Abstract
Background
Higher adherence to the Mediterranean diet (MeDi) may protect from Alzheimer’s disease (AD) but its association with Mild Cognitive Impairment (MCI) has not been explored.
Objective
To investigate the association between MeDi and MCI.
Design, Setting, Patients, Outcomes
In a multiethnic community study in New York, we used Cox proportional hazards to investigate the association between adherence to the MeDi (0 – 9 scale; higher scores higher adherence) and (1) incidence of MCI and (2) progression from MCI to AD. All models were adjusted for cohort, age, gender, ethnicity, education, APOE genotype, caloric intake, body mass index and time duration between baseline dietary assessment and baseline diagnosis.
Results
There were 1393 cognitively normal participants, 275 of whom developed MCI during 4.5 (± 2.7, 0.9–16.4) years of follow-up. Compared to subjects in the lowest MeDi adherence tertile, subjects in the middle MeDi tertile had 17 % (HR, 0.83; 95% CI, 0.62 – 1.12; p=0.24) less risk of developing MCI, while those at the highest MeDi adherence tertile had 28 % (HR, 0.72; 95% CI, 0.52 – 1.00; p=0.05) less risk of developing MCI (trend HR, 0.85; 95% CI, 0.72 – 1.00; p for trend= 0.05). There were 482 subjects with MCI, 106 of whom developed AD during 4.3 (± 2.7, 1.0 – 13.8) years of follow-up. Compared to subjects in the lowest MeDi adherence tertile, subjects in the middle MeDi adherence tertile had 45 % (HR, 0.55; 95% CI, 0.34 – 0.90; p=0.01) less risk of developing AD, while those at the highest MeDi adherence tertile had 48 % (HR, 0.52; 95% CI, 0.30 – 0.91; p=0.02) less risk of developing AD (trend HR, 0.71; 95% CI, 0.53 – 0.95; p for trend= 0.02).
Conclusions
Higher adherence to the MeDi is associated with a trend for reduced risk for developing MCI and with reduced risk for MCI conversion to AD.
The concept of MCI was developed in order to capture subjects who are in the transitional stage between normal aging and dementia or AD. Because of the high conversion rates, MCI subjects constitute a population suited for study of dementia-AD risk factor epidemiology and for investigation of possible behavioral or pharmacological preventive interventions. Among behavioral traits, diet may play an important role in the causation and prevention of AD. However, epidemiological data on diet and AD have been conflicting 1, 2. Moreover, there is paucity of research on the effect of dietary factors on either rates of development of MCI or on rates of MCI conversion to AD.
We recently demonstrated that higher adherence to the MeDi (a diet characterized by high intake of fish, vegetables, legumes, fruits, cereals, unsaturated fatty acids [mostly in the form of olive oil], low intake of dairy products, meat, saturated fatty acids and a regular but moderate amount of ethanol 3) is associated with lower AD risk 4, 5. Some studies have investigated the effect of some individual elements of the MeDi (i.e. alcohol, fatty acids, fish) in MCI and age-related cognitive decline6–12 with conflicting results. Nevertheless, potential associations between the whole MeDi pattern and MCI has not been explored. We examined the association between MeDi and MCI using data from the Washington Heights-Inwood Columbia Aging Project (WHICAP). We hypothesized that cognitively normal participants with higher adherence to the MeDi would have lower risk for future development of MCI and that MCI participants with higher MeDi adherence would have lower risk for developing future AD.
SUBJECTS AND METHODS
Sample and diagnoses
The sample for the current study has been described in detail in recent studies describing frequency and course of MCI in our cohorts 13, 14. The study included participants of 2 related cohorts recruited in 1992 (WHICAP 1992) and 1999 (WHICAP 1999) which were identified (via ethnicity and age stratification processes) from a probability sample of Medicare beneficiaries residing in an area of 3 contiguous census tracts within a geographically defined area of northern Manhattan 15. The same assessments and study procedures were used in both cohorts. At entry, a physician elicited each subject’s medical and neurological history and conducted a standardized physical and neurological examination. All available ancillary information (medical charts, CTs or MRIs) was considered in the evaluation.
Each subject also underwent a structured in-person interview including an assessment of health and function and a neuropsychological battery. Functional assessment of instrumental activities of daily living included a Disability and Functional Limitations Scale 16, 17. The neuropsychological battery 18 contained tests of memory (short and long-term verbal and nonverbal); orientation; abstract reasoning (verbal and non-verbal); language (naming, verbal fluency, comprehension and repetition); and construction (copying and matching). A global summary score on the Clinical Dementia Rating (CDR) 19 was also assigned.
A consensus diagnosis for the presence or absence of dementia was made at a diagnostic conference of neurologists and neuropsychologists where information of all the above evaluations was presented. Evidence of cognitive deficit (based on the neuropsychological scores as described above), evidence of impairment in social or occupational function (as assessed by the Blessed Dementia Rating Scale, the Schwab and England Activities of Daily Living Scale and the physician’s assessment), and evidence of cognitive and social-occupational function decline were the criteria used for the diagnosis of dementia as required by the Diagnostic and Statistical Manual of Mental Disorders, Revised Third Edition (DSM-III-R). The type of dementia was subsequently determined. For the diagnosis of probable or possible AD, the criteria of the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association 20 were used.
Because the current study had started before the concept of MCI was developed, diagnosis of MCI was not part of the standard consensus conference procedure but was retrospectively applied after the consensus conference for each visit among nondemented individuals. Details on MCI definition have been previously provided 13, 14. Briefly, consistent with standard criteria 21, those considered for MCI were required to have following. First, a subjective memory complaint (using items from the Disability and Functional Limitations Scale 16, 17, and the Blessed Functional Activities Scale 22). Second, objective impairment in at least one cognitive domain. Using selected items from the neuropsychological battery, 4 cognitive domains (memory, executive, language, and visuospatial) were defined 13, 14. Impairment was defined based on the average of the scores on the neuropsychological measures within that domain and a 1.5 SD cutoff using corrections for age, years of education, ethnicity, and sex and based on the previously established norms. Third, essentially preserved activities of daily living (defined above). Fourth, no diagnosis of dementia at the consensus conference. In order to further explore the association of MeDi to MCI, in the view of the original Petersen criteria 21, 23 (which focus on objective memory impairment), the overall MCI group was divided into two mutually exclusive MCI subtypes: (i) MCI with objective memory impairment (with or without objective impairment in other cognitive domains) and MCI without objective memory impairment (objective impairment in at least one of the three non-memory domains but memory domain composite score within norms). Dietary data were not available to the consensus panel and were not considered in the MCI diagnostic process. Subjects were followed at intervals of approximately 1.5 years, repeating the baseline examination and consensus diagnosis at each follow-up.
Evaluation
Predictors
Diet
Dietary data regarding average food consumption over the past year were obtained using a 61-item version of Willett’s semi-quantitative food frequency questionnaire (SFFQ) (Channing Laboratory, Cambridge, MA) 24. Trained interviewers administered the SFFQ in English or Spanish. We have previously reported validity (using two 7-day food records) and reliability (using two 3-month frequency assessments) of various components of the SSFQ in WHICAP 25–27.
Similarly to our previous work 4, 5, 28, we followed a method previously described 3 for the construction of the MeDi score. More specifically, we first regressed caloric intake (kcal) and calculated the derived residuals of daily gram intake 29 for each of the following seven categories 3: dairy, meat, fruits, vegetables, legumes, cereals and fish. A value of 0 or 1 was assigned to each of the seven above groups, using sex-specific medians as cut-offs. For beneficial components (fruits, vegetables, legumes, cereals and fish) persons whose consumption was below the median were assigned a value of 0, and persons whose consumption was at or above the median was assigned a value of 1. For components presumed to be detrimental (meat and dairy products) persons whose consumption was below the median were assigned a value of 1, and persons whose consumption was at or above the median was assigned a value of 0. For fat intake (8th food category) we used the ratio of daily consumption (in grams) of monounsaturated lipids to saturated lipids 3 (again using sex-specific median cutoffs for assignment values of 0 for low and 1 for high). For alcohol intake (9th food category), subjects were assigned a score of 0 for either no (0 g/day) or more than moderate (≥30 g/day) consumption, and a value of 1 for mild-moderate alcohol consumption (>0 to <30 g/day). This is in agreement with previous reports 3, that consider moderate amount of alcohol consumption as another characteristic component of the MeDi. We classified alcohol consumption dichotomously, also because of the skewed distribution of alcohol in our population (68% reporting no alcohol intake, 31% reporting less than 30 gm/day [mild to moderate intake], and 1% reporting ≥30 g/day [heavy intake]). The MeDi score was generated for each participant by adding the scores in the food categories (theoretically ranging 0–9) with higher score indicating higher adherence to the MeDi.
