Abstract
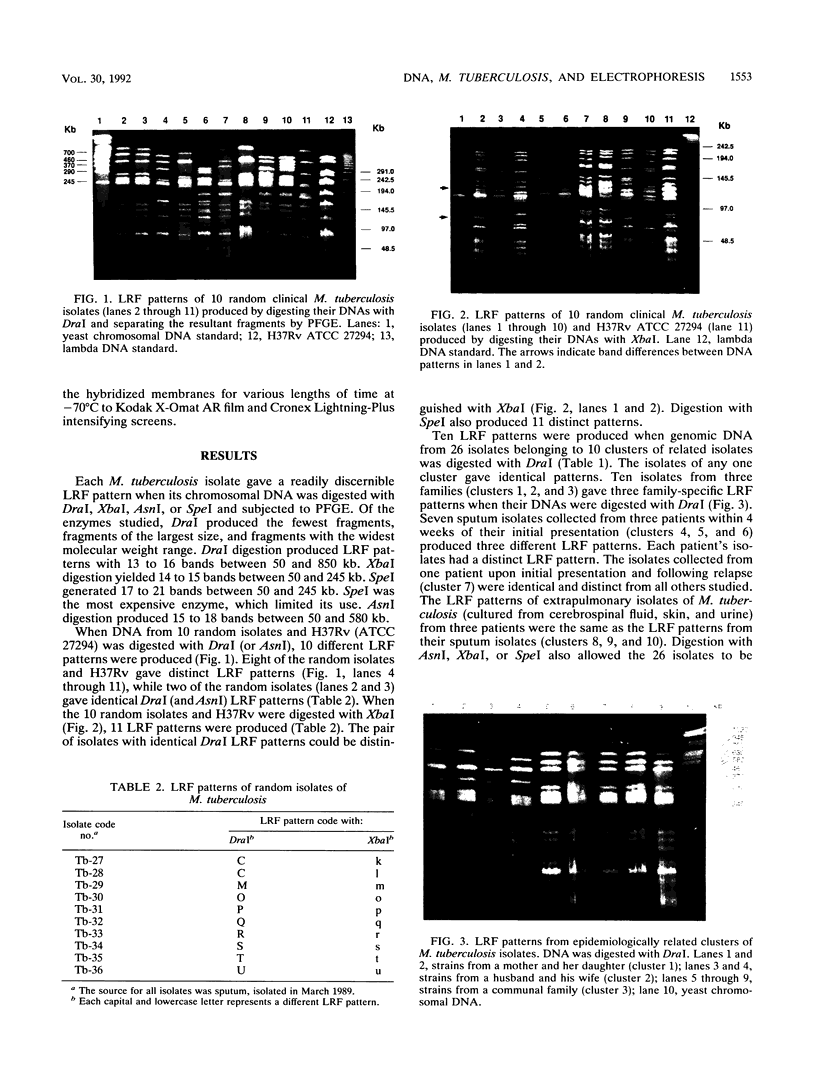
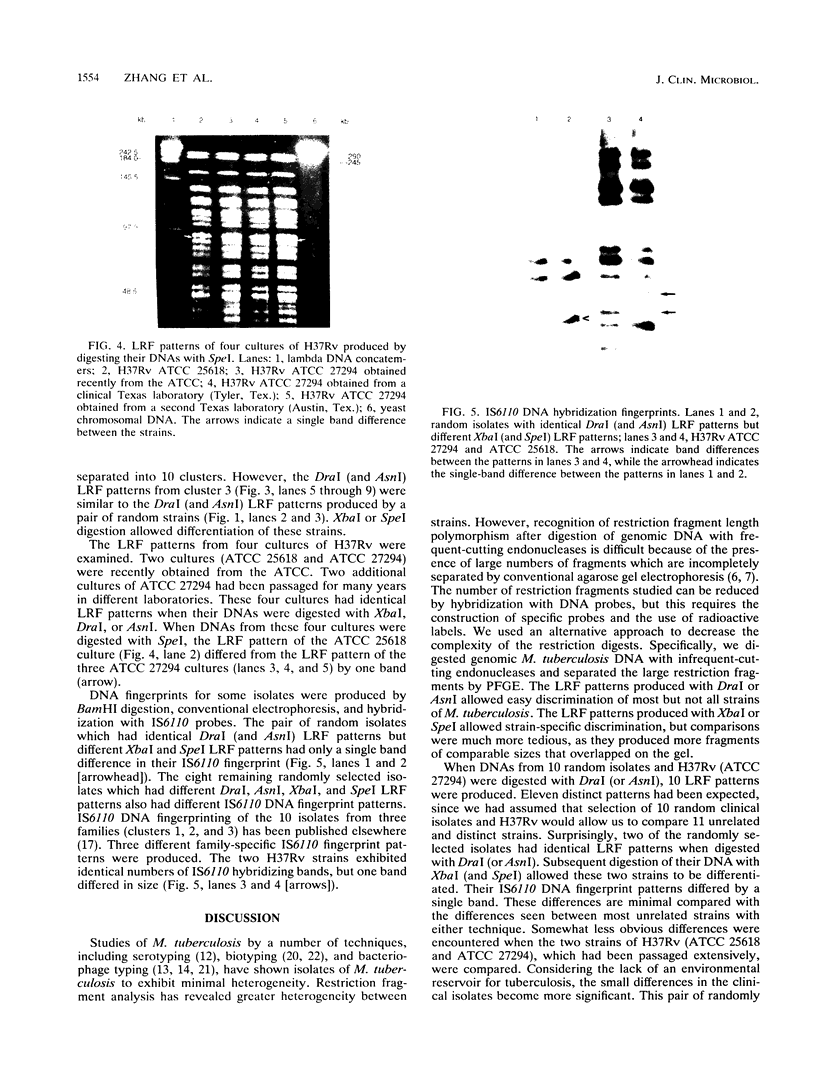
Mycobacterium tuberculosis isolates were studied by comparing large restriction fragment (LRF) patterns produced by digestion of chromosomal DNA with infrequent-cutting endonucleases and pulsed-field gel electrophoresis. Four cultures of H37Rv and 36 clinical isolates of M. tuberculosis were compared by using DraI, AsnI, XbaI, and SpeI. DraI and AsnI allowed easy visual separation of 18 of 21 epidemiologically unrelated strains. XbaI and SpeI allowed discrimination of all 21 unrelated strains, including the 3 strains inseparable with DraI and AsnI, but comparison of LRF patterns was more tedious because of overlapping fragments. A total of 26 isolates belonging to 10 clusters of related isolates were compared by pulsed-field gel electrophoresis, with all related isolates giving identical LRF patterns. These included multiple isolates from the same patient or the same family. The same grouping of clustered isolates was obtained when BamHI DNA digests were hybridized with two probes from the insertion sequence IS6110. Long-term laboratory passage of H37Rv produced minimal detectable changes in LRF patterns. LRF patterns are useful tools for epidemiologic studies of tuberculosis without the need for radioactive or specific DNA probes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allardet-Servent A., Bouziges N., Carles-Nurit M. J., Bourg G., Gouby A., Ramuz M. Use of low-frequency-cleavage restriction endonucleases for DNA analysis in epidemiological investigations of nosocomial bacterial infections. J Clin Microbiol. 1989 Sep;27(9):2057–2061. doi: 10.1128/jcm.27.9.2057-2061.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbeit R. D., Arthur M., Dunn R., Kim C., Selander R. K., Goldstein R. Resolution of recent evolutionary divergence among Escherichia coli from related lineages: the application of pulsed field electrophoresis to molecular epidemiology. J Infect Dis. 1990 Feb;161(2):230–235. doi: 10.1093/infdis/161.2.230. [DOI] [PubMed] [Google Scholar]
- Burns D. N., Wallace R. J., Jr, Schultz M. E., Zhang Y. S., Zubairi S. Q., Pang Y. J., Gibert C. L., Brown B. A., Noel E. S., Gordin F. M. Nosocomial outbreak of respiratory tract colonization with Mycobacterium fortuitum: demonstration of the usefulness of pulsed-field gel electrophoresis in an epidemiologic investigation. Am Rev Respir Dis. 1991 Nov;144(5):1153–1159. doi: 10.1164/ajrccm/144.5.1153. [DOI] [PubMed] [Google Scholar]
