Abstract
Proper regulation of the cyclic AMP-dependent protein kinase (PKA) pathway is required for normal growth and development in many fungi. We have reported that deletion of the PKA regulatory subunit gene, pkaR, in Aspergillus fumigatus leads to defects in germination and a hypersensitivity of conidia to oxidative stress. In this study, we further analyzed the defects of ΔpkaR conidia and found that a large proportion were abnormally larger than wild type. Because swelling and increased susceptibility to oxidative stress are characteristic of germinating conidia, we analyzed the metabolic activity of the conidia by mitochondrial staining. Whereas it required 4 h in rich medium for wild-type mitochondria to become active, ΔpkaR conidia harbored active mitochondria in the absence of a germinant. Furthermore, conidia of the mutant showed a dramatic loss in viability upon short-term storage in water, indicating starvation-induced death. Taken together, our data suggest that PKA activity regulates metabolic activation of resting conidia. Additionally, the ΔpkaR mutant displayed an abnormal abundance of hyphal nuclei and had increased transcript levels of several cell cycle regulatory genes. These data indicate an important role for PKA in the nuclear duplication cycle of A. fumigatus.
Aspergillus fumigatus is a ubiquitous saprophytic mold that can cause a severe and frequently fatal systemic infection in immunocompromised patients (17). Like many filamentous fungi, A. fumigatus undergoes an asexual developmental program following nutrient limitation, resulting in the formation of large numbers of clonal spores (conidia) that are metabolically quiescent and resistant to environmental stress. Conidia represent the infectious particle and, once inhaled, can reach the alveoli of the lung due to their small size (1 to 3 μm). In the presence of moisture and sufficient environmental nutrients, as in the context of the mammalian lung, resting conidia break metabolic and cell cycle dormancy. Following a period of nuclear division and isotropic growth (swelling), each conidium establishes an axis of polarity, which then develops into the invasive hypha (3). Therefore, conidial germination represents a critical process in the A. fumigatus life cycle and the first, essential step in the development of invasive aspergillosis following inhalation.
Little is known about the signaling mechanisms by which conidia relay external nutritional cues to the metabolic and cell cycle machinery within the cell. The cyclic AMP (cAMP)-dependent protein kinase (PKA) pathway has been among those of interest, as its involvement in various fungal processes, including germination and virulence, has been described for many fungi (reviewed in reference 5). The PKA holoenzyme is an inactive heterotetramer, with a dimer of regulatory subunits serving as a pseudosubstrate for two catalytic subunits. PKA activation occurs after the second messenger, cAMP, binds to the regulatory subunits and induces a conformational change that releases the active kinases. In yeast, cAMP production by adenylate cyclase occurs after extracellular glucose binds to a G-protein-coupled receptor, Gpr1p, which then activates the G-α protein, Gpa2p (14, 15). Although upstream signaling events in A. fumigatus are less well understood, several components of the cAMP pathway have been identified and characterized, including the adenlylate cyclase gene (acyA) and a G-protein α subunit gene (gpaB) (18).
We have previously generated a mutant of A. fumigatus that is deficient in the regulatory subunit of PKA (PkaR), leading to constitutive kinase activity. We have reported that the mutant demonstrates reduced rates of germination, radial growth, and conidiation compared to the wild type (wt). The conidia of the ΔpkaR mutant also show increased susceptibility to various forms of oxidative stress, and the mutant is attenuated for virulence in a neutropenic mouse model (35). These data underscore the importance of proper PKA regulation in the growth and pathogenesis of this opportunistic pathogen.
In this study, we sought to determine the mechanisms behind the increased susceptibility of ΔpkaR conidia to oxidative damage that we previously described. In doing so, we provide evidence that supports an important role for PKA signaling in the metabolic activation of resting conidia. Unlike the case for wt A. fumigatus conidia, we demonstrate that ΔpkaR conidia harbor active mitochondria in the absence of a germinant (i.e., no carbon or nitrogen source). We suggest that this premature metabolic activation accounts for the increased sensitivity of ΔpkaR conidia to oxidative stress, as well as for a decrease in conidial viability and germination. Interestingly, we also show that ΔpkaR hyphae display an increased abundance of nuclei, which may be partly due to deregulation of genes involved in the nuclear duplication cycle. Taken together, the data support a model in which PKA plays a role in both the metabolic activation of resting conidia and nuclear division in A. fumigatus.
MATERIALS AND METHODS
Organisms and growth conditions.
Aspergillus fumigatus strain H237, a clinical isolate, was used as the wt. Generation of the pkaR deletion (ΔpkaR) and complementation (pkaR C′) strains has been previously described (35). All strains were maintained on Aspergillus minimal medium (AMM), containing 10 mM ammonium tartrate as the nitrogen source. Conidia were harvested from 5-day-old AMM plates in sterile distilled water, filtered through two layers of Miracloth (EMD Biosciences), and counted using a hemacytometer. Subsequent experiments involved incubation of freshly harvested conidia in YG medium (2% glucose, 0.5% yeast extract) as described.
Quantification of conidial size.
Freshly harvested wt, ΔpkaR, and pkaR C′ conidia were adjusted to concentrations of >107 ml−1 in sterile distilled water. Prior to size analysis, conidial suspensions were placed in a water bath sonicator for 1 min. Particle size was then determined using a Coulter LS particle counter, and the data were reported as the percentages of conidia at size increments from 1 to 7 μm. One portion of each suspension was analyzed immediately, and one portion was analyzed following 24 h of refrigeration at 4°C.
