Synopsis
Atrial fibrillation (AF) is a complex arrhythmia with multiple possible mechanisms. It requires a trigger for initiation and a favorable substrate for maintenance. Pulmonary vein myocardial sleeves have the potential to generate spontaneous activity, and such arrhythmogenic activity is surfaced by modulation of intracellular calcium dynamics. Direct autonomic nerve recordings in canine models demonstrate that simultaneous sympathovagal discharges are the most common triggers of paroxysmal atrial tachycardia and paroxysmal AF. Autonomic modulation as a potential therapeutic strategy has been targeted clinically and experimentally, but its effectiveness as an adjunctive therapeutic modality to catheter ablation of AF has been inconsistent. Further studies are warranted before application can be widely implied for therapies of clinical AF.
Atrial fibrillation (AF) is a complex disease with multiple possible mechanisms.1 Many studies indicate that the arrhythmogenic foci within the thoracic veins are AF initiators. Once initiated, AF alters atrial electrical and structural properties (atrial remodeling) in a way that promotes its own maintenance and recurrences and may alter the response to antiarrhythmic drugs. The exact mechanisms by which the arrhythmogenic foci are triggered remained elusive, however. One possible immediate trigger is the paroxysmal autonomic nervous system (ANS) discharge. In normal dogs, sympathetic nerve stimulation rarely triggers AF. In dogs that undergo chronic rapid atrial pacing, however, sympathetic stimulation can lead to rapid repetitive activations in the isolated canine pulmonary vein (PV) and vein of Marshall preparations.2, 3 Sharifov and colleagues4 reported that a combined isoproterenol and acetylcholine infusion is more effective that acetylcholine alone in the induction of AF. Clinically, alterations of autonomic tone, involving the sympathetic and parasympathetic nervous systems, are implicated in initiating paroxysmal AF.5 These results suggest that simultaneous sympathetic and parasympathetic (sympathovagal) discharge is particularly profibrillatory. Also, there is evidence for heightened atrial sympathetic innervation in patients who have persistent AF,6 suggesting that potential autonomic substrate modification may serve as part of remodeled atrial substrate for AF maintenance.
Patterns of activation at the pulmonary vein and pulmonary vein-left atrial junction during sustained atrial fibrillation
AF is characterized by the coexistence of multiple activation wavelets within the atria. The mechanisms by which multiple wavefronts occur have been debated for many years. The focal source hypothesis states that a single rapidly focal driver underlies the mechanisms of AF. Alternatively, the multiple wavelet hypothesis posits that heterogeneous dispersion of repolarization is responsible for wavebreaks and the generation of multiple wavelets that sustain AF.7 Zipes and Knopel8, Spach and colleagues9 and Scherlag and colleagues10 provided the first pieces of evidence to support the importance of thoracic veins in the generation of electrical activity. The importance of these original works were proven by Haissaguerre and colleagues11, who demonstrated the critical role of PV in the generation and maintenance of AF in humans. Hamabe and colleagues12 reported that the PV- left atrial (LA) junction has segmental muscle disconnection and differential muscle narrowing in dogs. These changes combined with the complex fiber orientations within the PV provide robust anatomical bases for generating conduction disturbances at the PV-LA junction and complex intra-PV conduction patterns, to facilitate reentry formation. High-density (1-mm resolution) computerized mapping techniques have demonstrated that rapid PV focal discharges13-15 and PV-LA junction microreentry15 are present during sustained AF induced by rapid LA pacing. Fig. 1 shows an example. Fig. 1A shows the activation snapshots of right superior PV during sustained AF, showing three consecutive focal discharges (6081 ms, 6203 ms and 6316 ms). The focal discharge wavefronts met lines of functional conduction block (dotted lines) followed by the formation of complete reentry loops (6409 ms to 6595 ms). The wavefronts from LA also encountered a functional line of block, followed by the formation of reentry. After infusion of ibutilide (see Fig. 1B), a typical class III antiarrhythmic drug that is effective in prolonging the effective refractory period of atria, focal discharges (6344 ms, 6582 ms) and reentrant wavefronts (6035 ms to 6296 ms) activated at slower rates. The conducted wavefronts between the PV and LA were reduced significantly by ibutilide. The overall incidence of focal discharge in the PVs was not suppressed, however. A high dose of ibutilide may terminate all reentrant activity completely, thereby converting AF to PV tachycardia before conversion to sinus rhythm. These findings suggest that sustained AF is the result of a combination of PV focal discharge and PV-LA reentrant activity.
Figure 1.
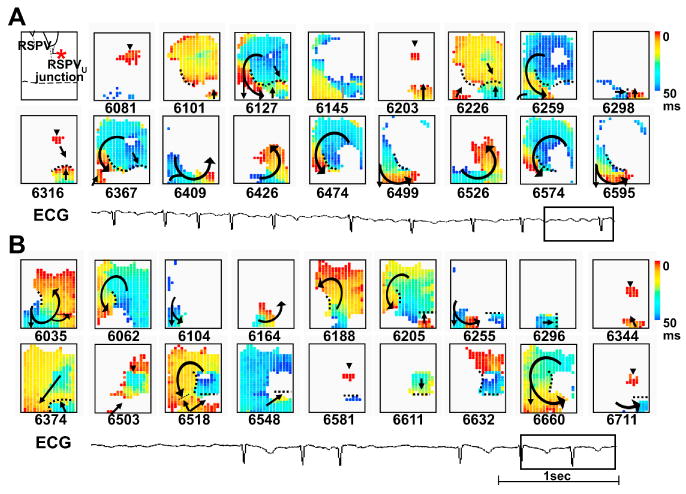
Patterns of activation during sustained AF induced by chronic rapid atrial pacing. (A) Snapshots of focal discharge and reentrant activation patterns within right super PV at baseline AF. Asterisk in the left upper corner indicates the anatomical location of focal discharge at the proximal right superior PV. Black horizontal dotted line indicates the PV-LA junction. The number below each snapshot represents the time in milliseconds, with the beginning of data acquisition as time zero. In snapshots, red color represents the wavefront; black arrows, the direction of wave propagation; black dotted line, line of block; arrowhead, site of focal discharge. The color bar on the right shows the time scale (0 to 50 ms). (B) Snapshots of focal discharge and reentrant activation patterns within right superior PV after ibutilide infusion (0.02 mg/kg). A rectangle towards the end of the ECG tracings shows the time period corresponding to the snapshots in (A) and (B). (From Chou CC, Zhou S, Tan AY, et al. High-density mapping of pulmonary veins and left atrium during ibutilide administration in a canine model of sustained atrial fibrillation. Am J Physiol Heart Circ Physiol 2005;289: H2704; used with permission)
A recent computational simulation study 16 showed that upregulation of the L-type Ca2+ current steepened restitution curves of the action potential duration (APD) and the conduction velocity. Spontaneous firing of ectopic foci, coupled with sinus activity, produced dynamic spatial dispersion of repolarization, including discordant alternans, which facilitated unidirectional conduction block and initiated reentrant atrial flutter or AF. The size of vulnerable window was larger for PV ectopic foci than the right atrial foci. These findings imply that the ectopic beats originated from PV are more likely to trigger AF than ectopic beats from elsewhere in the atria.
