Abstract
After glycosylphosphatidylinositols (GPIs) are added to GPI proteins of Saccharomyces cerevisiae, a fatty acid of the diacylglycerol moiety is exchanged for a C26:0 fatty acid through the subsequent actions of Per1 and Gup1. In most GPI anchors this modified diacylglycerol-based anchor is subsequently transformed into a ceramide-containing anchor, a reaction which requires Cwh43. Here we show that the last step of this GPI anchor lipid remodeling can be monitored in microsomes. The assay uses microsomes from cells that have been grown in the presence of myriocin, a compound that blocks the biosynthesis of dihydrosphingosine (DHS) and thus inhibits the biosynthesis of ceramide-based anchors. Such microsomes, when incubated with [3H]DHS, generate radiolabeled, ceramide-containing anchor lipids of the same structure as made by intact cells. Microsomes from cwh43Δ or mcd4Δ mutants, which are unable to make ceramide-based anchors in vivo, do not incorporate [3H]DHS into anchors in vitro. Moreover, gup1Δ microsomes incorporate [3H]DHS into the same abnormal anchor lipids as gup1Δ cells synthesize in vivo. Thus, the in vitro assay of ceramide incorporation into GPI anchors faithfully reproduces the events that occur in mutant cells. Incorporation of [3H]DHS into GPI proteins is observed with microsomes alone, but the reaction is stimulated by cytosol or bovine serum albumin, ATP plus coenzyme A (CoA), or C26:0-CoA, particularly if microsomes are depleted of acyl-CoA. Thus, [3H]DHS cannot be incorporated into proteins in the absence of acyl-CoA.
The lipid moieties of the glycosylphosphatidylinositol (GPI) lipid at the stage when it is transferred by the transamidase to GPI proteins are different from those found on mature GPI anchors of Saccharomyces cerevisiae. The free GPI lipids contain a phosphatidylinositol (PI) moiety, which comigrates in thin-layer chromatography (TLC) with the free PI of yeast membranes and therefore probably contains the typical C16:0 and C18:1 fatty acids found in yeast PI (7, 12, 26, 29, 31). In contrast, the majority of mature protein-linked GPI anchors contain a ceramide moiety, and a minor fraction contains a diacylglycerol modified to have C26:0 fatty acids in sn2 (9, 31). Ceramides of GPI anchors contain phytosphingosine (PHS) and C26 fatty acids, as do the bulk of the free sphingolipids in yeast membranes, which are inositolphosphorylceramides (IPCs) and derivatives thereof (9, 32). Thus, all mature GPI proteins of yeast contain large lipid moieties with C26 fatty acids, in the form of either a ceramide or a special diacylglycerol, and these lipids are introduced by remodeling enzymes (remodelases) that replace the primary lipid moiety of the anchor.
Recently, two gene products required for introducing C26:0 fatty acids into the primary GPI anchor have been identified (Fig. 1). PER1 encodes a phospholipase A2 that removes the C18:1 fatty acid of the primary anchor (13). GUP1 encodes an acyltransferase required for the addition of a C26:0 fatty acid to the liberated sn2 position, thus generating a pG1-type anchor (4, 13) (Fig. 1). pG1-type anchors may be the preferred substrate for the enzymes introducing ceramides, since the normal ceramide-containing anchor lipids are strongly reduced in per1Δ and gup1Δ mutants, but significant amounts of abnormal, more polar, base-resistant, inositol-containing anchors are observed in gup1Δ cells (4, 13; unpublished results).
FIG. 1.
GPI anchor lipid remodeling in the ER of Saccharomyces cerevisiae. Yeast genes implicated in the various steps are indicated in italic. Anchors are designated according to the lipid moiety they release upon nitrous acid treatment. BstI generates pG2-type anchors, which are gradually transformed into pG1- and IPC/B-type anchors over about 20 to 30 min (31). Upon arrival in the Golgi apparatus, a small fraction of GPI anchors with α-hydroxylated C26:0 fatty acid is generated (IPC/C-type anchors, not shown).
Yeast cells lacking CWH43 are unable to synthesize ceramide-containing GPI anchors, while the replacement of C18 by C26:0 fatty acids on the primary diacylglycerol anchor by Per1 and Gup1 is still intact (14, 34). CWH43 comprises an open reading frame encoding a 953-amino-acid protein with 19 predicted transmembrane domains. Single amino acid substitutions in the hydrophilic, lumenally exposed C-terminal part (amino acids 666 to 953) completely abolish the introduction of ceramides into GPI anchors, whereas mutations in the N-terminal part tend to destabilize the protein (14, 21, 34). The cwh43Δ mutants grow well in rich media and do not secrete GPI proteins, and some cwh43Δ strains are also able to grow in the presence of calcofluor white, quite unlike the per1Δ and gup1Δ mutants (14).
The yeast remodelase activity introducing ceramide (ceramide remodelase) can be monitored by metabolic labeling experiments using tritiated inositol ([3H]inositol) or tritiated dihydrosphingosine ([3H]DHS) (28, 31). When fed to intact cells, these tracers are rapidly taken up and incorporated into lipids and GPI proteins but not into other proteins. [3H]DHS labels only those GPI proteins which carry a ceramide in their anchors. All [3H]inositol- or [3H]DHS-derived label can be removed from the metabolically labeled proteins in the form of PIs or IPCs using nitrous acid, a reagent that releases the inositolphosphoryl-lipid moieties from GPI anchors by cleaving the link between glucosamine and inositol (10, 28, 31).
It presently is not clear what the substrates for the Cwh43-mediated remodelase reaction are. It appears that certain GPI proteins such as Gas1 do not receive ceramide anchors (9), whereas many others do. It also is not clear if this is because Cwh43 itself discriminates between different protein substrates or because only certain proteins get access to Cwh43. Furthermore, it is unclear if Cwh43 replaces the phosphatidic acid or the diacylglycerol moiety of the GPI proteins and if it introduces either a ceramide or only a long-chain base, the latter of which would have to be acylated through a second biosynthetic reaction. Here we report on a microsomal assay that recapitulates the findings previously made with living cells and allows these questions to be addressed under defined conditions in vitro.
MATERIALS AND METHODS
Strains, media, and materials.
Strains with single deletions of nonessential genes (CWH43, SUR2, and GUP1) were obtained from Euroscarf (http://web.uni-frankfurt.de/fb15/mikro/euroscarf/col_index.html) in BY4741 (MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0) or BY4742 (MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0). Other strains are shown in Table 1. Strains were cultured at 24, 30, or 37°C in yeast extract-peptone-dextrose medium or in minimal medium supplemented with glucose, galactose, or raffinose and amino acids, uracil, and adenine (30). Selection for integration of KanMX4-containing deletion cassettes was performed on yeast extract-peptone-dextrose plates containing 200 μg/ml G418 (Calbiochem). Pepstatin was obtained from Alexis, octyl-Sepharose and concanavalin A-Sepharose from Amersham Biosciences, Zymolyase from Seikagaku, and C26:0-coenzyme A (C26:0-CoA) from American Radiolabeled Chemicals; C26:0, myriocin, cerulenin, and other chemicals were purchased from Sigma. [3H]DHS (25 to 60 Ci/mmol) was from Anawa Trading SA, Switzerland, or RC Tritec AG, Teufen, Switzerland; the [3H]DHS used for Fig. 2A was from NEN (2.5 Ci/mmol).
