Abstract
Protein kinase CK2 (casein kinase 2) is a eukaryotic serine/threonine protein kinase with multiple substrates and roles in diverse cellular processes, including differentiation, proliferation, and translation. The mammalian holoenzyme consists of two catalytic alpha or alpha′ subunits and two regulatory beta subunits. We report the identification and characterization of a Plasmodium falciparum CK2α orthologue, PfCK2α, and two PfCK2β orthologues, PfCK2β1 and PfCK2β2. Recombinant PfCK2α possesses protein kinase activity, exhibits similar substrate and cosubstrate preferences to those of CK2α subunits from other organisms, and interacts with both of the PfCK2β subunits in vitro. Gene disruption experiments show that the presence of PfCK2α is crucial to asexual blood stage parasites and thereby validate the enzyme as a possible drug target. PfCK2α is amenable to inhibitor screening, and we report differential susceptibility between the human and P. falciparum CK2α enzymes to a small molecule inhibitor. Taken together, our data identify PfCK2α as a potential target for antimalarial chemotherapeutic intervention.
Malaria, caused by infection with intracellular protozoan parasites of the genus Plasmodium, is responsible for 300 million to 600 million clinical cases annually (49), resulting in the deaths of up to 3 million people every year (9, 10). The need for novel intervention strategies is rendered more acute by the rise of resistance of the parasites (especially Plasmodium falciparum, the causative agent of the most virulent form of the disease) to most available drugs. There is a clear need for further research aimed at identifying novel drug targets (43).
The parasite life cycle is complex, with a succession of proliferation and differentiation events, in the regulation of which protein phosphorylation is likely to play crucial roles. Reversible phosphorylation of proteins is a major regulatory mechanism in most cellular processes, and approximately 30% of all eukaryotic cellular proteins carry a phosphate group. Deregulation of protein phosphorylation underlies various pathologies, including cancers, and protein kinases are considered promising drug targets, comprising as much as 30% of all protein targets under investigation (13). The divergences between human and plasmodial protein kinases suggest that specific inhibition of the latter is an achievable goal (14, 15).
Protein kinase CK2, formerly known as casein kinase II, is a serine/threonine protein kinase ubiquitously expressed in eukaryotes. It has over 300 cellular substrates catalogued to date (34). Consistent with its multiple substrates, the enzyme plays a crucial role in many cellular processes, including differentiation, proliferation, survival, translation, apoptosis, cell cycle progression, and cellular responses to stress and DNA damage (1, 38); mammalian CK2 has even been found to act as an exokinase, phosphorylating several extracellular proteins (44, 55). CK2 is essential to viability in yeasts and slime molds (24, 37). The human CK2 holoenzyme consists of two catalytic alpha or alpha′ subunits and two regulatory beta subunits, and recent evidence indicates that the beta subunits interact with several protein kinases in addition to CK2α (reviewed in reference 6), pointing to a likely role in the integration of numerous signaling pathways. The human genome encodes three CK2α subunits and a single version of the beta subunit. Increasing evidence shows that CK2 is an attractive target for antineoplastic and antiviral drugs (46).
The genome of the P. falciparum strain 3D7 (18) has been fully sequenced, allowing the discovery of the entire complement of plasmodial protein kinases by analysis of the set of predicted peptides (3, 57). A putative CK2α orthologue and two predicted CK2β subunits were identified in these analyses. Here we present the biochemical characterization of the PfCK2α and both PfCK2β orthologues and demonstrate by using a reverse genetics approach that the catalytic subunit is essential for completion of the erythrocytic asexual cycle of the parasite.
MATERIALS AND METHODS
Expression and purification of the three PfCK2 subunits.
Oligonucleotides were designed to amplify the PfCK2α open reading frame (ORF) from P. falciparum (clone 3D7A) cDNA. The forward (5′-GGGGGGATCCATGTCGGTTAGCTCAATTAATAAA-3′) and reverse (5′-GGGGGTCGACTTATGATTCCTCACGGTCTTCTC-3′) primers carried BamHI and SalI sites, respectively (underlined). Oligonucleotides were also designed to amplify the PfCK2β1 and PfCK2β2 ORFs from P. falciparum (clone 3D7A) cDNA. The PfCK2β1 forward (5′-GGGGAGATCTATGGAAAATAGTGATTCGAATAAAGAC-3′) and reverse (5′-GGGGGTCGACTTACGTTTCAGAAATTTGTAGTTCTTCC-3′) primers carried BglII and SalI sites, respectively (underlined). The PfCK2β2 sequence has a long N-terminal extension. Oligonucleotides were designed to amplify the PfCK2β2 sequence, lacking the N-terminal extension, from P. falciparum (clone 3D7A) cDNA. The forward (5′-GGGGGGATCCATGGAAGCAACAGTGTCTTGGATTG-3′) and reverse (5′-GGGGGTCGACTCATTGACACTCTTCAGAGGATTCCG-3′) primers carried BamHI and SalI sites, respectively (underlined). The short version of PfCK2β2, lacking the N-terminal extension, was named shPfCK2β2. Catalytically inactive (“kinase dead”) PfCK2α was obtained by site-directed mutagenesis (K72M) of pGEX-PfCK2α by overlap extension PCR (23). All cloning primers are provided in Table S1 in the supplemental material. All PCR products were verified by sequencing in the vector pGEM-T Easy (Promega) and then subcloned into the plasmid pGEX-4T3 (GE Healthcare) to generate N-terminal glutathione S-transferase (GST) fusions. The shPfCK2β2 sequence was also inserted between the BamHI and NotI sites of the plasmid pQE-30 for expression with an N-terminal His6 tag. A pET29 vector containing P. falciparum CK2α in frame with a C-terminal His6 tag sequence was a kind gift from D. Chakrabarti. The pGEX4T3 constructs were expressed in Escherichia coli BL21 Gold cells, SG13009 cells were used for expression from the pQE30 plasmid, and the pET29 construct was expressed in E. coli BL21(DE3) cells for 20 h at 20°C with 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG). GST-tagged proteins were purified on glutathione-agarose beads (Sigma), and His-tagged proteins were purified on Ni2+-agarose beads (Qiagen), following the manufacturer's recommendations.
Construction of the knockout (KO) plasmid pCAM-BSD-KOPfCK2α.
A fragment from the PfCK2α ORF was amplified by PCR and inserted between the BamHI and NotI sites of the pCAM-BSD plasmid (47), which contains the Aspergillus terreus blasticidin-S-deaminase gene, whose gene product confers resistance to the drug blasticidin. Cloning primers are listed in Table S1 in the supplemental material.
Construction of the complementation plasmid pCHD-PfCK2a.
A plasmid for in vivo episomal expression of PfCK2α subunits was constructed as follows: the full-length PfCK2α coding sequence was first inserted between the BglII and NotI sites of the plasmid pHGB (51) and then transferred into the plasmid pCHD-1/2 (51) by a Gateway LR clonase reaction according to the manufacturer's instructions (Invitrogen). The plasmid pCHD-1/2 includes a cassette encoding human dihydrofolate reductase, conferring resistance to the antifolate drug WR99210. Parasites that were transfected with both a KO plasmid and a complementation plasmid were cultured under double-drug selection (see below).
3′-Tagging plasmid.
The 3′ end of the PfCK2α coding sequence (538 bp, omitting the stop codon) was amplified by PCR using primers incorporating PstI and BamHI restriction sites and inserted between the PstI and BamHI sites of the pCAM-BSD-hemagglutinin (HA) plasmid (42).
Kinase assays.
Standard kinase reactions (30 μl) occurred in kinase buffer (20 mM Tris-HCl [pH 7.5], 20 mM MgCl2, 2 mM MnCl2, and 10 μM ATP) containing 0.075 MBq [γ-32P]ATP (370 MBq/ml; GE Healthcare), 1 μg of recombinant kinase, and 5 μg of substrate. Reactions were carried out at 30°C for 30 min and terminated by the addition of Laemmli buffer. Samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The gels were dried and exposed for autoradiography.