Anatomic and neural substrates in the pulmonary veins
Zipes and Knope8 conclude that not only did atrial muscle extended for some distance into these the thoracic veins but also that these muscle sleeves received vagal innervation. It was possible that both the autonomic nerves and atrial muscles in the PVs were important in triggering AF. Subsequent works demonstrated significant heterogeneity of the cell types within the PV muscle sleeves. Masani et al17 showed that node-like cells were present in the myocardial layer of the PV of rats. Among the ordinary myocardial cells resembling those of the atrial myocardium, clear cells with structural features similar to those of sinus node cells were identified. They appeared singly or in small groups among ordinary myocardial cells. Cheung18 reported that isolated PVs were capable of independent pacemaking activity. Light and electron microscope studies suggest that cells morphologically akin to specialized conduction cells were present in human PVs.19 Chou and colleagues20 reported that canine PVs had a layer of large pale periodic-acid-Schiff (PAS)-positive cells at the site of focal discharge, supporting the notion that Purkinje-like cells were present in the PVs. A distinguishing feature of the sinus node, as compared with other parts of the atria, is the presence of rich autonomic innervation.21-23 In comparison, Tan and colleagues24 identified abundant sympathetic nerve fibers within the PV using immunohistochemical staining techniques. These findings are consistent with that reported by Masani,17 who observed that in PVs, nerve fibers containing small and large vesicles with and without dense cores were juxtaposed to the node-like cells. The close interaction between the nerve structures and the specialized muscle cells might play a role in the generation of ectopic activities
Cardiac autonomic innervation
Kawashima25 performed detailed anatomical studies of human cardiac autonomic innervation. The cardiac sympathetic ganglia include a superior cervical ganglion which communicates with C1-3, and the cervicothoracic (stellate) ganglion, which communicates with C7-8-T1-2. In addition, the thoracic ganglia (as low as the seventh thoracic ganglion) also contributes to the sympathetic innervation to the heart. The superior, middle and inferior cardiac nerves from these ganglia innervate the heart by following a simple course along the brachiocephalic trunk, common carotid, and subclavian arteries. Alternatively, the thoracic cardiac nerves in the posterior mediastinum have to follow a complex course to reach the heart in the middle mediastinum. The parasympathetic innervation comes from the vagus nerve and is divided into superior, middle and inferior branches. Although both sides of the autonomic branches run through the ventral and dorsal aspects of the aortic arch, the right autonomic cardiac nerves tend to follow a ventral course.
Many investigators have studied the macroscopic and microscopic anatomy of cardiac autonomic nerves within the atria. Among those that focused on PV autonomic nerves, Armour and colleagues 26 provided a detailed map of autonomic nerve distributions in human hearts. They found that autonomic nerves were concentrated in “ganglionic plexi” around great vessels, such as the PVs. Chiou and colleagues determined that these nerves converged functionally onto fat pads located around the superior vena cava-aortic junction, and that catheter ablation of this fat pad effectively denervated many regions of the atria but preserved innervation of the ventricle27. On a more microscopic scale, Chevalier and colleagues 28 discovered several gradients of PV autonomic innervation, with nerves more abundant in the proximal PV than distal PV and more abundant in the epicardium than endocardium. The PV-LA junction is rich in autonomic innervation24. Stimulation of the ganglionic plexi at the PV-LA junction can convert PV focal discharges into AF29 and radiofrequency ablation at these sites can potentially result in successful denervation and prevent AF inducibility30, 31. A recent experimental canine vagal AF study31 reported that ablation of the autonomic ganglia at the base of the PVs suppresses the effective refractory period-abbreviating and AF-promoting effects of cervical vagal stimulation, suggesting that ganglionic plexi ablation may contribute to the effectiveness of PV-directed ablation procedure.
Vagal influences on cardiac electrophysiology
It is well known that vagal nerve stimulation and acetylcholine infusion can result in significant changes of cardiac electrophysiology, including heterogeneous effects on atrial refractory period,32 on pacemaker activity and atrioventricular conduction,33 and on induction of AF.34 Cervical vagal stimulation shortens the atrial effective refractory period primarily in the high right atrium and facilitates induction of AF by single premature extrastimulus.35 Coumel and colleagues36 reported that vagal activity might predispose patients to develop paroxysmal atrial arrhythmias. The investigators studied 18 human cases and discovered sinus slowing often preceded the onset of atrial arrhythmias in these mostly middle-aged male. The investigators proposed that vagal activation might induce shortening of the APD, which in turn facilitates reentrant atrial arrhythmias.