TABLE 1.
Saccharomyces cerevisiae strains
| Strain | Genotype (reference) |
|---|---|
| X2180 (S288c) | MATasuc2 mal gal2 cup1 |
| 164-1C (sec18) | MATα sec18tshis4 leu2 |
| 2039 (wt) | MATaura3-52 trp1 leu2 his4 |
| 2039-sur2 (sur2Δ) | MATasur2::TRP1 ura3-52 trp1 leu2 his4 |
| 2039-scs7Δ (scs7Δ) | MATascs7::URA3 ura3-52 trp1 leu2 his4 |
| FBY4128 (mcd4Δ) | MATahis3Δ1 leu2Δ0 lys2Δ0 MET15 ura3Δ0 YKL165c::kanMX4 harboring p425met-TbGPI10 (36) |
| FBY4179 | cwh43Δ + pCWH43 (14) |
FIG. 2.
[3H]DHS incorporation into proteins in wt microsomes in vitro. (A) Native (n) or boiled (b) microsomes (micr.) were labeled with 100 μCi [3H]DHS at 30°C for 1 h (lanes 1 to 4) or 4 h (lanes 5 to 8) without (−) or with cytosol (cyt.) that either had been boiled (b) or not (n). Proteins were extracted, and glycoproteins were purified by concanavalin A-Sepharose affinity chromatography before analysis by SDS-PAGE and fluorography. (B) Lanes 1 to 5, microsomes from wt cells prepared as for panel A were labeled with 10 μCi [3H]DHS (D) under standard conditions (lane 1) or with different ingredients omitted as indicated at the bottom. Reaction mixtures in lanes 4 and 5 contained 10 nanomoles of C26:0-CoA (26CA), and lane 4 contained 0.5 U of apyrase in addition. Lanes 6 to 8, sec18 cells were grown at 24°C, aliquots of 21 × 106 cells were precultured with (+) or without (−) myriocin (40 μg/ml) for 90 min at 24°C, the preculture was continued at 37°C for a further 15 min, and cells were labeled for 2 h with 25 μCi [3H]DHS (D) or 25 μCi [3H]inositol (I) at 37°C under the same conditions as used during the preculture. Cells were broken using glass beads, and proteins were extensively delipidated with chloroform-methanol-water (10:10:3) and boiled in sample buffer before being analyzed by SDS-PAGE/fluorography. (C) Aliquots (10%) of lipid extracts from the microsomal labeling reactions shown in panel B, lanes 1, 3, 4, and 5, were deacylated with NaOH or control incubated, desalted, and analyzed by TLC in solvent 1, followed by radioimaging.
Preparation of microsomes.
Cells exponentially growing in minimal medium supplemented with glucose, amino acids, uracil, and adenine at 30°C were transferred to medium containing 40 μg/ml of myriocin and further incubated at 30°C for 90 min. Aliquots of 8.4 × 108 cells were resuspended in spheroplasting buffer (10 mM NaN3, 1.4 M sorbitol, 50 mM K2HPO4 [pH 7.5], 40 mM β-mercaptoethanol, 0.2 mg/ml of Zymolyase) and incubated at 30°C to make spheroplasts. The spheroplasts were then resuspended in lysis buffer (10 mM triethanolamine [pH 7.2], 0.8 M sorbitol, 1 mM EDTA, and protease inhibitor cocktail) and were lysed by being forced with a precooled syringe 10 times through a 0.4-mm-wide needle. The microsomes were filtered through a 2-μm-pore filter (Millipore, MillexAP no. SLAP02550) to avoid contamination with intact cells, centrifuged at 15,000 × g for 40 min at 4°C, and resuspended in lysis buffer. The supernatant was frozen to −20°C and utilized as a source of cytosol in the remodeling assays.
Microsomal ceramide-remodeling assay.
Microsomes from X2180 were used unless indicated otherwise. The standard conditions for the in vitro remodeling assay were as follows. Microsomes equivalent to 100 μg of microsomal proteins were incubated for 1 h at 30°C in 25 mM Tris-HCl (pH 7.5) containing 1 mM ATP, 1 mM GTP, 1 mM CoA, 30 mM creatine phosphate, 1 mg/ml of creatine kinase, cytosol (200 μg of proteins), 10 nmol of C26:0, 20 μg/ml myriocin, 200 μg/ml cycloheximide, and 10 μCi (0.17 nmol) of [3H]DHS in a final volume of 100 μl. Myriocin and cycloheximide were added to inhibit endogenous DHS production and block incorporation of [3H]DHS into newly made proteins during incubation. Where indicated, C26:0-CoA (10 nanomoles total) or MgCl2 (2 mM) was also included. “Final conditions” for the assay were determined through the various attempts to optimize incorporation and to make the assay more defined. When done under “final conditions,” assay mixtures contained 600 μg bovine serum albumin (BSA) instead of cytosol, and in addition to the ingredients of the standard assay mixture also contained MgCl2 (2 mM), glutathione (GSH) (5 mM), and NADPH (1 mM). To set up the assay, [3H]DHS and C26:0 or C26:0-CoA were dried in separate tubes, and [3H]DHS was resuspended in 25 μl of lysis buffer by vortexing and then transferred to the tube containing the dried C26:0 or C26:0-CoA. After vortexing, other ingredients were added. Reactions were started by adding microsomes and stopped by the addition of 600 μl of CHCl3-CH3OH (1:1). Protein pellets were extensively delipidated by repeated extraction with organic solvents as described for GPI proteins of metabolically labeled intact cells (16). Labeled proteins either were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)/fluorography or were further delipidated and purified by concanavalin A-Sepharose affinity chromatography as described previously (16). Bound proteins either were released from concanavalin A-Sepharose by boiling in SDS sample buffer and analyzed by SDS-PAGE/fluorography or were released from concanavalin A-Sepharose using pronase and the radioactivity of the thus-generated anchor peptides was detected by scintillation counting. In many assays, samples were divided into two parts to be analyzed separately by SDS-PAGE/fluorography and pronase treatment/scintillation counting, and the two methods always gave excellent agreement. For analytical purposes the anchor peptides were freed from hydrophobic peptides using octyl-Sepharose column chromatography, and labeled anchor peptides were eluted with 25% and then 50% propanol (16). Anchor peptides are normally found only in the 50% eluate, except for anchors from gup1Δ cells, which also contain abnormally polar anchor lipids so that the bulk of gup1Δ anchor peptides elute at 25% propanol (see Fig. 5C) (4). Lipid moieties were released from peptides by nitrous acid treatment for analysis by TLC (16). Lipids were resolved by TLC on Silica 60 plates (20 by 20 cm) using solvent 1 (chloroform-methanol-NH4OH at 40:10:1) or solvents 2 and 3 (chloroform-methanol-0.25% KCl at 55:45:10 and 55:45:5, respectively). Radioactivity was detected by fluorography or radioimaging using the Bio-Rad Molecular Imager FX. Radioactivity was quantified by one- or two-dimensional radioscanning in a Berthold radioscanner.
FIG. 5.