We used MeDi score from the first time the dietary assessment was performed as the main predictor in the survival analyses. Because the cognitively normal and MCI definitions were partially based on availability of neuropsychological assessment their ascertainment was not always synchronous with the dietary assessments. For the incident MCI analyses, the time of 1st dietary assessment coincided (+/− 1.5 years) with the time of 1st assessment of cognitively normal status for 82% of the subjects, was performed more than 1.5 years earlier for 14% of the subjects and more than 1.5 years later for only 4%. For the incident AD analyses the time of 1st dietary assessment coincided (+/− 1.5 years) with the time of 1st assessment of MCI status for 84% of the subjects, was performed more than 1.5 years earlier for 11% of the subjects and more than 1.5 years later for only 5%. Summarizing, the timing of MeDi adherence assessment and cognitively normal/MCI assessments overlapped to a significant degree. Despite the above, in all survival models we included a term adjusting for the difference in time between 1st diet and 1st cognitive status assessment.
Covariates
Age (years), education (years), caloric intake (kcal), and body mass index (BMI; weight in kilograms divided by height in square meters [kg/m2]) 30 were used as continuous variables. We also considered cohort (1992 cohort as reference), and gender (men as reference). Ethnic group was based on self-report using the format of the 1990 census 31. Participants were then assigned to one of four groups: Black (non-Hispanic), Hispanic, White (non-Hispanic) or Other. Ethnicity was used as a dummy variable with White (non-Hispanic) as the reference. Apolipoprotein (APOE) genotype was used dichotomously: absence of ε4 allele vs. presence of either one or two ε4 alleles.
Statistical analyses
Baseline characteristics of subjects by missing dietary data, by outcome of interest and by MeDi tertiles were compared using t-test or ANOVA for continuous variables and χ2 test for categorical variables.
Cognitively normal to incident MCI
We calculated Cox proportional hazards models with MCI as the dichotomous outcome. The time-to-event variable was time from 1st cognitive normal status to 1st visit of MCI diagnosis. Subjects diagnosed with MCI and then reverting back to normal were considered cases and their 1st MCI diagnostic visit was considered the event visit. Persons who did not develop MCI were censored at the time of their last follow-up (i.e. all subject-visits before the last follow-up were used in the analyses). The main predictor was MeDi score (from the 1st dietary evaluation) as a continuous variable initially and in tertiles form subsequently (used for trend test calculation). We simultaneously adjusted for the following variables: cohort, age at intake in the study, gender, ethnicity, education, APOE, BMI and time between 1st dietary and 1st cognitive status assessment. Although caloric intake adjusted residuals were used in the MeDi score calculation, we also included caloric intake as a covariate in the models (as recommended by Willet et al.29). All predictors were used as time-constant covariates. In additional analyses we recalculated the models using the two different types of MCI as the outcomes: MCI with and MCI without memory impairment.
MCI to incident AD
We calculated Cox proportional hazards models with AD as the dichotomous outcome. The time-to-event variable was time from 1st MCI status to 1st visit of AD diagnosis. Subjects diagnosed with AD and then reverting back to MCI, were considered cases and their 1st AD diagnostic visit was considered the event visit. Therefore, censored subjects never developed AD at any of their follow-up visits. Persons who did not develop AD were censored at the time of their last follow-up (i.e. all subject-visits before the last follow-up were used in the analyses). Predictors and covariates were the same as in the ‘cognitively normal to incident MCI’ models described above. In additional analyses we recalculated the models using the two different types of MCI (with and without memory impairment) as the starting point.
RESULTS
Missing data analyses
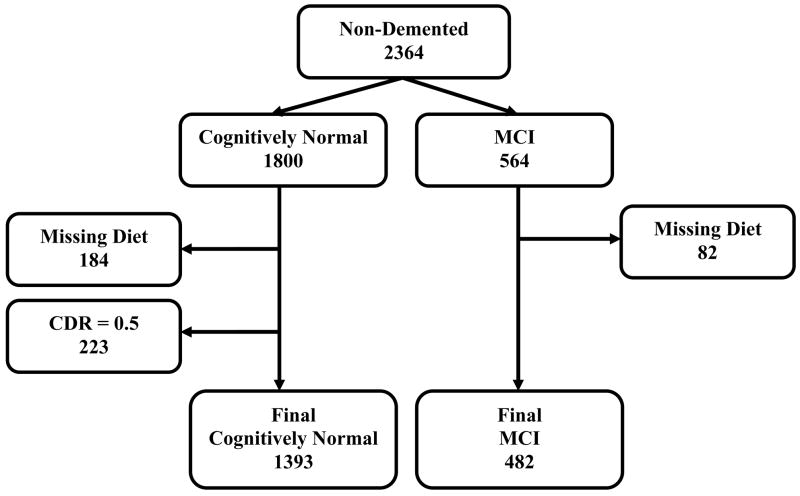
Among all potential participants in both WHICAP cohorts, 2364 individuals were considered for the present study because of sufficient data for MCI diagnosis and available follow-up 14 (figure 1). In comparison to 1329 subjects who were not considered because of missing data or lack of follow-up, these 2364 subjects were slightly younger, more likely to be women, had higher cognitive performance, while they did not differ in ethnicity. The above have been previously reported in detail 14. Among the 2364 subjects, 1800 were cognitively normal (non-demented, non-MCI) at initial evaluation (serving for calculation of the effect of MeDi on incidence of MCI) and 564 were diagnosed with MCI at initial evaluation (serving for calculation of the effect of MeDi on MCI conversion to AD).
Figure 1.
Flow chart describing sample size.
Cognitively normal to incident MCI analyses (figure 1)
Among the 1800 subjects who were cognitively normal at initial evaluation dietary information was missing for 184 (partially because the dietary assessment component was added after initiation of the study). In order to have an even cleaner sample for the incident MCI analyses we additionally excluded 223 subjects who despite not meeting our MCI criteria, had a rating of CDR = 0.5 at the 1st evaluation. Compared to the 184 subjects with missing dietary information, the 1393 cognitively normal subjects (with CDR=0 and available dietary information) used for the incident MCI analyses had lower BMI (27.5 vs. 28.5; p=0.02) and borderline higher cognitive performance (composite cognitive z-score 0.46 vs. 0.37; p=0.05). The groups did not differ in gender, age, ethnicity, education, or APOE status. As expected, compared to the 223 subjects with CDR=0.5, the 1393 cognitively normal subjects (with CDR=0 and available dietary information) used for the incident MCI analyses were younger (76.7 vs. 79.0; p<0.001), more educated (10.8 vs. 6.8 years; p<0.001), had higher cognitive performance (composite cognitive z-score 0.46 vs. −0.46; p<0.001) and were more likely to be Whites (31% vs. 11%) and less likely to be Hispanics (34% vs. 60%; p<0.001). The groups did not differ in gender, bmi, or APOE status.
MCI to incident AD analyses (figure 1)
Compared to the 82 MCI subjects with missing dietary information, the 482 MCI subjects with available dietary information used included for the incident AD analyses had borderline higher education (9.1 vs. 8.0 years of school; p=0.05) and borderline higher cognitive performance (composite cognitive z-score −0.27 vs. −0.40; p=0.05). The groups did not differ in gender, age, ethnicity, APOE status or BMI.
Clinical-demographic-dietary characteristics
At 1st evaluation, compared with subjects who were cognitively normal, MCI subjects were older, less educated, more likely to be Hispanic and less likely to be White (table 1). Cognitively normal and MCI subjects did not differ in gender, APOE genotype, BMI, total caloric intake and MeDi adherence.
Table 1.
Demographic and clinical characteristics during 1st evaluation for all subjects.
| Cognitively Normal N = 1393 | MCI N = 482 | All N = 1875 | P | |
|---|---|---|---|---|
| Gender-Men N (%) | 447 (32) | 156 (32) | 603 (32) | 0.91 |
| Age yrs, mean (SD) | 76.7 (6.48) | 77.5 (6.58) | 76.9 (6.5) | 0.02 |
| Ethnicity, N (%) White | 434 (31) | 124 (26) | 558 (30) | <0.001 |
| Black | 479 (34) | 144 (30) | 623 (33) | |
| Hispanic | 473 (34) | 214 (44) | 687 (36) | |
| Other | 7 (1) | 0 (0) | 7 (1) | |
| Education yrs, mean (SD) | 10.8 (4.6) | 9.1 (4.9) | 10.4 (4.7) | <0.001 |
| At least 1 ε4, N (%) | 327 (27) | 127 (30) | 454 (28) | 0.18 |
| BMI, mean (SD) | 27.5 (5.49) | 27.2 (5.28) | 27.4 (5.4) | 0.34 |
| Energy (kcal), mean (SD) | 1426.1 (498.0) | 1421.8 (591.9) | 1425.0 (523.6) | 0.88 |
| MeDi Score, mean (SD) | 4.37 (1.69) | 4.31 (1.62) | 4.36 (1.67) | 0.47 |
Hispanics adhered more to the MeDi, Blacks less, while Whites were in between (table 2). The higher the MeDi adherence the lower the total caloric intake. There was no association between MeDi score and age, gender, education, APOE genotype or BMI.
