Abstract
We demonstrate diverse roles of interferon–γ (IFN-γ) in the induction and regulation of immune-mediated inflammation using a transfer model of autoimmune diabetes. The diabetogenic CD4+BDC2.5 (BDC) T cell clone upon transfer into NOD.scid mice induced destruction of islets of Langerhans leading to diabetes. Administration of a neutralizing antibody to IFN-γ (H22) resulted in long term protection (LTP) from diabetes, with inflammation but persistence of a significant, albeit decreased numbers of β-cells. BDC T cells were a mixture of cells expressing high, intermediate and low levels of the T cell receptor. Clonotype-low BDC T cells were required for LTP. Furthermore, islet infiltrating leukocytes in the LTP mice contained Foxp3+CD4 T cells. Islet inflammation in both diabetic and LTP mice was characterized by heavy infiltration of macrophages. Gene expression profiles indicated that macrophages in diabetic mice were M1-type, while LTP mice contained M2-differentiated. The LTP was abolished if mice were treated with either an antibody depleting CD4 T cells, or a neutralizing antibody to CTLA-4, in this case, only at a late stage. Neutralization of IL-10, TGF-β, GITR or CD25 had no effect. Transfer of only clonotype-high expressing BDC T cells induced diabetes but in contrast, H22 antibodies did not inhibit diabetes. While clonotype high T cells induced diabetes even when IFN-γ was neutralized, paradoxically, there was reduced inflammation and no diabetes if host myeloid cells lacked IFN-γ receptor. Hence, using monoclonal CD4 T cells, IFN-γ can have a wide diversity of roles, depending on the setting of the immune process.
Keywords: Diabetes, T cells, cytokines, regulation
Introduction
The NOD mouse model is useful for testing strategies that modulate diabetogenic T cells: a number of manipulations in the NOD mouse can control the diabetic process (1–3). Although several proinflammatory cytokines may play a role in diabetogenesis, their precise mechanism and cellular targets are complex and remain to be defined. Indeed, the NOD mouse diabetes involves polyclonal sets of both CD8 and CD4 T cells, some of which develop into effector cells while other are regulatory in nature, making it difficult to dissect the effects of cytokines on any one defined pathogenic T cell.
The highly pleiotropic cytokine IFN-γ plays a major role in immune mediated inflammation (4, 5). Initially described as the major cytokine responsible for macrophage activation (6), IFN-γ, the prototype Th1 cytokine, also mediates a number of effects on lymphocytes (4). To examine the role of IFN-γ in immune inflammation an acute transfer model of diabetes mediated by the diabetogenic BDC2.5 (BDC) T cell was examined (7–9). This accelerated model allowed us to focus exclusively on how activated CD4 T cells induced islet inflammation and pathology without the participation of B cells or CD8 T cells. In general, IFN-γ appears to have a limited involvement in the spontaneous diabetes of NOD mice (10–14) but participates in the acute models induced by cyclophosphamide injection or diabetogenic cell transfers (14–17). In this acute diabetes transfer model, the cell responsible for the killing of β-cell is an activated macrophage (9): their selective depletion precludes β-cell death despite the persistence of inflammation containing mostly neutrophils and NK cells (9). We report that diabetogenic CD4 T cells triggered inflammation that could be modulated by IFN-γ with profound consequences on the development of diabetes.
Materials and Methods
Mice
The BDC2.5 (BDC) TCR transgenic mice on the NOD background, and BDC2.5/NOD.scid (BDC.scid) were established in our mouse colony at Washington University School of Medicine. NOD mice on the scid genetic background (NOD.CB17-Prkdcscid/J) or on the Rag-1−/− were obtained from The Jackson Laboratory (Bar Harbor, ME) and maintained in our mouse colony-referred to as NOD.scid and NOD.Rag-1−/− mice. To obtain NOD.Rag-1−/−.IFN-γR−/−, the NOD.Rag-1−/− and NOD.IFNγR−/− (NOD.129S1(B6)-Ifngr2tm1Pbro/DvsJ), obtained from The Jackson Laboratory were inter-crossed. IFNγR−/− mice were identified by polymerase chain reaction using the primers described by Serreze et al (11). All experimental mice were 6 to 8 weeks old except BDC.scid which were 3 to 4 weeks of age by the time they develop diabetes. (The NOD.IFNγR−/− came from the Jackson Laboratory which provided the information on its development. See Serreze et al (11)).
Adoptive transfer
Splenocytes from BDC mice were activated in culture with concanavalin A (Con A), (Sigma–Aldrich, St. Louis, MO) and in selected instances with a mimotope peptide (AVRPLWVRME) as previously described (9). Results with either were identical and were not separated from each other. Usually mice were injected intravenously (i.v.) with a dose of 4×106 activated BDC T cells, known to transfer diabetes to all recipients by day 8 (9). For the rechallenge experiments, mice were injected i.v. with an equal dose of activated BDC T cells (4×106) or a higher dose (1.5×107) which transfers diabetes in six days when injected into NOD.scid recipients. For BDC.scid transfers, splenic T cells were harvest and 106 cells were transferred i.v. CD4 T cells were sorted from splenocytes by their clonotype expression using an anti-BDC monoclonal antibody (mAb). The purity of BDC high clonotype (BDC-hi) and BDC low clonotype (BDC-lo) cells was 98% and 78% respectively (supplemental figure 1). Sorted cells were transferred i.v. (106) into recipients. The recipient mice were followed for diabetes incidence. Two consecutive readings of blood glucose of ≥250mg/dl, measured by a glucometer (Bayer, Elkhart, IN), was indicative of diabetes.
Antibody treatments
For most experiments involving IFN-γ neutralization in vivo, NOD.scid and rag-1−/− mice received 300µg intraperitoneally (i.p.) of H22 mAb (kindly provided by Dr. R. Schreiber, Washington University School of Medicine, St. Louis, MO) one day before and two days after cell transfer. Anti-CD25 (P61), Anti-TGF-β (1D11), anti-GITR (DTA-1), anti-IL-10 (JES5-2A5), anti-CD4 (YTS191.1) and anti-CTLA-4 (4F10) mAbs were injected twice with a dose of 500µg i.p. three days apart. A rat IgG isotype antibody (Sigma-Aldrich) was used as control.
Measurement of pancreatic insulin
Frozen pancreata form acute diabetes and long term protected mice at different times post BDC T cell transfer were weighed before homogenization in pre-chilled acid alcohol (80% alcohol in 12N sulfuric acid). One milliliter aliquots of the homogenate for each sample were stored at 4°C overnight and then microfuged at 10,000 rpm for 5 minutes at room temperature. Dilutions of 1:1000 to 1:5000 were prepared with Linco RIA buffer assay and measured with Linco Rat Insulin Kit (Linco Research, Inc., St. Charles, MO). Samples were obtained from 4 to 6 mice per time point.
Isolation of cells from infiltrated mouse islets
Infiltrated mouse islets were isolated and dispersed by trypsinization as previously described (9). In brief, seven mice were sacrificed per islet preparation by cervical dislocation. Hanks’ solution (Invitrogen, Carlsbad, CA) was injected into the common bile duct and then the pancreas was resected, cut into small evenly sized pieces, and digested in collagenase at 39°C. The tissue suspension was then centrifuged through a Ficoll (Sigma–Aldrich) gradient of 23, 20.5, and 11%. All Ficoll interfaces were collected (except for the bottom pellet) and the cells dispersed by trypsin digestion in a 37°C water bath for 3 minutes and then washed in CMRL medium 1066 (Invitrogen) several times. Infiltrating islet leukocytes were obtained from LTP and diabetic NOD.scid mice that received BDC T cells.
Flow Cytometry
Splenocytes and infiltrating islet leukocytes were stained for different cellular markers. Leukocytes were stained with a FITC-labeled anti-CD45(Ly-5) antibody (BD Biosciences, San Jose, CA); T cells were stained with either a FITC-labeled anti-CD4 (L3T4) or FITC-labeled anti-CD3ε chain antibody (145-2C11); Neutrophils were stained with a FITC-labeled anti-Gr-1(RB6-8C5) antibody. Macrophages were analyzed with a FITC-labeled anti-F4/80 antibody (BM8), while dendritic cells were analyzed with a FITC-labeled anti-CD11c antibody (HL3). NK cell analysis was performed with biotinylated anti-CD49b/Pan-NK (DX5) and secondary stain with Streptavidin-Allophycocyanin (APC). NK cells were defined as CD3ε negative and CD49b/Pan-NK positive. All antibodies were obtained from BD Biosciences, San Jose, CA. For Foxp3 staining, surface stained CD4 T cells were incubated in permeabilization buffer for 16–18hrs at 4°C before performing intracellular staining (FJK-16s) (eBioscience, San Diego, CA). All FACS analysis was performed on a FACSCalibur, and data analyzed using FlowJo software (Ashland, OR).
Histology
Mice were anesthetized with ketamine and sacrificed by cervical dislocation. The pancreata were fixed in 10% formalin. Slides were stained with hematoxylin and eosin (H&E). For detection of insulin, 5µm serial sections were de-paraffinized and stained with HistoMouse-SP Kit (AEC, Broad Spectrum, Bulk) (Invitrogen, Carlsbad, CA) using guinea pig anti-insulin polyclonal antibody (1:100) (Linco, St. Charles, MO). Insulitis scoring was performed according to the following criteria: extensive insulitis, >50% of the islet area is infiltrated; insulitis, <50% of the islet area; normal or peri-insulitis, islets are either intact or infiltration is restricted to the periphery.