Ceramide remodeling in vitro using microsomes from mutants deficient in in vivo ceramide remodeling. (A) Microsomes from wt (X2180), cwh43Δ (Δ), cwh43Δ complemented with a plasmid-borne Cwh43 (Δ+), mcd4Δ, or gup1Δ cells were used for an in vitro remodeling assay under standard conditions, except that cytosol was omitted in the reaction shown in lane 2. Proteins were extracted and analyzed by SDS-PAGE/fluorography. (B) Lipid extracts from the microsomal reactions of panel A, lanes 1 and 3, and from a further reaction using boiled (b) wt microsomes instead of native (n) ones were deacylated by mild NaOH treatment (+) or control incubated (−) and were analyzed by TLC in solvent 1 and radioimaging. (C) gup1Δ cells were labeled in vivo with [3H]inositol (lane 2), and gup1Δ-derived microsomes were labeled in vitro using [3H]DHS. Lipid moieties were released from polar anchor peptides eluting from the octyl-Sepharose column at 25% propanol (lanes 2 to 4) or from the routinely used ones eluting at 50% propanol (lane 1). Lipids were deacylated or not and analyzed by TLC in solvent 2 and fluorography.
RESULTS
Microsomes incorporate [3H]DHS into GPI proteins.
Cell-free microsomes were prepared from X2180 wild-type (wt) cells that had been treated for a few hours with myriocin, a specific inhibitor of serine palmitoyltransferase, the key enzyme for DHS biosynthesis (18, 23), and were labeled with [3H]DHS as described in Materials and Methods. As shown in Fig. 2A, many proteins were efficiently labeled with [3H]DHS without any significant difference between 1 h and 4 h (lanes 1 to 4 versus 5 to 8) of labeling time. The addition of cytosol increased the incorporation of radioactivity into the proteins, irrespective of whether it had been boiled (lanes 4 and 8) or not (lanes 1 and 5). However, when microsomes were boiled (lanes 3 and 7), labeling of the proteins was completely abolished. This strongly suggested that the remodeling, or at least the incorporation of DHS into proteins, is an enzymatic process and not a spontaneous reaction.
Myriocin pretreatment of cells was critical (Fig. 2B, lane 1 versus 2), and this was probably for two reasons: first, because the lack of sphingolipids, and specifically of ceramides, delays the transport of GPI proteins from the endoplasmic reticulum (ER) to the Golgi apparatus and causes an accumulation of immature GPI proteins in the ER (18, 28, 33, 35), and second, because myriocin pretreatment allows cells to be starved of DHS, PHS, and ceramides and thereby prevents the introduction of ceramides into GPI anchors by the ER-based GPI anchor ceramide remodelase Cwh43. The appearance of distinct bands on SDS-PAGE argued that most of the labeled glycoproteins were still localized in the ER and had not reached the Golgi apparatus, where the glycan elongation transforms most GPI proteins as well as many other secretory proteins into diffuse and poorly migrating proteins of high molecular mass (6). Figure 2B shows proteins labeled with [3H]DHS in vitro side by side with GPI proteins of sec18-1 cells metabolically labeled in vivo with [3H]DHS or [3H]inositol at 37°C, at which temperature the vesicular transport of proteins from the ER to the Golgi apparatus is blocked in this mutant. This shows that the proteins labeled by [3H]DHS in microsomes (Fig. 2B, lanes 1 to 5) were of similar mass as the ones labeled by [3H]DHS or [3H]inositol in intact cells (Fig. 2B, lanes 6 to 8). This is strong evidence that the microsomes incorporate [3H]DHS into immature GPI proteins of the ER. Omission of C26:0, CoA, and ATP significantly reduced he incorporation of [3H]DHS into proteins but did not abolish it, suggesting that a certain amount of these ingredients or of acyl-CoA was present the microsomes or the cytosol (Fig. 2B, lane 1 versus 3). Addition of C26:0-CoA improved the incorporation of [3H]DHS into proteins only slightly (Fig. 2B, lanes 3 to 5), although it strongly enhanced the Lac1/Lag1/Lip1-dependent ceramide synthesis in these microsomes (Fig. 2C, lane 1′ versus 4′ and 5′). This suggests that ATP and CoA may stimulate the attachment of [3H]DHS to GPI proteins not by allowing for acyl-CoA biosynthesis but via another mechanism.
Characterization of GPI anchors generated in vivo.
The inositolphosphoryl-lipid moieties of GPI-anchored proteins from wt cells labeled with [3H]inositol are of three kinds, as described before (31); pG1, a remodeled form of PI containing C26:0 in sn2 of glycerol, IPC/B, and IPC/C (Fig. 1 and 3A, lane 4). IPC/B- and IPC/C-type GPI anchor lipids are formed in the ER and the Golgi apparatus, respectively (28). The major IPC/B and IPC/C moieties of GPI anchors are believed to contain PHS-C26:0, and PHS-C26:0-OH ceramides, respectively, based on the chemical analysis of yeast GPI anchors, which were shown to contain PHS, C26:0, and smaller amounts of C26:0-OH (9, 28).
FIG. 3.
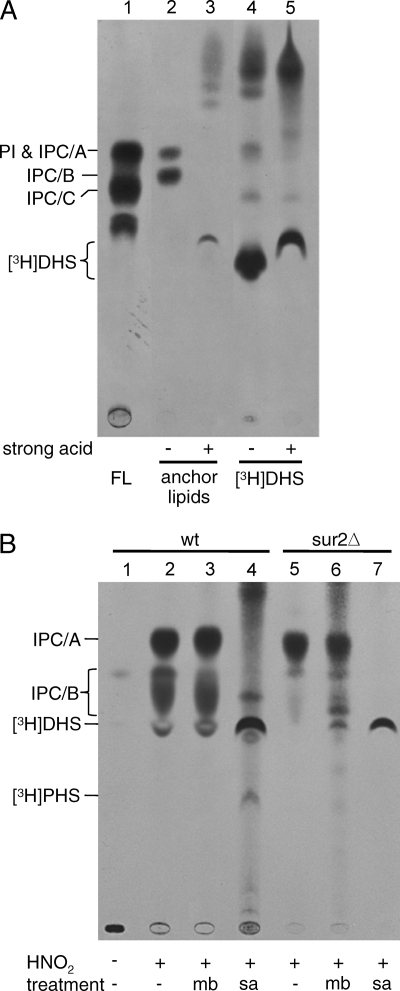
Characterization of GPI anchor lipids made by microsomes in vitro. (A) Exponentially growing wt, scs7Δ, and sur2Δ cells were metabolically labeled with [3H]inositol. After extensive delipidation, the proteins were concentrated by concanavalin A-Sepharose affinity chromatography and digested with pronase. GPI anchor peptides were purified over an octyl-Sepharose column, anchor lipids were released by nitrous acid (HNO2) treatment (16), and released anchor lipids were analyzed by TLC (solvent 3) and fluorography (16). Lane 1 in all panels contains an aliquot of the lipid extract of [3H]inositol-labeled wt cells (free lipids [FL]). (B) Lanes 1 to 4, X2180 cells were labeled with [3H]inositol at 30°C (lanes 1 and 2), and microsomes from the same cells were labeled for 1 h with 10 μCi [3H]DHS under standard conditions (lanes 3 and 4). Anchor lipids were prepared as for panel A. Part of the samples was deacylated with mild NaOH treatment as indicated. All samples were desalted before analysis on TLC (solvent 3) and fluorography. Lanes 5 to 8, 100,000-cpm aliquots of [3H]DHS-labeled anchor peptides generated in an in vitro assay were treated (+) or control incubated (−) for 3 h at 37°C with PI-specific phospholipase C (PI-PLC). Released ceramide moieties were analyzed by TLC (solvent 1). Lane 5, anchor peptides kept on ice; lane 6, incubation for 3 h at 37°C without PI-PLC. [3H]DHS used for the labeling of microsomes is in lane 8. (C) Microsomes were prepared from wt or sur2Δ cells either using the standard procedure or omitting EDTA from the lysis buffer used to break the cells (−EDTA). Microsomes were labeled with [3H]DHS, and anchor lipids were released, treated with NaOH for O deacylation, and analyzed as for panel A.