Analysis of conidial sensitivity to hydrogen peroxide.
Sterile coverslips were inoculated with 106 ml−1 of conidia in YG from each strain of the isogenic set. For analysis of resting conidia, the conidia were incubated for 45 min at room temperature to allow adherence to the coverslips. Following incubation, coverslips were washed with water and placed into H2O2 (0.01 or 0.025%) or water (control) for 1 h at 37°C. Following treatment, coverslips were washed again, incubated in YG for 4 h at 37°C, and then stained for viability with 5 μM of Fun-1 (Invitrogen) for 30 min at 30°C. Coverslips were then mounted onto microscope slides, and fluorescence microscopy was performed with an Olympus AX80 microscope fitted with fluorescein isothiocyanate and tetramethyl rhodamine isocyanate filters. Conidia that demonstrated red fluorescence were scored as viable. In order to analyze the sensitivity of swollen conidia, the 4-h incubation at 37°C was performed prior to the H2O2 treatment. For each sample, at least 100 conidia were scored. Chi-square analysis was used to demonstrate statistical significance (SigmaPlot software).
Analysis of metabolic activity.
Sterile coverslips were inoculated with 106 ml−1of wt or mutant conidia in a total of 3 ml prewarmed YG and then incubated for 4 h at 37°C. Following incubation, YG was removed and 3 ml of medium containing 20 nM Mitotracker Orange CM-H2 TMRos (Invitrogen) was added. The conidia were then incubated for 20 min at 37°C. The coverslips were washed twice with distilled water, placed on a microscope slide, and sealed. Samples were observed by fluorescence microscopy (tetramethyl rhodamine isocyanate filter), and optical images were analyzed using Magnafire 2.1 and Photoshop 6.0 software.
Conidial viability upon storage in water.
Conidia were freshly harvested from the isogenic set as described above and adjusted to a concentration of 1.5 × 103 ml−1 in distilled water. A 150-μl aliquot was spread onto AMM plates (in triplicate) on the same day as harvesting (day 0), after 24 h at 4°C (day 1), and after 7 days at 4°C. Colonies were counted following 2 days of incubation at 37°C. Mean CFU counts for days 1 and 7 are reported as percentages of counts on day 0.
Fluorescence staining of nuclei and cell walls.
Staining of nuclei and cell walls was performed by a modification of previously described methods (9). Briefly, conidia of the wt were incubated for 10 to 12 h at 37°C, while ΔpkaR conidia were incubated for 14 to 16 h to compensate for the reduced germination and growth kinetics of the mutant (35). Following incubation, coverslips were washed with sterile distilled water and fixed [8% formaldehyde, 50 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES) (pH 6.7), 25 mM EGTA (pH 7.0), 5 mM MgSO4, and 5% dimethyl sulfoxide] for 30 min. After fixation, coverslips were washed, treated with RNase (0.1 mg/ml) for 2 h, washed again, and stained with propidium iodide (12.5 μg/ml; Sigma) and calcofluor white (Fluorescent Brightener 28; 0.4 μg/ml; Sigma) for 5 min at room temperature. Fluorescence microscopy was performed as above with a DAPI (4′,6′-diamidino-2-phenylindole)/fluorescein isothiocyanate/tetramethyl rhodamine isocyanate filter cube.
Reverse transcription-PCR (RT-PCR).
The sequence of A. fumigatus nimA (AFUA_6G02670) was downloaded and used to design primers. The sequences for cln1 and pcl1 from Saccharomyces cerevisiae were used in a BlastP search against the A. fumigatus genome, and the top-scoring matches, AFUA_2G03920 (E value, 2e−14) for cln1 and AFUA_1G04750 (E value, 2e−31) for pcl1, were downloaded and similarly used for primer design. The primer sequences used in this study are listed in Table 1.
TABLE 1.
Oligonucleotides used in this study
| Primer | Sequence (5′ → 3′) |
|---|---|
| gpdA forward | TCATCAACGACAAGTTCGGC |
| gpdA reverse | ACAACACGGCGAGAGTAACC |
| Afcln1 forward | CTCTTTATGATGACGACATG |
| Afcln1 reverse | TTCATTTTCCGGTGTGATGG |
| Afpcl1 forward | GCAACAGCAGCAACACTACC |
| Afpcl1 reverse | GCAGCAGAAGCCAAGAATCG |
| AfnimA forward | TCAGCCAGTTGTCCATCGAG |
| AfnimA reverse | AGTTCAACCAATGTTCGGCC |
To determine relative steady-state transcript levels of the cell cycle genes, wt and ΔpkaR conidia were inoculated into YG and incubated at 37°C (250 rpm). Total RNA was extracted (RNeasy minikit; Qiagen) from cultures at various developmental time points, including resting conidia (5 min), swollen conidia (3.5 h for wt and 4.5 h for ΔpkaR), germlings (6.5 h for wt and 7.5 h for ΔpkaR), and mature hyphae (16 h for both strains). Total RNA (3 μg) from each time point were treated with DNase (RQ1 DNase; Qiagen) for 1 h at 37°C. Following the DNase incubation, 8 μl of each sample was used for first-strand cDNA synthesis (Superscript III; Invitrogen) or for a control reaction without reverse transcriptase. Two microliters of each sample was subsequently used in a PCR (50-μl mixture) with primers for either gpdA or cell cycle genes (nimA, pcl1, or cln1). After electrophoresis of the samples, gels were stained with Sybr green I (Molecular Probes) and images of the PCR products were acquired with a Storm 840 PhosphorImager (Molecular Dynamics). Band intensities were determined with ImageQuant 5.2 software. Band intensities of the cell cycle PCR products were normalized to amplified gpdA signal from the same template, and the ratios of these values are displayed. No PCR product was detected when reverse transcriptase was omitted from the cDNA synthesis reaction mixture (data not shown).