Kinase assays were also analyzed by the phosphocellulose method (20). A final assay volume of 18 μl contained 36 ng of PfCK2α, 167 mM of peptide substrate, 50 mM Tris-HCl (pH 7.5), 20 mM MgCl2, 25 μM ATP, 40 to 150 mM NaCl, and 0.037 MBq [γ-32P]ATP (220 TBq/mmol; GE Healthcare). Reactions took place for 10 min at room temperature and were terminated by the addition of 60 μl of 4% trichloroacetic acid. Reaction mixtures were centrifuged at 10,000 × g for 15 min, and then 60 μl of supernatant was spotted onto 4- by 4-cm Whatman P81 phosphocellulose paper squares. The squares were washed three times for 15 min in 0.5% orthophosphate, and then the amount of radiolabel incorporated into the peptide substrate was quantified by scintillation counting. Three peptides substrates were used in this study, the NEB peptide p6012 (RRRADDSDDDDD), the custom peptide (RRREDEESDDEE), obtained from NeoMPS, and the eIF2β-derived peptide (MSGDEMIFDPTMSKKKKKKKKP) (40, 45), obtained from GeneCust (Evry, France).
The Kms of PfCK2α for ATP and GTP were determined by performing kinase assays with ATP concentrations of 100 μM, 25 μM, 6.25 μM, and 1.5625 μM. Reactions were carried out in triplicate. The [γ-32P]ATP/GTP was added to the unlabeled ATP/GTP and diluted serially to ensure a constant ratio of labeled-to-unlabeled ATP/GTP. The NEB peptide RRADDSDDDDD (100 μM) was used as the substrate. The Kms of PfCK2α for ATP in the presence or absence of PfCK2β were determined by performing kinase assays in triplicate with ATP concentrations of 100 μM, 50 μM, or 25 μM, with the NeoMPS peptide (100 μM) as a substrate. Reaction mixtures contained 36 ng of PfCK2α alone or in combination with 36 ng of PfCK2β1/shPfCK2β2.
Measurement of 50% inhibitory concentrations (IC50s).
To test the effect of small molecule inhibitors on PfCK2α, kinase activity was measured in the presence of increasing concentrations of these molecules. Stocks of the molecules contained dimethyl sulfoxide or ethanol as a solvent, and negative controls for the reactions were provided by reaction mixtures containing ethanol or dimethyl sulfoxide without the small molecule inhibitor. Kinase reactions were performed by the phosphocellulose method as detailed above.
Interaction assay.
A mixture of 5 μg of each recombinant protein was incubated at 4°C for 30 min in 20 mM Tris-HCl (pH 7.5), 0.2 M NaCl, 0.1% Nonidet P40 (IGEPAL), and 10% glycerol. Glutathione-agarose beads were added to each reaction mixture. The tubes were rotated at 4°C for 1 h; the beads were recovered by centrifugation and washed four times in reaction buffer. Laemmli buffer was added to the beads, which were then heated to 100°C. Samples were separated by SDS-PAGE on 12% acrylamide gels and either stained or transferred to the membrane for Western blot analysis.
Western blot analysis.
Western blotting was performed according to conventional protocols. Briefly, samples were separated by SDS-PAGE on a 12% acrylamide gel and blotted onto nitrocellulose. The membranes were blocked according to standard methods and incubated with rabbit anti-His antibody (1:1000; Santa Cruz Biotechnology) or subunit-specific antibodies generated in rabbits by BioGenes (Germany) against the PfCK2α-derived peptide ADVNIHKPKEYYDY. A goat anti-rabbit secondary antibody coupled to horseradish peroxidase was used at a ratio of 1:10,000. Antigen was visualized using the enhanced chemiluminescence system (PerkinElmer).
Parasite culture and transfection.
Cultures of the P. falciparum strain 3D7A (54) were maintained at 37°C in RPMI 1640 medium (Gibco) supplemented with 25 mM sodium bicarbonate, 2 mM l-glutamine, 300 mM hypoxanthine, 10 μg/ml gentamicin, and AlbuMAX II (Sigma). Cultures were seeded at 5% hematocrit and maintained at a parasitemia of 1 to 10% with daily changes of medium. The incubator was flushed with a gas mixture containing 5% CO2.
For transfection, asexual blood stage parasites were synchronized by sorbitol treatment (26) to obtain a majority of ring stage parasites. Forty-eight hours later, ring stage parasites were transfected by electroporation with 100 μg of purified plasmid DNA in Cytomix buffer as described previously (16, 17, 47). Blasticidin (2.5 μg/ml) was added to the culture medium to select for transformed parasites. Parasites under double selection had WR99210 (5 nM) added to the culture medium in addition to the blasticidin. Parasites were maintained in this supplemented medium from 2 days posttransfection.
Preparation of parasite protein extract.
Parasite cultures were lysed in 0.15% saponin. After centrifugation and washing, the parasite pellets were sonicated in RIPA buffer (30 mM Tris, pH 8.0, 150 mM NaCl, 20 mM MgCl2, 1 mM EDTA, 0.5% Triton X-100, 1% Nonidet P40, 10 mM β-glycerophosphate, 10 mM NaF, 1 mM phenylmethylsulfonyl fluoride, 10 mM benzamidine hydrochlorate hydrate, and Roche complete cocktail protease inhibitors). The lysates were cleared by centrifugation (15,000 rpm for 15 min at 4°C), and the total amount of proteins in the supernatant was measured using the Bradford assay (8).
DNA extraction and Southern blotting.
Parasite cultures were lysed in 0.15% saponin. The parasite pellets were resuspended in cold phosphate-buffered saline and treated with proteinase K (150 μg/ml) and 2% SDS at 55°C for 2 to 3 h. The genomic DNA was extracted in phenol/chloroform/isoamyl alcohol (25:24:1) and precipitated in ethanol and 0.3 M sodium acetate. The DNA was digested with HindIII.
Primers used in genotype characterization.
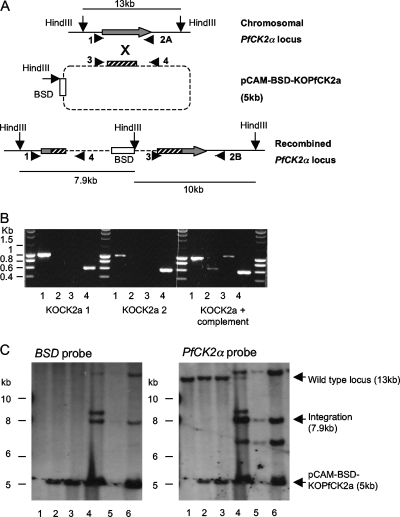
For the detection of integration of the plasmid pCAM-BSD-KOPfCK2α into genomic DNA, the following primers were used (see Fig. 6A for numbering and see Table S1 in the supplemental material for the sequences): primer 1, CK2a5primeF; primer 2a, CK2aR; primer 2b, CK2a3primeR; primer 3, pCAM-BSDF; and primer 4, pCAM-BSDR.
FIG. 6.
PfCK2α is necessary for erythrocytic-stage parasite viability. 3D7 parasites transfected with pCAM-BSD-KOPfCK2a with or without pCHD-PfCK2a were analyzed by PCR and Southern blotting. (A) Disruption strategy for PfCK2α. The locations of the primers used for PCR screening and the HindIII sites used in Southern blotting are shown. (B) PCR screening of genomic DNA (gDNA) from untransfected 3D7 parasites, two separate pCAM-BSD-KOPfCK2a-transfected lines (KOCK2a 1 and KOCK2a 2), and parasites transfected with both the KO plasmid and the complementation plasmid (KOCK2a + complement). Lane 1, amplification of the wild-type PfCK2α locus (primers 1 and 2A; expected size, 1,030 bp). Lane 2, amplification over the 5′ integration boundary (primers 1 and 4; expected size, 677 bp). Lane 3, amplification over the 3′ integration boundary (primers 2B and 3; expected size, 1,060 bp). Lane 4, amplification of the insert in the pCAM-BSD-KOPfCK2a plasmid (primers 3 and 4; expected size, 610 bp). Evidence of integration is seen only in gDNA from the doubly transfected parasite culture (KOCK2a + complement). (C) This parasite line was cloned by limiting dilution, and the gDNA was analyzed by Southern blotting. The restriction enzyme HindIII was used to digest the gDNA, and the fragments were analyzed by Southern blotting using BSD and PfCK2α as probes. Lanes: 1, untransfected 3D7; 2, KOCK2a1; 3, KOCK2a2; 4, KOCK2a plus complement; 5, KOCK2a plus complement clone E7; anf 6, KOCK2a plus complement clone G9.