As a prelude to the analysis of the in vitro-remodeled GPI lipids, we wanted to confirm by genetic means that the in vivo-generated, metabolically labeled anchor lipid previously named IPC/B indeed contains PHS and C26:0, not DHS and C26:0-OH. The genetic confirmation was sought using a sur2Δ mutant, which is deficient in the transformation of DHS into PHS, and a scs7Δ mutant, which is unable to hydroxylate the α carbon of the fatty acid in ceramides (17, 22). wt and mutant cells were labeled in vivo with [3H]inositol, and their GPI lipids were isolated for analysis by TLC. The GPI lipids of the wt strain showed the usual remodeled PI (pG1), IPC/B, and three minor bands, one of which may represent IPC/C (Fig. 3A, lane 4). The anchor lipids of scs7Δ also contained IPC/B as the main anchor lipid (Fig. 3A, lane 2 versus 4), confirming that the fatty acid of IPC/B is not hydroxylated. In contrast, IPC/B was no longer present in sur2Δ cells (Fig. 3A, lane 3 versus 4), confirming the presence of PHS in IPC/B. A more hydrophobic band named IPC/A was seen in this strain, which must represent DHS-C26:0. The result also indicates that DHS-containing ceramides can be utilized by the ER remodelase.
GPI anchors are remodeled to IPC/A and IPC/B in vitro.
Anchor lipids from [3H]DHS-labeled proteins generated in the in vitro assay were compared with anchor lipids generated in intact cells, as shown in Fig. 3B. Anchor lipids labeled in vitro in wt microsomes appeared as two bands, one comigrating with IPC/B and the other being more hydrophobic and running at the position of IPC/A (Fig. 3B, lane 3). Both lipids were resistant to mild base treatment (Fig. 3B, lanes 3 and 4, and 4B, lanes 3 and 6), as is expected for ceramide-containing anchor lipids. The two species were, however, destroyed by strong acid hydrolysis and yielded [3H]DHS and traces of [3H]PHS (Fig. 4A, lane 3, and B, lane 4). PHS was not formed upon strong acid hydrolysis of sur2Δ-derived anchor lipids (Fig. 4B, lane 7 versus 4). When anchor peptides obtained from in vitro-labeled GPI proteins were treated with PI-specific phospholipase C, two different anchor lipids were removed, which migrated in the region of ceramide standards on TLC (Fig. 3B, lane 7). This argues that the difference between IPC/A- and IPC/B-type anchor lipids resides in the ceramide moiety. To confirm that the two in vitro-generated anchor lipids comigrating with IPC/A and IPC/B contain DHS-C26:0 and PHS-C26:0, respectively, we repeated the in vitro experiment with sur2Δ cells. As seen in Fig. 3C, deletion of SUR2 eliminated the band comigrating with IPC/B, whereas IPC/A was still made. The predominance of IPC/A in anchor lipids generated by wt microsomes suggested that Sur2 hydroxylase may not be optimally working in the in vitro system. Sur2 belongs to a family of lipid desaturases and hydroxylases often requiring cytochrome b5 as an electron carrier. When EDTA was removed from the buffers used during cell lysis, we found that the proportion of IPC/B made in vitro was significantly increased (Fig. 3C, lanes 6 and 7 versus 2 and 3). This is compatible with the view that EDTA partially inactivates Sur2 activity by removing an iron atom from an essential component of the hydroxylase.
FIG. 4.
Strong acid hydrolysis destroys in vitro-labeled anchor lipids. (A) Microsomes from X2180 wt cells were labeled with [3H]DHS, and anchor lipids were subjected to strong acid hydrolysis (1 M HCl in methanol-H2O [9:1] for 16 h at 80°C) (32) along with a sample of [3H]DHS used for labeling. FL, lipid extract from [3H]inositol-labeled wt cells. Part of the [3H]DHS also is transformed into apolar material during hydrolysis (lanes 4 and 5). (B) The same wt as well as anchor lipids from sur2Δ cells were subjected to either mild base (mb) or strong acid (sa) hydrolysis. All lipids were analyzed by TLC in solvent 3 and fluorography. Acid hydrolysis leads to the appearance of DHS derived from IPC/A, while most PHS (contained in IPC/B) is probably destroyed.
Cwh43, Mcd4, and Gup1 are required for ceramide remodeling in vitro.
cwh43Δ cells lack the capacity to make ceramide-based GPI anchors, so that all GPI anchors of cwh43Δ cells are of the pG1 type (Fig. 1) (14, 34). cwh43Δ-derived microsomes were entirely unable to incorporate [3H]DHS into proteins (Fig. 5A, lane 3), although they made normal amounts of ceramides (Fig. 5B, lane 1′ versus 3′). Incorporation of [3H]DHS into proteins was restored, albeit only partially, by overexpressing Cwh43 in cwh43Δ cells from plasmid pCWH43 (Fig. 5A, lanes 3, 4). The same plasmid completely restored the incorporation of [3H]DHS into proteins in intact cwh43Δ cells (not shown). Incomplete restoration in vitro may be caused by the fact that the overexpression of Cwh43 from the GAL1 promoter may render the accumulation of not-yet-remodeled GPI proteins during preculture in myriocin less efficient.
The mdc4Δ strain lacks an ethanolamine-phosphate side chain on the α1,4-linked mannose of its GPI anchors, and this has been found to be correlated with a complete absence of ceramide remodeling (36). Similarly, the microsomes of this cell line do not incorporate any [3H]DHS into proteins (Fig. 5A, lanes 5 and 6).
The in vitro remodelase test also faithfully reproduced the ceramide remodelase defect of gup1Δ cells (4). Microsomes of gup1Δ cells still incorporated [3H]DHS into proteins, albeit with a lower efficiency than wt cells (Fig. 5A, lanes 9 and 10 versus 7 and 8), but most GPI proteins that were labeled in the wt were also labeled in gup1Δ cells. It was previously reported that metabolic labeling of gup1Δ cells with [3H]inositol yields GPI anchor peptides that elute from the preparative octyl-Sepharose column at 25% propanol and contain abnormally polar anchor lipids (4). This previous study showed that of the three polar anchor lipids of gup1Δ cells (Fig. 5C, lane 2), only the one of intermediate mobility is mild base sensitive, suggesting that it represents a lyso-PI (4). This lipid was not labeled with [3H]DHS in vitro, but the two lipids that previously were characterized as mild base resistant were labeled, and these were also mild base resistant when labeled in vitro (Fig. 5C, lanes 3 and 4). Thus, these two anchor lipids seem to contain a long-chain base and inositol but possibly lack a fatty acid.