RESULTS
Conidia of the ΔpkaR mutant have a larger mean diameter than wt conidia.
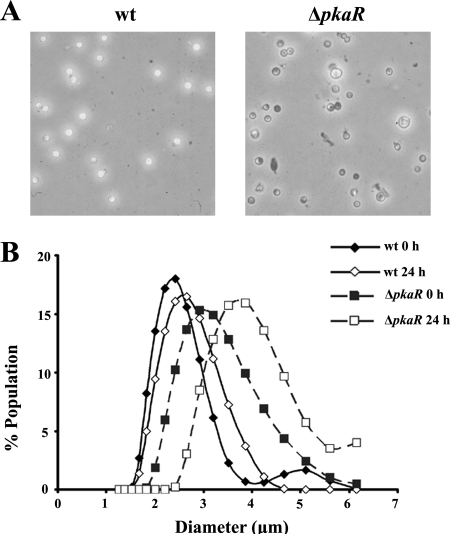
Whereas wt conidia appeared to be uniform in size upon microscopic examination, many of the ΔpkaR conidia were notably larger (Fig. 1A). To quantitate this size difference, conidial suspensions of the isogenic set were compared using a Coulter LS particle counter. Briefly, conidia that were freshly harvested in sterile distilled water were analyzed either immediately or after 24 h in water at 4°C. At the zero time point, the majority of wt conidia fit within a moderately narrow curve that peaked at 2.4 μm and ranged between 1.8 to 4.0 μm. In comparison, ΔpkaR conidia were more broadly distributed between 2 and 6 μm, with a peak at 2.9 μm (Fig. 1B). Therefore, these data were consistent with our microscopic observations.
FIG. 1.
Conidia of the ΔpkaR mutant are more variable in size than wt conidia. (A) Freshly harvested conidia visualized by phase-contrast microscopy. (B) Mean diameters of conidia from freshly harvested cultures (0 h) or after incubating for 24 h at 4°C, analyzed using an LS Coulter size particle counter.
A characteristic of wt A. fumigatus conidia is their stability during long-term refrigeration in distilled water, in terms of both viability and size (16). The size distribution of wt conidia showed a slight shift to the right following overnight incubation at 4°C. In contrast, the peak of the ΔpkaR conidia shifted more dramatically to 3.9 μm after 24 h of refrigeration. Results with the complemented strain were identical to those for the wt, both in terms of size and size stability (data not shown). These results demonstrate that the ΔpkaR conidia are more variable in size than the wt and that the size heterogeneity increases over the course of overnight storage in distilled water.
Mitochondria of the ΔpkaR mutant are active in the absence of a germinant.
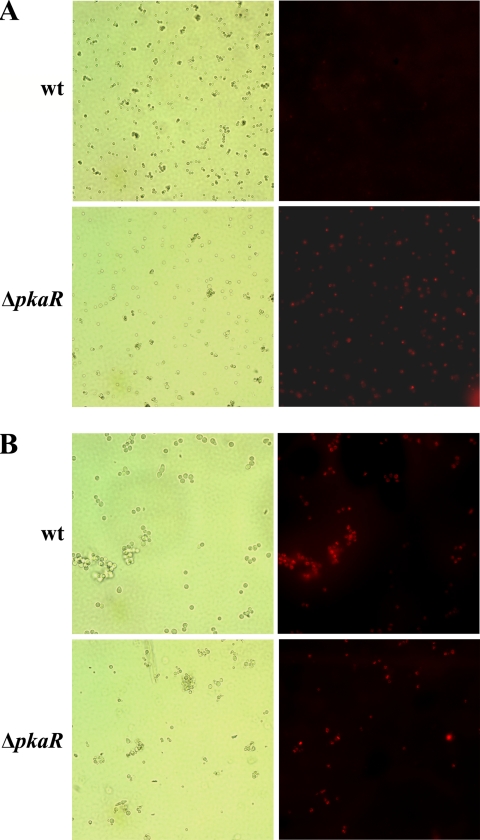
Conidial swelling is the earliest morphological indicator of germination (23). Therefore, we hypothesized that the observed increase in ΔpkaR conidial size was the result of precocious swelling in water. Because mitochondrial respiration is initiated at the onset of germination (3), we monitored the respiratory state of the mitochondria to distinguish between metabolically active (germinating) and metabolically quiescent (resting) conidia. We used the reduced Mitotracker Orange CMTMRos, a mitochondrion-selectable probe that requires oxidation by an actively respiring cell to become fluorescent. Whereas it required 4 h of incubation at 37°C in a complete growth medium (YG) for wt conidia to stain positively with Mitotracker Orange, the majority of ΔpkaR conidia were positive before incubation (Fig. 2A). Furthermore, the majority of wt conidia became activated at about the same time (4 h), suggesting that the conidia were uniformly responsive to the same environmental cues (Fig. 2B). In contrast, the mutant conidia became activated in a relatively asynchronous fashion, with gradually increasing numbers of conidia containing fluorescent mitochondria being detected over the 4 h of observation (Fig. 2B and data not shown).
FIG. 2.