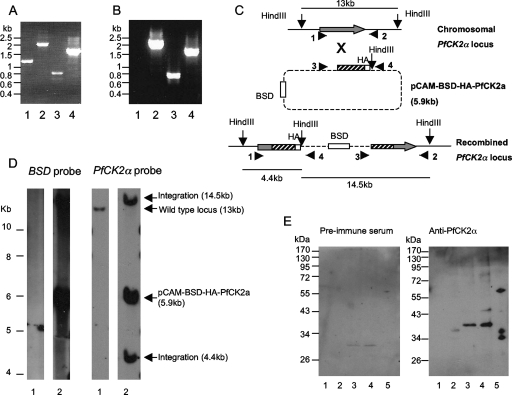
For the detection of integration of the plasmid pCAM-BSD-HA, the following primers were used (see Fig. 7D for numbering and see Table S1 in the supplemental material for the sequences): primer 1, CK2aF; primer 2, CK2a3primeR; primer 3, pCAM-BSDF; and primer 4, pCAM-BSDR.
FIG. 7.
PfCK2α can be targeted for recombination. (A) PCR screening for integration in genomic DNA (gDNA) from parasites transfected with pCAM-BSD-HA-PfCK2a revealed the presence of parasites in which integration events had occurred. Lane 1, amplification of the wild-type locus (primers 1 and 2; expected size, 1,176 bp). Lane 2, amplification over the 5′ integration boundary (primers 1 and 4; expected size, 1,973 bp). Lane 3, amplification over the 3′ integration boundary (primers 2 and 3; expected size, 810 bp). Lane 4, amplification of the insert in the pCAM-BSD-KOPfCK2a plasmid (primers 3 and 4; expected size, 610 bp). This culture still contained the wild-type locus, and therefore clonal lines were derived from the culture by limiting dilution. (B) PCR screening for integration in gDNA from one of the parasite clones revealed that the wild-type band had been lost (lane 1, 1,176 bp) and only the integration bands were seen. (D) This clonal line was further analyzed by Southern blotting. (C) A schematic of the chromosomal gene locus, the pCAM-BSD-HA-PfCK2a plasmid, and the recombined locus, showing the locations of oligonucleotide primers used for the PCR screens (A and B). Oligonucleotide identities are listed in Table S1 in the supplemental material. HindIII sites and the expected sizes of the fragments of gDNA after restriction digestion are shown. (D) Southern blotting. Lanes: 1, untransfected 3D7 parasites; 2, PfCK2αHA clone B3; and 3, PfCK2αHA clone E1. (E) Western blot analysis showing PfCK2α expression in erythrocytic stage parasites. Protein extract from unsynchronized erythrocytic-stage P. falciparum parasites was prepared from wild-type 3D7 parasites (lane 2) and from parasites with a sequence encoding a HA tag incorporated at the 3′ end of the PfCK2α gene locus (lane 3, clone B3; lane 4, clone E1). Protein extract from unparasitized red blood cells (lane 1) and recombinant GST-PfCK2α (lane 5) were included as negative and positive controls. Two identical Western blot analyses were performed with immunopurified rabbit anti-PfCK2α antibodies (right) or with preimmune serum from the same rabbit as a negative control (left). The expected sizes of the proteins are indicated with arrows.
RESULTS
Bioinformatics.
Phylogenetic analysis of P. falciparum protein kinases identified the PlasmoDB (http://plasmodb.org/plasmo) sequence PF11_0096 as that of a CK2α orthologue (3, 57), with 65% amino acid sequence identity to Homo sapiens CK2α. PF11_0096 was therefore named PfCK2α. An alignment of PfCK2α with CK2α subunits from H. sapiens and Zea mays (see Fig. S1 in the supplemental material) reveals that PfCK2α possesses all 11 of the subdomains conserved across eukaryotic protein kinases (21, 22) and the majority of the conserved features of CK2α subunits (2). Just downstream from subdomain II is a putative nuclear localization signal, Pro-Val-Lys-Lys-Lys-Lys-Ile, conserved across CK2α homologues. PfCK2α also possesses three invariant residues common to CK2 family members; the ATP binding motif present in most other protein kinases is Gly-X-Gly-X-X-Gly, whereas in the CK2 family the motif is Gly-X-Gly-X-X-Ser (PfCK2α, Gly50-Ser55). The most highly conserved amino acid motif specific to members of the CK2 family is Asp179-Trp-Gly181 (notation from PfCK2α; most protein kinases display Asp-Phe-Gly at this position). Likewise, Gly203-Pro-Glu205 (notation from PfCK2α) is a common feature of the family, which diverges from the Ala-Pro-Glu motif present in the vast majority of other protein kinases; thus, all three CK2-specific motifs are present in PfCK2α.
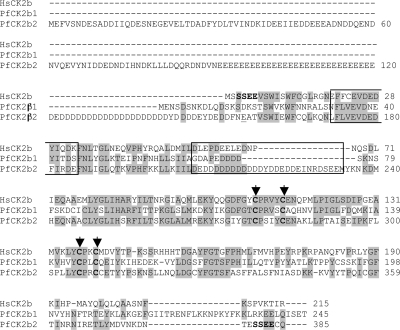
Two putative CK2β subunits were identified in P. falciparum (57), hereafter referred to as PfCK2β1 and PfCK2β2 (PlasmoDB identifiers PF11_0048 and PF13_0232, respectively). BLASTP searches using the putative PfCK2β1/PfCK2β2 amino acid sequences as queries confirmed their identities as CK2β orthologues. An alignment with the human CK2β sequence (HsCK2β) (Fig. 1) reveals that many of the conserved features of CK2β subunits, including the four cysteine residues responsible for zinc finger formation (12), are present in PfCK2β1 and PfCK2β2 (e.g., Cys117, -122, -145, and -148 for PfCK2β1) (Fig. 1). The human CK2β sequence has a well-documented CK2 phosphorylation site at the N terminus (SSEE). PfCK2β2 possesses several phosphorylatable residues in the N-terminal region that are surrounded by a number of acidic residues, which could therefore be phosphorylated by CK2, and a TESSEE sequence at the C terminus reminiscent of the HsCK2β N-terminal phosphorylation site (MSSEE). The stretch of amino acids found to be necessary for the export of CK2 as an ectokinase (CK2β amino acids E20 to K33) (44) are largely conserved in the PfCK2β sequences, leading to the intriguing possibility that PfCK2 may be exported from the parasite. The acidic stretch responsible for downregulation of CK2 activity and association with the plasma membrane (HsCK2β amino acids D55 to D64) (29, 32) is present in PfCK2β1 (D68 to D75) and extended in PfCK2β2 (D207 to E226). This insertion occurs in a region looping out from the main protein structure (12) and is not unique; for example, Saccharomyces cerevisiae CK2β has an insertion sequence of 30 amino acids in this location. Along with the insertion region, PfCK2β2 has a highly acidic and repetitive N-terminal extension that is not found in other CK2β subunits. The N-terminal region is at the periphery of the 3D structure of the human CK2β peptide (12, 35) and is not a conserved part of the CK2β structure and thus may possibly function as a docking site or region that interacts with binding partners or substrates of the PfCK2 holoenzyme. The human CK2β is phosphorylated at S209 in a cell cycle-dependent manner by p34cdc2 (19, 30, 33), although the function of this phosphorylation is unknown. Both PfCK2β subunits possess serine residues near the C terminus that could be phosphorylated.