Table 2.
Demographic and clinical characteristics during 1st evaluation for all subjects by MeDi tertiles.
| Low MeDi N = 609 | Middle MeDi N = 775 | High MeDi N = 491 | P | |
|---|---|---|---|---|
| Gender-Men N (%) | 442 (69) | 532 (69) | 318 (65) | 0.23 |
| Age yrs, mean (SD) | 76.9 (6.6) | 76.8 (6.5) | 77.2 (6.2) | 0.48 |
| Ethnicity, N (%) White | 182 (30) | 223 (29) | 153 (31) | 0.001 |
| Black | 234 (38) | 260 (33) | 139 (26) | |
| Hispanic | 190 (31) | 289 (37) | 208 (42) | |
| Other | 3 (1) | 3 (1) | 1 (1) | |
| At least 1 ε4, N (%) | 146 (27) | 179 (27) | 129 (29) | 0.70 |
| Education yrs, mean (SD) | 10.5 (4.5) | 10.3 (4.7) | 10.4 (4.9) | 0.77 |
| BMI, mean (SD) | 27.7 (5.8) | 27.4 (5.2) | 27.1 (5.3) | 0.19 |
| Energy (kcal), mean (SD) | 1494.9 (608.2)* | 1395.4 (472.5)* | 1385.1 (477.4)* | <0.001 |
p<0.05 for all MeDi tertiles according to post-hoc Scheffe’s test.
MeDi and risk for incident MCI
Despite somewhat lower scores in MCI subjects (as compared to cognitively normal) at baseline (table 1) the groups did not significantly differ cross-sectionally. Longitudinal analyses (particularly with use of survival models) is a much more powerful method of detecting predictor differences since they can combine in a single statistic two different risk dimensions: (i) proportion of subjects who reach an event (convert to MCI or AD) and (ii) time duration (how soon subjects convert or non-convert to MCI or AD).
Subjects who were cognitively normal at 1st evaluation were followed (until MCI incidence or last follow-up for subjects who remained cognitively normal) for an average of 4.5 (± 2.7, 0.9–16.4) years. Overall, 275 subjects developed incident MCI.
Higher adherence to the MeDi was associated with a borderline trend for lower risk of developing MCI (table 3 and figure 2). The results were similar in adjusted and unadjusted models. Each additional unit of the MeDi score was associated with 8% (p=0.04) less risk of developing MCI. Compared to subjects in the lowest MeDi adherence tertile, subjects in the middle MeDi tertile had 17 % (p = 0.24) less risk of developing MCI, while those at the highest tertile (high adherence to the MeDi) had 28 % (p = 0.05) less risk of developing MCI. Among the other covariates in the model younger age and higher education were the only protective factors for development of MCI.
Table 3.
Cox Proportional Hazard Ratios for incidence of MCI for subjects cognitively normal at 1st evaluation by MeDi score. Adjusted models include slightly lower number of subjects because of missing data in some of the covariates. Adjusted models simultaneously control for cohort, age, gender, ethnicity, education, APOE genotype, caloric intake, body mass index and time between 1st dietary and 1st cognitive assessment.
| Predictor | HR | 95 % CI | P | |
|---|---|---|---|---|
| Unadjusted: Cognitively Normal 1st evaluation ( N = 1393) Incident MCI (N = 275) | ||||
| MeDi continuous | 0.93 | 0.87 | 1.00 | 0.06 |
|
| ||||
| Low MeDi tertile | 1 (ref) | - | - | - |
| Middle MeDi tertile | 0.87 | 0.66 | 1.14 | 0.33 |
| High MeDi tertile | 0.73 | 0.53 | 1.00 | 0.05 |
|
| ||||
| MeDi tertile trend | 0.85 | 0.73 | 1.00 | 0.05 |
| Adjusted: Cognitively Normal 1st evaluation ( N = 1199) Incident MCI (N = 241) | ||||
| MeDi continuous | 0.92 | 0.85 | 0.99 | 0.04 |
|
| ||||
| Low MeDi tertile | 1 (ref) | - | - | - |
| Middle MeDi tertile | 0.83 | 0.62 | 1.12 | 0.24 |
| High MeDi tertile | 0.72 | 0.52 | 1.00 | 0.05 |
|
| ||||
| MeDi tertile trend | 0.85 | 0.72 | 1.00 | 0.05 |
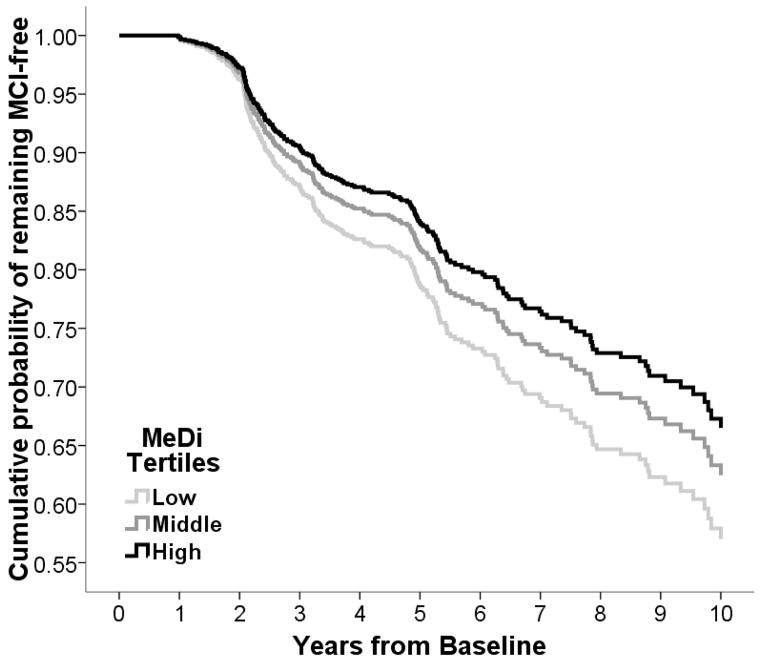
Figure 2.
Survival curves based on Cox analysis comparing cumulative MCI incidence in subjects cognitively normal at 1st evaluation by each Mediterranean diet (MeDi) tertile (p for trend 0.05). The figure is derived from a model that is adjusted for cohort, age, gender, ethnicity, education, APOE genotype, caloric intake, body mass index and time between 1st dietary and 1st cognitive assessment. Duration of follow-up is truncated at 10 years. Log-rank test for pair-wise comparisons: middle vs. low MeDi tertile x2 = 0.91, p = 0.33, low vs. high MeDi tertile x2 = 3.72, p = 0.05, middle vs. high x2 = 1.22, p = 0.26.
The effect of MeDi on development of different MCI subtypes is subject to power limitations, but it is still worth exploring. Overall 108 subjects developed incident MCI with memory impairment. In fully adjusted models, as compared to subjects in the lowest MeDi tertile, those in the middle MeDi tertile had a HR 0.90 (95% CI; 0.57 – 1.40; p = 0.64) for developing MCI with memory impairment, while those at the highest MeDi tertile had a HR 0.71 (95% CI: 0.43 – 1.17; p = 0.18). The overall MeDi HR trend was 0.84 (0.66 – 1.03); p for trend 0.18. MCI without memory impairment was developed by 133 subjects. In fully adjusted models, as compared to subjects in the lowest MeDi tertile, those in the middle MeDi tertile had a HR 0.79 (95% CI; 0.53 – 1.19; p = 0.27) for developing MCI without memory impairment, while those at the highest MeDi tertile had a HR 0.71 (95% CI: 0.46 – 1.12; p = 0.14). The overall MeDi HR trend was 0.84 (0.67 – 1.05); p for trend 0.13.
MeDi and Risk for MCI conversion to AD
Subjects with MCI at 1st evaluation were followed (until AD incidence or last follow-up for subjects who did not develop AD) for an average of 4.3 (± 2.7, 1.0 – 13.8) years. Overall, 106 subjects developed AD.