Laser capture microdissection
Whole pancreata were dissected from mice immediately (within 1–2 mins) following sacrifice with minimal direct handling of the pancreas itself. The pancreas was immediately immersed in O.C.T. (Sakura Finetek, Torrance, CA) and frozen in Cytocool II (Richard-Allen Scientific, 16 Kalamazoo, MI). For laser capture, serial 7 µm-thick cryosections were cut onto Superfrost slides (Fisher Scientific) and immediately fixed in methanol (~1 min) pre-cooled to −20°C and kept within the cryostat. After fixation, O.C.T. was removed manually from sections in the cryostat, and sections were washed twice in 95% ethanol, stained with Eosin (in 100% ethanol) at room temperature for 5 seconds then washed in several changes of 95% then 100% ethanol followed by multiple changes of tissue-grade xylenes. Slides were then incubated in desiccation chambers and microdissected within 3–4 hours. Extensive initial testing of RNA quality following different treatments indicated that abundant endogenous RNAses present in exocrine pancreas led to degradation of RNA: pancreata needed to be frozen within a few minutes of excision, laser capture performed within a few hours of sectioning and sections fixed immediately after transfer to slides. Problems developed if pancreata blocks were stored for more than a few months; or sections were fixed in ethanol, acetone, or formaldehyde; or methanol was not thoroughly pre-cooled and kept continuously at ≤−20°C; or sections were exposed to aqueous solution at any time (hence, staining only in alcohol-dissolved eosin).
Laser capture microdisection was performed on an Arcturus PixCell IIe LCM microscope in a specially designated climate-controlled room. Islets were recognized histologically and were laser-captured whole. RNA from dissected islets was purified using the PicoPure kit (Arcturus), and quality verified on an Agilent 2100 BioAnalyzer for assessment of picogram-level RNA. Dissection specificity was routinely verified by qRT-PCR for acinar- (Amylase), ductal-(carbonic anhydrase II), and islet-specific (Insulin) markers.
Microarray analysis
For GeneChips, RNA from the laser captured islets was pooled from untreated NOD.scid mice, diabetic and LTP mice at d3 to 8 (n=5 mice per group). Pooled RNA (30 ng/sample) was amplified, labeled, and fragmented (by one round of Arcturus RiboAmp HS kit amplification followed by the RNA Amplification and Labeling Kit from Enzo Life Sciences). The resulting biotinylated cRNA probes were hybridized to Affymetrix (Santa Clara, CA) MOE430v2 GeneChips; expression patterns analyzed using dChip (18, 19) and an in-house-developed analysis suite that annotates lists of genes by associated Gene Ontology (GO) terms and then clusters and analyzes the lists using the fractional representations of each GO term (20). As is standard for dChip analysis software, all GeneChips used to determine effects of time following transfer of T cells or experimental treatment were normalized to the GeneChip with total average intensity closest to the mean intensity of all chips in the experiment. Filtering of false positives generated by multiple comparison error (21) was also performed as a standard part of the dChip analysis software in computing model-based expression levels. Time course visualization and clustering of patterns of M1-related and M2-related gene expression identified using dChip were further confirmed using several clustering algorithms in the SpotFire DecisionSite software package (TIBCO Software Inc, Palo Alto, CA), and plotted using GraphPad Prism. Validation of selected gene expression changes was performed using qRT-PCR as previously described (22), except that 18s primers were used as standards, and analysis was performed on a Stratagene MX3000P machine. Gene chip data GEO accession number: GSE12389.
Bone marrow engraftment
Donor cells were isolated from IFNγR−/− and IFNγR+/+ NOD.Rag-1−/− mice treated with 5-fluorouracil (Sigma-Aldrich) for 3–5 days before mice were killed. Recipient mice (NOD.Rag.IFNγR−/− and NOD.Rag.IFNγR+/+) received a single lethal dose of 9.50-Gy using a cesium source and were engrafted with 5×106 bone marrow cells i.v. Transplanted mice were given water supplemented with trimethoprim-sulfamethoxazole (Hi-Tech Pharmacal, NY) ad libitum for 2 weeks and were allowed to engraft for a minimum of 70 days before transfer experiments. Bone marrow engrafted mice were analyzed by flow cytometry to corroborate effectiveness of engraftment by detecting intracellular phosphorylation of STAT-1 upon IFN-γ administration (1000U/mL for 15 minutes at room temperature) with an anti-STAT-1 mAb (BD Biosciences) (Supplemental Figure 2).
Results
Antibodies to IFN-γ induced long term protection (LTP) from diabetes
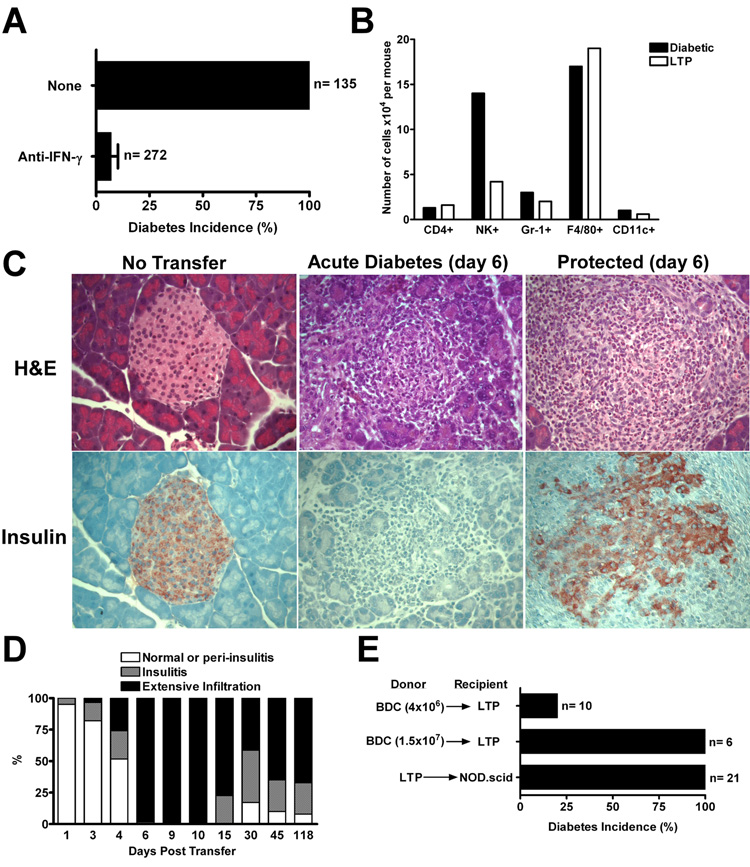
Transfer of BDC T cells into NOD.rag-1−/− or scid mice rapidly resulted in diabetes, the time of development depending on the amounts of T cells transferred (results with either were identical and thus are not separated). In all our transfer experiments BDC T cells were first activated for three days: pooling all experiments, all 135 injected mice became diabetic (Fig. 1A and supplemental figure 3A). Testing a total of 272 mice through several experiments, the mAb H22, to IFN-γ (23), inhibited the development of diabetes by 93%. In most experiments H22, at 300ug, was administered a day before the diabetogenic T cell transfer and, a second dose, two days after (Fig. 1A and supplemental figure 3A). The inhibition of diabetes persisted: most of the mice were tested up to 140 days after the injection of BDC T cells, and these were still normoglycemic.
FIGURE 1.
Treatment with antibodies to IFN-γ induces LTP from diabetes. A, Incidence of diabetes in NOD.Rag-1−/− or NOD.scid mice that received activated BDC T cells (4×106) alone, or along with the anti-IFN-γ (H22) mAb. Mice injected with BDC T cells had 100% diabetes incidence by day 7, while mice injected with BDC T cells plus the H22 antibody showed only 7% diabetes incidence over a period of 140 days. Results obtained were pooled from a total of eighteen experiments. B, Distribution of islet infiltrating leukocytes at day 7 from NOD.scid mice post BDC T cell transfer (“Diabetic”) and from H22 treated recipients (“LTP”). Leukocytes were recovered from pancreatic islets of six mice in each group and analyzed by flow cytometry for the various leukocyte markers. The total numbers of islet-infiltrating leukocytes recovered per mouse from diabetic versus H22 treated mice were 5.9×105 and 3.8×105, respectively. C, Histological analysis, Sections were stained with H&E and insulin from NOD.scid mice that were untreated (‘No Transfer’), that received activated BDC T cells alone (‘Acute Diabetes’) or in conjunction with the H22 mAb (‘Protected’). Magnification of 20x. D, Insulitis scores from H&E pancreata sections of LTP mice. Infiltration started at the same time as the acute diabetic mice (day 3) and by day 6 there was 100% infiltration without diabetes. Data obtained from 1,201 scored islets of 61 experimental mice. E, Upper and middle bars: rechallenge of LTP mice with 4×106 or 1.5×107 activated BDC T cells, respectively, at day 90 of LTP and followed for 60 days after rechallenge. Lower bar: transfer of bulk splenocytes from LTP mice (between 60 to 143 days of protection) into unmanipulated NOD.scid recipients (diabetes incidence of 100% by day 19).