FIG. 1.
Alignment of PfCK2β1 and PfCK2β2 with Homo sapiens CK2β. ClustalW alignments of the following proteins were performed: Homo sapiens CK2β (HsCK2β; AAM50092); PfCK2β1 (AAN35637; PlasmoDB PF11_0048; 33% identical to HsCK2β), PfCK2β2 (CAD52554; PlasmoDB PF13_0232; 39% identical to HsCK2β). Note the long N-terminal extension and acidic insertion sequence in PfCK2β2. The sequence of shPfCK2β2 begins (after an artificially introduced initiating methionine) with residue E156, underlined. The cysteine residues thought to hold the zinc finger in place are indicated in bold text and with arrowheads. The potential autophosphorylation site on PfCK2β2 (SSEE) is indicated in bold. The export of CK2 as an ectokinase is mediated by CK2β residues 20 to 33 (44) (boxed, along with the equivalent residues in PfCK2β1 and PfCK2β2). The acidic region mentioned in the text is also boxed (D107 to E133 of PfCK2β2).
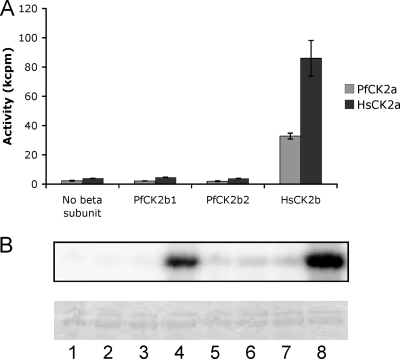
Microarray data available on PlasmoDB reveal that the mRNAs encoding all three subunits are detectable throughout the parasite life cycle (7, 28). Expression of the PfCK2α protein in asexual blood stage parasites was verified by Western blot analysis (see Fig. 7F).
In vitro activity of PfCK2α.
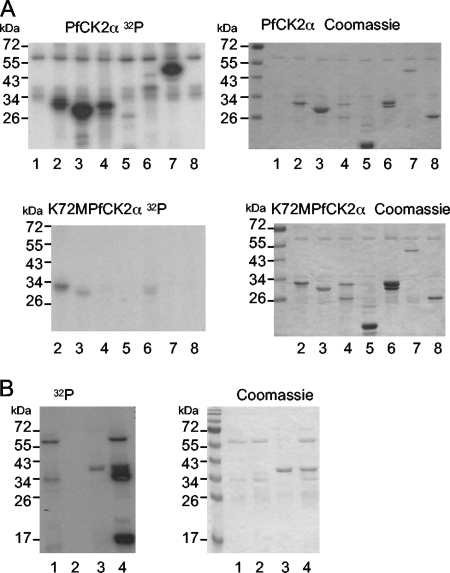
Kinase activity of bacterially expressed recombinant GST-PfCK2α was detected by an in vitro kinase assay (Fig. 2, top). GST-PfCK2α autophosphorylates (the 66-kDa band present in each lane in Fig. 2 corresponds to the size of the GST-tagged PfCK2α subunit) and is capable of phosphorylating a range of exogenous substrates, with strongest activity toward the caseins (Fig. 2, lanes 2 to 4) and recombinant GST-tagged shPfCK2β2, a short version of the PfCK2β2 subunit lacking the N-terminal extension (see Materials and Methods) (Fig. 2, lane 7). There was no activity against the GST moiety alone (Fig. 2, lane 8), indicating that the activity in lane 7 was against the beta subunit itself and that the autophosphorylation is against the PfCK2α subunit itself. We did not detect any activity against the PfCK2β1 subunit (data not shown). These observations are consistent with general preferences of CK2 homologues for substrates with highly acidic phosphoacceptor sites (11, 25, 39, 52, 56); several potential such sites are present on PfCK2β2. Parallel assays performed with an inactive PfCK2α mutant (Lys72Met; the Lys residue is required for correct orientation of the ATP molecule) were negative for kinase activity, confirming that activity is indeed due to PfCK2α (Fig. 2, bottom). PfCK2α autophosphorylates by a transreaction (Fig. 2B); GST-PfCK2α and PfCK2α-His autophosphorylate (Fig. 2, lanes 1 and 3), while GST-K72MPfCK2α does not (Fig. 2, lane 2) but is phosphorylated in the presence of PfCK2α-His, indicating that at least a proportion of the autophosphorylation of PfCK2α occurs by an intermolecular reaction.
FIG. 2.
GST-PfCK2α kinase activity. (A) Autoradiograms (left) and Coomassie blue-stained gels (right) of kinase assays performed with GST-PfCK2α (top) or catalytically inactive GST-K72MPfCK2α (bottom) and the following substrates: lane 1, no substrate; lane 2, α-casein; lane 3, β-casein; lane 4, mixed dephosphorylated caseins; lane 5, myelin basic protein; lane 6, histone H1; lane 7, GST-shPfCK2β2; and lane 8, GST. (B) Autophosphorylation can occur by a transreaction. Left, autoradiogram; right, Coomassie blue-stained gel. The band observed at roughly 66 kDa is the GST-PfCK2α autophosphorylation band, and the band at roughly 40 kDa is the PfCK2α-His autophosphorylation band. Lane 1, GST-PfCK2α; lane 2, GST-K72MPfCK2α; lane 3, PfCK2α-His; and lane 4, GST-K72MPfCK2α plus PfCK2α-His. Radioactive phosphate is incorporated into catalytically inactive PfCK2α (K72MPfCK2α) in the presence of PfCK2α-His, indicating that autophosphorylation can occur by an intermolecular reaction.
PfCK2α shares features in common with CK2α from other systems.
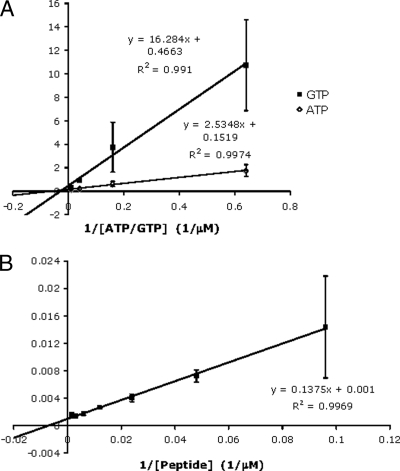
A feature often cited as being characteristic of CK2 enzymes is that they have similar affinities for GTP and ATP. PfCK2α has a Km of 16.7 μM and Vmax of 6.6 nmol/min for ATP and a Km of 34.9 μM and Vmax of 2.1 nmol/min for GTP (Fig. 3A). The enzyme displays a number of other features that confirm it as a true member of the CK2α family: (i) PfCK2α is able to phosphorylate the CK2 substrate peptide RRRADDSDDDDD (NEB), with a Km of 137.5 μM (Fig. 3B); (ii) CK2α enzymes are known to have a wide variety of substrates (34), and correspondingly, PfCK2α (but not the K72M mutant protein used as a negative control) phosphorylates a number of proteins within heat-inactivated parasite protein extract (data not shown); (iii) the activity of PfCK2α is inhibited by the well-established CK2-specific inhibitor TBB (3,4,5,6-tetrabromobenzotriazole), with a similar IC50 curve to that of human CK2α (IC50 for PfCK2α, 2 μM; IC50 for HsCK2α, 1.5 μM) (see Fig. 8C); and (iv) it can be recruited by the human CK2β subunit to phosphorylate the eIF2β-derived peptide (40, 45) and the Olig2 protein (27) (Fig. 4). This is in line with the established ability of human CK2, but not CK2α alone, to phosphorylate the substrates used in this experiment (27, 40).
FIG. 3.
PfCK2α kinetics. The enzyme kinetics of PfCK2α in a Lineweaver-Burke presentation. The experiments were performed in triplicate, the data points represent the means, and the error bars represent three standard deviations. (A) The graph was obtained by linear regression of the enzyme kinetic data for ATP and GTP. The intercepts on the x axis give the negative reciprocal of the Km, and the intercepts on the y axis give the reciprocal of the Vmax. (B) The graph was obtained by linear regression of the enzyme kinetic data for the NEB peptide RRRADDSDDDDD. The intercept on the x axis gives the negative reciprocal of the Km.