The incorporation of [3H]DHS into proteins in microsomes from per1Δ cells was also severely (>5-fold) reduced, whereas the synthesis of ceramides was not affected (not shown).
Altogether, it appears that the microsomal ceramide-remodeling assay faithfully reproduces the events that have been observed in intact cells.
Definition of optimal conditions for the microsomal ceramide remodelase activity.
Numerous experiments were carried out in an attempt to optimize incorporation of [3H]DHS into proteins. Many ingredients were found to consistently either enhance or inhibit the incorporation of [3H]DHS into proteins, but for some of them the effect was somewhat variable from one experiment to the next. For instance, omission of cytosol had very drastic effects in some experiments but less drastic effects in others. Only those parameters which gave consistent results in many experiments are described in the following.
Freezing microsomes prior to the assays resulted in an 80% loss of activity. The standard in vitro reaction was done in the presence of myriocin to prevent the biosynthesis of cold DHS from serine and NADPH present in the added cytosol, but omission of myriocin from the in vitro assay did not diminish the incorporation of [3H]DHS (not shown). When cold DHS was added to the reaction mixture, the incorporation of [3H]DHS was strongly reduced, as shown in Fig. 6A and B. Calculations show that the chemical amounts of DHS incorporated into GPI proteins increased when the amount of DHS was raised from 0.17 nmol to 4.32 nmol (= 1.3 μg/ml) (Fig. 6B).
FIG. 6.
The concentration of DHS limits the ceramide-remodeling reaction. X2180 wt cells were preincubated with myriocin, and microsomes were prepared. (A) Lane 1, microsomal remodeling assays were preformed under standard conditions with 10 μCi (0.17 nanomole) of [3H]DHS. Lane 2 contained boiled microsomes; various amounts of cold DHS were added in lanes 3 to 5. Eighty percent of the reaction product was used for SDS-PAGE/fluorography. Chemical amounts of exogenously added DHS (including [3H]DHS) are indicated at the bottom. (B) Anchor peptides were prepared from the remaining 20% and their radioactivity determined by scintillation counting. The chemical amounts of DHS incorporated into proteins were calculated by multiplying the specific activity of added DHS ([3H]DHS plus cold DHS) by the total incorporated radioactivity for those values which were significantly above background. This amount is a minimal estimate because the microsomes potentially contained endogenous DHS or PHS as well. (C) Microsomal remodeling assays were preformed under final conditions (see Materials and Methods) using 25 μCi (1 nanomole) of [3H]DHS and 30 or 100 μg of microsomal protein per assay. Incubations lasted 4, 10, 24, 60, or 120 min. Anchor peptides were prepared and their radioactivity determined by scintillation counting. All conditions were tested in duplicate, and standard deviations are indicated.
As can be seen in Fig. 6C, using 100 μg of microsomal protein per assay we can observe a constant, close-to-linear increase of incorporated radioactivity during the first 30 to 60 min of incubation. Using less protein does not significantly reduce the rate of incorporation of [3H]DHS (Fig. 6C). A likely explanation of this fact is that with fewer microsomes and hence less cold DHS and PHS, the specific activity of [3H]DHS in the assay becomes higher. The close-to-linear time course, however, and the total absence of incorporation with boiled microsomes (Fig. 2A and 6C) argue that under our standard conditions (60 min of incubation and 100 μg of protein) we will observe less incorporation if one of the required enzyme activities or substrates becomes limiting.
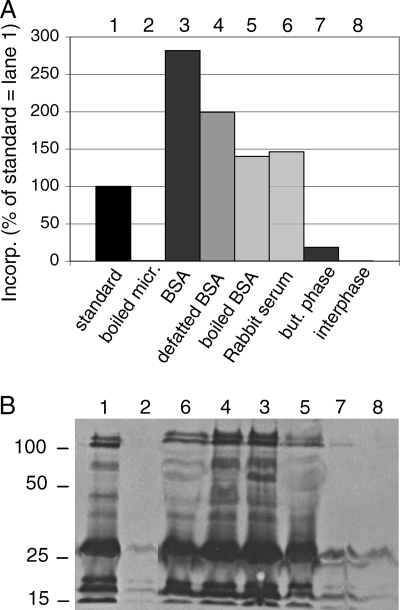
On the technical side, adding [3H]DHS to assays not directly, but incorporated into phosphatidylcholine-containing liposomes reduced its incorporation into proteins by a factor of about 2 (not shown). The addition of cytosol was strongly stimulatory (see Fig. 8A, bar 2 versus 1, and 8B, bar 7 versus 1), but [3H]DHS was incorporated into the same proteins in the presence or absence of cytosol (Fig. 2A). As boiled cytosol had the same enhancing effect (Fig. 2A), we initially assumed that the active factor might be an ion or small molecule. Fractionation of cytosol by gel filtration on Biogel-P2 (separating in the range of 100 to 1,800 Da) and testing individual fractions in the microsomal remodeling assay did not, however, reveal any stimulatory activity in the low-molecular-weight range (not shown). We also were unable to extract a stimulatory lipid from cytosol using an organic solvent (Fig. 7, bars 7 and 8). Interestingly, cytosol was efficiently replaced by other proteins, such as BSA, defatted or boiled BSA, or rabbit serum (Fig. 7, bars 3 to 6). In our view, the data suggest that proteins might stabilize the microsomes, e.g., by preventing their aggregation during the incubation, or that proteins protect the remodelases and/or GPI protein substrates from proteolytic degradation.
FIG. 8.
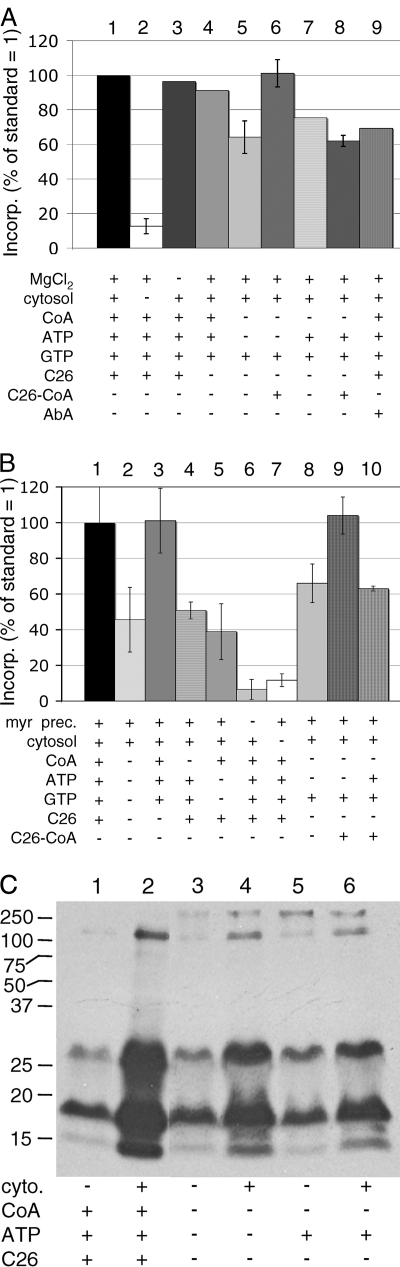
Dependence of microsomal remodeling assay on exogenous ATP, CoA, and C26:0. (A) X2180 wt cells were preincubated with myriocin, and microsomes were prepared. Microsomal remodeling assays were preformed under standard conditions with Mg2+ (bar 1) or with ingredients omitted or added as indicated at the bottom. The amount of radioactivity in anchor peptides was determined by scintillation counting and plotted as a percentage of incorporation under standard conditions (bar 1). Error bars indicate standard deviations, where the assay conditions were tested in duplicate or triplicate assays. Reaction 1 contained a total of 36,000 cpm of anchor peptides. (B) As for panel A, but assays were carried out in the absence of Mg2+, reaction 5 contained apyrase, and microsomes for reaction 6 were from cells not precultured with myriocin. The bars indicate the means from three to five independent assays for reactions 1 to 7 and from duplicate assays for reactions 8 to 10. Reaction 1 contained a mean of 54,000 cpm of anchor peptides. (C) Standard assays using microsomes from X2180 cells were performed with some ingredients omitted as indicated at the bottom. Labeled proteins were analyzed by SDS-PAGE and fluorography.
