Abstract
Human tuberculosis (TB) principally involves the lungs, where local immunity impacts on the load of Mycobacterium tuberculosis (M.tb). Because concomitants of local Th1 immunity are still under-explored in humans, we characterized immune responses in bronchoalveolar cells (BAC) and systemically in peripheral blood mononuclear cells (PBMC) in persons with active pulmonary TB and in healthy community controls. PPD and live M.tb-induced IFN-γ-production was observed in CD4+, CD8+, γδ TCR+, and CD56+ alveolar T cell subpopulations and NK cells (CD3−CD56+). IFN-γ-producing CD4+ T cells (mostly CD45RO+) were more abundant (p<0.05). M.tb-induced IL-12p70, but interestingly also IL-4, were increased (p<0.05) in BAC from TB patients. Constitutive expression of IL-12Rβ1 and IL-12Rβ2 mRNA in BAC and PBMC and IFN-γR1 in BAC was similar in both study groups. Data were normalized to account for differences between the study groups in proportions of alveolar T cells and macrophages. IFN-γ production and its induction by IL-12R engagement occur virtually unimpaired in the bronchoalveolar spaces of patients with pulmonary TB. The reasons for the apparent failure of M. tuberculosis growth control during active pulmonary TB disease may be several including the expression of locally active immunosuppressive mechanisms that subvert the antimycobacterial effects of IFN-γ.
Keywords: Human pulmonary tuberculosis, BAL cells, interferon gamma, interleukin 12, interleukin 4
INTRODUCTION
Mycobacterium tuberculosis (M.tb), the etiological agent of TB causes latent infection in a third of the world’s population. In the majority of cases, protective immune responses contain the primary M.tb infection in an asymptomatic latent state. Only 5–10% of infected individuals actually develop active TB disease during their lifetime 1. Despite its ability to affect many organs, TB most commonly presents as a pulmonary disease.
Although readily measured, systemic immunity does not accurately reflect pulmonary immune responses in the bronchoalveolar spaces at the entry site of aerosolized M.tb in humans. Systemic immune responses during human TB are characterized by reduced production of IFN-γ upon in vitro stimulation of PBMC with M.tb 2 or M.tb antigens 3;4, and suppression of antigen-specific lymphocyte proliferation 5–7, or IL-2 and IFN-γ production 8–10 by products of monocytes and macrophages 3;5;9;11–14.
Our group has previously shown the presence of an alveolitis of compartmentalized activated αβ TCR -bearing lymphocytes (CD69+, HLA-DR+, transferrin receptor+), and of immature alveolar macrophages 15 during adult pulmonary TB disease. We have also shown increased lymphocyte proliferation and IFN-γ release upon PPD stimulation of whole bronchoalveolar cells (BAC) 15;16 and together with others 17;18 increased constitutive IFN-γ levels in BAC. Similarly constitutive IFN-γ levels are increased in sputum 19 during adult TB and in BAC from pediatric TB patients 20.
Alveolar macrophages are among the first cells to come into contact with M.tb from inhaled droplet nuclei and are involved in innate and the initiation of adaptive immune mechanisms. Alveolar macrophages 21 and dendritic cells 22–24 produce IL-12 and additional cyto, - and chemokines such as TNF-α 25;26, IL-1, IL-6 26, IL-15, and IL-18 27;28.
IL-12 serves as a master regulator of the Th1 response by inducing the production of IFN-γ 29–31. IL-12 binds to its high affinity receptor (IL-12R) 32;33, a heterodimer that consists of a β1 (IL-12Rβ1) and a β2 (IL-12Rβ2) subunit 34. IL-12Rβ1 is involved in IL-12 binding and expressed on both Th1 and Th2 cells 29. In contrast IL-12Rβ2 is expressed on Th1 cells only 35–37, is involved in IL-12 binding and induces STAT-4 mediated intracellular signaling for IFN-γ production 29;32–34.