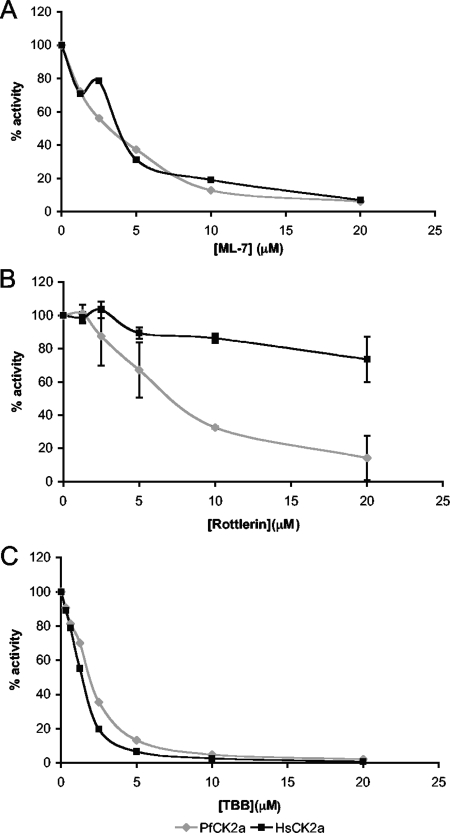
FIG. 8.
PfCK2α can be distinguished from HsCK2α by small molecule inhibitors. Two small molecules, ML-7 and Rottlerin, were identified in a primary screen as inhibiting the activity of PfCK2α to below 10% of the uninhibited enzyme. The inhibitors Rottlerin and ML-7 were included in increasing concentrations in kinase assays with 25 μM ATP, 36 ng of enzyme, and the peptide RRREDEESDDEE as substrate. Activity was measured using the phosphocellulose assay method, and results were scored as a percentage of the control (no inhibitor). (A) ML-7. (B) Rottlerin. Mean values from two experiments are shown, with the error bars representing the standard deviations. (C) The classical CK2 inhibitor, TBB, has a similar inhibitory profile for PfCK2α and HsCK2α.
FIG. 4.
PfCK2α and HsCK2β interact in vitro. To further test the interactions of the alpha and beta subunits, two substrates that are phosphorylated by the CK2 holoenzyme and not by the CK2α subunit alone were included in kinase assays with mixtures of human and P. falciparum alpha and beta subunits. (A) Phosphorylation of the eIF2β[1-22] peptide (40, 45) by PfCK2α-His or HsCK2α in the presence and absence of GST-PfCK2β1, GST-shPfCK2β2, or HsCK2β was measured by kinase assays, and the amount of radiolabel incorporated into the peptide was counted by scintillation. Results are shown as the means of two experiments, with the error bars representing the standard deviations. (B) Phosphorylation of the GST-Olig2[1-177] protein (27) by PfCK2α-His (lanes 1 to 4) or HsCK2α (lanes 5 to 8) alone (lanes 1 and 5) or in the presence of GST-PfCK2β1 (lanes 2 and 6), GST-shPfCK2β2 (lanes 3 and 7), or HsCK2β (lanes 4 and 8). Top, autoradiogram; bottom, corresponding Coomassie blue-stained gel of the kinase assay.
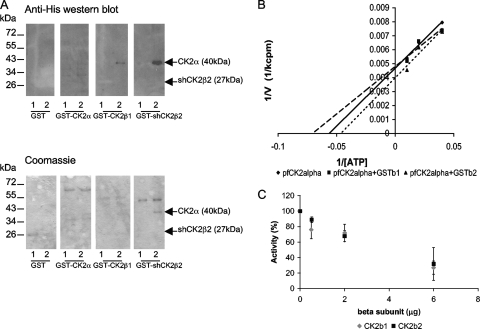
PfCK2α and the PfCK2 beta subunits interact in vitro.
To assess whether the two regulatory PfCK2 subunits are able to associate with PfCK2α in vitro, all three subunits were expressed in E. coli as His- or GST-tagged proteins and used in pull-down experiments. Mixtures of His- and GST-tagged proteins were prepared, from which proteins were pulled down using glutathione beads. The pulled-down proteins were then subjected to Western blot analysis using an anti-His antibody to detect any bound His-tagged protein that was copurified with the GST-tagged proteins. PfCK2α-His was copurified with both GST-tagged beta subunits but not with GST alone (Fig. 5A). The interaction does not significantly alter the Km for ATP (Fig. 5B) or the phosphorylation of calmodulin or the peptide RRRSDDSDDDDD (data not shown). However, the interaction of the beta subunits with the kinase has functional significance, at least in vitro, since the activity of the kinase toward β-casein is reduced with increasing amounts of GST-PfCK2β present in the reaction mixtures (Fig. 5C).
FIG. 5.
PfCK2α and PfCK2β subunits interact in vitro. (A) Pull-down assays. Each of the GST-tagged subunits (indicated at the bottom of each panel) was incubated with PfCK2α-His (lane 1) or His-shPfCK2β2 (lane 2). Complexes containing the GST-tagged subunits were then purified using glutathione agarose beads, and any bound His-tagged subunits were detected by Western blot analysis. Top, anti-His Western blots. Bottom, Coomassie blue-stained gels. (B) The PfCK2α Km for ATP is not greatly altered in the presence of the PfCK2β subunits. ATP concentrations of 100 μM, 50 μM, and 25 μM were used. Reaction mixtures contained PfCK2α alone or with equimolar amounts of PfCK2β1 or shPfCK2β2. (C) Phosphorylation of β-casein by PfCK2αHis in the presence of increasing concentrations of GST-tagged PfCK2α subunits (and concomitant decreases in the concentration of GST) was quantified by phosphorimaging. Standard kinase assays were performed with 1 μg of β-casein, 1 μg of PfCK2αHis, and 6 μg of GST/GST-PfCK2β in various proportions. The graph represents the combined results of three experiments (means and standard deviations shown).
PfCK2α is essential for completion of the erythrocytic asexual cycle.
We next wanted to determine whether PfCK2α plays essential functions in parasite survival. To generate a plasmid able to disrupt the PfCK2α gene, an internal fragment of the coding sequence, excluding the critical motifs Gly-X-Gly-X-X-Ser (subdomain I, involved in anchoring of the ATP molecule) and Gly-Pro-Glu (subdomain VIII, required for structural stability of the C-terminal lobe) (see Fig. S1 in the supplemental material), was amplified and cloned into the transfection vector pCAM-BSD (47), which confers resistance to blasticidin. Integration of this construct (pCAM-BSD-KOPfCK2a) into the genomic locus by single-crossover homologous recombination is expected to result in a pseudodiploid configuration, where both truncated copies will be unable to express a functional enzyme, since both will lack one of the essential motifs (Fig. 6A).
After two independent transfections of pCAM-BSD-KOPfCK2a into 3D7 parasites, integration was monitored in the blasticidin-resistant populations by PCR (Fig. 6B), using primer combinations that allow discrimination between the episome, the wild-type locus, and the disrupted locus. Only the episome and the wild-type locus were detectable, with no sign of integration even after prolonged culturing (16 weeks); in contrast, we regularly observe disruption of nonessential genes 6 to 7 weeks posttransfection (for an example, see reference 42). This might be due either to the fact that the presence of an intact PfCK2α gene is crucial for parasite asexual multiplication or to the possible nonrecombinogenicity of the locus. To verify that the PfCK2α locus is indeed recombinogenic, we proceeded to transfect wild-type parasites with a “3′-tagging” construct whose integration was expected not to cause loss of function of the target protein. We readily observed integration of the tagging construct (Fig. 7A to E) and size increase in the PfCK2α protein caused by the HA tag (1.1 kDa) (Fig. 7F). This demonstrates that the locus is accessible to recombination if no loss of function is incurred, as is presumably the case with HA tagging, and therefore strengthens the case that PfCK2α is essential for the parasite's asexual cycle.