The LTP was not dependent on the presence of circulating H22 antibody. ELISA measurements of H22 mAb in the sera of 31 mice showed a range between 0.3 to 2.8µg/ml at days 72 to 89 post-antibody injection. We then determined whether the levels of H22 found in LTP mice were enough to afford protection from diabetes. Administration of a dose lesser than 25µg did not give protection (data not shown). In conclusion, the serum concentration of antibody in the protected mice at later time points was too far below the neutralizing levels, indicating that LTP depended only on neutralizing IFN-γ during an early, critical window of time. Both sets of mice that received the BDC T cells, that is, with or without injections of H22 mAb, showed a similar severe inflammatory response made up mostly of mononuclear phagocytes. Figure 1B shows the distribution of cells in the islet exudates from each group: both mostly comprised F4/80+ cells, with a smaller fraction of neutrophils, dendritic cells and NK cells. Histopathologically, the extensive inflammatory reaction in the diabetic mice was accompanied by complete disruption of islets with loss of insulin-positive cells (Fig. 1C). A recent study of ours, using depleting antibodies, indicated that of the three main cells of the exudate, neutrophils, NK cells and macrophages, the last were the only cells responsible for killing the β-cells (9).
During the first week, the inflammatory reaction in the BDC T cells plus H22 mAb injected mice disrupted the islets which became lobulated with inflammatory cells separating the lobules. Insulin staining was not detected in the diabetic mice while LTP mice showed decreased insulin staining (Fig. 1C). The total islet mass as determined by the insulin content of the pancreas was absent in acute diabetic mice and substantially reduced in the LTP cohort (Fig. 2). In the LTP mice, the inflammatory reaction persisted but became less intense throughout the observation time (Fig. 1D).
FIGURE 2.
Insulin content from total pancreas measured by radioimmuno assay form acute diabetes and LTP mice at different times post BDC T cell transfer. Each point represents the average insulin content of 4 to 6 mice per time point.
H22 treated mice were more resistant to the induction of diabetes when a second dose of BDC T cells was administered, even if the second dose occurred well after H22 concentration had dropped below neutralizing levels. In these experiments, when 4×106 T cells were injected into untreated NOD.scid mice, all mice became diabetic by the sixth day. However only 2 of 10 mice previously injected with H22 mAb became diabetic, even though, in this case, the mice had been injected 90 days before with H22 and BDC T cells (Fig. 1E, upper bar and supplemental figure 3B). In another set of experiments, increasing the dose of T cells to 1.5 × 107 did induce diabetes after twenty five days in 6 of 6 mice, while all the six control mice were diabetic by the fourth day (Fig. 1E, middle bar and supplemental figure 3B). Hence, the H22 treated recipients were not conducive to induction of disease when limiting numbers of diabetogenic CD4 T cells were reintroduced.
The LTP mice however still harbored diabetogenic T cells in their spleens. Sixty to 143 days after injection of BDC T cells plus H22 mAb, the protected mice were sacrificed and their spleen cells isolated and transferred into unmanipulated NOD.scid recipients. All 21 mice from six different experiments became diabetic by 8–19 days (Fig. 1E, lower bar and supplemental figure 3C).
In brief, treatment with anti-IFN-γ mAb protected mice from diabetes by reducing the mass of islet β-cells that died following the injection of BDC T cells. Protection was prolonged and accompanied by a persistent inflammatory reaction predominantly made up of macrophages. Importantly, the LTP mice contained diabetogenic T cells indicating an active immunomodulatory environment that kept the pathogenic T cells in check.
Gene expression analysis of islets from protected versus diabetic recipient mice showed different macrophage profiles
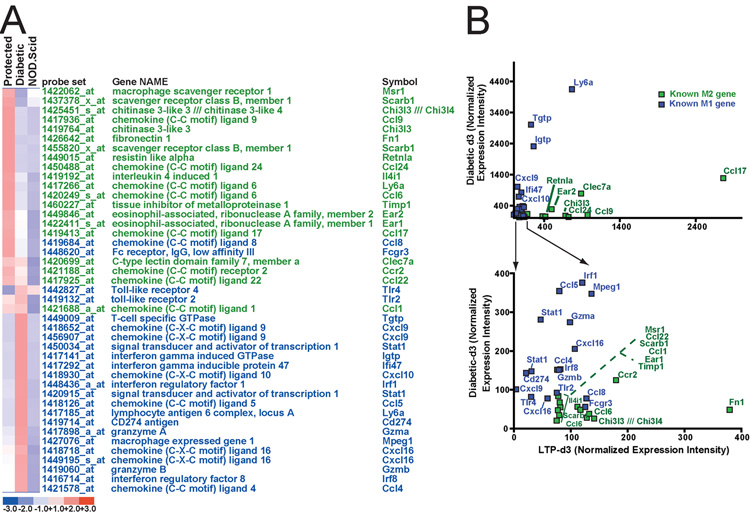
Initially, gene expression was assayed in pancreas from control untreated mice as well as LTP and diabetic mice at day 3 following BDC T cell injection (n=5 mice per condition, with RNA pooled for each GeneChip). We identified 1279 genes whose expression was increased in diabetic mice relative to untreated NOD.scid (lower bound of 90% confidence interval set at 1.3, intensity difference >= 50), 1162 increased in LTP vs NOD.scid and 1098 that decreased from NOD.scid to either diabetic or LTP mice. Gene Ontology analysis (20, 24) showed that the most significant patterns of changes in both diabetic and LTP islets relative to untreated mice at d3 were increases in genes classified by the GO term “Immune response” (0.45% of genes preferentially expressed in control mice vs. 4.8% of LTP and 5.9% of diabetic genes). Many of the genes increased in both diabetic and LTP islets were associated with macrophages. [Comparing to 247 genes specifically increased in activated macrophages (25, 26), we found that 60 representing 4.7% of the diabetic transcripts and 48, representing 4.1% of the LTP transcripts were characteristic of activated macrophages vs only 0.5% of transcripts enriched in untreated NOD.scid islets]. Altogether, 31% of the select group of genes representing macrophage activation was enriched in either the unprotected or protected islet population.
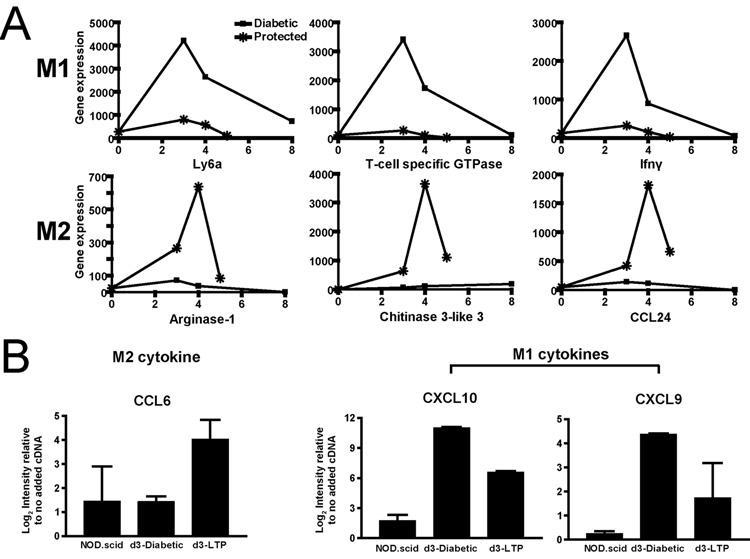
A distinct pattern was found in the profile of macrophage genes upregulated in islets of diabetic compared to LTP mice. When the LTP and diabetic expression profiles were compared directly to each other, using relatively high threshold for difference (fold change >=3.5, intensity difference >=75), those genes enriched in diabetic islets were characteristic of the activated M1-type associated with inflammatory responses, whereas genes enriched in LTP mice were characteristic of the M2-phenotype, activated by the alternative pathway (25–35). M1 macrophages are activated via IL-12 and IFN-γ-induced pathways that depend on downstream signaling via Stat1 and Stat4. Stat1 gene expression was enriched in diabetic mice relative to islets from LTP mice (Fig. 3A and B). Multiple target genes of Stat1-induced pathways were also expressed preferentially in islets from the diabetic mice. Examples include: Ly6a, Igtp, Ifi47, Irf1, Tgtp, as well as the chemokines CXCL9 and CXCL10 (Fig. 3A and B). In islets from LTP mice the following genes were expressed: chitinase 3-like 3 (Chil3l, aka YM-1), CCL24, CCL9 (aka Scya9), Ear1, Timp1, IL4i1, and CCL6 (Fig. 3A and B). Interestingly, expression of Arginase I, a key M2-associated gene was statistically high in LTP at d3, but much higher at the fourth day (Fig. 4A). Finally, q-RT-PCR on laser-captured islets at day 3 confirmed for selected cytokines that the M1 cytokines CXCL9 and CXCL10 were increased in diabetic vs LTP and the M2 cytokine CCL6 was increased in LTP vs. diabetic (Fig. 4B).
FIGURE 3.
Laser-capture microdissected LTP islets preferentially express M2 macrophage genes, whereas diabetic islets express M1 genes. GeneChip expression profiles were generated by directly comparing d3 LTP to d3 diabetic GeneChips. Those genes with profoundly differing patterns of expression (3.5-fold difference between LTP and diabetic, expressed at >75 intensity level) were identified. All genes, increased in either direction, were then classified by literature search as M1-associated or M2-associated. A, All differentially expressed M1 (blue) and M2 (green) genes from this analysis are depicted in the heat map. Note how almost all the M2 genes have higher expression in LTP and vice versa; none of the genes are expressed at the highest level in control islets. B, The same genes are plotted according to intensity levels. Genes with high expression (upper plot) and low expression (lower) are separated for clarity. Follow up experiments with additional cohorts of mice and using qRT-PCR confirms the pattern of association of M1 genes with diabetic islets and M2 with LTP.