Conidia of the ΔpkaR mutant harbor respiring mitochondria in the absence of a germinant. Conidia that were either freshly harvested (A) or incubated for 4 h in YG medium at 37°C (B) were stained with the fluorescent dye Mitotracker Orange. Active mitochondria stain orange. Corresponding bright-field and fluorescent images are shown.
Conidia of the ΔpkaR mutant are hypersensitive to nutritional and oxidative stress.
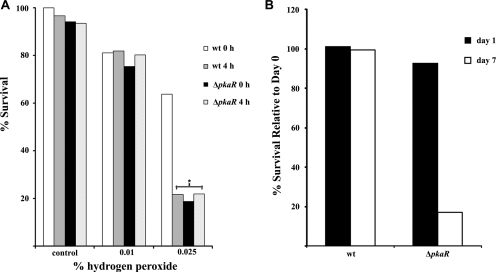
Conidia require an external nutrient source for survival once they have broken metabolic dormancy and depleted their intracellular carbohydrate stores. Therefore, we hypothesized that the prematurely germinating conidia of the ΔpkaR mutant would lose viability after relatively short-term storage in the absence of exogenous nutrients, relative to the wt. To test this, conidia of the wt and ΔpkaR strains were harvested, diluted, and plated for isolation at various time points. After 24 h of storage at 4°C (day 1), there was no significant change in viability for either the wt or the mutant, relative to CFU counts taken at day 0. However, after 7 days of storage, ΔpkaR conidia retained only 18% viability, compared to 100% for wt (Fig. 3B). The complemented mutant similarly showed no reduction in viability after 7 days (data not shown).
FIG. 3.
Conidia of the ΔpkaR mutant are hypersensitive to oxidative and nutritional stress. (A) Conidia were treated with H2O2 upon harvesting or after 4 h of incubation in YG. Conidia were then stained with FUN-1 to determine viability (n > 100). Statistical significance was determined by chi-square analysis (P < 0.01). (B) Conidia were harvested and diluted to a concentration of 1.5 × 103/ml. A 150-μl aliquot was then plated onto AMM at the time of harvesting (day 0) or after incubation at 4°C for 24 h (day 1) or 7 days (day 7). CFU were counted after 2 days of incubation at 37°C and are displayed as a percentage of CFU at day 0.
We previously reported that freshly harvested conidia of the ΔpkaR mutant were more susceptible to treatment with H2O2 than wt conidia. To determine whether this increased susceptibility could be due to the premature metabolic activity of the ΔpkaR conidia, we compared the H2O2 susceptibilities of resting and germinated conidia of the wt strain. Here we show that after the 4-h incubation in YG, all members of the isogenic set showed equivalent killing by the H2O2 treatment, whereas resting wt conidia remained significantly more resistant (Fig. 3A). Therefore, freshly harvested conidia of the ΔpkaR mutant behaved similarly to germinating conidia of the wt with respect to their sensitivity to oxidative stress. These findings suggest that the increased killing of ΔpkaR conidia upon short-term storage in water and exposure to H2O2 is a consequence of their premature initiation of germination.
Deletion of pkaR leads to an increase in the number of hyphal nuclei.
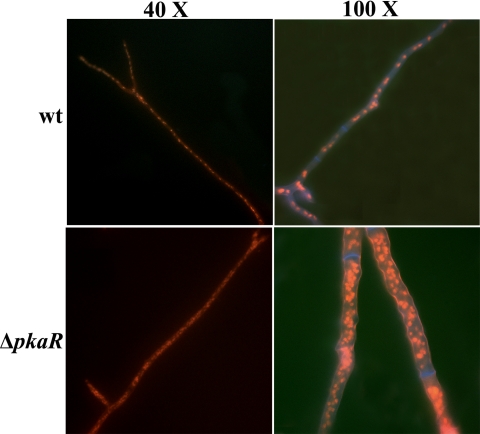
Because PKA is known to be a major regulator of the cell cycle in yeast (29), we next looked at whether deletion of pkaR led to anomalous changes in nuclear number or distribution in A. fumigatus. Conidia of the wt, ΔpkaR, or ΔpkaR C′ strain were incubated on coverslips until hyphae were of comparable length. Samples were subsequently stained with propidium iodide along with calcofluor white to visualize the nuclei and cell wall, respectively. In the wt, septa were regularly spaced and 4 to 10 nuclei could typically be seen within each hyphal compartment. In sharp contrast, the ΔpkaR mutant demonstrated irregularly spaced septa, and each hyphal segment contained considerably more nuclei, the number of which varied among the segments (Fig. 4). In addition, an increase in hyphal diameter was also evident in the mutant. The complemented strain was indistinguishable from the wt (data not shown). This result suggests that deletion of pkaR leads to some degree of deregulation with respect to the nuclear duplication cycle.
FIG. 4.
Hyphae of the ΔpkaR mutant contain an increased number of nuclei relative to those of the wt. Strains were grown in YG at 37°C. Hyphae were fixed and stained with propidium iodide and calcofluor white to visualize the nuclei and cell walls, respectively.
Deletion of pkaR leads to a deregulation of various cell cycle genes.