Sympathetic activation and the “Cai-transient triggering” hypothesis
Two recent works have enhanced the understanding of the mechanisms by which sympathovagal activation facilitates the onset of paroxysmal AF. Burashnikov and Antzelevitch37 infused acetylcholine to abbreviate atrial APD and permit rapid pacing in isolated coronary-perfused canine right atrium, which led to Cai accumulation. If this is coupled with a long pause (such as that occurred after AF), then a large Ca2+ release from the sarcoplasmic reticulum could induce late phase 3 early afterdepolarizations (EADs) and extrasystoles that initiated AF. This novel late phase 3 EAD mechanism is observed only in association with marked APD abbreviation. Patterson and colleagues38 showed that simultaneous infusion of norepinephrine and acetylcholine during rapid pacing facilitated the development of EADs and triggered atrial tachycardias. They also measured tension development and discovered that the persistent diastolic elevation of tension was associated with EADs. Assuming that tension is a good measure of Cai, then diastolic Cai elevation underlies the mechanisms of EADs. The investigators named this phenomenon “Cai transient triggering” and suggested that increased forward Na+-Ca2+ exchanger current might contribute to the generation of EADs.
The muscle sleeves of thoracic veins are capable of developing automaticity and triggered activity during sympathetic stimulation39. Ryanodine at low concentrations (0.5-2 μmol/L) causes a Ca-independent Cai release and facilitates the development of pacemaker activity in rabbit PVs40. The importance of Cai transient in atrial arrhythmogenesis is supported by a study that used isolated, Langendorff-perfused canine PV-LA preparations and two cameras to map membrane potential and Cai simultaneously20. Rapid PV firing was induced by rapid atrial pacing, low dose ryanodine and isoproterenol infusion, and the rise of Cai preceded the action potential upstroke during focal discharge. There was clustering of PAS-positive large cells around the PV focal discharge sites. To determine further the interaction between sympathetic nerves and the PAS-positive cells, Tan and colleagues41 performed a study in normal dogs. After sinus node crushing, left stellate ganglion stimulation caused PV tachycardias. The focus of tachycardia was determined by multichannel computerized mapping. PAS staining at the site of PV ectopy showed abundant pale-looking, glycogen-rich specialized conducting (Purkinje) cells. In addition, immunostaining showed abundant sympathetic (tyrosine hydroxylase positive) nerves at those sites. These results support the notion that sympathetic simulation induced PV focal discharge from sites with juxtapositioning of specialized conducting cells and autonomic nerves.
Structural Anatomy of the Atrial and PV Autonomic Nerves
Pappone et al42 hypothesized that the induction of bradycardia was due to vagal nerve stimulation, whereas the abolition of bradycardia with continued RF application suggests vagal denervation. The distribution of adrenergic and cholinergic nerves in this region were not delineated, however, so it is unclear whether sympathetic nerves were also eliminated during RF application. Tan et al24 performed immunostaining of 192 PV-LA segments harvested from 32 veins of eight human autopsied hearts using anti-tyrosine hydroxylase antibodies to label adrenergic nerves and anticholine acetyltransferase antibodies to label cholinergic nerves. Nerve densities were analyzed along the longitudinal and circumferential axes of the PV-LA junction. Longitudinally, adrenergic and cholinergic nerve densities were highest in the LA within 5 mm from the PV-LA junction versus further distally in the PV or more proximally in the LA proper. Circumferentially, both nerve densities were higher in the superior aspect of LSPV, anterosuperior aspect of RSPV and inferior aspects of the both inferior PVs than diametrically opposite and higher in the epicardial than endocardial half of the tissue. Significantly, no area of discrete adrenergic or cholinergic predominance was noted. Rather both nerve types have similar macroscopic distributions in and around PVs. Additionally, confocal microscopy of dual-stained sections showed that at cellular levels, up to 25% of all nerve fiber bundles contained both adrenergic and cholinergic nerves, more than 90% of ganglia contain both adrenergic and cholinergic elements within the same ganglion, and up to 30% of ganglion cell bodies may express adrenergic and cholinergic enzymes simultaneously within its neuroplasm. These data indicate that adrenergic and cholinergic nerves are highly colocated not only at tissue but also at cellular levels.
Implications of neural anatomy of the pulmonary vein
If both sympathetic and parasympathetic nerves are co-stimulated/ablated, why is bradycardia the dominant response elicited during ganglionic stimulation/ablation rather than tachycardia? Several explanations are proposed. Firstly, complex extracardiac neural pathways27 43 involved in the generation of bradycardic reflexes during stimulation/ablation around the PVs project to vagal nuclei centrally but do not involve sympathetic tracts.43 Second, a paracrine mechanism might be in operation, as ganglion cells predominantly are cholinergic24 and release mostly acetylcholine when stimulated/ablated. Third, adrenergic nerves are distributed more widely than cholinergic nerves.24 44 Hence, radiofrequency ablation is more likely to eliminate adrenergic nerves than the cholinergic nerves, resulting in a relatively heightened vagal tone and bradycardia. Clinical reports 42 30 show that autonomic reflexes are elicited most commonly within approximately 1cm of PV-LA junction. The anatomic colocalization of adrenergic and cholinergic innervations implies that it virtually would be impossible to eliminate only sympathetic or parasympathetic nerves selectively during catheter ablation of AF. The coexistence of adrenergic and cholinergic phenotypes within ganglionic cell neuroplasm also suggests that when ganglion cells are stimulated, adrenergic and cholinergic mediators may be released simultaneously, affecting cellular electrophysiology in ways that may predispose to triggered activity38.
