elements including photoreceptors. Pathogenic risk factors for uveitic foveal atrophy can be identified using clinical examination, and ancillary testing such as HD-OCT can help identify atrophy of the IS/OS junction. Careful attention to these factors is important in identifying patients who may be at risk of developing foveal atrophy or those who have already started to show signs of photoreceptor degeneration. Monitoring the progression of potential conditions that may predispose individuals to future foveal atrophy may be important in prompting early and aggressive therapy to avoid this vision-threatening complication. Furthermore, status of the IS/OS junction may serve as an important outcome measure in clinical trials in uveitis.
Acknowledgments
This work has been supported by the National Eye Institute Intramural Research Program.
References
- 1.Nussenblatt RB. Macular alterations secondary to intraocular inflammatory disease. Ophthalmology. 1986;93:984–8. doi: 10.1016/s0161-6420(86)33654-6. [DOI] [PubMed] [Google Scholar]
- 2.Rothova A, Suttorp-van Schulten MS, Frits Treffers W, et al. Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol. 1996;80:332–6. doi: 10.1136/bjo.80.4.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lardenoye CW, van Kooij B, Rothova A. Impact of macular edema on visual acuity in uveitis. Ophthalmology. 2006;113:1446–9. doi: 10.1016/j.ophtha.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 4.van Kooij B, Probst K, Fijnheer R, et al. Risk factors for cystoid macular oedema in patients with uveitis. Eye. 2008;22:256–60. doi: 10.1038/sj.eye.6702595. [DOI] [PubMed] [Google Scholar]
- 5.Rothova A, Berendschot TT, Probst K, et al. Birdshot chorioretinopathy: long-term manifestations and visual prognosis. Ophthalmology. 2004;111:954–9. doi: 10.1016/j.ophtha.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 6.Tugal-Tutkun I, Onal S, Altan-Yaycioglu R, et al. Uveitis in Behcet disease: an analysis of 880 patients. Am J Ophthalmol. 2004;138:373–80. doi: 10.1016/j.ajo.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 7.Dana MR, Merayo-Lloves J, Schaumberg DA, et al. Prognosticators for visual outcome in sarcoid uveitis. Ophthalmology. 1996;103:1846–53. doi: 10.1016/s0161-6420(96)30417-x. [DOI] [PubMed] [Google Scholar]
- 8.Deane JS, Rosenthal AR. Course and complications of intermediate uveitis. Acta Ophthalmol Scand. 1997;75:82–4. doi: 10.1111/j.1600-0420.1997.tb00256.x. [DOI] [PubMed] [Google Scholar]
- 9.Freeman G, Matos K, Pavesio CE. Cystoid macular oedema in uveitis: an unsolved problem. Eye. 2001;15:12–7. doi: 10.1038/eye.2001.5. [DOI] [PubMed] [Google Scholar]
- 10.Monnet D, Levinson RD, Holland GN, et al. Longitudinal cohort study of patients with birdshot chorioretinopathy. III Macular imaging at baseline. Am J Ophthalmol. 2007;144:818–828. doi: 10.1016/j.ajo.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Markomichelakis NN, Halkiadakis I, Pantelia E, et al. Patterns of macular edema in patients with uveitis: qualitative and quantitative assessment using optical coherence tomography. Ophthalmology. 2004;111:946–53. doi: 10.1016/j.ophtha.2003.08.037. [DOI] [PubMed] [Google Scholar]
- 12.Gallagher MJ, Yilmaz T, Cervantes-Castaneda RA, et al. The characteristic features of optical coherence tomography in posterior uveitis. Br J Ophthalmol. 2007;91:1680–5. doi: 10.1136/bjo.2007.124099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta V, Gupta P, Singh R, et al. Spectral-Domain Cirrus High-Definition Optical Coherence Tomography Is Better than Time-Domain Stratus Optical Coherence Tomography for Evaluation of Macular Pathologic Features in Uveitis. Am J Ophthalmol. 2008 doi: 10.1016/j.ajo.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 14.Witkin AJ, Duker JS, Ko TH, et al. Ultrahigh resolution optical coherence tomography of birdshot retinochoroidopathy. Br J Ophthalmol. 2005;89:1660–1. doi: 10.1136/bjo.2005.076570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bentley CR, Stanford MR, Shilling JS, et al. Macular ischaemia in posterior uveitis. Eye. 1993;7(Pt 3):411–4. doi: 10.1038/eye.1993.81. [DOI] [PubMed] [Google Scholar]