We nevertheless wanted to ascertain that PfCK2α can be disrupted if the enzyme is provided through expression of an extraneous copy of the gene. To this effect, a complementation plasmid was constructed, containing the full-length PfCK2α coding region under the control of the Pfhsp86 promoter and preceding a 3′ untranslated region (namely, the Plasmodium berghei dihydrofolate reductase terminator sequence). The P. falciparum hsp86 gene (PF07_0029) displays a similar mRNA expression profile to the PfCK2α gene (28); therefore, its promoter is presumably appropriate to drive expression of the complementing protein. In parallel with the transfection of the pCAM-BSD-KOPfCK2a plasmid alone, further populations of parasites were cotransfected with both pCAM-BSD-KOPfCK2a and the complementation plasmid. PCR analysis (Fig. 6B, right) showed that disruption of the targeted locus occurred only in the doubly transfected, doubly resistant parasites. Southern blot analysis independently confirmed that integration occurred only in the doubly transfected parasites (Fig. 6C). The 13-kb band that represents the wild-type locus dramatically decreased in the doubly transfected parasites and was undetectable in two clonal lines (E7 and G9) that were derived from this culture by limiting dilution. There are multiple possibilities for the recombination of the KO and complementation plasmids with each other before or after integration, which could account for the additional bands of unexpected size observed (6 kb and 14 kb). The most important observation is that the wild-type band disappears only in the doubly transfected parasites.
Taken together, these data provide strong evidence that PfCK2α is essential to viability of the asexual erythrocytic stage parasites.
PfCK2α kinase activity is amenable to inhibition.
Using a kinase-directed inhibitor library, we conducted a screen for compounds that inhibit PfCK2α. This screen identified the compounds Rottlerin and ML-7 as inhibitors of PfCK2α. The IC50s of these compounds were determined for both PfCK2α and HsCK2α (Fig. 8). While ML-7 inhibits both enzymes with an IC50 of roughly 3 to 4 μM, Rottlerin exhibits differential effects on the orthologues, inhibiting PfCK2α with an IC50 of 7 μM and HsCK2α with an IC50 of ≫20 μM. This result indicates that differential inhibition is possible, despite the high percent identity (65%) between the CK2α amino acid sequences of P. falciparum and Homo sapiens.
DISCUSSION
We have characterized a P. falciparum CK2α orthologue and confirmed that the recombinant enzyme exhibits kinase activity in vitro and exhibits features in common with other CK2α enzymes. PfCK2α contains the major motifs conserved across CK2 catalytic subunits, phosphorylates acidic sequences, interacts with the putative PfCK2β subunits and the HsCK2β subunit, is inhibited by the classic CK2 inhibitor TBB (3,4,5,6-tetrabromobenzotriazole), with a similar IC50 to that of HsCK2α, and is able to utilize GTP or ATP as a cosubstrate. We have also confirmed the identity of two PfCK2β subunits. The N-terminal extension of PfCK2β2 is unusually long for CK2β proteins, with 160 amino acids before the first conserved residue (Trp161 in PfCK2β2). Most CK2β subunits from vertebrates have only eight amino acids prior to this conserved residue (Homo sapiens, Gallus gallus, Mus musculus, Xenopus tropicalis, Bos taurus, and Danio rerio); this N-terminal extension is expanded in yeast (Saccharomyces cerevisiae, 37 residues), trypanosomatids (Trypanosoma brucei, 27 residues; Leishmania major, 21 residues), plants (Arabidopsis thaliana, 100 residues; Oryza sativa, 92 residues), and alveolates (Cryptosporidium parvum, 27 residues; Theileria parva, 34 residues). Within the alveolates, Plasmodium yoelii yoelii (125 residues) and Plasmodium vivax (157 residues) also have long extensions, but the extension of P. falciparum is the longest known. Homorepeat-containing proteins make up 35.7% of the proteome of P. falciparum, although the majority of these homorepeats are asparagines and lysines (48), unlike the polymers of acidic residues present in PfCK2β2. One hypothesis for the function of this extension is the downregulation of the alpha subunit. Polyglutamate is a potent CK2 inhibitor (50), and the N-terminal extension of PfCK2β2 is rich in polyglutamate and polyaspartate. This beta subunit also possesses an insertion of extra acidic residues (including a stretch of 11 consecutive aspartates) (Fig. 1) in the acidic domain known to downregulate CK2 (32). We have not been able to purify PfCK2β2 with the N-terminal extension, and therefore this hypothesis remains to be tested. However, we showed that the presence of either beta subunit reduces the activity of PfCK2α toward β-casein; such modulation of activity has been seen for other CK2s, often in a substrate-dependent fashion, For example, CK2β stimulates human CK2 activity toward topoisomerase II and p53 and inhibits activity toward calmodulin (6). Thus, the presence of two beta subunit species in P. falciparum (whereas there is only one in human cells) is likely to allow exquisite control of the activity of the catalytic subunit. The interactions we detected in vitro between the recombinant catalytic and regulatory subunits suggest that the P. falciparum CK2 subunits may form a similar holoenzyme structure to that seen in other organisms, although the stoichiometry of the complex in vivo will require detailed analysis of parasite extracts in nondenaturing conditions.
The existence of a number of discrete subpopulations of mammalian CK2 associated with different cellular compartments has been recognized (36), and it has been proposed that this is mediated by assembly of CK2 subunits as well as interaction with many other proteins. Our observation that the alpha and beta subunits possess putative signals for nuclear localization and protein export, respectively, suggests that in Plasmodium spp., like in other eukaryotes, CK2 may localize to a variety of compartments. Work is in progress to address this issue.
CK2α has been shown to be essential for life for a variety of organisms (24, 31, 37). We have demonstrated here that PfCK2α is required for parasite viability. We show that parasites lacking the enzyme are unable to survive or are impaired in their growth rate to such an extent that they are outcompeted by the parasites which retain wild-type genes. Our approach allows us to conclude that PfCK2α plays an important role during erythrocytic schizogony but does not provide any information about the molecular basis for essentiality. We are addressing this issue in a number of ways, including conditional expression based on a destabilization domain (4), localization (see above), and identification of interacting partners. Nevertheless, our data validate PfCK2 as a potential drug target. We have also demonstrated that PfCK2α is amenable to inhibition assays. Active HsCK2α is present in erythrocytes (53); this raises the question of selectivity of antimalarial inhibitors based on PfCK2α inhibition. We demonstrated that a small molecule inhibitor, Rottlerin, has a much lower IC50 for PfCK2α than for HsCK2α. Although we have identified in Rottlerin a compound that can distinguish between the human and plasmodial CK2α enzymes, it is unlikely to represent a suitable starting point for antimalarial drug discovery, since Rottlerin has multiple targets (41) and is too weak and nonspecific an inhibitor even to be used in cellular assays (5). However, we have established that differential inhibition is possible, despite the 65% identity between the primary sequences of PfCK2α and HsCK2α, which suggests that specific inhibition of the plasmodial (versus host) enzyme should be feasible. The level of activity of recombinant PfCK2α is such that the development of a high-throughput assay should be possible, opening the way for screening of chemical libraries as a first step toward antimalarial drug discovery based on PfCK2α inhibition.
Supplementary Material
Acknowledgments
We are grateful to D. Fidock (Columbia University, NY), who provided the pCAM-BSD plasmid, G. McFadden (University of Melbourne) for providing the pHGB vector system, D. Chakrabarti for providing the PfCK2a-pET29 expression plasmid, and D. Jacobus (Princeton) for WR99210. We are grateful to L. Reininger and D. Dorin-Semblat for frequent discussions and to J. Chevalier (Service Scientifique de l'Ambassade de France à Londres) for continuing interest and support.
Work in the C.D. laboratory is supported by INSERM, the FP6 (SIGMAL and ANTIMAL projects and the BioMalPar Network of Excellence) and FP7 (MALSIG project) programs of the European Commission, and a grant from the Novartis Institute for Tropical Diseases. Work in the C.C. laboratory is supported by INSERM, the Ligue Nationale Contre le Cancer, and the INCA. Z.H. is the recipient of a Wellcome Trust Ph.D. studentship.