Autonomic nervous system and AF in human patients
Several observations suggest the ANS plays an important role in the initiation and maintenance of AF in humans. Most patients who have idiopathic paroxysmal AF seem vagally dependent, with a heightened susceptibility to vasovagal cardiovascular response. In contrast, in most patients who have organic heart diseases, the paroxysmal AF episodes seem more sympathetically dependent.45 A shift toward an increase in sympathetic tone or toward a loss of vagal tone has been observed before postoperative paroxysmal AF46, before the onset of atrial flutter47 and before paroxysmal AF occurring during sleep48; whereas a shift toward vagal predominance was observed in young patients with lone AF and nocturnal episodes of paroxysmal AF49. More recently, a primary increase in adrenergic drive followed by marked modulation toward vagal predominance immediately before the onset of paroxysmal AF was observed.5, 50, 51 The ANS activity in all these studies was indirectly evaluated indirectly, however, by the analysis of heart rate variability parameters on continuous ECG recordings. Heart rate variability measures changes in the relative degree of ANS, not the absolute level of sympathetic or parasympathetic discharges. It is necessary, therefore, to perform direct recording of sympathetic and vagal nerve activity to prove or disprove these observations in ambulatory animals.
Sympathetic nerve recordings in animal models of paroxysmal atrial fibrillation
Barrett and colleagues52 first reported successful recording of renal sympathetic nerve activity in conscious rabbits continuously for more than 7 days. The renal sympathetic nerve activity may not predict the cardiac sympathetic nerve activity, however. To record cardiac sympathetic nerve activity, Jung et al53 used Data Sciences International transmitters to record stellate ganglion nerve activity, 24 hr a day, 7 days a week, for an average of 41.5±16.6 days in normal ambulatory dogs. The results showed a circadian variation of sympathetic outflow. Normal dogs rarely develop paroxysmal AF, however. To test the hypothesis that spontaneous ANS discharges can serve as triggers of paroxysmal AF, it is necessary to develop an animal model of paroxysmal AF. Wijffels and colleagues54 previously demonstrated that intermittent rapid pacing could induce progressively increased electrophysiological remodeling, leading to persistent AF. Rapid atrial pacing also causes significant neural remodeling characterized by heterogeneous increase of sympathetic innervation55 and extensive nerve sprouting56. Tan and colleagues57 implanted Data Sciences International transmitters to directly record left stellate ganglion nerve activity, left vagal nerve activity and LA local bipolar electrograms or surface ECG simultaneously in ambulatory dogs over several weeks. Intermittent rapid atrial pacing was performed and ANS activity was monitored when the pacemaker was turned off. Paroxysmal atrial tachycardia and paroxysmal AF were documented and that simultaneous sympathovagal discharges were the most common triggers of paroxysmal atrial tachycardia and paroxysmal AF. Cryoablation of the stellate ganglia and the superior cardiac branches of vagal nerve eliminated all episodes of paroxysmal AF and atrial tachycardias. These results further support the hypothesis that ANS activity is important in the generation of paroxysmal AF. Histological examinations of cryoablated dogs showed cardiac nerve sprouting and sympathetic hyperinnervation in the atria. These findings suggest decentralization rather than denervation of the sympathovagal nerves underlies the antiarrhythmic mechanism of stellate ganglion and vagal nerve ablation.
Cai dynamics and vagal atrial fibrillation in heart failure
The Framingham Heart Study58 concluded that in heart failure subjects, late development of AF was associated with increased mortality. Heart failure-related atrial arrhythmias appear to arise from macroreentrant sources, primarily by increasing atrial size and promoting interstitial fibrosis59. In addition to macroreentry, Stambler and colleagues60 reported that DADs-induced triggered activity may also be a mechanism of focal atrial tachycardias in pacing-induced heart failure dogs. Okuyama and colleagues61 demonstrated that some AF episodes were characterized by focal activations in the PVs and vein of Marshall, and by complex, fractionated wavefronts within the PVs in a canine heart failure model, suggesting the occurrence of significant proarrhythmic remodeling in the PVs during heart failure. A major arrhythmogenic mechanism in heart failure results from altered ryanodine receptor function62. A combination of abnormal ryanodine receptor and increased sympathetic tone during exercise can cause triggered activity63. Alternatively, direct autonomic nerve recordings in a canine heart failure model showed that not only sympathetic but also vagal nerves discharges were increased in heart failure dogs, and simultaneous sympathovagal discharges were common triggers of atrial arrhythmias64. A computer simulation study65 has suggested that vagal AF may arise from acetylcholine-induced stabilization of the primary spiral-wave generator and disorganization of propagation by repolarization gradient that causes fibrillatory dynamics. As IKACh activation shortens APD and hyperpolarizes the cell membrane, Atienza and colleagues66 reported that adenosine activates IKACh and accelerates AF by promoting reentry rather than triggered activity in human. However, Chou and colleagues67 reported that acetylcholine facilitates both PV focal discharges and PV-LA microreentry during vagal AF in a canine heart failure model. By using isolated, Langendorff-perfused canine PV-LA preparations and two cameras to map membrane potential and Cai simultaneously, it was demonstrated that pause-related large Cai elevation is associated with focal discharges in the PVs. A long preceding pause increases the Cai accumulation, leading to a greater SR Ca2+ release at the first beat after the pause.37 Because the APD was reduced by acetylcholine, this large rise of Cai resulted in persistent Cai elevation into late phase 3 to induce late phase 3 EADs and PV focal discharges37, 38, 68. These triggered beats followed by sustained PV-LA microreentry can induce atrial tachycardia and AF, suggesting that both triggered and reentrant activities are important during vagal AF. Failing hearts have increased INCX current,69 which renders them more susceptible to the late phase 3 EADs. Acetylcholine may increase Na+ conductance and intracellular Na+ activity, leading to altered INCX, reduced Cai efflux70, 71 and further enhanced Cai accumulation. The hypothesis is also supported by the suppression of late phase 3 EADs by ryanodine and thapsigargin infusion. Parasympathetic activation and acetylcholine release could be important mechanisms in the pathophysiology and atrial arrhythmogenesis in the heart failure status. Livanis et al72 reported that neurally mediated mechanisms may be implicated in the pathophysiology of syncope in patients with dilated cardiomyopathy. In that study, both sympathetic and parasympathetic heart rate parameters were markedly stimulated.