- 16.Chin MS, Caruso RC, Detrick B, et al. Autoantibodies to p75/LEDGF, a cell survival factor, found in patients with atypical retinal degeneration. J Autoimmun. 2006;27:17–27. doi: 10.1016/j.jaut.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509–16. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcher C, Bielefeld P, Desvaux C, et al. Bilateral loss of vision and macular ischemia related to Behcet disease. Am J Ophthalmol. 1997;124:116–7. doi: 10.1016/s0002-9394(14)71659-9. [DOI] [PubMed] [Google Scholar]
- 19.Yilmaz G, Akova Y, Aydin P. Macular ischaemia in Behcet’s disease. Eye. 2000;14(Pt 5):717–20. doi: 10.1038/eye.2000.190. [DOI] [PubMed] [Google Scholar]
- 20.Lardenoye CW, Probst K, DeLint PJ, et al. Photoreceptor function in eyes with macular edema. Invest Ophthalmol Vis Sci. 2000;41:4048–53. [PubMed] [Google Scholar]
- 21.Leal EC, Manivannan A, Hosoya K, et al. Inducible nitric oxide synthase isoform is a key mediator of leukostasis and blood-retinal barrier breakdown in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2007;48:5257–65. doi: 10.1167/iovs.07-0112. [DOI] [PubMed] [Google Scholar]
- 22.Yilmaz G, Sizmaz S, Yilmaz ED, et al. Aqueous humor nitric oxide levels in patients with Behcet disease. Retina. 2002;22:330–5. doi: 10.1097/00006982-200206000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Hsu WM, Chen SS, Peng CH, et al. Elevated nitric oxide level in aqueous humor of AIDS patients with cytomegalovirus retinitis. Ophthalmologica. 2003;217:298–301. doi: 10.1159/000070639. [DOI] [PubMed] [Google Scholar]
- 24.Liversidge J, Dick A, Gordon S. Nitric oxide mediates apoptosis through formation of peroxynitrite and Fas/Fas-ligand interactions in experimental autoimmune uveitis. Am J Pathol. 2002;160:905–16. doi: 10.1016/S0002-9440(10)64913-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajendram R, Saraswathy S, Rao NA. Photoreceptor mitochondrial oxidative stress in early experimental autoimmune uveoretinitis. Br J Ophthalmol. 2007;91:531–7. doi: 10.1136/bjo.2006.101576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rao NA, Wu GS. Free radical mediated photoreceptor damage in uveitis. Prog Retin Eye Res. 2000;19:41–68. doi: 10.1016/s1350-9462(99)00003-8. [DOI] [PubMed] [Google Scholar]
- 27.Dees C, Arnold JJ, Forrester JV, et al. Immunosuppressive treatment of choroidal neovascularization associated with endogenous posterior uveitis. Arch Ophthalmol. 1998;116:1456–61. doi: 10.1001/archopht.116.11.1456. [DOI] [PubMed] [Google Scholar]
- 28.Kedhar SR, Thorne JE, Wittenberg S, et al. Multifocal choroiditis with panuveitis and punctate inner choroidopathy: comparison of clinical characteristics at presentation. Retina. 2007;27:1174–9. doi: 10.1097/IAE.0b013e318068de72. [DOI] [PubMed] [Google Scholar]
- 29.Grossniklaus HE, Green WR. Choroidal neovascularization. Am J Ophthalmol. 2004;137:496–503. doi: 10.1016/j.ajo.2003.09.042. [DOI] [PubMed] [Google Scholar]
- 30.Clarkson JG, Blumenkranz MS, Culbertson WW, et al. Retinal detachment following the acute retinal necrosis syndrome. Ophthalmology. 1984;91:1665–8. doi: 10.1016/s0161-6420(84)34107-0. [DOI] [PubMed] [Google Scholar]
- 31.Moorthy RS, Inomata H, Rao NA. Vogt-Koyanagi-Harada syndrome. Surv Ophthalmol. 1995;39:265–92. doi: 10.1016/s0039-6257(05)80105-5. [DOI] [PubMed] [Google Scholar]
- 32.Kerkhoff FT, Lamberts QJ, van den Biesen PR, et al. Rhegmatogenous retinal detachment and uveitis. Ophthalmology. 2003;110:427–31. doi: 10.1016/S0161-6420(02)01744-X. [DOI] [PubMed] [Google Scholar]
- 33.Arroyo JG, Yang L, Bula D, et al. Photoreceptor apoptosis in human retinal detachment. Am J Ophthalmol. 2005;139:605–10. doi: 10.1016/j.ajo.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 34.Chan CC, Palestine AG, Nussenblatt RB, et al. Anti-retinal auto-antibodies in Vogt-Koyanagi-Harada syndrome, Behcet’s disease, and sympathetic ophthalmia. Ophthalmology. 1985;92:1025–8. doi: 10.1016/s0161-6420(85)33911-8. [DOI] [PubMed] [Google Scholar]
- 35.Adamus G. Autoantibody-induced apoptosis as a possible mechanism of autoimmune retinopathy. Autoimmun Rev. 2003;2:63–8. doi: 10.1016/s1568-9972(02)00127-1. [DOI] [PubMed] [Google Scholar]