FIGURE 4.
Follow up experiments with additional cohorts of mice and using qRT-PCR confirms the pattern of association of M1 genes with diabetic islets and M2 with LTP. A, Plots of representative, selected M1 (upper) and M2 (lower) genes at later time points. B, Plots of qRT-PCR of laser-captured islets for selected M1 and M2 genes showing the same pattern of expression as on GeneChip. Results are means±SD from duplicate, independent experiments. Note that the scale is Log2.
Evidence for regulatory T cell-dependent protection upon neutralization of IFN-γ
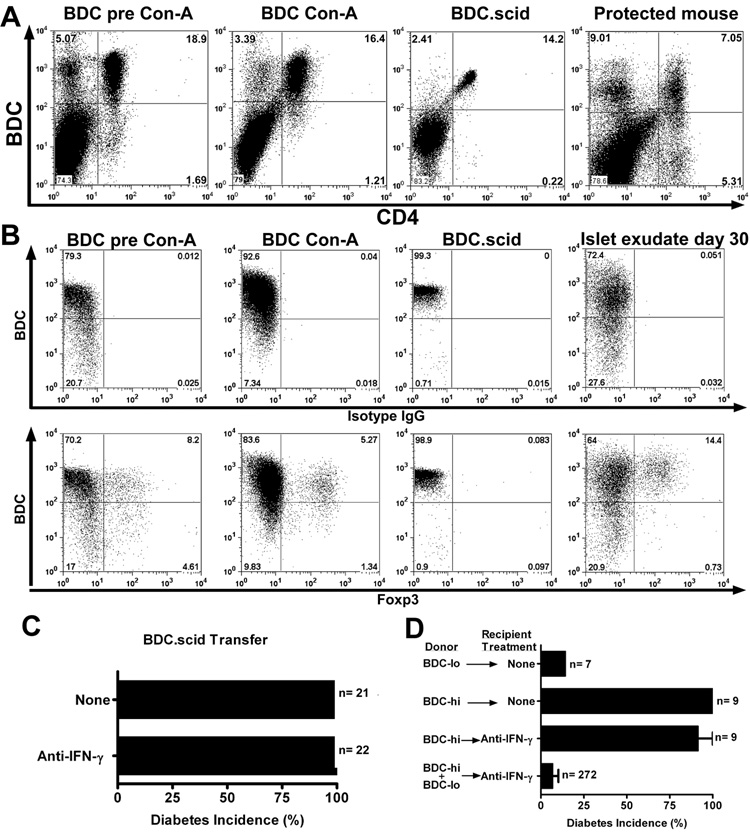
Flow cytometry analysis from 5 independent experiments from LTP mice showed expression of the BDC TCR at intermediate or low levels in 29 to 49% of them BDC-lo, while the remaining cells expressed high levels of the TCR , BDC-hi (Fig 5A). A previous report by Kanagawa et al, indicated that the BDC-lo T cells were regulatory in nature in that they either delayed or inhibited the onset of diabetes (36). In addition, the BDC2.5 TCR transgenic mice genetically crossed into the NOD.scid mice contained a homogeneous set of T cells only expressing high levels of the clonotype TCR (Fig 5A): all these mice became spontaneously diabetic within 28 days post birth.
FIGURE 5.
Anti-IFN-γ treatment did not inhibit diabetes induced by the transfer of BDC-hi T cells. A, Flow cytometry analysis of spleen CD4+ T cells from BDC pre-activation, post-activation, BDC.scid and LTP mice (day 70 of protection from diabetes) and their clonotype BDC hi and -lo expression. B, Flow cytometry analysis (on gated CD4+) looking at BDC and Foxp3+ expression from splenocytes of BDC pre-activation, post-activation, BDC.scid and islet exudate (day 30) of LTP mice. Upper panels stained with BDC plus the isotype IgG control antibody. Lower panels stained with BDC plus the anti-Foxp3 mAb. C, Incidence of diabetes in mice that received BDC.scid T cells (1×106) alone, or together with the H22 mAb treatment: both sets of mice developed diabetes 10 to 15 days post transfer. D, Transfer of sorted CD4+ T cells for their hi and -lo clonotype expression of BDC into NOD.scid recipients alone, or with the H22 mAb treatment and followed for 30 days for diabetes incidence. The lower bar represents the combined transfer of BDC-hi and -lo that leads to LTP from Figure 1A.
To ascertain whether H22-mediated protection required CD4 T cells that expressed endogenous TCRs in addition to the BDC2.5 TCR, transfer experiments were performed using only the BDC-hi CD4 T cells. BDC-hi T cells isolated from either BDC or BDC.scid mice transferred diabetes but this transfer of diabetes was not affected by injection of H22 (Fig 5C and supplemental figure 3D). Experiments were done administering once a very high dose of 1.5 mg of H22, or giving three injections in seven days with 300ug each. The diabetic process was not inhibited; hyperglycemia was delayed in a few mice by 4 to 20 days compared to the untreated mice but once diabetes developed it had the same features of severity and degree of inflammation (Fig. 5C and D; supplemental figure 3D and E). BDC-lo CD4 T cells did not transfer diabetes and, a mixture of BDC-hi and –lo T cells was needed for the protective effect of anti-IFN-γ (Fig. 5D). Flow cytometry analysis of BDC-hi T cells showed their high expression of IFN-γ but very limited expression of IL-17 (not shown).
Thus neutralization of IFN-γ did not directly control islet β-cell destruction mediated by BDC-hi T cells. The results pointed to a BDC-lo T cell regulating the response of BDC-hi T cells: at face value in the absence of IFN-γ the negative effects of this cell predominated over those of the BDC-hi T cells. The BDC-lo T cells directly or indirectly regulated the pathogenic potential of BDC-hi T cells or could control the final effector activated macrophage (e.g., by regulating its differentiation along M1 or M2 lineages) that kills islet β-cells.
BDC T cells were examined for the expression of Foxp3. As shown in figure 5B, CD4 T cells from BDC.NOD mice but not BDC.scid mice contained Foxp3+ cells. The CD4+Foxp3+ population in the BDC.NOD mice pre and post- activation predominated in the high and intermediate clonotype population. Moreover, flow cytometry analysis of islet infiltrating T cells from H22 treated mice at day 30 of protection (i.e. from fig. 1A that are protected from diabetes) showed the presence of CD4 T cells that expressed Foxp3 with about the same distribution of the BDC-clonotype. (Fig. 5B).
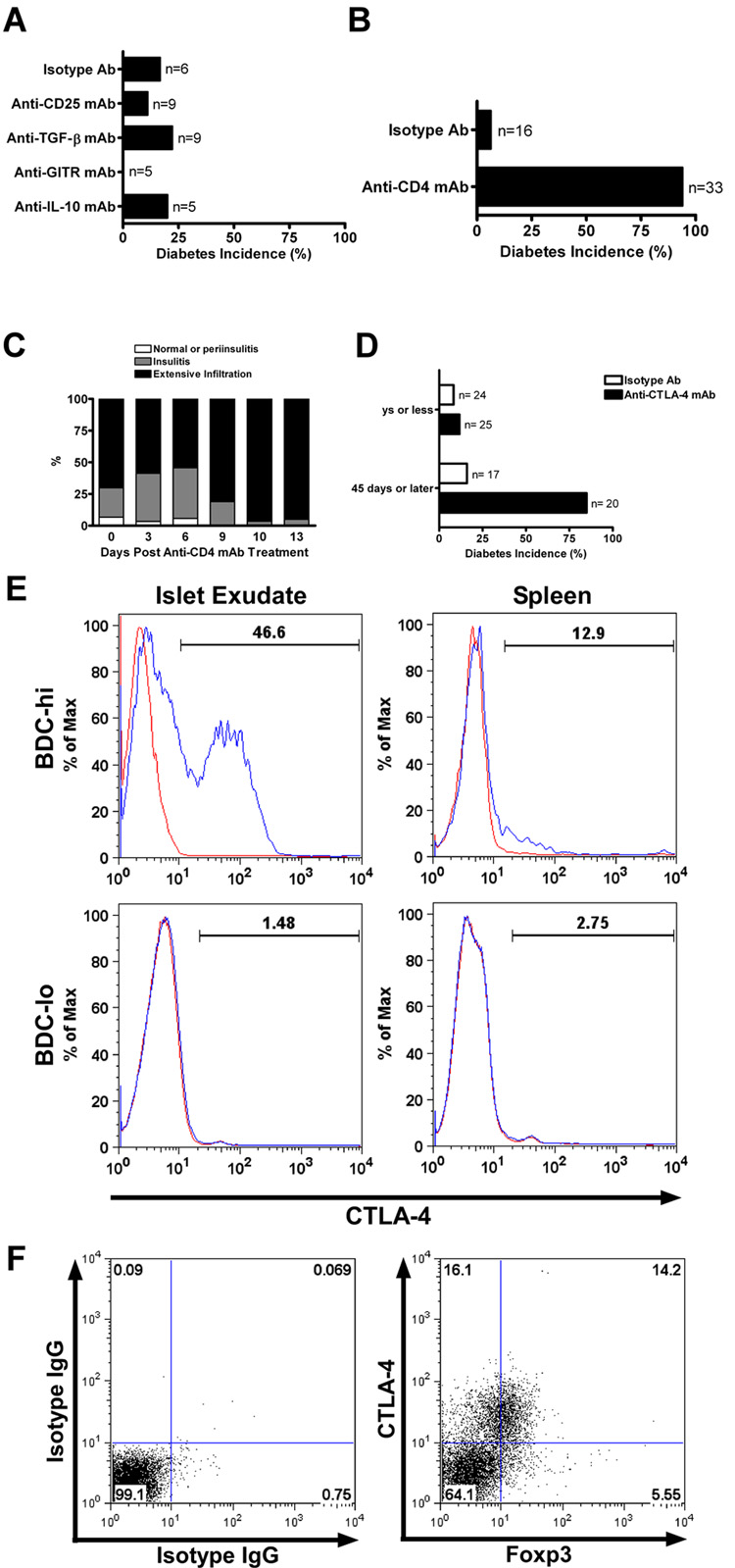
The mode of regulation was next examined by using a variety of neutralizing mAbs. Those that neutralized IL-10 or TGF-β had no effect i.e. LTP mice injected with H22 were still normoglycemic when given the neutralizing antibodies (Fig. 6A). Also injection of PC61, an antibody to CD25, which inhibits regulatory T cells and an antibody to the glucortocid-induced TNF receptor (GITR) had no effect (Fig. 6A). The efficacy of all these antibodies was ascertained in different experimental systems tested in the laboratory (37).