FIG. 7.
Dependence of microsomal remodeling assay on cytosol. (A) X2180 wt cells were precultured with myriocin, and microsomes were prepared. Microsomal remodeling assays were preformed under standard conditions (bar 1) or with cytosol replaced by various kinds of BSA or rabbit serum (all added at 600 μg/assay, bars 3 to 6). Cytosol was extracted with chloroform-methanol-water (10:10:3), and the extract was dried in a rotary evaporator and desalted by butanol-water partitioning. Apolar lipids of the butanol phase or polar lipids of the butanol-water interphase were used instead of cytosol for bars 7 and 8. Anchor peptides were prepared from 20% of the reaction products and their radioactivity determined by scintillation counting. (B) The remaining 80% of reaction product was analyzed by SDS-PAGE/fluorography.
Preculture of cells with myriocin was found to be crucial (Fig. 8B, bar 6 versus 1), as discussed above (Fig. 2B, lane 2).
Further experiments were done to evaluate the importance of CoA, ATP, GTP, and C26:0-CoA. The simultaneous omission of CoA and ATP reduced the incorporation of [3H]DHS into proteins by about 35% (Fig. 8A, bar 5, and B, bar 8), whereas omission of C26:0 was in most cases of no consequence (Fig. 8A, bar 4 versus 1, and B, bar 3 versus 1). This suggests that microsomal membranes contain sufficient C26:0-CoA or precursors thereof to attach a certain amount of [3H]DHS to GPI proteins but that exogenously added CoA and ATP can enhance the reaction. Addition of C26:0-CoA to the standard reaction mixture usually had no effect (not shown). However, C26:0-CoA usually enhanced the incorporation of [3H]DHS when CoA and ATP were lacking (Fig. 8A, bar 6 versus 5, and B, bar 9 versus 8), but not to levels higher than observed under the standard conditions. Curiously, C26:0-CoA consistently had little effect if added to reaction mixtures that contained ATP (Fig. 8A, bar 8 versus 7, and B, bar 10 versus 8). Aureobasidin A, a specific inhibitor of IPC synthase (24), could be expected to increase the amount of ceramide available for the remodeling reaction by blocking the further metabolism of ceramide, but it did not stimulate the incorporation of [3H]DHS into GPI proteins (Fig. 8A, bar 9 versus 1, and data not shown).
While many tests showed stimulation of the remodeling reaction by ATP, this stimulation was not dependent on the presence of Mg2+, suggesting that ATP, not Mg2+-ATP, is required. As shown in Fig. 8B (without Mg2+), the simultaneous omission of C26:0, CoA, ATP, and GTP again reduced the incorporation of [3H]DHS into proteins quite significantly (Fig. 8B, bars 1, 2, and 8). Furthermore, the omission of either ATP and GTP or CoA caused a similar reduction (Fig. 8B, bars 1 to 5). Gel electrophoresis experiments also showed that the profile of labeled proteins was not significantly different when microsomes were deprived of the possibility to make acyl-CoA (Fig. 8C). Reactions became more dependent on exogenously added CoA and ATP, and became even dependent on C26:0, when microsomes were derived from cells that had been precultured in the presence not only of myriocin but also of cerulenin, a drug which blocks fatty acid biosynthesis by inhibiting the β-ketoacyl-acyl carrier protein synthase (1, 20). As shown in Fig. 9, omission of C26:0 reduced the incorporation of [3H]DHS into proteins significantly (Fig. 9, bar 4 versus 3), omission of CoA or ATP led to a severe reduction (Fig. 9, bars 5 and 6 versus 3), and C26-CoA could restore some activity even though ATP was present (Fig. 9, bar 9 versus 5). Thus, after preculture of cells with cerulenin, the microsomes may be low in acyl-CoA so that the remodeling reaction becomes more dependent on acyl-CoA synthesis. The very-long-chain fatty acid-specific acyl-CoA synthase Fat1 has recently been localized in the ER (25). We further investigated whether fatty acids other than C26:0 would enhance the standard reaction (using microsomes from cells incubated with myriocin but not cerulenin). Compared to the reaction without added fatty acid, the addition of C26:0 or C16:0 was of no consequence; only C24:0 slightly stimulated the incorporation of [3H]DHS into proteins (not shown). Addition of physiological electron donors such as GSH or NADPH did not stimulate the reaction (not shown). The TLC mobility of anchor lipids also did not change when GSH, NADH, or NADPH was added to standard reaction mixture (not shown). After these studies, we now utilize a slightly modified standard assay including Mg2+, C24:0, NADPH, GSH, and BSA instead of cytosol (final conditions; see Materials and Methods).
FIG. 9.
Cerulenin enhances the need for ATP and C26:0 in the microsomal assay. X2180 wt cells were precultured for 180 min with cerulenin (10 μg/ml) and for the last 90 min with myriocin (40 μg/ml) in addition (bars 3 to 9) or only with myriocin for 90 min (bars 1 and 2). Microsomal remodeling assays were run in duplicate under standard conditions (bars 1 and 3) or with ingredients omitted or added as indicated at the bottom. The amount of radioactivity in anchor peptides was determined by scintillation counting and plotted as a percentage of incorporation under standard conditions (bar 1). Reaction 6 contained 0.5 U of apyrase in addition, and 2 or 10 nanomoles of C26:0-CoA was added in conjunction with 0.1 mg of purified yeast acyl-CoA binding protein.
DISCUSSION
Ceramides are found in the GPI anchors of certain plants (e.g., pears), Trypanosoma cruzi, Paramecium, Aspergillus fumigatus, and Dictyostelium, sometimes as the sole anchor lipid (3, 27). Recent studies show that, similar to the case in yeast, the first steps of GPI biosynthesis in A. fumigatus and T. cruzi do not use ceramide as the lipid support, suggesting that ceramide is added by remodeling at a later step not only in yeast but also in other species (2, 11).
In a recent report we described a microsomal assay for the Gup1-mediated addition of fatty acids in the sn2 position of GPI anchors, which revealed that the GUP1 homologue of Trypanosoma brucei can remodel free GPI lipids as well as GPI anchors of proteins (19). Here we describe a further assay allowing measurement of the replacement of diacylglycerol-based GPI anchors by ceramide-based anchors. These assays set the stage for further biochemical investigation and for reconstitution experiments of the various remodeling reactions.