- Cave M. D., Eisenach K. D., McDermott P. F., Bates J. H., Crawford J. T. IS6110: conservation of sequence in the Mycobacterium tuberculosis complex and its utilization in DNA fingerprinting. Mol Cell Probes. 1991 Feb;5(1):73–80. doi: 10.1016/0890-8508(91)90040-q. [DOI] [PubMed] [Google Scholar]
- Clark S. M., Lai E., Birren B. W., Hood L. A novel instrument for separating large DNA molecules with pulsed homogeneous electric fields. Science. 1988 Sep 2;241(4870):1203–1205. doi: 10.1126/science.3045968. [DOI] [PubMed] [Google Scholar]
- Collins D. M., De Lisle G. W. DNA restriction endonuclease analysis of Mycobacterium tuberculosis and Mycobacterium bovis BCG. J Gen Microbiol. 1984 Apr;130(4):1019–1021. doi: 10.1099/00221287-130-4-1019. [DOI] [PubMed] [Google Scholar]
- Eisenach K. D., Crawford J. T., Bates J. H. Genetic relatedness among strains of the Mycobacterium tuberculosis complex. Analysis of restriction fragment heterogeneity using cloned DNA probes. Am Rev Respir Dis. 1986 Jun;133(6):1065–1068. doi: 10.1164/arrd.1986.133.6.1065. [DOI] [PubMed] [Google Scholar]
- Eisenach K. D., Crawford J. T., Bates J. H. Repetitive DNA sequences as probes for Mycobacterium tuberculosis. J Clin Microbiol. 1988 Nov;26(11):2240–2245. doi: 10.1128/jcm.26.11.2240-2245.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goering R. V., Duensing T. D. Rapid field inversion gel electrophoresis in combination with an rRNA gene probe in the epidemiological evaluation of staphylococci. J Clin Microbiol. 1990 Mar;28(3):426–429. doi: 10.1128/jcm.28.3.426-429.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grothues D., Koopmann U., von der Hardt H., Tümmler B. Genome fingerprinting of Pseudomonas aeruginosa indicates colonization of cystic fibrosis siblings with closely related strains. J Clin Microbiol. 1988 Oct;26(10):1973–1977. doi: 10.1128/jcm.26.10.1973-1977.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hector J. S., Pang Y., Mazurek G. H., Zhang Y., Brown B. A., Wallace R. J., Jr Large restriction fragment patterns of genomic Mycobacterium fortuitum DNA as strain-specific markers and their use in epidemiologic investigation of four nosocomial outbreaks. J Clin Microbiol. 1992 May;30(5):1250–1255. doi: 10.1128/jcm.30.5.1250-1255.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans P. W., van Soolingen D., Dale J. W., Schuitema A. R., McAdam R. A., Catty D., van Embden J. D. Insertion element IS986 from Mycobacterium tuberculosis: a useful tool for diagnosis and epidemiology of tuberculosis. J Clin Microbiol. 1990 Sep;28(9):2051–2058. doi: 10.1128/jcm.28.9.2051-2058.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones W. D., Jr, Good R. C., Thompson N. J., Kelly G. D. Bacteriophage types of Mycobacterium tuberculosis in the United States. Am Rev Respir Dis. 1982 Jun;125(6):640–643. doi: 10.1164/arrd.1982.125.6.640. [DOI] [PubMed] [Google Scholar]