IFN-γ plays a central role in M.tb immunity by regulating the expression of a host of genes involved in antimycobacterial effector functions. By binding to its receptor (IFN-γR), IFN-γ activates macrophages to produce bactericidal superoxide, and reactive nitrogen intermediates, as well as TNF-α, IL-12, IL-1 and IL-6 38. Consequently IFN-γ knockout mice fail to produce reactive nitrogen intermediates and succumb rapidly to experimental infection with M.tb 39;40. Similarly, humans with hereditary failures of the IFN-γ/IL-12/IL-23 axis are at increased risk for mycobacterial infection 41–44 mostly with avirulent 45;46 but also virulent mycobacteria 42;47;48.
As demonstrated by the shedding of viable M.tb into the airways and progressive lung pathology during active untreated pulmonary TB disease, there is deficient M.tb growth control in the bronchoalveolar compartment despite enhanced local Th1 immunity. We thus hypothesized that BAC of TB patients would be characterized by abnormalities in the protein or mRNA expression of IFN-γ, IL -12p70, IL-12Rβ1, IL-12Rβ2 or IFN-γR1 or by release of immunosuppressive cytokines.
The current study was therefore designed to explore (1) the phenotype of IFN-γ-releasing resident lung T cell populations, (2) the M.tb-specific and IL-12R-mediated IFN-γ-production, (3) the production of IL-12p70, (4) the expression of IL-12Rβ1, IL-12Rβ2 and IFN-γR1, and (5) immunosuppressive cytokines (IL-4, IL-9, IL-10 and TGF-β) in the bronchoalveolar spaces and the systemic compartment during active pulmonary TB and in comparison with healthy community controls (CC).
PPD,- and live M.tb-induced IFN-γ-production was found in BAC from TB patients and all major alveolar T cell subpopulations and NK cells. IFN-γ and IL-12p70 responses to mycobacterial antigens were significantly increased in BAC from TB patients in comparison to autologous PBMC and to BAC and PBMC from CC. There was no evidence of reduced or altered expression of the IL-12R subunits or of IFNγR1 nor was there functional impairment of the receptors. However, we found elevated IL-4 levels in supernatants of M.tb stimulated BAC from patients with TB. This and data from other studies suggest the presence of distinct local immunosuppressive mechanisms that interfere with Th1-mediated effector functions in patients with active pulmonary TB who are unable to control M.tb growth in the bronchoalveolar environment.
MATERIAL AND METHODS
Approval to obtain BAC by bronchoalveolar lavage (BAL), to perform venipunctures and to obtain personal health information from the study subjects was obtained from the Institutional Review Boards of the Instituto Nacional de Enfermedades Respiratorias in Mexico City, and the University of Medicine and Dentistry of New Jersey, Newark.
Study Subjects
Informed written consent was obtained from all study subjects prior to performing any procedures. Study subjects willing to undergo BAL, venipuncture, chest radiographs, HIV-1 serology and tuberculin skin test (TST) were recruited at the Instituto Nacional de Enfermedades Respiratorias.
Eight subjects (four male, four female) with acid fast bacilli sputum smears that were positive and confirmed by culture representing drug-sensitive, active pulmonary TB were included in this study. Two of the patients had moderately-advanced TB, and six had far-advanced TB according to ATS radiographic criteria 49. All patients were TST positive (10 to 35 mm induration at 72hrs). Median duration of antituberculous chemotherapy prior to the research BAL procedures was five days.
Fourteen healthy persons (nine male, five female) were recruited as CC. CC had no clinical or radiographic evidence of respiratory diseases and no known concurrent or past contact with TB patients. Six CC were TST negative (0 mm), three had TST induration of ≤ 10 mm and five of >10mm. TST positive and TST negative CC are presented as a single subject group. Differences identified in this study between CC and TB patients were independent of the TST status of the CC. Patients with TB and CC had a median age of 29 years (range 19–60 years). Seven of the eight TB patients and 13 of 14 CC had a scar indicating childhood BCG vaccination.