Activation of the PKA pathway is required for yeast cells to exit G1 (29). Since PKA activity influences the transcription of several yeast cell cycle regulators (34), we hypothesized that constitutive PKA activity in the ΔpkaR mutant may lead to a deregulation of A. fumigatus cell cycle genes, resulting in the abnormal accumulation of nuclei. To test this, we compared mRNA levels of genes that are known to be upregulated during mitosis in yeast or Aspergillus spp. A. fumigatus cln1 is a homologue to the S. cerevisiae CLN1 gene, encoding a cyclin that shows increased expression at the end of G1. This transcriptional upregulation is controlled by the Cln3-Cdc28 complex, the protein levels of which are controlled by PKA phosphorylation. Additionally, pcl1 is the homologue of another yeast cyclin gene that is upregulated at the end of G1 (6, 21). We also analyzed the mRNA levels of nimA, which encodes the homologue of the NimA kinase in Aspergillus nidulans. Expression of nimA is highest during mitosis, and NimA activity is required for mitotic entry (22, 24).
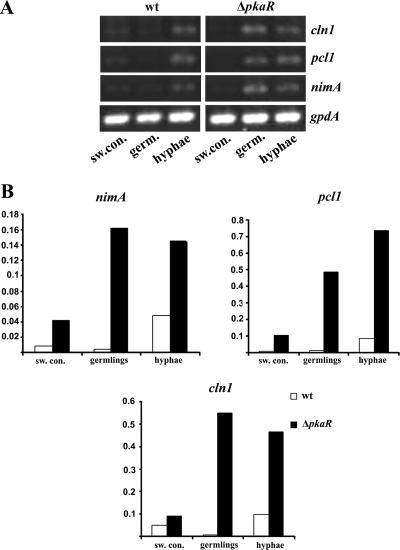
As nuclear division is linked to the A. fumigatus developmental cycle (19), we chose to compare the relative transcript levels of these genes at various developmental time points. Conidia of either the wt or the ΔpkaR mutant were inoculated into YG medium, and total RNA was extracted from cultures corresponding to resting conidia, swollen conidia, germlings, or mature hyphae. The levels of cln1, pc11, and nimA were then compared by RT-PCR, using the constitutively expressed gene gpdA as a control. In the wt, the abundance of the cell cycle mRNAs did not markedly increase until the hyphal stage of growth. In contrast, a premature and sustained increase in the mRNAs for all three genes was apparent in the ΔpkaR mutant, beginning at the germling stage (Fig. 5). Even at the hyphal stage, where the wt demonstrated a clear increase in transcript abundance, levels appeared to be higher in the ΔpkaR mutant compared to the respective gpdA control (Fig. 5B). These data suggest a role for PkaR in regulating several genes involved in the cell cycle, which may, in part, explain the increased accumulation of hyphal nuclei observed for the ΔpkaR mutant.
FIG. 5.
Genes involved in the cell cycle are upregulated in the ΔpkaR mutant. Conidia of the wt or the ΔpkaR mutant were incubated in YG at 37°C, and total RNA was extracted from cultures that corresponded to various developmental time points (swollen conidia, germlings, and hyphae). RT-PCR was performed for cell cycle genes (cln1, pcl1, and nimA) and for gpdA as a control. Reaction mixtures (50 μl) were divided into two portions. (A) PCR products are displayed on an ethidium bromide gel. (B) PCR products were stained with Sybr green I (Molecular Probes), and band intensities were determined with a phosphorimager. Expression levels for cln1, pcl1, and nimA are presented as ratios to gpdA levels for the respective strain and time point.
DISCUSSION
In filamentous fungi, the major extracellular mediators of development and metabolism are nutrients. The sensing of such nutrients occurs through highly specific and regulated signal transduction pathways. The inability to temporally coordinate the sensing and effecter mechanisms of these pathways can lead to an abnormal cellular state, resulting in reduced growth or viability of the fungus (1). Along these lines, we have previously demonstrated the importance of proper PKA regulation to the growth and virulence of the mold pathogen Aspergillus fumigatus (35). In this study, we provide evidence that PKA has a role in the metabolic activation of resting conidia and that inappropriate initiation of this signaling leads to an increased susceptibility to oxidative and nutritional stress. Furthermore, we demonstrate that loss of pkaR deregulates cell cycle gene expression and increases nuclear abundance, suggesting a role for PKA in the nuclear duplication cycle of A. fumigatus.
In the context of nutritional stress, many filamentous fungi produce conidia, which remain metabolically inactive until appropriate nutrients are present. Therefore, tight regulation of conidial germination ensures the survival of the organism under adverse environmental conditions. Several pathways have been implicated in relaying nutritional cues to the germination machinery. For example, the mitogen-activated protein (MAP) kinase pathway regulates germination in response to both carbon and nitrogen in A. fumigatus. Deletion of the MAP kinase gene sakA leads to more robust germination than wt on media containing a poor nitrogen source, such as nitrate or nitrite. This suggests that SakA negatively regulates germination in the presence of these suboptimal nutrients (32). Another MAP kinase, MpkC, positively regulates germination in the presence of polyalcohol sugars, as evidenced by germination and growth defects of an mpkC deletion strain in the presence of mannitol or sorbitol as the sole carbon source (26).
Similarly, a variety of genetic analyses implicate the Ras pathway in the germination of Aspergillus spp. Overexpression of a dominant-active RasA bypasses the carbon source requirement and leads to giant swollen conidia that do not form germ tubes in A. nidulans. It has been suggested that a decrease in Ras activity may be subsequently required for germ tube emergence, implicating involvement of this GTPase at a defined step in the germination process (28). In A. fumigatus, expression of dominant negative alleles of RasA or RasB leads to reduced or delayed germination, respectively, and expression of dominant-active RasA leads to germ tube formation in the absence of a carbon source (9). In line with these observations, deletion of rasB or rasA leads to a delayed germination phenotype (8, 10).