- Jones W. D., Jr, Kubica G. P. Fluorescent antibody techniques with mycobacteria. 3. Investigation of five serologically homogenous groups of mycobacteria. Zentralbl Bakteriol Orig. 1968 May;207(1):58–62. [PubMed] [Google Scholar]
- Kochi A. The global tuberculosis situation and the new control strategy of the World Health Organization. Tubercle. 1991 Mar;72(1):1–6. doi: 10.1016/0041-3879(91)90017-m. [DOI] [PubMed] [Google Scholar]
- Lévy-Frébault V. V., Thorel M. F., Varnerot A., Gicquel B. DNA polymorphism in Mycobacterium paratuberculosis, "wood pigeon mycobacteria," and related mycobacteria analyzed by field inversion gel electrophoresis. J Clin Microbiol. 1989 Dec;27(12):2823–2826. doi: 10.1128/jcm.27.12.2823-2826.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurek G. H., Cave M. D., Eisenach K. D., Wallace R. J., Jr, Bates J. H., Crawford J. T. Chromosomal DNA fingerprint patterns produced with IS6110 as strain-specific markers for epidemiologic study of tuberculosis. J Clin Microbiol. 1991 Sep;29(9):2030–2033. doi: 10.1128/jcm.29.9.2030-2033.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E., Singh K. V., Heath J. D., Sharma B. R., Weinstock G. M. Comparison of genomic DNAs of different enterococcal isolates using restriction endonucleases with infrequent recognition sites. J Clin Microbiol. 1990 Sep;28(9):2059–2063. doi: 10.1128/jcm.28.9.2059-2063.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. A., Pyle L. E., Stemke G. W., Finch L. R. Human ureaplasmas show diverse genome sizes by pulsed-field electrophoresis. Nucleic Acids Res. 1990 Mar 25;18(6):1451–1455. doi: 10.1093/nar/18.6.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman M. C., Sicilia M. J. Preliminary investigation of Mycobacterium tuberculosis biovars. J Clin Microbiol. 1984 Nov;20(5):1015–1016. doi: 10.1128/jcm.20.5.1015-1016.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider D. E., Jr, Jones W. D., Good R. C. The usefulness of phage typing Mycobacterium tuberculosis isolates. Am Rev Respir Dis. 1984 Dec;130(6):1095–1099. doi: 10.1164/arrd.1984.130.6.1095. [DOI] [PubMed] [Google Scholar]
- Wayne L. G. Numerical taxonomy and cooperative studies: roles and limits. Rev Infect Dis. 1981 Sep-Oct;3(5):822–828. doi: 10.1093/clinids/3.5.822. [DOI] [PubMed] [Google Scholar]
- Yan W., Chang N., Taylor D. E. Pulsed-field gel electrophoresis of Campylobacter jejuni and Campylobacter coli genomic DNA and its epidemiologic application. J Infect Dis. 1991 May;163(5):1068–1072. doi: 10.1093/infdis/163.5.1068. [DOI] [PubMed] [Google Scholar]