FIGURE 6.
Mode of regulation in LTP mice by neutralizing antibodies. A, LTP mice after 75 days were treated with an irrelevant IgG or with depleting antibodies to CD25, TGF-β, GITR or IL-10 and followed for 30 days for diabetes incidence. B, LTP mice at day 75 were treated with either an irrelevant IgG or anti-CD4 depleting mAb and followed for diabetes incidence. C, Insulitis score analysis from H&E pancreata sections of anti-CD4 treated LTP mice. The extensive infiltration increased post anti-CD4 mAb treatment correlated with diabetes onset. Data obtained from 212 scored islets of 14 experimental mice. D, LTP mice at different times were treated with either an irrelevant IgG or anti-CTLA-4 blocking antibody (4F10) and followed for 30 days. Treatment before day 35 did not show an increase in diabetes, while treatment performed after 45 days of protection increased diabetes incidence. E, Histogram analysis by flow cytometry for total expression of CTLA-4 in BDC-hi and BDC-lo T cells isolated from the islet exudate or the spleen of LTP mice at day 60 of protection. The blue line represents CTLA-4 and the red line the isotype control stain. F, Flow cytometry analysis for total expression of CTLA-4 and Foxp3 (gated on BDC-hi T cells) from islets of LTP mice after 60 days of protection. Left panel shows isotype control staining and right panel shows CTLA-4 and Foxp3 staining.
Two antibodies reversed the LTP. Injection of the YTS191.1 mAb, an antibody that depleted CD4 T cells, rapidly induced diabetes in LTP mice. LTP mice at day 75 post-treatment with H22 mAb were injected with the YTS191.1 mAb (two injections of 500µg each, 3 days apart); 31 of 33 (or 94%) mice developed diabetes 10 days after the treatment (Fig. 6B and supplemental figure 3F). Histopathological analysis showed islets with an extensive leukocyte infiltration by day 10, time of diabetes onset (Fig. 6C). FACS analysis of YTS191.1-treated mice demonstrated depletion of both BDC-hi and –lo T cells (data not shown).
A neutralizing antibody to CTLA-4 also reversed the protected state induced by H22 mAb administration. Protected mice (those given BDC T cells plus H22 mAb) after 45 days, or later, reverted to diabetes when injected with anti-CTLA-4 mAb: 17 of 20 mice became diabetic, while in contrast, 2 out of 17 injected with control antibodies became diabetic, a difference from 85% to 12% (Fig. 6D and supplemental figure 3G). However the capacity of anti-CTLA-4 mAb to reverse LTP protection was not found in mice at days 9, 25 or 35 after administration of BDC T cells plus H22. From a total of 25 mice given anti-CTLA-4, only four became diabetic, in comparison to two of 24 given control antibodies (Fig. 6D). Thus the early stages of H22 protection were independent of engagement of CTLA-4 while the later stage was influenced by CTLA-4-dependent inhibition. To note, flow cytometry analysis of CTLA-4 total expression in the exudates of LTP mice indicated noticeable levels in the BDC-hi but not BDC-lo T cells (Fig. 6E). CTLA-4 expression was not found in the splenocytes of LTP mice.
We analyzed the correlation of CTLA-4 and Foxp3 expression in BDC-hi T cells from the islet exudates of LTP mice. As shown in figure 6F, 30% of the BDC-hi gated T cells expressed CTLA-4 and from this, close to 50% were positive for the expression of Foxp3. Only 5.5% of the BDC-hi T cells were positive for Foxp3 and negative for CTLA-4.
Transfer of diabetes requires host myeloid cells to express the IFN-γ receptor
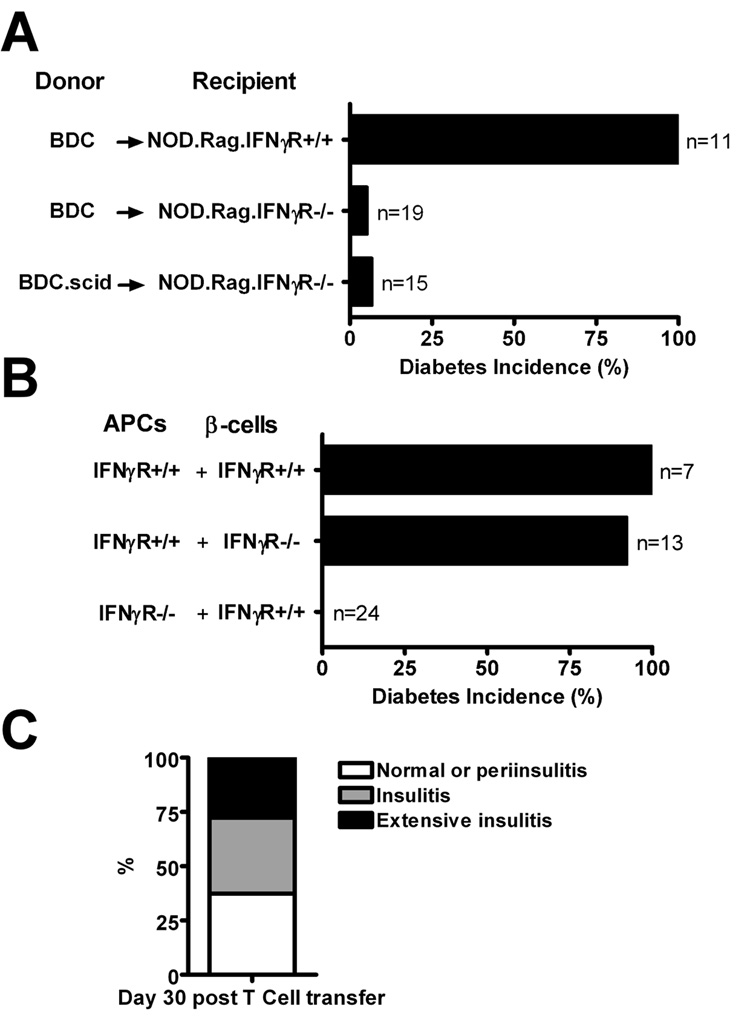
As was noted, the BDC-hi CD4 T cells induced diabetes resistant to H22 mAb treatment implying that IFN-γ was not required to mediate the effector reactions. But surprising, and paradoxically, BDC T cells, sorted for clonotype-high or from BDC.scid mice which only express high clonotype, or BDC T cells from NOD mice bearing BDC-hi and BDC-lo cells, did not transfer diabetes into mice lacking the IFNγR (Fig 7A and Table I). By making bone marrow chimeras, mice were examined in which the bone marrow derived cells or the β-cells expressed or did not express IFNγR. In agreement with the published work from Katz’s laboratory (38), BDC T cells induced diabetes in mice in which the β-cells lacked the receptor. But diabetes was not induced in mice where the myeloid compartment lacked the IFNγR (Fig. 7B, Table I and supplemental figure 3H). Mice were followed up to 60 days after transfers. All mice had an inflammatory reaction: while about 30% of islets were clean and not infiltrated, 40% showed a mixed infiltrate surrounding the islet which appeared normal; and the remaining 30% had inflammation penetrating the islets (Fig. 7C). In the case of the bone marrow chimeras their peripheral blood cells were confirmed to lack the IFNγR by testing Stat-1 movement to the nucleus after incubation with IFN-γ (Supplemental Figure 2). Thus the lack of IFNγR in the myeloid lineage translates in a reduction in the inflammatory reaction that kills β-cells implying that some IFN-γ interactions are amenable to H22 neutralization while others are not.
FIGURE 7.