Higher adherence to the MeDi was associated with lower risk of developing AD (table 4 and figure 3). The results were similar in adjusted and unadjusted models. Each additional unit of the MeDi score was associated with 11% (p=0.09) less risk of developing AD. Compared to subjects in the lowest MeDi adherence tertile, subjects in the middle MeDi tertile had 45 % (p = 0.01) less risk of developing AD, while those at the highest tertile (high adherence to the MeDi) had 48 % (p = 0.02) less risk of developing AD. Among the other covariates in the model younger age, higher education and higher BMI were the only protective factors for development of AD.
Table 4.
Cox Proportional Hazard Ratios for incidence of AD for subjects with MCI at the 1st evaluation by MeDi score. Adjusted models include slightly lower number of subjects because of missing data in some of the covariates. Adjusted models simultaneously control for cohort, age, gender, ethnicity, education, APOE genotype, caloric intake, body mass index and time between 1st dietary and 1st cognitive assessment.
| Predictor | HR | 95 % CI | P | |
|---|---|---|---|---|
| Unadjusted: MCI 1st evaluation ( N = 482) Incident AD ( N = 106) | ||||
| MeDi continuous | 0.95 | 0.85 | 1.07 | 0.48 |
|
| ||||
| Low MeDi tertile | 1 (ref) | - | - | - |
| Middle MeDi tertile | 0.62 | 0.39 | 0.98 | 0.04 |
| High MeDi tertile | 0.69 | 0.41 | 1.14 | 0.15 |
|
| ||||
| MeDi tertile trend | 0.82 | 0.63 | 1.07 | 0.15 |
| Adjusted: MCI 1st evaluation ( N = 409) Incident AD ( N = 96) | ||||
| MeDi continuous | 0.89 | 0.78 | 1.02 | 0.09 |
|
| ||||
| Low MeDi tertile | 1 (ref) | - | - | - |
| Middle MeDi tertile | 0.55 | 0.34 | 0.90 | 0.01 |
| High MeDi tertile | 0.52 | 0.30 | 0.91 | 0.02 |
|
| ||||
| MeDi tertile trend | 0.71 | 0.53 | 0.95 | 0.02 |
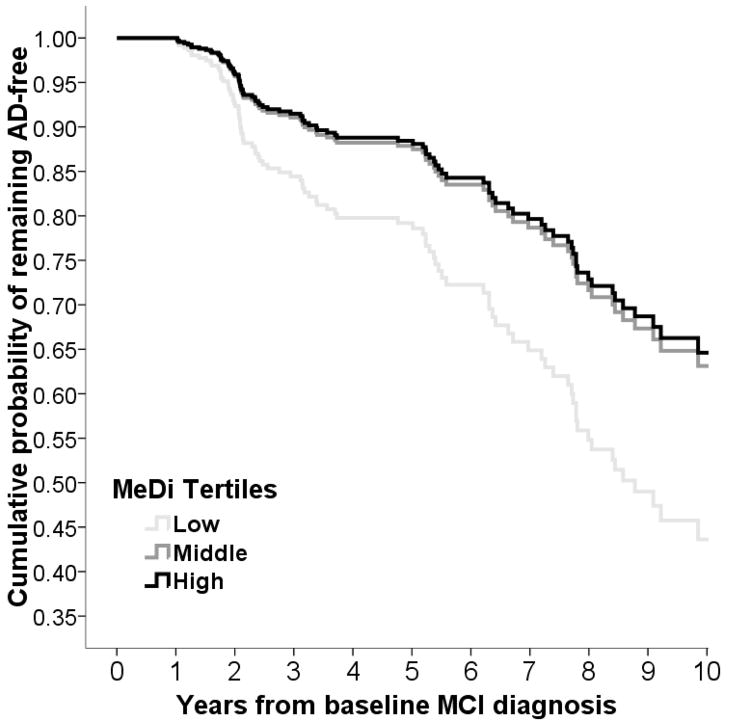
Figure 3.
Survival curves based on Cox analysis comparing cumulative AD incidence in subjects with MCI at 1st evaluation by Mediterranean diet (MeDi) tertile (p for trend 0.02). The figure is derived from a model that is adjusted for cohort, age, gender, ethnicity, education, APOE genotype, caloric intake, body mass index and time between 1st dietary and 1st cognitive assessment. Duration of follow-up is truncated at 10 years. Log-rank test for pair-wise comparisons was as follows: middle vs. low MeDi tertile x2 = 4.2, p = 0.03, low vs. high MeDi tertile x2 = 1.3, p = 0.23, middle vs. high x2 = 0.12, p = 0.72.
At 1st evaluation 175 subjects had MCI with memory impairment and 49 of them developed AD at follow-up. In fully adjusted models, as compared to MCI subjects with memory impairment belonging to the lowest MeDi tertile, MCI subjects with memory impairment in the middle MeDi tertile had a HR 0.48 (95% CI; 0.22 – 1.04; p = 0.06) for developing AD, while those MCI subjects with memory impairment at the highest MeDi tertile had a HR 0.71 (95% CI: 0.32 – 1.59; p = 0.41). The overall MeDi HR trend was 0.84 (0.55 – 1.29) p for trend 0.45. At 1st evaluation 234 subjects had MCI without memory impairment and 47 of them developed AD at follow-up. In fully adjusted models, as compared to MCI subjects without memory impairment belonging to the lowest MeDi tertile, MCI subjects without memory impairment in the middle MeDi tertile had a HR 0.49 (95% CI; 0.24 – 1.01; p = 0.05) for developing AD, while those MCI subjects without memory impairment at the highest MeDi tertile had a HR 0.25 (95% CI: 0.10 – 0.63; p = 0.003). The overall MeDi HR trend was 0.50 (0.35 – 0.79); p for trend 0.003.
Supplementary analyses
Considering the possibility of an overall healthier lifestyle behavior accounting for the MeDi effect, we considered a measure of overall comorbidity in the analyses: a modified version32, 33 of the Charlson Index of Comorbidity 34. This measure includes items for myocardial infarct, congestive heart failure, peripheral vascular disease, hypertension, chronic renal disease, chronic obstructive pulmonary disease, arthritis, gastrointestinal disease, mild liver disease, diabetes, and systemic malignancy. There was no difference in comorbidities between subjects who remained cognitively normal during follow-up and those who developed MCI during follow-up (1.9 vs. 1.9; p = 0.91) or between those who had MCI at baseline and those who developed AD during follow-up (2.0 vs. 2.1; p = 0.66). Similarly, the comorbidity index did not differ among different levels of MeDi adherence: 1.9 for lowest, 1.9 for middle and 1.9 for highest adherence tertiles; p = 0.75). Including the comorbidity index (simultaneously with all the other covariates) in the incident MCI analyses did not appreciably change the results: middle MeDi adherence tertile HR 0.83 [0.61–1.12], p=0.23; highest tertile HR 0.74 [0.53–1.04], p=0.08; overall MeDi tertile HR trend 0.86 [0.72–1.02], p for trend=0.08. Including the comorbidity index (simultaneously with all the other covariates) in the incident AD analyses produced similar results: middle tertile HR 0.59 [0.36–0.97], p=0.04, highest tertile HR 0.56 [0.32–0.97], p=0.04; overall MeDi tertile HR trend 0.74 [0.55–0.98], p for trend=0.04.
In additional supplementary analyses we used age (rather than duration from cognitive assessment) as the time-to-event variable. In these analyses the directionality of the effect was similar but the strength was significantly attenuated: incident MCI HR MeDi continuous 0.94 [0.87–1.0], p=0.09; MeDi middle tertile 0.91 [0.69–1.19], p=0.49; MeDi highest tertile 0.76 [0.56–1.04], p=0.09; MeDi tertile trend 0.87 [0.75–1.02], p for trend=0.09.
DISCUSSION
This study suggests that higher adherence to the MeDi is associated with (i) a borderline reduction in risk for developing MCI and (ii) a reduction in risk for conversion from MCI to AD. The gradual reduction in risks for higher tertiles of MeDi adherence also suggests a possible dose-response effect. The associations between MeDi and risk for development of MCI and of MCI conversion to AD did not attenuate even when simultaneously adjusting for many commonly considered potential confounders, such as age, gender, ethnicity, education, APOE genotype, caloric intake and BMI. Adherence to MeDi did not seem to differentially affect risk for development of MCI with or without memory impairment. The association between higher adherence to the MeDi and lower risk for conversion to AD was much more prominent for MCI subjects without memory impairment.