Neural Modulation as a Potential Therapeutic Strategy
The effectiveness of autonomic modulation as an adjunctive therapeutic strategy to catheter ablation of AF has been inconsistent. Although favorable results have been obtained by Nakagawa and colleagues and Pappone and colleagues30, 42, others found no beneficial 73 or deleterious74 outcomes in patients who had denervation compared to those who did not, a finding also underlined by animal studies by Hirose and colleagues35, where partial vagal denervation of the high right atrium was found to increase inducibility of AF. These conflicting studies suggest that the interactions between the ANS and AF are more complex than currently understood. Perhaps a degree of individual variability accounts for these discrepancies, with some patients having more pronounced autonomic triggers than others. As an illustration, Scanavacca and colleagues75 recently found that in a small number of patients who had “autonomic” paroxysmal AF, denervation alone without substrate modification in the atria was effective in preventing AF recurrence in 2 of 11 patients, these two patients having the most pronounced and persistent changes in heart rate variability. In summary, the evidence to date suggests that autonomic modulation does have an adjunctive role to play in catheter AF ablation, especially when applied selectively. Further mechanistic and clinical studies are warranted before a wider application can be recommended.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Allessie MA, Boyden PA, Camm AJ, et al. Pathophysiology and prevention of atrial fibrillation. Circulation. 2001 February 6;103(5):769–77. doi: 10.1161/01.cir.103.5.769. [DOI] [PubMed] [Google Scholar]
- 2.Chen YJ, Chen SA, Chang MS, Lin CI. Arrhythmogenic activity of cardiac muscle in pulmonary veins of the dog: implication for the genesis of atrial fibrillation. Cardiovasc Res. 2000 November;48(2):265–73. doi: 10.1016/s0008-6363(00)00179-6. [DOI] [PubMed] [Google Scholar]
- 3.Doshi RN, Wu TJ, Yashima M, et al. Relation between ligament of Marshall and adrenergic atrial tachyarrhythmia. Circulation. 1999;100:876–83. doi: 10.1161/01.cir.100.8.876. [DOI] [PubMed] [Google Scholar]
- 4.Sharifov OF, Fedorov VV, Beloshapko GG, Glukhov AV, Yushmanova AV, Rosenshtraukh LV. Roles of adrenergic and cholinergic stimulation in spontaneous atrial fibrillation in dogs. J Am Coll Cardiol. 2004 February 4;43(3):483–90. doi: 10.1016/j.jacc.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 5.Bettoni M, Zimmermann M. Autonomic tone variations before the onset of paroxysmal atrial fibrillation. Circulation. 2002 June 11;105(23):2753–9. doi: 10.1161/01.cir.0000018443.44005.d8. [DOI] [PubMed] [Google Scholar]
- 6.Gould PA, Yii M, McLean C, et al. Evidence for increased atrial sympathetic innervation in persistent human atrial fibrillation. Pacing Clin Electrophysiol. 2006 August;29(8):821–9. doi: 10.1111/j.1540-8159.2006.00447.x. [DOI] [PubMed] [Google Scholar]
- 7.Moe GK, Abildskov JA. Atrial fibrillation as a self-sustaining arrhythmia independent of focal discharge. Am Heart J. 1959;58(1):59–70. doi: 10.1016/0002-8703(59)90274-1. [DOI] [PubMed] [Google Scholar]
- 8.Zipes DP, Knope RF. Electrical properties of the thoracic veins. Am J Cardiol. 1972 March;29:372–6. doi: 10.1016/0002-9149(72)90533-4. [DOI] [PubMed] [Google Scholar]
- 9.Spach MS, Barr RC, Jewett PH. Spread of excitation from the atrium into thoracic veins in human beings and dogs. Am J Cardiol. 1972 December;30:844–54. doi: 10.1016/0002-9149(72)90009-4. [DOI] [PubMed] [Google Scholar]
- 10.Scherlag BJ, Yeh BK, Robinson MJ. Inferior interatrial pathway in the dog. Circ Res. 1972;31:18–35. doi: 10.1161/01.res.31.1.18. [DOI] [PubMed] [Google Scholar]
- 11.Haissaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998 September 3;339:659–66. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 12.Hamabe A, Okuyama Y, Miyauchi Y, et al. Correlation between anatomy and electrical activation in canine pulmonary veins. Circulation. 2003;107:1550–5. doi: 10.1161/01.cir.0000056765.97013.5e. [DOI] [PubMed] [Google Scholar]
- 13.Zhou S, Chang CM, Wu TJ, et al. Nonreentrant focal activations in pulmonary veins in canine model of sustained atrial fibrillation. Am J Physiol Heart Circ Physiol. 2002;283(3):H1244–H1252. doi: 10.1152/ajpheart.01109.2001. [DOI] [PubMed] [Google Scholar]
- 14.Chou CC, Zhou S, Miyauchi Y, et al. Effects of procainamide on electrical activity in thoracic veins and atria in canine model of sustained atrial fibrillation. Am J Physiol Heart Circ Physiol. 2004 May;286(5):H1936–H1945. doi: 10.1152/ajpheart.00754.2003. [DOI] [PubMed] [Google Scholar]
- 15.Chou CC, Zhou S, Tan AY, Hayashi H, Nihei M, Chen PS. High Density Mapping of Pulmonary Veins and Left Atrium During Ibutilide Administration in a Canine Model of Sustained Atrial Fibrillation. Am J Physiol Heart Circ Physiol. 2005 July;289:H2704–H2713. doi: 10.1152/ajpheart.00537.2005. [DOI] [PubMed] [Google Scholar]