The cAMP-PKA pathway also plays a critical role in environmental sensing in yeast and filamentous fungi. In S. cerevisiae, both Ras and a canonical G-protein-coupled receptor to G-alpha cascade act upstream to activate adenylate cyclase upon addition of glucose to growth-repressed cells. Interestingly, Ras signaling through cyclase does not appear to be conserved in filamentous fungi (7). Once activated, PKA has several important roles in yeast activation and conidial germination. For instance, the breakdown of the storage carbohydrate trehalose by the enzyme trehalase is one of the earliest metabolic events observed upon the initiation of germination (3). In Neurospora crassa and A. nidulans, trehalase is activated by PKA-dependent phosphorylation, as is also seen in S. cerevisiae and Schizosaccharomyces pombe (2, 4, 30). Furthermore, increased protein synthesis is required at the onset of germination, and PKA activity is known to increase the expression of ribosomal proteins in yeast and A. fumigatus (11, 13). In A. nidulans and A. fumigatus, deletion of the gene encoding the major PKA catalytic subunit (pkaA and pkaC1, respectively) leads to delayed germ tube emergence (18, 20). Interestingly, we have previously reported that the ΔpkaR mutant of A. fumigatus also demonstrates reduced and delayed germination, indicating that proper regulation of PKA is required for normal germination kinetics (35).
The results in this report further demonstrate a role for PKA signaling in the germination of A. fumigatus. Unlike wt conidia, freshly harvested ΔpkaR conidia harbor active mitochondria in the absence of a germinant. We therefore suggest that constitutive PKA activity bypasses the requirement for environmental nutrients to trigger metabolic activation. This interpretation can account for several phenotypes observed with the ΔpkaR mutant (summarized in Fig. 6). First, our analyses revealed that the ΔpkaR conidial population is larger than that of the wt or the complemented strain, which can likely be explained by precocious isotropic growth in water. We did not observe germ tube formation in water alone (data not shown), suggesting either that additional signaling inputs are required for initiating polarized growth or that there were insufficient nutrients to support the demands of apical extension. In support of the latter possibility, we also observed that ΔpkaR conidia lose viability upon storage in water. Initially, the energy required for early events in germination comes from the breakdown of intracellular carbohydrate stores in the form of trehalose or glycogen. However, once these stores are depleted, extracellular nutrients are required for survival. Because wt conidia maintain metabolic quiescence, they can remain fully viable in water for a year or longer (16). In contrast, the reduced viability of the ΔpkaR mutant can likely be explained by an inability of the activated cells to survive once they have depleted their intracellular trehalose stores. Moreover, the inability of the ΔpkaR conidia to reach 100% germination, as previously reported (35), is consistent with the interpretation that there is a loss in viability among metabolically active cells.
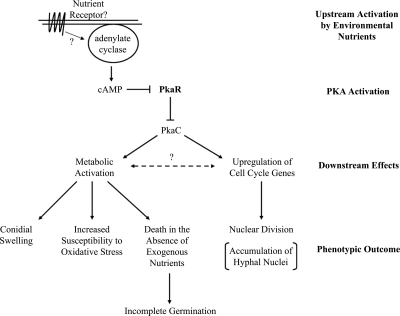
FIG. 6.
Summary. Upstream activation: a resting conidium senses environmental nutrients by an unknown signaling mechanism, ultimately leading to activation of adenylate cyclase and production of cAMP. PKA activation: binding of cAMP to PkaR leads to release and activation of PKA catalytic subunits. Downstream effects: phosphorylation of downstream targets leads to metabolic and cell cycle activation. Phenotypic outcome: metabolic activation leads to swelling and increased susceptibility to oxidative stress. In a ΔpkaR mutant, PKA is constitutively active, leading to these phenotypes in the absence of an extracellular trigger (i.e., nutrients). Also, in the mutant unregulated nuclear division leads to an increased accumulation of hyphal nuclei. It is possible that PKA lies upstream of only one branch, while an unknown pathway connects the two (dashed line).
Swelling is a prerequisite for effective killing of A. fumigatus conidia by macrophages (25). Here we show that freshly harvested conidia of the ΔpkaR mutant are as susceptible to H2O2 as wt conidia that have been incubated in rich medium for several hours (Fig. 3). This suggests that the increased killing of ΔpkaR conidia is additionally a consequence of a premature initiation of germination. Taken together, these findings underscore the importance of tight PKA regulation in ensuring that germination takes place only in an environment capable of supporting growth. The importance of the PKA regulatory subunit is demonstrated in Colletotrichum trifolii, where PkaR accumulates within conidia but then decreases as germination proceeds (33).
PKA signaling is a well-characterized regulator of the cell cycle in yeast. Our nuclear staining and RT-PCR data (Fig. 4 and 5) are among the first lines of evidence implicating PKA in the nuclear duplication cycle of A. fumigatus. Although we demonstrate deregulation of cell cycle genes as a consequence of pkaR deletion, the mechanism by which PKA influences the expression of the genes tested here remains unclear. However, PKA's influence on Cln1 has been previously described for S. cerevisiae, and our data suggest that a similar regulatory interaction may be present in A. fumigatus. In support of our interpretation, an A. fumigatus strain overexpressing the PKA catalytic subunit (pkaC1) demonstrates increased protein levels of the cell division control protein Cdc48 (11).