Higher adherence to the MeDi has been related to lower risk for AD 4, 5. MCI has been described as a predictor or a transitional stage between normal cognition and AD 35, 36. Thus, we expected that higher adherence to the MeDi would be related to MCI. The association between MeDi and MCI incidence was similar for MCI with and without memory impairment, while the protective MeDi effect for AD conversion was stronger for MCI subjects without memory impairment. Vascular comorbidity, including diabetes, hypertension, dyslipidemia and white matter abnormalities have been related to MCI 37–47 and it has been proposed that non-amnestic MCI in particular may be related to cerebrovascular disease 35, 36, 48. There is an increasing recognition of vascular comorbidity regarding AD risk 49, 50 and there is strong evidence relating the MeDi to lower risk of vascular risk factors such as dyslipidemia 51, 52, hypertension 51–54 and coronary heart disease 3, 52, 55, 56. Therefore the stronger effect of the MeDi for non-memory MCI conversion rates to AD may relate to underlying vascular mechanisms. Nevertheless, non-vascular biological mediating mechanisms (i.e. metabolic, oxidative and inflammatory) may also potentially mediate the epidemiological MeDi MCI associations4.
Both MCI and the MeDi have been associated with metabolic abnormalities. MCI has been linked to dysregulation of glucose and insulin homeostasis 57–61 and diabetes 37–41. At the same time, according to the vast majority of the literature (but see 62, 63), higher adherence to the MeDi seems to improve carbohydrate metabolism and in interventional studies it has been associated with significant reductions in plasma glucose 52, 54, serum insulin and insulin resistance 54, 64.
Oxidative stress could be another biological mechanisms relating MCI and the MeDi. Higher oxidative stress has been clearly invoked in MCI 65–70. Complex phenols and many other substances with important antioxidant properties such as olive oil 71, 72, wine, fruits and vegetables, vitamins C, E and carotenoids 73–78 are found in high concentrations in the typical components of the MeDi 79. Typical Mediterranean meals 80 or meals rich in typical Mediterranean food elements 81, 82 have been shown to increase enzymes with antioxidant properties such as paroxonase and plasma carotenoids 80. Intervention studies with Mediterranean-type foods, have indicated significant reductions of markers of oxidative stress, such as isoprostanes 79, 83.
Finally, the protective effect of the MeDi for MCI may be mediated via inflammatory pathways. Links between MCI and higher inflammatory states have been demonstrated 84–88. Two small studies reported no effect of MeDi on inflammatory markers such as CRP 62, 63 or IL-6 62. Higher adherence to the MeDi has been associated with lower CRP in multiple large both observational 51, 89, 90 and interventional 54, 80 studies. As another example, tyrosol and caffeic acid, both found in extra virgin olive oil and in wine, (which are essential components of the MeDi), have been shown to significantly reduce IL-6 production from peripheral blood mononuclear cells of healthy volunteers 91. Higher adherence to the MeDi has been associated with lower IL-6 levels in both observational 51 90 and interventional 54 studies. Higher adherence to the MeDi is in general associated with significant reduction in a series of other inflammatory markers including white blood cell counts etc 51.
The potentially beneficial effect of the MeDi may be the result of some of its individual food components. For example, potentially beneficial effects for MCI or MCI conversion to AD have been reported for alcohol 7, 11, fish 10, PUFA 10, 11 (also for age-related cognitive decline 8), and lower SFA 10. Interestingly, in other studies alcohol 7, 12, PUFA 6 or other nutrients such as vitamin E 92, 93 have failed to be associated with protection for MCI or MCI conversion to AD. Differences in the definition of the outcome (MCI [objective cognitive cutoffs in different cognitive domains + memory complaint + absence of functional impairment + absence of dementia] vs. age-related cognitive decline [only a particular cognitive cutoff on a summary cognitive score such as the MMSE]) may partially account for the discrepancies. Nevertheless, it is also possible that a composite dietary pattern such as the MeDi may better capture nutritional dimensions that may be missed by single nutrients (i.e. potential additive and interactive effects among nutritional components).
Although Hispanics reported higher adherence to the MeDi they also have higher risk for MCI 14 and AD 94. A particular ethnic group may have a mixture of multiple protective and risk factors, the overall interaction of which determine the probability of developing a complex disease, such as AD. For example, the Hispanics may be placed at risk by their low education and by their SORL1 gene status 95, while they may be protected by their dietary habits and by the lack of detrimental effect of the APOE genotype 94. At the same time there may be multiple other genes or behavioral traits unique to the Hispanics that may contribute to AD, resulting in an overall higher risk in this ethnic group.
Study limitations regarding duration of follow-up, demographics of subjects with either missing data for MCI diagnostic assignment or missing follow-up have been discussed in detail in a previous publication 14. Subjects excluded from the present analyses because of missing dietary information had slightly lower cognitive performance, were less educated and had higher BMI. Worse cognition and lower educational level indicate that these subjects were more likely at higher risk for MCI and AD, but higher BMI indicates the opposite. Most important, these subjects did not differ in most other characteristics. Potential confounding from associations between adherence to the MeDi and total caloric intake or ethnicity was addressed by adjusting for these factors. Limitations, relating to the construction of the MeDi score (i.e. use of an a priori dietary pattern score, equal weighing of underlying food categories, underestimation of total food and caloric intake etc) have been discussed in detail in previous publications 4, 5. All models were adjusted for total caloric intake (largely determined by (i) metabolic efficiency, (ii) BMI and (iii) physical activity). Given that metabolic efficiency is unmeasurable and that BMI was included as a covariate, when we adjust for total caloric intake we essentially adjust for physical activity29. Nevertheless, we cannot completely exclude the possibility that physical activity may partially account for some of the MeDi’s effect. Although adherence to the MeDi was not related to education or to overall level of medical comorbidities in our data, it is possible that a better diet is related to higher socioeconomic status or to other habits or characteristics related to better health. Therefore, despite adjusting for multiple variables the study is observational and we cannot completely exclude residual confounding or ‘healthy person bias’ (that can be only addressed via the randomization of an interventional study). In conservative (for the study size and follow-up) models using age as the timing variable of survival models the associations were significantly attenuated. Although age was not related to MeDi we cannot completely exclude the possibility that the noted associations are confounded by age. Finally, because the current study had started before the concept of MCI was developed, diagnosis of MCI was retrospectively applied in already collected data (rather than being applied synchronously with the diagnostic consensus conference).
Because of the synchronous timing of dietary and cognitive assessments and the relatively short follow-up we cannot completely exclude reverse causation (i.e. dietary habits being affected by cognitive status rather than the opposite). Nevertheless, in two previous publications, using a subset of 390 subjects with repeated (2 – 4) dietary assessments over a course of ~8 (and up to 13) years, we demonstrated that adherence to the MeDi is remarkably stable over time 4, 5. Therefore, we consider it more likely that the MeDi adherence reported reflects our population’s longstanding dietary habits. Finally, since this is the 1st study demonstrating an association between the MeDi and MCI, replication in other populations is necessary.
Confidence in our findings is strengthened by the following factors. The study is community-based and the population is multiethnic, increasing the external validity of the findings. Dietary data were collected with an instrument that has been previously validated and has been used widely in epidemiological studies. The diagnosis of MCI and AD took place in a University hospital with expertise in such disorders and was based on comprehensive assessment and standard research criteria. The patients were followed prospectively at relatively short intervals. Measures for multiple potential confounders were carefully recorded and adjusted for in the analyses.
Overall, the effects of dietary habits in MCI have not been adequately explored. These results provide support that MeDi-type habits may affect risk for both developing MCI for MCI conversion to AD. Possible biological mechanisms underlying this association remain to be explored. Exploration of such mechanisms and potential future interventional studies will provide a more complete and convincing picture of the conceivably important role of a healthy diet in risk of cognitive impairment and AD.
Acknowledgments
Federal NIA grants AG028506, P01-AG07232, RR00645.
Footnotes
Disclosure: The authors report no conflicts of interest.