The SDS-PAGE/fluorography profile of GPI proteins labeled in vitro is very similar to that of GPI proteins labeled in vivo when the exit from the ER is blocked. This argues that the microsomal assay measures the GPI-remodeling event occurring in the ER. While in vivo remodeling in the ER generates IPC/B-containing anchors, the in vivo remodeling in the Golgi apparatus generates IPC/C-containing anchors (28). The fact that the in vitro-labeled GPI anchors contain IPC/B but not IPC/C suggests that the in vitro test reproduces ER remodeling or else that Scs7, the enzyme hydroxylating the fatty acid moiety of sphingolipids and required for the generation of IPC/C, is not operative in our assay. The current knowledge suggests that Scs7 is localized outside the ER in vesicles, whereas Sur2, generating PHS from DHS and required for the generation of IPC/B-containing anchors, is localized to the ER. (25).
The increase of DHS incorporation upon addition of cold DHS (Fig. 6B) might be thought to be due to a detergent effect of DHS, but the 4.3 nmol giving the highest incorporation corresponds to a concentration of 0.0013% (wt/vol) in the assay; 4.3 nmol/assay correspond to 1.3 μg of DHS mixing in with a total of approximately 100 μg of membrane lipids. While 4.32 nmol of long-chain bases/100 μg membrane lipid is a 15-fold-higher concentration than the physiological 42 pmol/A600 unit of cells (8), ceramide synthase-deficient lag1Δ lac1Δ strains have >20-fold-increased long-chain base levels and are living (15). Thus, adding 4.32 nanomoles to the assay mixture is not expected to significantly alter the membrane structure. Moreover, adding 1.3 μg of lyso-phosphatidic acid, a natural detergent, had no influence on the incorporation (not shown). A more likely interpretation of the results in Fig. 6 and 8 is that the DHS concentration in the standard assay somewhat limits the rate of DHS incorporation, whereas the C26:0 concentration does not. The concentration of nonremodeled GPI proteins that can serve as substrates is most likely also rate limiting, but it is impossible to test this by varying their concentration in our microsomal assay. C26:0-CoA or C26:0, required for synthesis of C26-CoA by the ER-based acyl-CoA synthase Fat1 (25), had major effects only if the cells had previously been depleted of acyl-CoA by cerulenin treatment (Fig. 9), but the addition of C26:0-CoA to the standard assay was usually of no consequence. In contrast, addition of C26:0-CoA strongly enhanced the biosynthesis of [3H]ceramide from [3H]DHS in the standard assay (Fig. 2C) (15). This argues that in this in vitro system, the acyl-CoA-dependent ceramide synthesis pathway does not feed directly into the ceramide pool that is utilized by the GPI ceramide remodelase activity. The same idea is supported by findings obtained with intact cells: (i) the type of ceramides predominating in GPI anchors (PHS-C26:0) is different from that predominating in IPCs (PHS-C26:0-OH) (28) (Fig. 3) and (ii) GPI anchor lipids made by a lag1Δ lac1Δ ydc1Δ ypc1Δ strain kept alive by the murine LAG1 homologue Lass5 contain the typical IPC/B moiety although the free sphingolipids of this strain almost exclusively contain C16:0 and C18:0 fatty acids in their ceramide moiety (5). These data suggest that Cwh43 does not just transfer ceramides made by the acyl-CoA- and Lag1- or Lac1-dependent ceramide synthase but may generate ceramides on GPI anchors through a different mechanism. However, the significant dependence on CoA and ATP of the incorporation of [3H]DHS into proteins in microsomes from acyl-CoA-depleted cells (Fig. 9, bars 5 and 6) suggests that [3H]DHS cannot be incorporated as such, as proposed in the introduction, but that it has to be acylated before being added to proteins. Further studies are necessary to fully understand the mode of operation of Cwh43.
Acknowledgments
This work was supported by grants 31-67188.01 and 3100AO-116802/1 from the Swiss National Science Foundation.
We thank Vikram Ghugtyal for helpful comments.
Footnotes
Published ahead of print on 12 December 2008.
REFERENCES
- 1.Awaya, J., T. Ohno, H. Ohno, and S. Omura. 1975. Substitution of cellular fatty acids in yeast cells by the antibiotic cerulenin and exogenous fatty acids. Biochim. Biophys. Acta 409267-273. [DOI] [PubMed] [Google Scholar]
- 2.Bertello, L. E., M. J. Alves, W. Colli, and R. M. de Lederkremer. 2004. Inositolphosphoceramide is not a substrate for the first steps in the biosynthesis of glycoinositolphospholipids in Trypanosoma cruzi. Mol. Biochem. Parasitol. 13371-80. [DOI] [PubMed] [Google Scholar]
- 3.Bosson, R., and A. Conzelmann. 2007. Multiple functions of inositolphosphorylceramides in the formation and intracellular transport of glycosylphosphatidylinositol-anchored proteins in yeast. Biochem. Soc. Symp. 74199-209. [DOI] [PubMed] [Google Scholar]
- 4.Bosson, R., M. Jaquenoud, and A. Conzelmann. 2006. GUP1 of Saccharomyces cerevisiae encodes an O-acyltransferase involved in remodeling of the GPI anchor. Mol. Biol. Cell 172636-2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cerantola, V., C. Vionnet, O. F. Aebischer, T. Jenny, J. Knudsen, and A. Conzelmann. 2007. Yeast sphingolipids do not need to contain very long chain fatty acids. Biochem. J. 401205-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conzelmann, A., C. Fankhauser, and C. Desponds. 1990. Myoinositol gets incorporated into numerous membrane glycoproteins of Saccharomyces cerevisiae; incorporation is dependent on phosphomannomutase (sec53). EMBO J. 9653-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conzelmann, A., A. Puoti, R. L. Lester, and C. Desponds. 1992. Two different types of lipid moieties are present in glycophosphoinositol-anchored membrane proteins of Saccharomyces cerevisiae. EMBO J. 11457-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dickson, R. C., E. E. Nagiec, M. Skrzypek, P. Tillman, G. B. Wells, and R. L. Lester. 1997. Sphingolipids are potential heat stress signals in Saccharomyces. J. Biol. Chem. 27230196-30200. [DOI] [PubMed] [Google Scholar]
- 9.Fankhauser, C., S. W. Homans, J. E. Thomas-Oates, M. J. McConville, C. Desponds, A. Conzelmann, and M. A. Ferguson. 1993. Structures of glycosylphosphatidylinositol membrane anchors from Saccharomyces cerevisiae. J. Biol. Chem. 26826365-26374. [PubMed] [Google Scholar]
- 10.Ferguson, M. A., S. W. Homans, R. A. Dwek, and T. W. Rademacher. 1988. Glycosyl-phosphatidylinositol moiety that anchors Trypanosoma brucei variant surface glycoprotein to the membrane. Science 239753-759. [DOI] [PubMed] [Google Scholar]