- 16.Gong Y, Xie F, Stein KM, et al. Mechanism Underlying Initiation of Paroxysmal Atrial Flutter/Atrial Fibrillation by Ectopic Foci. A Simulation Study. Circulation. 2007 April;115(16):2094–102. doi: 10.1161/CIRCULATIONAHA.106.656504. [DOI] [PubMed] [Google Scholar]
- 17.Masani F. Node-like cells in the myocardial layer of the pulmonary vein of rats: an ultrastructural study. J Anat. 1986 April;145:133–42. [PMC free article] [PubMed] [Google Scholar]
- 18.Cheung DW. Electrical activity of the pulmonary vein and its interaction with the right atrium in the guinea-pig. J Physiol. 1981 May;314:445–56. doi: 10.1113/jphysiol.1981.sp013718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perez-Lugones A, McMahon JT, Ratliff NB, et al. Evidence of specialized conduction cells in human pulmonary veins of patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2003 August;14(8):803–9. doi: 10.1046/j.1540-8167.2003.03075.x. [DOI] [PubMed] [Google Scholar]
- 20.Chou CC, Nihei M, Zhou S, et al. Intracellular calcium dynamics and anisotropic reentry in isolated canine pulmonary veins and left atrium. Circulation. 2005;111:2889–297. doi: 10.1161/CIRCULATIONAHA.104.498758. [DOI] [PubMed] [Google Scholar]
- 21.Crick SJ, Sheppard MN, Anderson RH, Polak JM, Wharton J. A quantitative study of nerve distribution in the conduction system of the guinea pig heart. J Anat. 1996;188:403–16. [PMC free article] [PubMed] [Google Scholar]
- 22.Crick SJ, Wharton J, Sheppard MN, et al. Innervation of the human cardiac conduction system. A quantitative immunohistochemical and histochemical study. Circulation. 1994 April;89(4):1697–708. doi: 10.1161/01.cir.89.4.1697. [DOI] [PubMed] [Google Scholar]
- 23.Miyauchi Y, Zhou S, Okuyama Y, et al. Altered atrial electrical restitution and heterogeneous sympathetic hyperinnervation in hearts with chronic left ventricular myocardial infarction: implications for atrial fibrillation. Circulation. 2003 July 22;108(3):360–6. doi: 10.1161/01.CIR.0000080327.32573.7C. [DOI] [PubMed] [Google Scholar]
- 24.Tan AY, Li H, Wachsmann-Hogiu S, Chen LS, Chen PS, Fishbein MC. Autonomic innervation and segmental muscular disconnections at the human pulmonary vein-atrial junction: implications for catheter ablation of atrial-pulmonary vein junction. J Am Coll Cardiol. 2006;48:132–43. doi: 10.1016/j.jacc.2006.02.054. [DOI] [PubMed] [Google Scholar]
- 25.Kawashima T. The autonomic nervous system of the human heart with special reference to its origin, course, and peripheral distribution. Anat Embryol (Berl) 2005 July;209(6):425–38. doi: 10.1007/s00429-005-0462-1. [DOI] [PubMed] [Google Scholar]
- 26.Armour JA, Murphy DA, Yuan BX, Macdonald S, Hopkins DA. Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat Rec. 1997 February;247(2):289–98. doi: 10.1002/(SICI)1097-0185(199702)247:2<289::AID-AR15>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 27.Chiou CW, Eble JN, Zipes DP. Efferent vagal innervation of the canine atria and sinus and atrioventricular nodes--the third fat pad. Circulation. 1997;95:2573–84. doi: 10.1161/01.cir.95.11.2573. [DOI] [PubMed] [Google Scholar]
- 28.Chevalier P, Tabib A, Meyronnet D, et al. Quantitative study of nerves of the human left atrium. Heart Rhythm. 2005 May;2(5):518–22. doi: 10.1016/j.hrthm.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 29.Scherlag BJ, Yamanashi W, Patel U, Lazzara R, Jackman WM. Autonomically induced conversion of pulmonary vein focal firing into atrial fibrillation. J Am Coll Cardiol. 2005 June 7;45(11):1878–86. doi: 10.1016/j.jacc.2005.01.057. [DOI] [PubMed] [Google Scholar]
- 30.Nakagawa H, Scherlag BJ, Wu R, et al. Addition of selective ablation of autonomic ganglia to pulmonary vein antrum isolation for treatment of paroxysmal and persistent atrial fibrillation. Circulation. 2006;110:III-459. [Google Scholar]
- 31.Lemola K, Chartier D, Yeh YH, et al. Pulmonary vein region ablation in experimental vagal atrial fibrillation: role of pulmonary veins versus autonomic ganglia. Circulation. 2008 January;117(4):470–7. doi: 10.1161/CIRCULATIONAHA.107.737023. [DOI] [PubMed] [Google Scholar]
- 32.Zipes DP, Mihalick MJ, Robbins GT. Effects of selective vagal and stellate ganglion stimulation of atrial refractoriness. Cardiovasc Res. 1974 September;8:647–55. doi: 10.1093/cvr/8.5.647. [DOI] [PubMed] [Google Scholar]
- 33.Spear JF, Moore EN. Influence of brief vagal and stellate nerve stimulation on pacemaker activity and conduction within the atrioventricular conduction system of the dog. Circ Res. 1973;32:27–41. doi: 10.1161/01.res.32.1.27. [DOI] [PubMed] [Google Scholar]
- 34.Goldberger AL, Pavelec RS. Vagally-mediated atrial fibrillation in dogs: conversion with bretylium tosylate. Int J Cardiol. 1986 October;13:47–55. doi: 10.1016/0167-5273(86)90078-1. [DOI] [PubMed] [Google Scholar]
- 35.Hirose M, Leatmanoratn Z, Laurita KR, Carlson MD. Partial vagal denervation increases vulnerability to vagally induced atrial fibrillation. J Cardiovasc Electrophysiol. 2002 December;13(12):1272–9. doi: 10.1046/j.1540-8167.2002.01272.x. [DOI] [PubMed] [Google Scholar]