References
- 1.Luchsinger JA, Mayeux R. Dietary factors and Alzheimer’s disease. Lancet Neurol. 2004 Nov;3(10):579–587. doi: 10.1016/S1474-4422(04)00878-6. [DOI] [PubMed] [Google Scholar]
- 2.Luchsinger JA, Noble JM, Scarmeas N. Diet and Alzheimer’s Disease. Curr Neurol Neurosci Rep. 2007 Sep;7(5):366–372. doi: 10.1007/s11910-007-0057-8. [DOI] [PubMed] [Google Scholar]
- 3.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003 Jul 26;348(26):2599–2608. doi: 10.1056/NEJMoa025039. [DOI] [PubMed] [Google Scholar]
- 4.Scarmeas N, Stern Y, Mayeux R, Luchsinger JA. Mediterranean diet, Alzheimer disease, and vascular mediation. Arch Neurol. 2006 Dec;63(12):1709–1717. doi: 10.1001/archneur.63.12.noc60109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scarmeas N, Stern Y, Tang MX, Mayeux R, Luchsinger JA. Mediterranean diet and risk for Alzheimer’s disease. Ann Neurol. 2006 Apr 18;59(6):912–921. doi: 10.1002/ana.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solfrizzi V, Colacicco AM, D’Introno A, et al. Dietary fatty acids intakes and rate of mild cognitive impairment. The Italian Longitudinal Study on Aging. Exp Gerontol. 2006 Jun;41(6):619–627. doi: 10.1016/j.exger.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 7.Solfrizzi V, D’Introno A, Colacicco AM, et al. Alcohol consumption, mild cognitive impairment, and progression to dementia. Neurology. 2007 May 22;68(21):1790–1799. doi: 10.1212/01.wnl.0000262035.87304.89. [DOI] [PubMed] [Google Scholar]
- 8.Solfrizzi V, Panza F, Torres F, et al. High monounsaturated fatty acids intake protects against age-related cognitive decline. Neurology. 1999 May 12;52(8):1563–1569. doi: 10.1212/wnl.52.8.1563. [DOI] [PubMed] [Google Scholar]
- 9.Panza F, Solfrizzi V, Colacicco AM, et al. Mediterranean diet and cognitive decline. Public Health Nutr. 2004 Oct;7(7):959–963. doi: 10.1079/phn2004561. [DOI] [PubMed] [Google Scholar]
- 10.Eskelinen MH, Ngandu T, Helkala EL, et al. Fat intake at midlife and cognitive impairment later in life: a population-based CAIDE study. Int J Geriatr Psychiatry. Jan 10 2008; doi: 10.1002/gps.1969. [DOI] [PubMed] [Google Scholar]
- 11.Anttila T, Helkala EL, Viitanen M, et al. Alcohol drinking in middle age and subsequent risk of mild cognitive impairment and dementia in old age: a prospective population based study. BMJ. 2004 Sep 4;329(7465):539. doi: 10.1136/bmj.38181.418958.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Espeland MA, Gu L, Masaki KH, et al. Association between Reported Alcohol Intake and Cognition: Results from the Women’s Health Initiative Memory Study. Am J Epidemiol. 2005 February 1, 2005;161(3):228–238. doi: 10.1093/aje/kwi043. [DOI] [PubMed] [Google Scholar]
- 13.Manly JJ, Bell-McGinty S, Tang MX, Schupf N, Stern Y, Mayeux R. Implementing diagnostic criteria and estimating frequency of mild cognitive impairment in an urban community. Arch Neurol. 2005 Nov;62(11):1739–1746. doi: 10.1001/archneur.62.11.1739. [DOI] [PubMed] [Google Scholar]
- 14.Manly JJ, Tang MX, Schupf N, Stern Y, Vonsattel JP, Mayeux R. Frequency and course of mild cognitive impairment in a multiethnic community. Ann Neurol. 2008 Apr;63(4):494–506. doi: 10.1002/ana.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scarmeas N, Levy G, Tang MX, Manly J, Stern Y. Influence of leisure activity on the incidence of Alzheimer’s disease. Neurology. 2001 Dec 26;57(12):2236–2242. doi: 10.1212/wnl.57.12.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Golden RR, Teresi JA, Gurland BJ. Development of indicator scales for the Comprehensive Assessment and Referral Evaluation (CARE) interview schedule. 1984 Mar;:138–146. doi: 10.1093/geronj/39.2.138. [DOI] [PubMed] [Google Scholar]
- 17.Gurland B, Kuriansky J, Sharpe L, Simon R, Stiller P, Birkett P. The Comprehensive assessment and Referral Evaluation (CARE)--rationale, development and reliability. Int J Aging Hum Dev. 1977;8(1):9–42. doi: 10.2190/cl3j-0e20-97xx-mv5l. [DOI] [PubMed] [Google Scholar]
- 18.Stern Y, Andrews H, Pittman J, et al. Diagnosis of dementia in a heterogeneous population. Development of a neuropsychological paradigm-based diagnosis of dementia and quantified correction for the effects of education. Arch Neurol. 1992;49(5):453–460. doi: 10.1001/archneur.1992.00530290035009. [DOI] [PubMed] [Google Scholar]
- 19.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 20.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 21.Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001 Dec;58(12):1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 22.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114(512):797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 23.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 24.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 25.Luchsinger JA, Tang MX, Shea S, Mayeux R. Caloric intake and the risk of Alzheimer disease. Arch Neurol. 2002 Aug;59(8):1258–1263. doi: 10.1001/archneur.59.8.1258. [DOI] [PubMed] [Google Scholar]
- 26.Luchsinger JA, Tang MX, Shea S, Mayeux R. Antioxidant vitamin intake and risk of Alzheimer disease. Arch Neurol. 2003 Mar;60(2):203–208. doi: 10.1001/archneur.60.2.203. [DOI] [PubMed] [Google Scholar]
- 27.Luchsinger JA, Tang MX, Siddiqui M, Shea S, Mayeux R. Alcohol intake and risk of dementia. J Am Geriatr Soc. 2004 Apr;52(4):540–546. doi: 10.1111/j.1532-5415.2004.52159.x. [DOI] [PubMed] [Google Scholar]
- 28.Scarmeas N, Luchsinger JA, Mayeux R, Stern Y. Mediterranean diet and Alzheimer disease mortality. Neurology. 2007 Sep 11;69(11):1084–1093. doi: 10.1212/01.wnl.0000277320.50685.7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willett W, Stampfer M. Implications of total energy intake for epidemiological analyses. In: Willett W, editor. Nutritional Epidemiology. New York: Oxford University Press; 1998. pp. 273–301. [Google Scholar]
- 30.Kuczmarski R, Carroll M, Flegal K, Troiano R. Varying body mass index cutoff points to describe overweight prevalence among U.S. adults: NHANES III (1988 to 1994) Obes Res. 1997 November 1, 1997;5(6):542–548. doi: 10.1002/j.1550-8528.1997.tb00575.x. [DOI] [PubMed] [Google Scholar]
- 31.Census of Population and Housing. Summary Tape File1, Technical Documentation. Washington, DC: Bureau of the consensus; 1991. [Google Scholar]
- 32.Scarmeas N, Brandt J, Albert M, et al. Delusions and Hallucinations Are Associated With Worse Outcome in Alzheimer Disease. Arch Neurol. 2005 Oct;62(10):1601–1608. doi: 10.1001/archneur.62.10.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scarmeas N, Albert M, Brandt J, et al. Motor signs predict poor outcomes in Alzheimer disease. Neurology. 2005 May 24;64(10):1696–1703. doi: 10.1212/01.WNL.0000162054.15428.E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 35.Petersen RC. Mild cognitive impairment: current research and clinical implications. Semin Neurol. 2007 Feb;27(1):22–31. doi: 10.1055/s-2006-956752. [DOI] [PubMed] [Google Scholar]
- 36.Luis CA, Loewenstein DA, Acevedo A, Barker WW, Duara R. Mild cognitive impairment: directions for future research. Neurology. 2003 Aug 26;61(4):438–444. doi: 10.1212/01.wnl.0000080366.90234.7f. [DOI] [PubMed] [Google Scholar]
- 37.Yaffe K, Blackwell T, Whitmer RA, Krueger K, Barrett Connor E. Glycosylated hemoglobin level and development of mild cognitive impairment or dementia in older women. J Nutr Health Aging. 2006 Jul-Aug;10(4):293–295. [PubMed] [Google Scholar]