Footnotes
Published ahead of print on 29 December 2008.
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1.Ahmed, K., D. A. Gerber, and C. Cochet. 2002. Joining the cell survival squad: an emerging role for protein kinase CK2. Trends Cell Biol. 12226-230. [DOI] [PubMed] [Google Scholar]
- 2.Allende, J. E., and C. C. Allende. 1995. Protein kinases 4. Protein kinase CK2: an enzyme with multiple substrates and a puzzling regulation. FASEB J. 9313-323. [DOI] [PubMed] [Google Scholar]
- 3.Anamika, N. Srinivasan, and A. Krupa. 2005. A genomic perspective of protein kinases in Plasmodium falciparum. Proteins 58180-189. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong, C. M., and D. E. Goldberg. 2007. An FKBP destabilization domain modulates protein levels in Plasmodium falciparum. Nat. Methods 41007-1009. [DOI] [PubMed] [Google Scholar]
- 5.Bain, J., L. Plater, M. Elliott, N. Shpiro, C. J. Hastie, H. McLauchlan, I. Klevernic, J. S. Arthur, D. R. Alessi, and P. Cohen. 2007. The selectivity of protein kinase inhibitors: a further update. Biochem. J. 408297-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bibby, A. C., and D. W. Litchfield. 2005. The multiple personalities of the regulatory subunit of protein kinase CK2: CK2 dependent and CK2 independent roles reveal a secret identity for CK2β. Int. J. Biol. Sci. 167-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bozdech, Z., M. Llinas, B. L. Pulliam, E. D. Wong, J. Zhu, and J. L. DeRisi. 2003. The transcriptome of the intraerythrocytic developmental cycle of Plasmodium falciparum. PLoS Biol. 1E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72248-254. [DOI] [PubMed] [Google Scholar]
- 9.Breman, J. G. 2001. The ears of the hippopotamus: manifestations, determinants, and estimates of the malaria burden. Am. J. Trop. Med. Hyg. 641-11. [DOI] [PubMed] [Google Scholar]
- 10.Breman, J. G., M. S. Alilio, and A. Mills. 2004. Conquering the intolerable burden of malaria: what's new, what's needed: a summary. Am. J. Trop. Med. Hyg. 711-15. [PubMed] [Google Scholar]
- 11.Chan, P. K., M. Aldrich, R. G. Cook, and H. Busch. 1986. Amino acid sequence of protein B23 phosphorylation site. J. Biol. Chem. 2611868-1872. [PubMed] [Google Scholar]
- 12.Chantalat, L., D. Leroy, O. Filhol, A. Nueda, M. J. Benitez, E. M. Chambaz, C. Cochet, and O. Dideberg. 1999. Crystal structure of the human protein kinase CK2 regulatory subunit reveals its zinc finger-mediated dimerization. EMBO J. 182930-2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen, P. 2002. Protein kinases—the major drug targets of the twenty-first century? Nat. Rev. Drug Discov. 1309-315. [DOI] [PubMed] [Google Scholar]
- 14.Doerig, C. 2004. Protein kinases as targets for anti-parasitic chemotherapy. Biochim. Biophys. Acta 1697155-168. [DOI] [PubMed] [Google Scholar]
- 15.Doerig, C., and L. Meijer. 2007. Antimalarial drug discovery: targeting protein kinases. Expert Opin. Ther. Targets. 11279-290. [DOI] [PubMed] [Google Scholar]
- 16.Fidock, D. A., T. Nomura, R. A. Cooper, X. Su, A. K. Talley, and T. E. Wellems. 2000. Allelic modifications of the cg2 and cg1 genes do not alter the chloroquine response of drug-resistant Plasmodium falciparum. Mol. Biochem. Parasitol. 1101-10. [DOI] [PubMed] [Google Scholar]
- 17.Fidock, D. A., and T. E. Wellems. 1997. Transformation with human dihydrofolate reductase renders malaria parasites insensitive to WR99210 but does not affect the intrinsic activity of proguanil. Proc. Natl. Acad. Sci. USA 9410931-10936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gardner, M. J., N. Hall, E. Fung, O. White, M. Berriman, R. W. Hyman, J. M. Carlton, A. Pain, K. E. Nelson, S. Bowman, I. T. Paulsen, K. James, J. A. Eisen, K. Rutherford, S. L. Salzberg, A. Craig, S. Kyes, M. S. Chan, V. Nene, S. J. Shallom, B. Suh, J. Peterson, S. Angiuoli, M. Pertea, J. Allen, J. Selengut, D. Haft, M. W. Mather, A. B. Vaidya, D. M. Martin, A. H. Fairlamb, M. J. Fraunholz, D. S. Roos, S. A. Ralph, G. I. McFadden, L. M. Cummings, G. M. Subramanian, C. Mungall, J. C. Venter, D. J. Carucci, S. L. Hoffman, C. Newbold, R. W. Davis, C. M. Fraser, and B. Barrell. 2002. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 419498-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gietz, R. D., K. C. Graham, and D. W. Litchfield. 1995. Interactions between the subunits of casein kinase II. J. Biol. Chem. 27013017-13021. [DOI] [PubMed] [Google Scholar]
- 20.Glass, D. B., R. A. Masaracchia, J. R. Feramisco, and B. E. Kemp. 1978. Isolation of phosphorylated peptides and proteins on ion exchange papers. Anal. Biochem. 87566-575. [DOI] [PubMed] [Google Scholar]
- 21.Hanks, S. K., and T. Hunter. 1995. Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J. 9576-596. [PubMed] [Google Scholar]
- 22.Hanks, S. K., and A. M. Quinn. 1991. Protein kinase catalytic domain sequence database: identification of conserved features of primary structure and classification of family members. Methods Enzymol. 20038-62. [DOI] [PubMed] [Google Scholar]
- 23.Ho, S. N., H. D. Hunt, R. M. Horton, J. K. Pullen, and L. R. Pease. 1989. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 7751-59. [DOI] [PubMed] [Google Scholar]
- 24.Kikkawa, U., S. K. O. Mann, R. A. Firtel, and T. Hunter. 1992. Molecular cloning of casein kinase II α subunit from Dictyostelium discoideum and its expression in the life cycle. Mol. Cell. Biol. 125711-5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuenzel, E. A., J. A. Mulligan, J. Sommercorn, and E. G. Krebs. 1987. Substrate specificity determinants for casein kinase II as deduced from studies with synthetic peptides. J. Biol. Chem. 2629136-9140. [PubMed] [Google Scholar]
- 26.Lambros, C., and J. P. Vanderberg. 1979. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 65418-420. [PubMed] [Google Scholar]
- 27.Laudet, B. A., C. Barette, V. Dulery, O. Renaudet, P. Dumy, A. Metz, R. Prudent, A. Deshiere, O. Dideberg, O. Filhol, and C. Cochet. 2007. Structure-based design of small peptide inhibitors of protein kinase CK2 subunit interaction. Biochem. J. 408363-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le Roch, K. G., Y. Zhou, P. L. Blair, M. Grainger, J. K. Moch, J. D. Haynes, P. De La Vega, A. A. Holder, S. Batalov, D. J. Carucci, and E. A. Winzeler. 2003. Discovery of gene function by expression profiling of the malaria parasite life cycle. Science 3011503-1508. [DOI] [PubMed] [Google Scholar]
- 29.Leroy, D., O. Filhol, N. Quintaine, D. Sarrouilhe, P. Loue-Mackenbach, E. M. Chambaz, and C. Cochet. 1999. Dissecting subdomains involved in multiple functions of the CK2β subunit. Mol. Cell. Biochem. 19143-50. [PubMed] [Google Scholar]