Alternatively, it has been suggested that an increase in hyphal nuclei may be a generalized response to an increase in cytoplasmic volume, a phenotype that is seen in our ΔpkaR mutant as well as in other mutants of Aspergillus spp., including Rac and Ras mutants (8, 12, 31). However, the expression of cell cycle regulatory genes has not been tested in any of these other strains, and thus it is not clear whether increased expression levels would be a common phenomenon. Moreover, Ras and Rac GTPases have known roles in yeast and mammalian cell cycle control (27, 29); therefore, it is possible that these Aspergillus mutants share the nuclear phenotype due, in part, to the conserved role of these genes in nuclear division. Taken together, our data are consistent with a direct role for PKA signaling in cell cycle control, as proposed for yeast, although additional mechanisms may contribute to the nuclear phenotype observed in A. fumigatus.
Resting conidia must break both metabolic and cell cycle dormancy at the onset of germination. Here we provide evidence that supports the PKA signaling pathway as an upstream regulator of both processes. However, as there may be cross talk between metabolism and cell cycle progression, it is possible that PKA signaling lies upstream of only one process, while an unknown pathway, connecting the two, activates the other (Fig. 6). Future studies will be aimed at further characterizing the physiological processes under the control of PKA in A. fumigatus and the downstream effectors through which PKA mediates such processes.
Acknowledgments
This work was supported by NIH grants AI061497 (J.C.R.) and AI06145 (D.S.A.).
We thank Lauren Fox and Timothy Stephens for technical assistance, Daryl Richie for helpful discussions, and Jay Card for assistance with photography and preparation of the figures.
Footnotes
Published ahead of print on 5 January 2009.
REFERENCES
- 1.Bahn, Y., C. Xue, A. Idnurm, J. C. Rutherford, J. Heitman, and M. E. Cardenas. 2007. Sensing the environment: lessons from fungi. Nat. Rev. Microbiol. 557-69. [DOI] [PubMed] [Google Scholar]
- 2.Carrillo, D., J. Vicente-Soler, and M. Gacto. 1994. Cyclic AMP signalling pathway and trehalase activation in the fission yeast Schizosaccharomyces pombe. Microbiology 1401467-1472. [DOI] [PubMed] [Google Scholar]
- 3.d'Enfert, C. 1997. Fungal spore germination: insights from the molecular genetics of Aspergillus nidulans and Neurospora crassa. Fungal Genet. Biol. 21163-172. [Google Scholar]
- 4.d'Enfert, C., B. Bonini, P. Zapella, T. Fontaine, A. da Silva, and H. Terenzi. 1999. Neutral trehalases catalyse intracellular trehalose breakdown in the filamentous fungi Aspergillus nidulans and Neurospora crassa. Mol. Microbiol. 32471-483. [DOI] [PubMed] [Google Scholar]
- 5.D'Souza, C. A., and J. Heitman. 2001. Conserved cAMP signaling cascades regulate fungal development and virulence. FEMS Microbiol. Rev. 25349-364. [DOI] [PubMed] [Google Scholar]
- 6.Espinoza, F. H., J. Ogas, I. Herskowitz, and D. O. Morgan. 1994. Cell cycle control by a complex of the cyclin HCS26 (PCL1) and the kinase PHO85. Science 2661388-1391. [DOI] [PubMed] [Google Scholar]
- 7.Fillinger, S., M. Chaveroche, K. Shimizu, N. Keller, and C. d'Enfert. 2002. cAMP and ras signalling independently control spore germination in the filamentous fungus Aspergillus nidulans. Mol. Microbiol. 441001-1016. [DOI] [PubMed] [Google Scholar]
- 8.Fortwendel, J. R., K. K. Fuller, T. J. Stephens, W. C. Bacon, D. S. Askew, and J. C. Rhodes. 2008. Aspergillus fumigatus RasA regulates asexual development and cell wall integrity. Eukaryot. Cell 71530-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fortwendel, J. R., J. C. Panepinto, A. E. Seitz, D. S. Askew, and J. C. Rhodes. 2004. Aspergillus fumigatus rasA and rasB regulate the timing and morphology of asexual development. Fungal Genet. Biol. 41129-139. [DOI] [PubMed] [Google Scholar]
- 10.Fortwendel, J. R., W. Zhao, R. Bhabhra, S. Park, D. S. Perlin, D. S. Askew, and J. C. Rhodes. 2005. A fungus-specific Ras homolog contributes to the hyphal growth and virulence of Aspergillus fumigatus. Eukaryot. Cell 41982-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grosse, C., T. Heinekamp, O. Kniemeyer, A. Gehrke, and A. A. Brakhage. 2008. Protein kinase A regulates growth, sporulation, and pigment formation in Aspergillus fumigatus. Appl. Environ. Microbiol. 744923-4933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris, S. D., J. L. Morrell, and J. E. Hamer. 1994. Identification and characterization of Aspergillus nidulans mutants defective in cytokinesis. Genetics 136517-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klein, C., and K. Struhl. 1994. Protein kinase A mediates growth-regulated expression of yeast ribosomal protein genes by modulating RAP1 transcriptional activity. Mol. Cell. Biol. 141920-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kraakman, L., K. Lemaire, P. Ma, A. W. R. H. Teunissen, M. C. V. Donaton, P. Van Dijck, J. Winderickx, J. H. de Winde, and J. M. Thevelein. 1999. A Saccharomyces cerevisiae G-protein coupled receptor, Gpr1, is specifically required for glucose activation of the cAMP pathway during the transition to growth on glucose. Mol. Microbiol. 321002-1012. [DOI] [PubMed] [Google Scholar]