- 38.Yaffe K, Krueger K, Cummings SR, et al. Effect of raloxifene on prevention of dementia and cognitive impairment in older women: the Multiple Outcomes of Raloxifene Evaluation (MORE) randomized trial. Am J Psychiatry. 2005 Apr;162(4):683–690. doi: 10.1176/appi.ajp.162.4.683. [DOI] [PubMed] [Google Scholar]
- 39.Yaffe K, Blackwell T, Kanaya AM, Davidowitz N, Barrett-Connor E, Krueger K. Diabetes, impaired fasting glucose, and development of cognitive impairment in older women. Neurology. 2004 Aug 24;63(4):658–663. doi: 10.1212/01.wnl.0000134666.64593.ba. [DOI] [PubMed] [Google Scholar]
- 40.Das SK, Bose P, Biswas A, et al. An epidemiologic study of mild cognitive impairment in Kolkata, India. Neurology. 2007 Jun 5;68(23):2019–2026. doi: 10.1212/01.wnl.0000264424.76759.e6. [DOI] [PubMed] [Google Scholar]
- 41.Luchsinger JA, Reitz C, Patel B, Tang MX, Manly JJ, Mayeux R. Relation of diabetes to mild cognitive impairment. Arch Neurol. 2007 Apr;64(4):570–575. doi: 10.1001/archneur.64.4.570. [DOI] [PubMed] [Google Scholar]
- 42.Solomon A, Kareholt I, Ngandu T, et al. Serum cholesterol changes after midlife and late-life cognition: twenty-one-year follow-up study. Neurology. 2007 Mar 6;68(10):751–756. doi: 10.1212/01.wnl.0000256368.57375.b7. [DOI] [PubMed] [Google Scholar]
- 43.Tervo S, Kivipelto M, Hanninen T, et al. Incidence and risk factors for mild cognitive impairment: a population-based three-year follow-up study of cognitively healthy elderly subjects. Dement Geriatr Cogn Disord. 2004;17(3):196–203. doi: 10.1159/000076356. [DOI] [PubMed] [Google Scholar]
- 44.Lopez OL, Jagust WJ, Dulberg C, et al. Risk factors for mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 2. Arch Neurol. 2003 Oct;60(10):1394–1399. doi: 10.1001/archneur.60.10.1394. [DOI] [PubMed] [Google Scholar]
- 45.DeCarli C. Mild cognitive impairment: prevalence, prognosis, aetiology, and treatment. Lancet Neurol. 2003 Jan;2(1):15–21. doi: 10.1016/s1474-4422(03)00262-x. [DOI] [PubMed] [Google Scholar]
- 46.DeCarli C, Miller BL, Swan GE, Reed T, Wolf PA, Carmelli D. Cerebrovascular and brain morphologic correlates of mild cognitive impairment in the National Heart, Lung, and Blood Institute Twin Study. Arch Neurol. 2001 Apr;58(4):643–647. doi: 10.1001/archneur.58.4.643. [DOI] [PubMed] [Google Scholar]
- 47.Wolf H, Ecke GM, Bettin S, Dietrich J, Gertz HJ. Do white matter changes contribute to the subsequent development of dementia in patients with mild cognitive impairment? A longitudinal study. Int J Geriatr Psychiatry. 2000 Sep;15(9):803–812. doi: 10.1002/1099-1166(200009)15:9<803::aid-gps190>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 48.Gauthier S, Reisberg B, Zaudig M, et al. Mild cognitive impairment. Lancet. 2006 Apr 15;367(9518):1262–1270. doi: 10.1016/S0140-6736(06)68542-5. [DOI] [PubMed] [Google Scholar]
- 49.Breteler MM. Vascular risk factors for Alzheimer’s disease: an epidemiologic perspective. Neurobiol Aging. 2000;21(2):153–160. doi: 10.1016/s0197-4580(99)00110-4. [DOI] [PubMed] [Google Scholar]
- 50.Luchsinger J, Mayeux R. Cardiovascular risk factors and Alzheimer’s disease. Curr Atheroscler Rep. 2004;6(4):261–266. doi: 10.1007/s11883-004-0056-z. [DOI] [PubMed] [Google Scholar]
- 51.Chrysohoou C, Panagiotakos DB, Pitsavos C, Das UN, Stefanadis C. Adherence to the Mediterranean diet attenuates inflammation and coagulation process in healthy adults: The ATTICA Study. J Am Coll Cardiol. 2004 Jul 7;44(1):152–158. doi: 10.1016/j.jacc.2004.03.039. [DOI] [PubMed] [Google Scholar]
- 52.Singh RB, Dubnov G, Niaz MA, et al. Effect of an Indo-Mediterranean diet on progression of coronary artery disease in high risk patients (Indo-Mediterranean Diet Heart Study): a randomised single-blind trial. Lancet. 2002 Nov 9;360(9344):1455–1461. doi: 10.1016/S0140-6736(02)11472-3. [DOI] [PubMed] [Google Scholar]
- 53.Psaltopoulou T, Naska A, Orfanos P, Trichopoulos D, Mountokalakis T, Trichopoulou A. Olive oil, the Mediterranean diet, and arterial blood pressure: the Greek European Prospective Investigation into Cancer and Nutrition (EPIC) study. Am J Clin Nutr. 2004 October 1, 2004;80(4):1012–1018. doi: 10.1093/ajcn/80.4.1012. [DOI] [PubMed] [Google Scholar]
- 54.Esposito K, Marfella R, Ciotola M, et al. Effect of a Mediterranean-Style Diet on Endothelial Dysfunction and Markers of Vascular Inflammation in the Metabolic Syndrome: A Randomized Trial. JAMA. 2004 September 22, 2004;292(12):1440–1446. doi: 10.1001/jama.292.12.1440. [DOI] [PubMed] [Google Scholar]
- 55.de Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation. 1999 Feb 16;99(6):779–785. doi: 10.1161/01.cir.99.6.779. [DOI] [PubMed] [Google Scholar]
- 56.Knoops KT, de Groot LC, Kromhout D, et al. Mediterranean diet, lifestyle factors, and 10-year mortality in elderly European men and women: the HALE project. Jama. 2004 Sep 22;292(12):1433–1439. doi: 10.1001/jama.292.12.1433. [DOI] [PubMed] [Google Scholar]
- 57.Odetti P, Piccini A, Giliberto L, et al. Plasma levels of insulin and amyloid beta 42 are correlated in patients with amnestic Mild Cognitive Impairment. J Alzheimers Dis. 2005 Dec;8(3):243–245. doi: 10.3233/jad-2005-8303. [DOI] [PubMed] [Google Scholar]
- 58.Reger MA, Watson GS, Green PS, et al. Intranasal Insulin Administration Dose-Dependently Modulates Verbal Memory and Plasma Amyloid-beta in Memory-Impaired Older Adults. J Alzheimers Dis. 2008 Jun;13(3):323–331. doi: 10.3233/jad-2008-13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reger MA, Watson GS, Green PS, et al. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology. 2008 Feb 5;70(6):440–448. doi: 10.1212/01.WNL.0000265401.62434.36. [DOI] [PubMed] [Google Scholar]
- 60.Watson GS, Cholerton BA, Reger MA, et al. Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary study. Am J Geriatr Psychiatry. 2005 Nov;13(11):950–958. doi: 10.1176/appi.ajgp.13.11.950. [DOI] [PubMed] [Google Scholar]
- 61.Zhao Z, Xiang Z, Haroutunian V, Buxbaum JD, Stetka B, Pasinetti GM. Insulin degrading enzyme activity selectively decreases in the hippocampal formation of cases at high risk to develop Alzheimer’s disease. Neurobiol Aging. 2007 Jun;28(6):824–830. doi: 10.1016/j.neurobiolaging.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 62.Ambring A, Johansson M, Axelsen M, Gan L, Strandvik B, Friberg P. Mediterranean-inspired diet lowers the ratio of serum phospholipid n-6 to n-3 fatty acids, the number of leukocytes and platelets, and vascular endothelial growth factor in healthy subjects. Am J Clin Nutr. 2006 Mar;83(3):575–581. doi: 10.1093/ajcn.83.3.575. [DOI] [PubMed] [Google Scholar]
- 63.Michalsen A, Lehmann N, Pithan C, et al. Mediterranean diet has no effect on markers of inflammation and metabolic risk factors in patients with coronary artery disease. Eur J Clin Nutr. 2006 Apr;60(4):478–485. doi: 10.1038/sj.ejcn.1602340. [DOI] [PubMed] [Google Scholar]
- 64.Jula A, Marniemi J, Huupponen R, Virtanen A, Rastas M, Ronnemaa T. Effects of diet and simvastatin on serum lipids, insulin, and antioxidants in hypercholesterolemic men: a randomized controlled trial. Jama. 2002 Feb 6;287(5):598–605. doi: 10.1001/jama.287.5.598. [DOI] [PubMed] [Google Scholar]
- 65.Butterfield DA, Sultana R. Redox proteomics identification of oxidatively modified brain proteins in Alzheimer’s disease and mild cognitive impairment: insights into the progression of this dementing disorder. J Alzheimers Dis. 2007 Aug;12(1):61–72. doi: 10.3233/jad-2007-12107. [DOI] [PubMed] [Google Scholar]