Transfer of diabetes requires host myeloid cells to express the IFNγR. A, Transfer of 4×106 activated BDC T cells or BDC.scid T cells (106) into NOD.Rag.IFNγR+/+ or NOD.Rag.IFNγR−/− recipients and followed for diabetes incidence. While activated BDC T cells transferred diabetes into NOD.Rag.IFNγR+/+, the transfer of either activated BDC.NOD T cells or BDC.scid cells into NOD.Rag.IFNγR−/− did not increase diabetes incidence. Experimental mice were followed for 60 days post T cell transfer. B, Bone marrow chimeric mice in which the bone marrow derived cells or the β-cells expressed or did not express IFNγR were transferred with activated BDC, T cells (4×106) and followed for diabetes incidence for 30 days. Only recipient mice that contained bone marrow derived cells that lacked IFNγR and β-cells-expressing IFNγR were free of diabetes. C, Islets from previous mice that were free of diabetes (day 30, last day of observation) were examined for insulitis by H&E sections. Data obtained from 24 experimental mice.
TABLE I.
Summary of IFNγR transfer experiments.
| T cells | NOD.scid or rag recipients | Diabetes incidence | Number of experiments | ||
|---|---|---|---|---|---|
| Donor | Treatment | Strain | Treatment | ||
| BDC.IFNγR+/+ | Con-A | IFNγR+/+ | None | 135/135 | 36 |
| BDC.IFNγR+/+ | Con-A | IFNγR−/− | None | 1/19 | 3 |
| BDC.scid | None | IFNγR+/+ | None | 27/27 | 9 |
| BDC.scid | None | IFNγR−/− | None | 1/15 | 2 |
| BDC.scid | None | IFNγR−/− | H22 (day 0 & 2) |
1/10 | 1 |
| BDChi (sorted) | None | IFNγR−/− | None | 1/5 | 1 |
| BDChi (sorted) | None | IFNγR−/− | H22 (day -1 & 2) |
0/5 | 1 |
| BDC.IFNγR+/+ | Con-A |
APC: IFNγR+/+ β-cells: IFNγR−/− |
None | 12/13 | 2 |
| BDC.IFNγR+/+ | Con-A |
APC: IFNγR−/− β-cells: IFNγR+/+ |
None | 0/24 | 2 |
Mice that did not develop diabetes were followed from 30 to 60 days post T cell transfer in all experiments.
Discussion
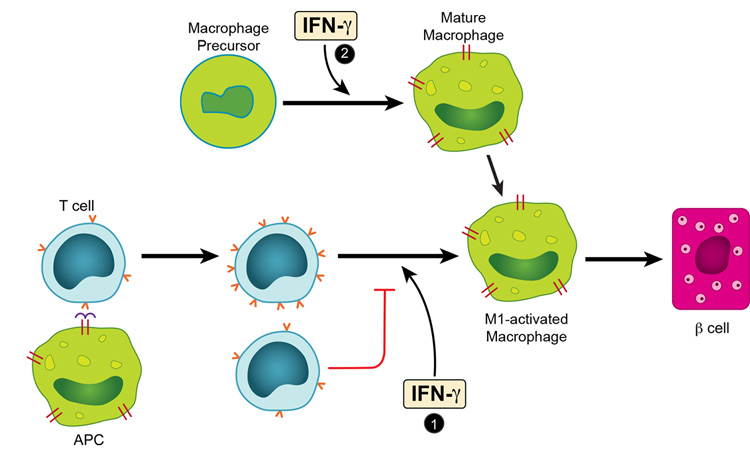
The acute transfer model pointed to several notable effects of IFN-γ in autoimmune diabetes mediated by a clonal set of diabetogenic CD4 T cells. In general the results together with those of others, outline the various distinct and complex cellular interactions taking place in autoimmune diseases where there is chronic antigenic stimulation and involvement of practically all the cells of the immune system. The experiments identified two situations that IFN-γ participated in the induction of the inflammatory response and the consequent diabetes mediated by BDC T cells (Fig.8).
FIGURE 8.
IFN-γ effects in the induction of the inflammatory response and the consequent diabetes mediated by BDC T cells. As a result of the interaction between an APC containing a diabetogenic antigen and a diabetogenic CD4 T cells, two sets of activated CD4 T cells are generated, differing in the extent of expression of their TCR (noted by the numbers of “v” in red in the figure). In the islet tissue these cells generated an inflammatory exudate rich in M1-activated macrophages. IFN-γ, exerts an effect in this step, marked as # 1 allowing the TCR high T cell to predominate. In the absence or blocking of IFN-γ in the step 1, the TCR-low predominates and the resulting exudate has features of the M2-activated macrophages, and β-cell function is preserved. The macrophage precursor differentiates in a process influenced by IFN-γ, see # 2, to generate macrophages that respond to inflammatory cues.
First when inflammation was transferred by BDC T cells bearing high and low levels of the TCR, IFN-γ was required to induce an M1 exudate that led to β-cell death; in its absence, the BDC T cells entered into a regulatory pathway in which the exudate was typical of the M2 macrophages. The M1 exudate is typical of IFN-γ mediated processes. Among the various genes modulated by IFN-γ, that encoding for CXCL10 was previously implicated in the progression of diabetes: neutralizing antibody to CXCL10 in a cyclophosphamide-exacerbated NOD model suppressed diabetes (39). The M2 macrophages are associated with the alternative pathway activated by the cytokines such as IL-4/13 which can promote allergic inflammation and wound healing in target tissues, rather than cytotoxicity (40, 41). YM-1 and arginase epitomize the genes expressed in the alternative pathway. Arginase competes with iNOS for nitrogen, shunting it away from production of cytotoxic nitric oxide (a presumed mediator of β-cell death in diabetes) (42) and toward production of extracellular matrix and/or proliferation-stimulating polyamines. Another transcript enriched in the LTP islets was CCL6 shown to be induced in macrophages by IL-4, inhibited by IFN-γ and a key chemotactic factor for fibrogenic (i.e., non cytotoxic) macrophages (40, 41).
Activated macrophages in the M1-exudate were in close contact with β-cells and were responsible for their death. In culture the same cells killed β-cells (9). The mechanism of action of the activated macrophages has not been determined. Activated macrophages produce peroxynitrate and β-cells have been shown to be highly sensitive to oxidative damage (42). In contrast the alternative activated macrophages in the M-2 exudates show considerably less cytocidal molecules. To note that the response to an M1 or M2 exudate is not all or none: indeed LTP mice did have a reduced mass of β-cells, but sufficient to maintain euglycemia. In unpublished experiments no proliferation of β-cells was discerned in the LTP mice.
The key role of the clonotype-low BDC T cell was apparent: it did not transfer diabetes yet it influenced profoundly the behavior of the clonotype-high BDC T cells. Thus, in a situation in which T cells bearing different amounts of the TCR were together, IFN-γ was a key cytokine that allowed the clonotype-high T cells to dominate, induce the M1 exudate and diabetes (Fig. 8, #1). Pari passu, in the absence of IFN-γ, the modulatory action of the clonotype-low T cells predominated, influenced the type of exudate (M-2), and diabetes was controlled. How the BDC-lo cell deviated the exudate to an M2 type, is not apparent. We could not find evidence for the involvement of IL-10 or TGF-β. The finding that foxp3 T cells distributed among the T cells bearing different amounts of the clonotype (Fig. 5B) does not allow us to determine which set, if any, was involved in the protection. It is to be noted that neither anti-CD25 nor anti-GITR mAb affected the LTP. However, CTLA-4 was definitely involved but at a later stage of the chronic LTP.
CTLA-4 has been implicated in some of the effects mediated by regulatory T cells (43) and in NOD diabetes (44, 45), in our case, CTLA-4 expression was found within the Foxp3 population of the BDC-hi T cells. CTLA-4 could be participating in two effects: 1) direct inhibition of the effector T cell (46–48) and 2), direct influence on the macrophage as it has been suggested, for example by increasing the production of indoleamine 2–3 dioxygenase (44, 49–54). Current ongoing experiments are focused to solve these questions.
An important feature of the LTP pathway is that the effector diabetogenic T cells were not eliminated but were present, not in islets but in spleen. In contrast to the BDC T cells in islets, which expressed CTLA-4, those in the spleen were negative. A comparable situation was previously reported in NOD mice given Freund’s adjuvant and protected from diabetes (55). It all implies that much of the regulation is local.
Another finding that calls our attention is that of the rapid reversal of the LTP when BDC T cells were depleted. Our expectation was that the depletion would simply abrogate the inflammatory process, that is elimination of the BDC-M2 axis would require new interaction leading to a BDC-M1 axis. Yet, reversal was fast, implying that the LTP is a continous process of control, and that elimination of the regulatory pathway immediately converts the process back to the original M1 exudate.
Finally, a highly paradoxical result emerged from the analysis of the requirements for IFN-γ signals in the host leukocytes. By performing bone-marrow chimeras, we noted that the host APC compartment but not the islet β-cells needed IFN-γ signals to allow BDC T cells to induce the full inflammatory response that leads to diabetes (Fig. 8, # 2). So while diabetes in the presence of clonotype-hi T cells could not be neutralized by H22, it still required APC to express the IFN-γ receptor. It could be that the H22 treatment is not effective at neutralizing the IFN-γ signals transmitted between T cells and APCs at close proximity. Another possibility is that tonic levels of IFN-γ signaling may be necessary for macrophages to differentiate into effector cells that kill islet β-cells. If there is already a pool of such cells, lowering IFN-γ at or around the time of the BDC transfer may not have any effect on the recruitment of these killer APCs into the islets.