- 36.Coumel P, Attuel P, Lavallee J, Flammang D, Leclercq JF, Slama R. The atrial arrhythmia syndrome of vagal origin. Arch Mal Coeur Vaiss. 1978 June;71(6):645–56. [PubMed] [Google Scholar]
- 37.Burashnikov A, Antzelevitch C. Reinduction of atrial fibrillation immediately after termination of the arrhythmia is mediated by late phase 3 early afterdepolarization-induced triggered activity. Circulation. 2003 May 13;107(18):2355–60. doi: 10.1161/01.CIR.0000065578.00869.7C. [DOI] [PubMed] [Google Scholar]
- 38.Patterson E, Lazzara R, Szabo B, et al. Sodium-calcium exchange initiated by the Ca2+ transient: an arrhythmia trigger within pulmonary veins. J Am Coll Cardiol. 2006 March 21;47(6):1196–206. doi: 10.1016/j.jacc.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 39.Wit AL, Cranefield PF. Triggered and automatic activity in the canine coronary sinus. Circ Res. 1977 October;41:434–45. doi: 10.1161/01.res.41.4.434. [DOI] [PubMed] [Google Scholar]
- 40.Honjo H, Boyett MR, Niwa R, et al. Pacing-induced spontaneous activity in myocardial sleeves of pulmonary veins after treatment with ryanodine. Circulation. 2003 April 15;107(14):1937–43. doi: 10.1161/01.CIR.0000062645.38670.BD. [DOI] [PubMed] [Google Scholar]
- 41.Tan AY, Zhou S, Jung BC, et al. Ectopic Atrial Arrhythmias Arising from Canine Thoracic Veins During In-vivo Stellate Ganglia Stimulation. Am J Physiol Heart Circ Physiol. 2008 doi: 10.1152/ajpheart.01321.2007. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pappone C, Santinelli V, Manguso F, et al. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation. 2004 January 27;109(3):327–34. doi: 10.1161/01.CIR.0000112641.16340.C7. [DOI] [PubMed] [Google Scholar]
- 43.Aviado DM, Guevara AD. The Bezold-Jarisch reflex. A historical perspective of cardiopulmonary reflexes. Ann N Y Acad Sci. 2001 June;940:48–58. [PubMed] [Google Scholar]
- 44.Marron K, Wharton J, Sheppard MN, et al. Distribution, morphology, and neurochemistry of endocardial and epicardial nerve terminal arborizations in the human heart. Circulation. 1995 October 15;92(8):2343–51. doi: 10.1161/01.cir.92.8.2343. [DOI] [PubMed] [Google Scholar]
- 45.Huang JL, Wen ZC, Lee WL, Chang MS, Chen SA. Changes of autonomic tone before the onset of paroxysmal atrial fibrillation. Int J Cardiol. 1998 October 30;66:275–83. doi: 10.1016/s0167-5273(98)00241-1. [DOI] [PubMed] [Google Scholar]
- 46.Dimmer C, Tavernier R, Gjorgov N, Van Nooten G, Clement DL, Jordaens L. Variations of autonomic tone preceding onset of atrial fibrillation after coronary artery bypass grafting. Am J Cardiol. 1998 July 1;82(1):22–5. doi: 10.1016/s0002-9149(98)00231-8. [DOI] [PubMed] [Google Scholar]
- 47.Wen ZC, Chen SA, Tai CT, Huang JL, Chang MS. Role of autonomic tone in facilitating spontaneous onset of typical atrial flutter. J Am Coll Cardiol. 1998 March 1;31(3):602–7. doi: 10.1016/s0735-1097(97)00555-x. [DOI] [PubMed] [Google Scholar]
- 48.Coccagna G, Capucci A, Bauleo S, Boriani G, Santarelli A. Paroxysmal atrial fibrillation in sleep. Sleep. 1997 June;20(6):396–8. doi: 10.1093/sleep/20.6.396. [DOI] [PubMed] [Google Scholar]
- 49.Herweg B, Dalal P, Nagy B, Schweitzer P. Power spectral analysis of heart period variability of preceding sinus rhythm before initiation of paroxysmal atrial fibrillation. Am J Cardiol. 1998 October 1;82(7):869–74. doi: 10.1016/s0002-9149(98)00494-9. [DOI] [PubMed] [Google Scholar]
- 50.Zimmermann M, Kalusche D. Fluctuation in autonomic tone is a major determinant of sustained atrial arrhythmias in patients with focal ectopy originating from the pulmonary veins. J Cardiovasc Electrophysiol. 2001 March;12(3):285–91. doi: 10.1046/j.1540-8167.2001.00285.x. [DOI] [PubMed] [Google Scholar]
- 51.Amar D, Zhang H, Miodownik S, Kadish AH. Competing autonomic mechanisms precede the onset of postoperative atrial fibrillation. J Am Coll Cardiol. 2003 October 1;42(7):1262–8. doi: 10.1016/s0735-1097(03)00955-0. [DOI] [PubMed] [Google Scholar]
- 52.Barrett CJ, Ramchandra R, Guild SJ, Lala A, Budgett DM, Malpas SC. What sets the long-term level of renal sympathetic nerve activity: a role for angiotensin II and baroreflexes? Circ Res. 2003 June 27;92(12):1330–6. doi: 10.1161/01.RES.0000078346.60663.A0. [DOI] [PubMed] [Google Scholar]
- 53.Jung BC, Dave AS, Tan AY, et al. Circadian variations of stellate ganglion nerve activity in ambulatory dogs. Heart Rhythm. 2005;3:78–85. doi: 10.1016/j.hrthm.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 54.Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995 October 1;92:1954–68. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 55.Jayachandran JV, Sih HJ, Winkle W, Zipes DP, Hutchins GD, Olgin JE. Atrial fibrillation produced by prolonged rapid atrial pacing is associated with heterogeneous changes in atrial sympathetic innervation. Circulation. 2000 March 14;101:1185–91. doi: 10.1161/01.cir.101.10.1185. [DOI] [PubMed] [Google Scholar]