- 30.Litchfield, D. W., F. J. Lozeman, M. F. Cicirelli, M. Harrylock, L. H. Ericsson, C. J. Piening, and E. G. Krebs. 1991. Phosphorylation of the beta subunit of casein kinase II in human A431 cells. Identification of the autophosphorylation site and a site phosphorylated by p34cdc2. J. Biol. Chem. 26620380-20389. [PubMed] [Google Scholar]
- 31.Lou, D. Y., I. Dominguez, P. Toselli, E. Landesman-Bollag, C. O'Brien, and D. C. Seldin. 2008. The alpha catalytic subunit of protein kinase CK2 is required for mouse embryonic development. Mol. Cell. Biol. 28131-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meggio, F., B. Boldyreff, O. G. Issinger, and L. A. Pinna. 1994. Casein kinase 2 down-regulation and activation by polybasic peptides are mediated by acidic residues in the 55-64 region of the beta-subunit. A study with calmodulin as phosphorylatable substrate. Biochemistry 334336-4342. [DOI] [PubMed] [Google Scholar]
- 33.Meggio, F., B. Boldyreff, O. Marin, O. G. Issinger, and L. A. Pinna. 1995. Phosphorylation and activation of protein kinase CK2 by p34cdc2 are independent events. Eur. J. Biochem. 2301025-1031. [DOI] [PubMed] [Google Scholar]
- 34.Meggio, F., and L. A. Pinna. 2003. One-thousand-and-one substrates of protein kinase CK2? FASEB J. 17349-368. [DOI] [PubMed] [Google Scholar]
- 35.Niefind, K., B. Guerra, I. Ermakowa, and O. G. Issinger. 2001. Crystal structure of human protein kinase CK2: insights into basic properties of the CK2 holoenzyme. EMBO J. 205320-5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olsten, M. E., and D. W. Litchfield. 2004. Order or chaos? An evaluation of the regulation of protein kinase CK2. Biochem. Cell Biol. 82681-693. [DOI] [PubMed] [Google Scholar]
- 37.Padmanabha, R., J. L. Chen-Wu, D. E. Hanna, and C. V. Glover. 1990. Isolation, sequencing, and disruption of the yeast CKA2 gene: casein kinase II is essential for viability in Saccharomyces cerevisiae. Mol. Cell. Biol. 104089-4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pinna, L. A. 2002. Protein kinase CK2: a challenge to canons. J. Cell Sci. 1153873-3878. [DOI] [PubMed] [Google Scholar]
- 39.Pinna, L. A., A. Donella-Deana, and F. Meggio. 1979. Structural features determining the site specificity of a rat liver cAMP-independent protein kinase. Biochem. Biophys. Res. Commun. 87114-120. [DOI] [PubMed] [Google Scholar]
- 40.Poletto, G., J. Vilardell, O. Marin, M. A. Pagano, G. Cozza, S. Sarno, A. Falques, E. Itarte, L. A. Pinna, and F. Meggio. 2008. The regulatory beta subunit of protein kinase CK2 contributes to the recognition of the substrate consensus sequence. A study with an eIF2 beta-derived peptide. Biochemistry 478317-8325. [DOI] [PubMed] [Google Scholar]
- 41.Redwood, C., S. L. Davies, N. J. Wells, A. M. Fry, and I. D. Hickson. 1998. Casein kinase II stabilizes the activity of human topoisomerase IIalpha in a phosphorylation-independent manner. J. Biol. Chem. 2733635-3642. [DOI] [PubMed] [Google Scholar]
- 42.Reininger, L., O. Billker, R. Tewari, A. Mukhopadhyay, C. Fennell, D. Dorin-Semblat, C. Doerig, D. Goldring, L. Harmse, L. Ranford-Cartwright, J. Packer, and C. Doerig. 2005. A NIMA-related protein kinase is essential for completion of the sexual cycle of malaria parasites. J. Biol. Chem. 28031957-31964. [DOI] [PubMed] [Google Scholar]
- 43.Ridley, R. G. 2002. Medical need, scientific opportunity and the drive for antimalarial drugs. Nature 415686-693. [DOI] [PubMed] [Google Scholar]
- 44.Rodriguez, F. A., C. Contreras, V. Bolanos-Garcia, and J. E. Allende. 2008. Protein kinase CK2 as an ectokinase: the role of the regulatory CK2beta subunit. Proc. Natl. Acad. Sci. USA 1055693-5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salvi, M., S. Sarno, O. Marin, F. Meggio, E. Itarte, and L. A. Pinna. 2006. Discrimination between the activity of protein kinase CK2 holoenzyme and its catalytic subunits. FEBS Lett. 5803948-3952. [DOI] [PubMed] [Google Scholar]
- 46.Sarno, S., S. Moro, F. Meggio, G. Zagotto, D. Dal Ben, P. Ghisellini, R. Battistutta, G. Zanotti, and L. A. Pinna. 2002. Toward the rational design of protein kinase casein kinase-2 inhibitors. Pharmacol. Ther. 93159-168. [DOI] [PubMed] [Google Scholar]
- 47.Sidhu, A. B., S. G. Valderramos, and D. A. Fidock. 2005. pfmdr1 mutations contribute to quinine resistance and enhance mefloquine and artemisinin sensitivity in Plasmodium falciparum. Mol. Microbiol. 57913-926. [DOI] [PubMed] [Google Scholar]
- 48.Singh, G. P., B. R. Chandra, A. Bhattacharya, R. R. Akhouri, S. K. Singh, and A. Sharma. 2004. Hyper-expansion of asparagines correlates with an abundance of proteins with prion-like domains in Plasmodium falciparum. Mol. Biochem. Parasitol. 137307-319. [DOI] [PubMed] [Google Scholar]
- 49.Snow, R. W., C. A. Guerra, A. M. Noor, H. Y. Myint, and S. I. Hay. 2005. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 434214-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tellez, R., M. Gatica, C. C. Allende, and J. E. Allende. 1990. Copolymers of glutamic acid and tyrosine are potent inhibitors of oocyte casein kinase II. FEBS Lett. 265113-116. [DOI] [PubMed] [Google Scholar]
- 51.Tonkin, C. J., G. G. van Dooren, T. P. Spurck, N. S. Struck, R. T. Good, E. Handman, A. F. Cowman, and G. I. McFadden. 2004. Localization of organellar proteins in Plasmodium falciparum using a novel set of transfection vectors and a new immunofluorescence fixation method. Mol. Biochem. Parasitol. 13713-21. [DOI] [PubMed] [Google Scholar]
- 52.Tuazon, P. T., E. W. Bingham, and J. A. Traugh. 1979. Cyclic nucleotide-independent protein kinases from rabbit reticulocytes. Site-specific phosphorylation of casein variants. Eur. J. Biochem. 94497-504. [DOI] [PubMed] [Google Scholar]
- 53.Uhle, S., O. Medalia, R. Waldron, R. Dumdey, P. Henklein, D. Bech-Otschir, X. Huang, M. Berse, J. Sperling, R. Schade, and W. Dubiel. 2003. Protein kinase CK2 and protein kinase D are associated with the COP9 signalosome. EMBO J. 221302-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walliker, D., I. A. Quakyi, T. E. Wellems, T. F. McCutchan, A. Szarfman, W. T. London, L. M. Corcoran, T. R. Burkot, and R. Carter. 1987. Genetic analysis of the human malaria parasite Plasmodium falciparum. Science 2361661-1666. [DOI] [PubMed] [Google Scholar]
- 55.Walter, J., M. Schnolzer, W. Pyerin, V. Kinzel, and D. Kubler. 1996. Induced release of cell surface protein kinase yields CK1- and CK2-like enzymes in tandem. J. Biol. Chem. 271111-119. [DOI] [PubMed] [Google Scholar]
- 56.Walton, G. M., J. Spiess, and G. N. Gill. 1985. Phosphorylation of high mobility group protein 14 by casein kinase II. J. Biol. Chem. 2604745-4750. [PubMed] [Google Scholar]
- 57.Ward, P., L. Equinet, J. Packer, and C. Doerig. 2004. Protein kinases of the human malaria parasite Plasmodium falciparum: the kinome of a divergent eukaryote. BMC Genomics 579. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.