Previous studies had shown that the natural NOD diabetogenesis was affected to a minor degree in situations where the IFN-γ receptor or the structural genes for IFN-γ were mutated: at the most a slower development was found with a small reduction in incidence (10–14). It suggests other pathways of activation and effector functions taking place during this chronic process. On the other hand in the various manipulations where the diabetogenic process was dissected, such as shown in these studies, a role of IFN-γ is apparent (15–17). In the acute cyclophosphamide model, antibodies to IFN-γ were highly effective in stopping diabetogenesis (15, 16) as well as the absence of the IFN-γ receptor in the myeloid cell compartment (56). Gene expression profiles disclosed high expression of IFN-γ induced genes (57). Other systems have also shown other roles of IFN-γ when involving other cells. In the experiments reported by Cain et al (58), the BDC T cells derived from BDC.scid ie with high clonotype could be inhibited by NKT cells and in this instance, IFN-γ was required in a process where the target tissue appeared to be that of the host. The take home message is that as more cells join the process, there are more diverse interactions taking place.
Supplementary Material
Aknowlegments
We thank Katherine Frederick, Patrice Bittner and Shirley Petzold for their technical assistance; members of the Unanue laboratory, particularly Javier A. Carrero and Roger Belizaire; members of the Mills laboratory; Robert D. Schreiber for his valuable reagent and the critical analysis.
Footnotes
Disclosures
The authors declare that they have no competing financial interest.
This work was supported by research grants from the National Institutes of Health (ERU: DK058177; JCM: K08 DK066062 and DRTC 5 P60 DK20579), the Juvenile Diabetic Research Foundation, the Kilo Diabetes and Vascular Research Foundation and the Multiplex Gene Analysis Core of the Siteman Cancer Center (supported in part by NCI grant#P30 CA91842).
Abbreviations used in this paper: LTP, long term protected: BDC, BDC2.5; NOD.Rag, NOD.Rag-1−/−; NOD.scid, NOD.CB17-Prkdcscid/J; BDC.scid, BDC2.5/NOD.scid, IFN-γR−/−, IFN-γ receptor−/−.
References
- 1.Tisch R, McDevitt H. Insulin-dependent diabetes mellitus. Cell. 1996;85:291–297. doi: 10.1016/s0092-8674(00)81106-x. [DOI] [PubMed] [Google Scholar]
- 2.Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu Rev Immunol. 2005;23:447–485. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- 3.Shoda LK, Young DL, Ramanujan S, Whiting CC, Atkinson MA, Bluestone JA, Eisenbarth GS, Mathis D, Rossini AA, Campbell SE, Kahn R, Kreuwel HT. A comprehensive review of interventions in the NOD mouse and implications for translation. Immunity. 2005;23:115–126. doi: 10.1016/j.immuni.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Schoenborn JR, Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol. 2007;96:41–101. doi: 10.1016/S0065-2776(07)96002-2. [DOI] [PubMed] [Google Scholar]
- 5.Bach EA, Aguet M, Schreiber RD. The IFN gamma receptor: a paradigm for cytokine receptor signaling. Annu Rev Immunol. 1997;15:563–591. doi: 10.1146/annurev.immunol.15.1.563. [DOI] [PubMed] [Google Scholar]
- 6.Schreiber RD, Pace JL, Russell SW, Altman A, Katz DH. Macrophage-activating factor produced by a T cell hybridoma: physiochemical and biosynthetic resemblance to gamma-interferon. J Immunol. 1983;131:826–832. [PubMed] [Google Scholar]
- 7.Katz JD, Wang B, Haskins K, Benoist C, Mathis D. Following a diabetogenic T cell from genesis through pathogenesis. Cell. 1993;74:1089–1100. doi: 10.1016/0092-8674(93)90730-e. [DOI] [PubMed] [Google Scholar]
- 8.Suri A, Katz JD. Dissecting the role of CD4+ T cells in autoimmune diabetes through the use of TCR transgenic mice. Immunol Rev. 1999;169:55–65. doi: 10.1111/j.1600-065x.1999.tb01306.x. [DOI] [PubMed] [Google Scholar]
- 9.Calderon B, Suri A, Unanue ER. In CD4+ T-cell-induced diabetes, macrophages are the final effector cells that mediate islet beta-cell killing: studies from an acute model. Am J Pathol. 2006;169:2137–2147. doi: 10.2353/ajpath.2006.060539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hultgren B, Huang X, Dybdal N, Stewart TA. Genetic absence of gamma-interferon delays but does not prevent diabetes in NOD mice. Diabetes. 1996;45:812–817. doi: 10.2337/diab.45.6.812. [DOI] [PubMed] [Google Scholar]
- 11.Serreze DV, Post CM, Chapman HD, Johnson EA, Lu B, Rothman PB. Interferon-gamma receptor signaling is dispensable in the development of autoimmune type 1 diabetes in NOD mice. Diabetes. 2000;49:2007–2011. doi: 10.2337/diabetes.49.12.2007. [DOI] [PubMed] [Google Scholar]
- 12.Kanagawa O, Xu G, Tevaarwerk A, Vaupel BA. Protection of nonobese diabetic mice from diabetes by gene(s) closely linked to IFN-gamma receptor loci. J Immunol. 2000;164:3919–3923. doi: 10.4049/jimmunol.164.7.3919. [DOI] [PubMed] [Google Scholar]
- 13.Wang B, Andre I, Gonzalez A, Katz JD, Aguet M, Benoist C, Mathis D. Interferon-gamma impacts at multiple points during the progression of autoimmune diabetes. Proc Natl Acad Sci U S A. 1997;94:13844–13849. doi: 10.1073/pnas.94.25.13844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicoletti F, Zaccone P, Di Marco R, Di Mauro M, Magro G, Grasso S, Mughini L, Meroni P, Garotta G. The effects of a nonimmunogenic form of murine soluble interferon-gamma receptor on the development of autoimmune diabetes in the NOD mouse. Endocrinology. 1996;137:5567–5575. doi: 10.1210/endo.137.12.8940385. [DOI] [PubMed] [Google Scholar]
- 15.Debray-Sachs M, Carnaud C, Boitard C, Cohen H, Gresser I, Bedossa P, Bach JF. Prevention of diabetes in NOD mice treated with antibody to murine IFN gamma. J Autoimmun. 1991;4:237–248. doi: 10.1016/0896-8411(91)90021-4. [DOI] [PubMed] [Google Scholar]
- 16.Campbell IL, Kay TW, Oxbrow L, Harrison LC. Essential role for interferon-gamma and interleukin-6 in autoimmune insulin-dependent diabetes in NOD/Wehi mice. J Clin Invest. 1991;87:739–742. doi: 10.1172/JCI115055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trembleau S, Penna G, Gregori S, Giarratana N, Adorini L. IL-12 administration accelerates autoimmune diabetes in both wild-type and IFN-gamma-deficient nonobese diabetic mice, revealing pathogenic and protective effects of IL-12-induced IFN-gamma. J Immunol. 2003;170:5491–5501. doi: 10.4049/jimmunol.170.11.5491. [DOI] [PubMed] [Google Scholar]
- 18.Schadt EE, Li C, Ellis B, Wong WH. Feature extraction and normalization algorithms for high-density oligonucleotide gene expression array data. J Cell Biochem Suppl. 2001;37 Suppl:120–125. doi: 10.1002/jcb.10073. [DOI] [PubMed] [Google Scholar]
- 19.Zhong S, Li C, Wong WH. ChipInfo: Software for extracting gene annotation and gene ontology information for microarray analysis. Nucleic Acids Res. 2003;31:3483–3486. doi: 10.1093/nar/gkg598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doherty JM, Carmichael LK, Mills JC. GOurmet: a tool for quantitative comparison and visualization of gene expression profiles based on gene ontology (GO) distributions. BMC Bioinformatics. 2006;7:151. doi: 10.1186/1471-2105-7-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. Series B (Methodological) 1995;57:289–300. [Google Scholar]
- 22.Mills JC, Andersson N, Stappenbeck TS, Chen CC, Gordon JI. Molecular characterization of mouse gastric zymogenic cells. J Biol Chem. 2003;278:46138–46145. doi: 10.1074/jbc.M308385200. [DOI] [PubMed] [Google Scholar]
- 23.Schreiber RD, Hicks LJ, Celada A, Buchmeier NA, Gray PW. Monoclonal antibodies to murine gamma-interferon which differentially modulate macrophage activation and antiviral activity. J Immunol. 1985;134:1609–1618. [PubMed] [Google Scholar]
- 24.Harris MA, Clark J, Ireland A, Lomax J, Ashburner M, Foulger R, Eilbeck K, Lewis S, Marshall B, Mungall C, Richter J, Rubin GM, Blake JA, Bult C, Dolan M, Drabkin H, Eppig JT, Hill DP, Ni L, Ringwald M, Balakrishnan R, Cherry JM, Christie KR, Costanzo MC, Dwight SS, Engel S, Fisk DG, Hirschman JE, Hong EL, Nash RS, Sethuraman A, Theesfeld CL, Botstein D, Dolinski K, Feierbach B, Berardini T, Mundodi S, Rhee SY, Apweiler R, Barrell D, Camon E, Dimmer E, Lee V, Chisholm R, Gaudet P, Kibbe W, Kishore R, Schwarz EM, Sternberg P, Gwinn M, Hannick L, Wortman J, Berriman M, Wood V, de la Cruz N, Tonellato P, Jaiswal P, Seigfried T, White R. The Gene Ontology (GO) database and informatics resource. Nucleic Acids Res. 2004;32(Database issue):D258–D261. doi: 10.1093/nar/gkh036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lang R, Patel D, Morris JJ, Rutschman RL, Murray PJ. Shaping gene expression in activated and resting primary macrophages by IL-10. J Immunol. 2002;169:2253–2263. doi: 10.4049/jimmunol.169.5.2253. [DOI] [PubMed] [Google Scholar]