- 15.Kubler, E., H. U. Mosch, S. Rupp, and M. P. Lisanti. 1997. Gpa2p, a G-protein alpha-subunit, regulates growth and pseudohyphal development in Saccharomyces cerevisiae via a cAMP-dependent mechanism. J. Biol. Chem. 27220321-20323. [DOI] [PubMed] [Google Scholar]
- 16.Lamarre, C., S. Sokol, J. Debeaupuis, C. Henry, C. Lacroix, P. Glaser, J. Coppee, J. Francois, and J. Latge. 2008. Transcriptomic analysis of the exit from dormancy of Aspergillus fumigatus conidia. BMC Genomics 9417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Latge, J. 1999. Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 12310-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liebmann, B., M. Muller, A. Braun, and A. A. Brakhage. 2004. The cyclic AMP-dependent protein kinase A network regulates development and virulence in Aspergillus fumigatus. Infect. Immun. 725193-5203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Momany, M., and I. Taylor. 2000. Landmarks in the early duplication cycles of Aspergillus fumigatus and Aspergillus nidulans: polarity, germ tube emergence and septation. Microbiology 1463279-3284. [DOI] [PubMed] [Google Scholar]
- 20.Ni, M., S. Rierson, J. Seo, and J. Yu. 2005. The pkaB gene encoding the secondary protein kinase A catalytic subunit has a synthetic lethal interaction with pkaA and plays overlapping and opposite roles in Aspergillus nidulans. Eukaryot. Cell 41465-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogas, J., B. J. Andrews, and I. Herskowitz. 1991. Transcriptional activation of CLN1, CLN2, and a putative new G1 cyclin (HCS26) by SWI4, a positive regulator of G1-specific transcription. Cell 661015-1026. [DOI] [PubMed] [Google Scholar]
- 22.O'Regan, L., J. Blot, and A. Fry. 2007. Mitotic regulation by NIMA-related kinases. Cell Div. 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osherov, N., and G. S. May. 2001. The molecular mechanisms of conidial germination. FEMS Microbiol. Lett. 199153-160. [DOI] [PubMed] [Google Scholar]
- 24.Osmani, S., G. May, and N. Morris. 1987. Regulation of the mRNA levels of nimA, a gene required for the G2-M transition in Aspergillus nidulans. J. Cell Biol. 1041495-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Philippe, B., O. Ibrahim-Granet, M. C. Prevost, M. A. Gougerot-Pocidalo, M. Sanchez Perez, A. Van der Meeren, and J. P. Latge. 2003. Killing of Aspergillus fumigatus by alveolar macrophages is mediated by reactive oxidant intermediates. Infect. Immun. 713034-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reyes, G., A. Romans, C. K. Nguyen, and G. S. May. 2006. Novel mitogen-activated protein kinase MpkC of Aspergillus fumigatus is required for utilization of polyalcohol sugars. Eukaryot. Cell 51934-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt, A., J. Durgan, A. Magalhaes, and A. Hall. 2007. Rho GTPases regulate PRK2/PKN2 to control entry into mitosis and exit from cytokinesis. EMBO J. 261624-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Som, T., and V. S. Kolaparthi. 1994. Developmental decisions in Aspergillus nidulans are modulated by Ras activity. Mol. Cell. Biol. 145333-5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thevelein, J. M. 1991. Fermentable sugars and intracellular acidification as specific activators of the RAS-adenylate cyclase signalling pathway in yeast: the relationship to nutrient-induced cell cycle control. Mol. Microbiol. 51301-1307. [DOI] [PubMed] [Google Scholar]
- 30.Thevelein, J. M. 1988. Regulation of trehalase activity by phosphorylation-dephosphorylation during developmental transitions in fungi. Exp. Mycol. 121-7. [Google Scholar]
- 31.Virag, A., M. P. Lee, H. Si, and S. D. Harris. 2007. Regulation of hyphal morphogenesis by cdc42 and rac1 homologues in Aspergillus nidulans. Mol. Microbiol. 661579-1596. [DOI] [PubMed] [Google Scholar]
- 32.Xue, T., C. K. Nguyen, A. Romans, and G. S. May. 2004. A mitogen-activated protein kinase that senses nitrogen regulates conidial germination and growth in Aspergillus fumigatus. Eukaryot. Cell 3557-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang, Z., and M. B. Dickman. 1999. Molecular cloning and characterization of Ct-PKAR, a gene encoding the regulatory subunit of cAMP-dependent protein kinase in Colletotrichum trifolii. Arch. Microbiol. 171249-256. [DOI] [PubMed] [Google Scholar]
- 34.Zambon, A. C., L. Zhang, S. Minovitsky, J. R. Kanter, S. Prabhakar, N. Salomonis, K. Vranizan, I. Dubchak, B. R. Conklin, and P. A. Insel. 2005. Gene expression patterns define key transcriptional events in cell-cycle regulation by cAMP and protein kinase A. Proc. Natl. Acad. Sci. USA 1028561-8566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao, W., J. C. Panepinto, J. R. Fortwendel, L. Fox, B. G. Oliver, D. S. Askew, and J. C. Rhodes. 2006. Deletion of the regulatory subunit of protein kinase A in Aspergillus fumigatus alters morphology, sensitivity to oxidative damage, and virulence. Infect. Immun. 744865-4874. [DOI] [PMC free article] [PubMed] [Google Scholar]