- 11.Fontaine, T., T. K. Smith, A. Crossman, J. S. Brimacombe, J. P. Latge, and M. A. Ferguson. 2004. In vitro biosynthesis of glycosylphosphatidylinositol in Aspergillus fumigatus. Biochemistry 4315267-15275. [DOI] [PubMed] [Google Scholar]
- 12.Fujita, M., and Y. Jigami. 2008. Lipid remodeling of GPI-anchored proteins and its function. Biochim. Biophys. Acta 1780410-420. [DOI] [PubMed] [Google Scholar]
- 13.Fujita, M., T. Yoko-O, and Y. Jigami. 2006. Inositol deacylation by Bst1p is required for the quality control of glycosylphosphatidylinositol-anchored proteins. Mol. Biol. Cell 17834-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghugtyal, V., C. Vionnet, C. Roubaty, and A. Conzelmann. 2007. CWH43 is required for the introduction of ceramides into GPI anchors in Saccharomyces cerevisiae. Mol. Microbiol. 651493-1502. [DOI] [PubMed] [Google Scholar]
- 15.Guillas, I., P. A. Kirchman, R. Chuard, M. Pfefferli, J. C. Jiang, S. M. Jazwinski, and A. Conzelmann. 2001. C26-CoA-dependent ceramide synthesis of Saccharomyces cerevisiae is operated by Lag1p and Lac1p. EMBO J. 202655-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guillas, I., M. Pfefferli, and A. Conzelmann. 2000. Analysis of ceramides present in glycosylphosphatidylinositol anchored proteins of Saccharomyces cerevisiae. Methods Enzymol. 312506-515. [DOI] [PubMed] [Google Scholar]
- 17.Haak, D., K. Gable, T. Beeler, and T. Dunn. 1997. Hydroxylation of Saccharomyces cerevisiae ceramides requires Sur2p and Scs7p. J. Biol. Chem. 27229704-29710. [DOI] [PubMed] [Google Scholar]
- 18.Horvath, A., C. Sutterlin, U. Manning-Krieg, N. R. Movva, and H. Riezman. 1994. Ceramide synthesis enhances transport of GPI-anchored proteins to the Golgi apparatus in yeast. EMBO J. 133687-3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaquenoud, M., M. Pagac, A. Signorell, M. Benghezal, J. Jelk, P. Butikofer, and A. Conzelmann. 2008. The Gup1 homologue of Trypanosoma brucei is a GPI glycosylphosphatidylinositol remodelase. Mol. Microbiol. 67202-212. [DOI] [PubMed] [Google Scholar]
- 20.Magnuson, K., S. Jackowski, C. O. Rock, and J. E. J. Cronan. 1993. Regulation of fatty acid biosynthesis in Escherichia coli. Microbiol. Rev. 57522-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin-Yken, H., A. Dagkessamanskaia, P. De Groot, A. Ram, F. Klis, and J. Francois. 2001. Saccharomyces cerevisiae YCRO17c/CWH43 encodes a putative sensor/transporter protein upstream of the BCK2 branch of the PKC1-dependent cell wall integrity pathway. Yeast 18827-840. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell, A. G., and C. E. Martin. 1997. Fah1p, a Saccharomyces cerevisiae cytochrome b5 fusion protein, and its Arabidopsis thaliana homolog that lacks the cytochrome b5 domain both function in the alpha-hydroxylation of sphingolipid-associated very long chain fatty acids. J. Biol. Chem. 27228281-28288. [DOI] [PubMed] [Google Scholar]
- 23.Miyake, Y., Y. Kozutsumi, S. Nakamura, T. Fujita, and T. Kawasaki. 1995. Serine palmitoyltransferase is the primary target of a sphingosine-like immunosuppressant, ISP-1/myriocin. Biochem. Biophys. Res. Commun. 211396-403. [DOI] [PubMed] [Google Scholar]
- 24.Nagiec, M. M., E. E. Nagiec, J. A. Baltisberger, G. B. Wells, R. L. Lester, and R. C. Dickson. 1997. Sphingolipid synthesis as a target for antifungal drugs. Complementation of the inositol phosphorylceramide synthase defect in a mutant strain of Saccharomyces cerevisiae by the AUR1 gene. J. Biol. Chem. 2729809-9817. [DOI] [PubMed] [Google Scholar]
- 25.Natter, K., P. Leitner, A. Faschinger, H. Wolinski, S. McCraith, S. Fields, and S. D. Kohlwein. 2005. The spatial organization of lipid synthesis in the yeast Saccharomyces cerevisiae derived from large scale green fluorescent protein tagging and high resolution microscopy. Mol. Cell Proteomics 4662-672. [DOI] [PubMed] [Google Scholar]
- 26.Pittet, M., and A. Conzelmann. 2007. Biosynthesis and function of GPI proteins in the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta 1771405-420. [DOI] [PubMed] [Google Scholar]
- 27.Pittet, M., D. Uldry, M. Aebi, and A. Conzelmann. 2006. The N-glycosylation defect of cwh8Delta yeast cells causes a distinct defect in sphingolipid biosynthesis. Glycobiology 16155-164. [DOI] [PubMed] [Google Scholar]
- 28.Reggiori, F., E. Canivenc-Gansel, and A. Conzelmann. 1997. Lipid remodeling leads to the introduction and exchange of defined ceramides on GPI proteins in the ER and Golgi of Saccharomyces cerevisiae. EMBO J. 163506-3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneiter, R., B. Brugger, R. Sandhoff, G. Zellnig, A. Leber, M. Lampl, K. Athenstaedt, C. Hrastnik, S. Eder, G. Daum, F. Paltauf, F. T. Wieland, and S. D. Kohlwein. 1999. Electrospray ionization tandem mass spectrometry (ESI-MS/MS) analysis of the lipid molecular species composition of yeast subcellular membranes reveals acyl chain-based sorting/remodeling of distinct molecular species en route to the plasma membrane. J. Cell Biol. 146741-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sherman, F. 2002. Getting started with yeast. Methods Enzymol. 3503-41. [DOI] [PubMed] [Google Scholar]
- 31.Sipos, G., F. Reggiori, C. Vionnet, and A. Conzelmann. 1997. Alternative lipid remodelling pathways for glycosylphosphatidylinositol membrane anchors in Saccharomyces cerevisiae. EMBO J. 163494-3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith, S. W., and R. L. Lester. 1974. Inositol phosphorylceramide, a novel substance and the chief member of a major group of yeast sphingolipids containing a single inositol phosphate. J. Biol. Chem. 2493395-3405. [PubMed] [Google Scholar]
- 33.Sutterlin, C., T. L. Doering, F. Schimmoller, S. Schroder, and H. Riezman. 1997. Specific requirements for the ER to Golgi transport of GPI-anchored proteins in yeast. J. Cell Sci. 1102703-2714. [DOI] [PubMed] [Google Scholar]
- 34.Umemura, M., M. Fujita, T. Yoko-O, A. Fukamizu, and Y. Jigami. 2007. Saccharomyces cerevisiae CWH43 is involved in the remodeling of the lipid moiety of GPI anchors to ceramides. Mol. Biol. Cell 184304-4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watanabe, R., K. Funato, K. Venkataraman, A. H. Futerman, and H. Riezman. 2002. Sphingolipids are required for the stable membrane association of glycosylphosphatidylinositol-anchored proteins in yeast. J. Biol. Chem. 27749538-49544. [DOI] [PubMed] [Google Scholar]
- 36.Zhu, Y., C. Vionnet, and A. Conzelmann. 2006. Ethanolaminephosphate side chain added to glycosylphosphatidylinositol (GPI) anchor by mcd4p is required for ceramide remodeling and forward transport of GPI proteins from endoplasmic reticulum to Golgi. J. Biol. Chem. 28119830-19839. [DOI] [PubMed] [Google Scholar]