- 66.Ding Q, Dimayuga E, Keller JN. Oxidative damage, protein synthesis, and protein degradation in Alzheimer’s disease. Curr Alzheimer Res. 2007 Feb;4(1):73–79. doi: 10.2174/156720507779939788. [DOI] [PubMed] [Google Scholar]
- 67.Keller JN, Schmitt FA, Scheff SW, et al. Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology. 2005 Apr 12;64(7):1152–1156. doi: 10.1212/01.WNL.0000156156.13641.BA. [DOI] [PubMed] [Google Scholar]
- 68.Lovell MA, Markesbery WR. Oxidative damage in mild cognitive impairment and early Alzheimer’s disease. J Neurosci Res. 2007 Nov 1;85(14):3036–3040. doi: 10.1002/jnr.21346. [DOI] [PubMed] [Google Scholar]
- 69.Mecocci P. Oxidative stress in mild cognitive impairment and Alzheimer disease: a continuum. J Alzheimers Dis. 2004 Apr;6(2):159–163. doi: 10.3233/jad-2004-6207. [DOI] [PubMed] [Google Scholar]
- 70.Rinaldi P, Polidori MC, Metastasio A, et al. Plasma antioxidants are similarly depleted in mild cognitive impairment and in Alzheimer’s disease. Neurobiol Aging. 2003 Nov;24(7):915–919. doi: 10.1016/s0197-4580(03)00031-9. [DOI] [PubMed] [Google Scholar]
- 71.Fito M, Cladellas M, de la Torre R, et al. Antioxidant effect of virgin olive oil in patients with stable coronary heart disease: a randomized, crossover, controlled, clinical trial. Atherosclerosis. 2005 Jul;181(1):149–158. doi: 10.1016/j.atherosclerosis.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 72.Alarcon de la Lastra C, Barranco MD, Motilva V, Herrerias JM. Mediterranean diet and health: biological importance of olive oil. Curr Pharm Des. 2001 Jul;7(10):933–950. doi: 10.2174/1381612013397654. [DOI] [PubMed] [Google Scholar]
- 73.Szeto YT, Tomlinson B, Benzie IF. Total antioxidant and ascorbic acid content of fresh fruits and vegetables: implications for dietary planning and food preservation. Br J Nutr. 2002 Jan;87(1):55–59. doi: 10.1079/BJN2001483. [DOI] [PubMed] [Google Scholar]
- 74.Joshipura KJ, Hu FB, Manson JE, et al. The effect of fruit and vegetable intake on risk for coronary heart disease. Ann Intern Med. 2001 Jun 19;134(12):1106–1114. doi: 10.7326/0003-4819-134-12-200106190-00010. [DOI] [PubMed] [Google Scholar]
- 75.Witztum JL. The oxidation hypothesis of atherosclerosis. Lancet. 1994 Sep 17;344(8925):793–795. doi: 10.1016/s0140-6736(94)92346-9. [DOI] [PubMed] [Google Scholar]
- 76.Byers T, Perry G. Dietary carotenes, vitamin C, and vitamin E as protective antioxidants in human cancers. Annu Rev Nutr. 1992;12:139–159. doi: 10.1146/annurev.nu.12.070192.001035. [DOI] [PubMed] [Google Scholar]
- 77.Owen RW, Haubner R, Wurtele G, Hull E, Spiegelhalder B, Bartsch H. Olives and olive oil in cancer prevention. Eur J Cancer Prev. 2004 Aug;13(4):319–326. doi: 10.1097/01.cej.0000130221.19480.7e. [DOI] [PubMed] [Google Scholar]
- 78.Stupans I, Kirlich A, Tuck KL, Hayball PJ. Comparison of radical scavenging effect, inhibition of microsomal oxygen free radical generation, and serum lipoprotein oxidation of several natural antioxidants. J Agric Food Chem. 2002 Apr 10;50(8):2464–2469. doi: 10.1021/jf0112320. [DOI] [PubMed] [Google Scholar]
- 79.Mancini M, Parfitt VJ, Rubba P. Antioxidants in the Mediterranean diet. Can J Cardiol. 1995 Oct;11(Suppl G):105G–109G. [PubMed] [Google Scholar]
- 80.Blum S, Aviram M, Ben-Amotz A, Levy Y. Effect of a Mediterranean meal on postprandial carotenoids, paraoxonase activity and C-reactive protein levels. Ann Nutr Metab. 2006;50(1):20–24. doi: 10.1159/000089560. [DOI] [PubMed] [Google Scholar]
- 81.Wallace AJ, Sutherland WH, Mann JI, Williams SM. The effect of meals rich in thermally stressed olive and safflower oils on postprandial serum paraoxonase activity in patients with diabetes. Eur J Clin Nutr. 2001 Nov;55(11):951–958. doi: 10.1038/sj.ejcn.1601250. [DOI] [PubMed] [Google Scholar]
- 82.Aviram M, Dornfeld L, Rosenblat M, et al. Pomegranate juice consumption reduces oxidative stress, atherogenic modifications to LDL, and platelet aggregation: studies in humans and in atherosclerotic apolipoprotein E-deficient mice. Am J Clin Nutr. 2000 May;71(5):1062–1076. doi: 10.1093/ajcn/71.5.1062. [DOI] [PubMed] [Google Scholar]
- 83.Sanchez-Moreno C, Cano MP, de Ancos B, et al. Mediterranean vegetable soup consumption increases plasma vitamin C and decreases F2-isoprostanes, prostaglandin E2 and monocyte chemotactic protein-1 in healthy humans. J Nutr Biochem. 2006 Mar;17(3):183–189. doi: 10.1016/j.jnutbio.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 84.Galimberti D, Fenoglio C, Lovati C, et al. Serum MCP-1 levels are increased in mild cognitive impairment and mild Alzheimer’s disease. Neurobiol Aging. 2006 Dec;27(12):1763–1768. doi: 10.1016/j.neurobiolaging.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 85.Galimberti D, Schoonenboom N, Scheltens P, et al. Intrathecal chemokine synthesis in mild cognitive impairment and Alzheimer disease. Arch Neurol. 2006 Apr;63(4):538–543. doi: 10.1001/archneur.63.4.538. [DOI] [PubMed] [Google Scholar]
- 86.Guerreiro RJ, Santana I, Bras JM, Santiago B, Paiva A, Oliveira C. Peripheral inflammatory cytokines as biomarkers in Alzheimer’s disease and mild cognitive impairment. Neurodegener Dis. 2007;4(6):406–412. doi: 10.1159/000107700. [DOI] [PubMed] [Google Scholar]
- 87.Magaki S, Mueller C, Dickson C, Kirsch W. Increased production of inflammatory cytokines in mild cognitive impairment. Exp Gerontol. 2007 Mar;42(3):233–240. doi: 10.1016/j.exger.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tarkowski E, Andreasen N, Tarkowski A, Blennow K. Intrathecal inflammation precedes development of Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2003 Sep;74(9):1200–1205. doi: 10.1136/jnnp.74.9.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Paschos GK, Rallidis LS, Liakos GK, et al. Background diet influences the anti-inflammatory effect of alpha-linolenic acid in dyslipidaemic subjects. Br J Nutr. 2004 Oct;92(4):649–655. doi: 10.1079/bjn20041230. [DOI] [PubMed] [Google Scholar]
- 90.Fung TT, McCullough ML, Newby P, et al. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. 2005 July 1, 2005;82(1):163–173. doi: 10.1093/ajcn.82.1.163. [DOI] [PubMed] [Google Scholar]
- 91.Bertelli AA, Migliori M, Panichi V, et al. Oxidative stress and inflammatory reaction modulation by white wine. Ann N Y Acad Sci. 2002 May;957:295–301. doi: 10.1111/j.1749-6632.2002.tb02929.x. [DOI] [PubMed] [Google Scholar]
- 92.Petersen RC, Thomas RG, Grundman M, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005 Jun 9;352(23):2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 93.Dunn JE, Weintraub S, Stoddard AM, Banks S. Serum alpha-tocopherol, concurrent and past vitamin E intake, and mild cognitive impairment. Neurology. 2007 Feb 27;68(9):670–676. doi: 10.1212/01.wnl.0000255940.13116.86. [DOI] [PubMed] [Google Scholar]
- 94.Tang MX, Stern Y, Marder K, et al. The APOE-epsilon4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. Journal of the American Medical Association. 1998;279(10):751–755. doi: 10.1001/jama.279.10.751. [DOI] [PubMed] [Google Scholar]
- 95.Rogaeva E, Meng Y, Lee JH, et al. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet. 2007 Feb;39(2):168–177. doi: 10.1038/ng1943. [DOI] [PMC free article] [PubMed] [Google Scholar]