- 26.Pull SL, Doherty JM, Mills JC, Gordon JI, Stappenbeck TS. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc Natl Acad Sci U S A. 2005;102:99–104. doi: 10.1073/pnas.0405979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73:209–212. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 28.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 29.Welch JS, Escoubet-Lozach L, Sykes DB, Liddiard K, Greaves DR, Glass CK. TH2 cytokines and allergic challenge induce Ym1 expression in macrophages by a STAT6-dependent mechanism. J Biol Chem. 2002;277:42821–42829. doi: 10.1074/jbc.M205873200. [DOI] [PubMed] [Google Scholar]
- 30.Cormier SA, Yuan S, Crosby JR, Protheroe CA, Dimina DM, Hines EM, Lee NA, Lee JJ. T(H)2-mediated pulmonary inflammation leads to the differential expression of ribonuclease genes by alveolar macrophages. Am J Respir Cell Mol Biol. 2002;27:678–687. doi: 10.1165/rcmb.4882. [DOI] [PubMed] [Google Scholar]
- 31.Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005;23:344–346. doi: 10.1016/j.immuni.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Rauh MJ, Ho V, Pereira C, Sham A, Sly LM, Lam V, Huxham L, Minchinton AI, Mui A, Krystal G. SHIP represses the generation of alternatively activated macrophages. Immunity. 2005;23:361–374. doi: 10.1016/j.immuni.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Herbert DR, Holscher C, Mohrs M, Arendse B, Schwegmann A, Radwanska M, Leeto M, Kirsch R, Hall P, Mossmann H, Claussen B, Forster I, Brombacher F. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity. 2004;20:623–635. doi: 10.1016/s1074-7613(04)00107-4. [DOI] [PubMed] [Google Scholar]
- 34.Zhu Z, Zheng T, Homer RJ, Kim YK, Chen NY, Cohn L, Hamid Q, Elias JA. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science. 2004;304:1678–1682. doi: 10.1126/science.1095336. [DOI] [PubMed] [Google Scholar]
- 35.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 36.Kanagawa O, Militech A, Vaupel BA. Regulation of diabetes development by regulatory T cells in pancreatic islet antigen-specific TCR transgenic nonobese diabetic mice. J Immunol. 2002;168:6159–6164. doi: 10.4049/jimmunol.168.12.6159. [DOI] [PubMed] [Google Scholar]
- 37.Suri A, Shimizu J, Katz JD, Sakaguchi S, Unanue ER, Kanagawa O. Regulation of autoimmune diabetes by non-islet-specific T cells - a role for the glucocorticoid-induced TNF receptor. Eur J Immunol. 2004;34:447–454. doi: 10.1002/eji.200324599. [DOI] [PubMed] [Google Scholar]
- 38.Pakala SV, Chivetta M, Kelly CB, Katz JD. In autoimmune diabetes the transition from benign to pernicious insulitis requires an islet cell response to tumor necrosis factor alpha. J Exp Med. 1999;189:1053–1062. doi: 10.1084/jem.189.7.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morimoto J, Yoneyama H, Shimada A, Shigihara T, Yamada S, Oikawa Y, Matsushima K, Saruta T, Narumi S. CXC chemokine ligand 10 neutralization suppresses the occurrence of diabetes in nonobese diabetic mice through enhanced beta cell proliferation without affecting insulitis. J Immunol. 2004;173:7017–7024. doi: 10.4049/jimmunol.173.11.7017. [DOI] [PubMed] [Google Scholar]
- 40.Orlofsky A, Lin EY, Prystowsky MB. Selective induction of the beta chemokine C10 by IL-4 in mouse macrophages. J Immunol. 1994;152:5084–5091. [PubMed] [Google Scholar]
- 41.Orlofsky A, Wu Y, Prystowsky MB. Divergent regulation of the murine CC chemokine C10 by Th(1) and Th(2) cytokines. Cytokine. 2000;12:220–228. doi: 10.1006/cyto.1999.0535. [DOI] [PubMed] [Google Scholar]
- 42.Heitmeier MR, Scarim AL, Corbett JA. Interferon-gamma increases the sensitivity of islets of Langerhans for inducible nitric-oxide synthase expression induced by interleukin 1. J Biol Chem. 1997;272:13697–13704. doi: 10.1074/jbc.272.21.13697. [DOI] [PubMed] [Google Scholar]
- 43.Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, Shimizu J, Takahashi T, Nomura T. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 44.Fallarino F, Bianchi R, Orabona C, Vacca C, Belladonna ML, Fioretti MC, Serreze DV, Grohmann U, Puccetti P. CTLA-4-Ig activates forkhead transcription factors and protects dendritic cells from oxidative stress in nonobese diabetic mice. J Exp Med. 2004;200:1051–1062. doi: 10.1084/jem.20040942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luhder F, Chambers C, Allison JP, Benoist C, Mathis D. Pinpointing when T cell costimulatory receptor CTLA-4 must be engaged to dampen diabetogenic T cells. Proc Natl Acad Sci U S A. 2000;97:12204–12209. doi: 10.1073/pnas.200348397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 2000;164:183–190. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 47.Taylor PA, Lees CJ, Fournier S, Allison JP, Sharpe AH, Blazar BR. B7 expression on T cells down-regulates immune responses through CTLA-4 ligation via T-T interactions [corrections] J Immunol. 2004;172:34–39. doi: 10.4049/jimmunol.172.1.34. [DOI] [PubMed] [Google Scholar]
- 48.Tang Q, Boden EK, Henriksen KJ, Bour-Jordan H, Bi M, Bluestone JA. Distinct roles of CTLA-4 and TGF-beta in CD4+CD25+ regulatory T cell function. Eur J Immunol. 2004;34:2996–3005. doi: 10.1002/eji.200425143. [DOI] [PubMed] [Google Scholar]
- 49.Grohmann U, Fallarino F, Bianchi R, Orabona C, Vacca C, Fioretti MC, Puccetti P. A defect in tryptophan catabolism impairs tolerance in nonobese diabetic mice. J Exp Med. 2003;198:153–160. doi: 10.1084/jem.20030633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Puccetti P, Grohmann U. IDO and regulatory T cells: a role for reverse signalling and non-canonical NF-kappaB activation. Nat Rev Immunol. 2007;7:817–823. doi: 10.1038/nri2163. [DOI] [PubMed] [Google Scholar]
- 51.Keskin DB, Marshall B, Munn D, Mellor AL, Gearhart DA. Decreased protein nitration in macrophages that overexpress indoleamine 2, 3-dioxygenase. Cell Mol Biol Lett. 2007;12:82–102. doi: 10.2478/s11658-006-0048-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mellor AL, Chandler P, Baban B, Hansen AM, Marshall B, Pihkala J, Waldmann H, Cobbold S, Adams E, Munn DH. Specific subsets of murine dendritic cells acquire potent T cell regulatory functions following CTLA4-mediated induction of indoleamine 2,3 dioxygenase. Int Immunol. 2004;16:1391–1401. doi: 10.1093/intimm/dxh140. [DOI] [PubMed] [Google Scholar]
- 53.Mellor AL, Baban B, Chandler P, Marshall B, Jhaver K, Hansen A, Koni PA, Iwashima M, Munn DH. Cutting edge: induced indoleamine 2,3 dioxygenase expression in dendritic cell subsets suppresses T cell clonal expansion. J Immunol. 2003;171:1652–1655. doi: 10.4049/jimmunol.171.4.1652. [DOI] [PubMed] [Google Scholar]
- 54.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 55.Ulaeto D, Lacy PE, Kipnis DM, Kanagawa O, Unanue ER. A T-cell dormant state in the autoimmune process of nonobese diabetic mice treated with complete Freund's adjuvant. Proc Natl Acad Sci U S A. 1992;89:3927–3931. doi: 10.1073/pnas.89.9.3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mori Y, Kato T, Kodaka T, Kanagawa EM, Hori S, Kanagawa O. Protection of IFN-{gamma} signaling-deficient NOD mice from diabetes by cyclophosphamide. Int Immunol. 2008 doi: 10.1093/intimm/dxn080. (Advance Access published July 21, 2008). [DOI] [PubMed] [Google Scholar]
- 57.Matos M, Park R, Mathis D, Benoist C. Progression to islet destruction in a cyclophosphamide-induced transgenic model: a microarray overview. Diabetes. 2004;53:2310–2321. doi: 10.2337/diabetes.53.9.2310. [DOI] [PubMed] [Google Scholar]
- 58.Cain JA, Smith JA, Ondr JK, Wang B, Katz JD. NKT cells and IFN-gamma establish the regulatory environment for the control of diabetogenic T cells in the nonobese diabetic mouse. J Immunol. 2006;176:1645–1654. doi: 10.4049/jimmunol.176.3.1645. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.