- 56.Chang CM, Wu TJ, Zhou SM, et al. Nerve sprouting and sympathetic hyperinnervation in a canine model of atrial fibrillation produced by prolonged right atrial pacing. Circulation. 2001;103:22–5. doi: 10.1161/01.cir.103.1.22. [DOI] [PubMed] [Google Scholar]
- 57.Tan AY, Zhou S, Ogawa M, et al. Neural mechanisms of paroxysmal atrial fibrillation and paroxysmal atrial tachycardia in ambulatory canines. Circulation. 2008 doi: 10.1161/CIRCULATIONAHA.108.776203. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang TJ, Larson MG, Levy D, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003 June 17;107(23):2920–5. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 59.Li D, Fareh S, Leung TK, Nattel S. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation. 1999 July 6;100:87–95. doi: 10.1161/01.cir.100.1.87. [DOI] [PubMed] [Google Scholar]
- 60.Stambler BS, Fenelon G, Shepard RK, Clemo HF, Guiraudon CM. Characterization of sustained atrial tachycardia in dogs with rapid ventricular pacing-induced heart failure. J Cardiovasc Electrophysiol. 2003 May;14(5):499–507. doi: 10.1046/j.1540-8167.2003.02519.x. [DOI] [PubMed] [Google Scholar]
- 61.Okuyama Y, Miyauchi Y, Park AM, et al. High resolution mapping of the pulmonary vein and the vein of Marshall during induced atrial fibrillation and atrial tachycardia in a canine model of pacing-induced congestive heart failure. J Am Coll Cardiol. 2003;42:348–60. doi: 10.1016/s0735-1097(03)00586-2. [DOI] [PubMed] [Google Scholar]
- 62.Marx SO, Reiken S, Hisamatsu Y, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000 May 12;101(4):365–76. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 63.Wehrens XH, Lehnart SE, Reiken SR, et al. Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin2. Science. 2004 April 9;304(5668):292–6. doi: 10.1126/science.1094301. [DOI] [PubMed] [Google Scholar]
- 64.Ogawa M, Zhou S, Tan AY, et al. Left stellate ganglion and vagal nerve activity and cardiac arrhythmias in ambulatory dogs with pacing-induced congestive heart failure. J Am Coll Cardiol. 2007;50:335–43. doi: 10.1016/j.jacc.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 65.Kneller J, Zou R, Vigmond EJ, Wang Z, Leon LJ, Nattel S. Cholinergic atrial fibrillation in a computer model of a two-dimensional sheet of canine atrial cells with realistic ionic properties. Circ Res. 2002 May 17;90(9):E73–E87. doi: 10.1161/01.res.0000019783.88094.ba. [DOI] [PubMed] [Google Scholar]
- 66.Atienza F, Almendral J, Moreno J, et al. Activation of inward rectifier potassium channels accelerates atrial fibrillation in humans: evidence for a reentrant mechanism. Circulation. 2006 December 5;114(23):2434–42. doi: 10.1161/CIRCULATIONAHA.106.633735. [DOI] [PubMed] [Google Scholar]
- 67.Chou CC, Nguyen BL, Tan AY, et al. Intracellular calcium dynamics and acetylcholine-induced triggered activity in the pulmonary veins of dogs with pacing-induced heart failure. Heart Rhythm. 2008 August;5(8):1170–7. doi: 10.1016/j.hrthm.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Patterson E, Po SS, Scherlag BJ, Lazzara R. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm. 2005 June;2(6):624–31. doi: 10.1016/j.hrthm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 69.Li D, Melnyk P, Feng J, et al. Effects of experimental heart failure on atrial cellular and ionic electrophysiology. Circulation. 2000 June 6;101(22):2631–8. doi: 10.1161/01.cir.101.22.2631. [DOI] [PubMed] [Google Scholar]
- 70.Tajima T, Tsuji Y, Sorota S, Pappano AJ. Positive vs. negative inotropic effects of carbachol in avian atrial muscle: role of Ni-like protein. Circ Res. 1987 October;61(4 Pt 2):I105–I111. [PubMed] [Google Scholar]
- 71.Matsumoto K, Pappano AJ. Sodium-dependent membrane current induced by carbachol in single guinea-pig ventricular myocytes. J Physiol. 1989 August;415:487–502. doi: 10.1113/jphysiol.1989.sp017733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Livanis EG, Kostopoulou A, Theodorakis GN, et al. Neurocardiogenic mechanisms of unexplained syncope in idiopathic dilated cardiomyopathy. Am J Cardiol. 2007 February 15;99(4):558–62. doi: 10.1016/j.amjcard.2006.09.098. [DOI] [PubMed] [Google Scholar]
- 73.Lemery R, Birnie D, Tang AS, Green M, Gollob M. Feasibility study of endocardial mapping of ganglionated plexuses during catheter ablation of atrial fibrillation. Heart Rhythm. 2006 April;3(4):387–96. doi: 10.1016/j.hrthm.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 74.Cummings JE, Gill I, Akhrass R, Dery M, Biblo LA, Quan KJ. Preservation of the anterior fat pad paradoxically decreases the incidence of postoperative atrial fibrillation in humans. J Am Coll Cardiol. 2004 March 17;43(6):994–1000. doi: 10.1016/j.jacc.2003.07.055. [DOI] [PubMed] [Google Scholar]
- 75.Scanavacca M, Pisani CF, Hachul D, et al. Selective atrial vagal denervation guided by evoked vagal reflex to treat patients with paroxysmal atrial fibrillation. Circulation. 2006 August 29;114(9):876–85. doi: 10.1161/CIRCULATIONAHA.106.633560. [DOI] [PubMed] [Google Scholar]