Preparation of BAC
BAC were obtained by BAL. Briefly, after local anesthesia of the upper airways with 2% lidocaine solution, and additional instillation of 1% lidocaine in the lower airways, a flexible fiberoptic bronchoscope (P30, Olympus BF, New Hyde Park, NY) was wedged consecutively into two segments of the radiographically involved lungs in the TB patients and into two segments of the right middle lobe in CC. A total of 300mL sterile saline solution (150mL per segment) was instilled in 20mL aliquots. Median recovery of bronchoalveolar lavage fluid (BAL fluid) was 72% and 68% in TB patients and CC respectively. Following centrifugation, BAC were re-suspended, counted and adjusted at 106 per milliliter in complete culture medium [RPMI 1640 medium (BioWhitaker, Walkersville, MD), supplemented with two mM L-glutamine (Sigma Chemical Co., St Louis, MO), 50 μg/mL Gentamycin sulfate (BioWhittaker) and 10% heat-inactivated pooled AB human serum (Gemini, Woodland, CA)]. Viability of cells was 95% (median, range 93% – 99%) by trypan blue exclusion (Gibco Life Technologies, Grand Island, NY). Yield of BAC from TB patients (median, 55 × 106) was greater than that from CC (median, 31 × 106). The cell-free BAL fluid was stored at −20°C for measurement of IL-10 and TGF-β by ELISA.
Preparation of PBMC
PBMC were prepared by centrifugation (300g, 45 min, 21°C) of heparinized venous whole blood (WB) diluted 1:1 with RPMI alone over a Ficoll-Paque (Axis-Shield PoC As, Oslo, Norway)50 density gradient. PBMC were removed from the interface, washed and re-suspended in complete culture medium and then counted and adjusted at 106 per milliliter. Viability of PBMC was 100% by trypan blue exclusion.
Preparation of M.tb H37Ra for in vitro Stimulation
Suspensions of M.tb strain H37Ra (ATCC # 25177, Manassas, VA) were prepared in Middlebrook 7H9 broth medium (Difco Laboratories, Detroit, MI) supplemented with 10% albumin dextrose catalase (Difco Laboratories) and 0.2% glycerol. Following a 21-day incubation period at 37°C on an orbital shaking incubator, M.tb stock suspensions were harvested, aliquoted and stored at −70°C until use. Concentrations of the M.tb stock suspensions were determined by CFU counts from serial dilutions after 21 days of incubation on 7H10 solid medium. For the preparation of unclumped single M.tb suspensions, M.tb stock cultures were re-suspended in infection medium (RPMI with L-glutamine and 30% pooled AB human serum) and declumped by vortexing for five minutes in the presence of sterile three-millimeter glass beads. Remaining M.tb clumps were removed with an additional centrifugation step (82g, 10 min). Appropriate volumes of declumped M.tb stock suspensions (and thus constant CFU numbers) were used for each in vitro stimulation to obtain the desired MOI (M.tb per cell) of 10 per BAC or monocytes.
Cell Subset Analysis in BAC and PBMC
For fluorescence activated cell sorting (FACS) analysis, 2 × 105 BAC or PBMC in complete culture medium were stained with PerCp-labeled anti-human CD3, and FITC-labeled anti-human CD4, CD8, CD56, or γδ TCR monoclonal antibodies (BD Biosciences, San José, CA) during a 15 minute incubation at room temperature in the dark. Cells were then washed with PBS and fixed with 1% paraformaldehyde (Sigma Chemical Co.). Isotype antibodies labeled with γ1PerCp, γ1FITC and γ2aFITC were included to assess nonspecific binding and define quadrant positions for analysis. Cells were acquired on a FACS Calibur® flow cytometer (Becton Dickinson, San Jose, CA) and analyzed in lymphocyte gates (CD3+ vs. SSC or FSC vs. SSC) using Cell Quest® research software version 3.3 (1996–1999). Alveolar T cell subset distributions are reported as percentages of positive cells within CD3+ alveolar cells and NK cells reported as CD56+ cells within a lymphocyte gate (FSC vs. SSC).
Intracellular IFN-γ Production
IFN-γ production was assessed by intracellular staining and FACS analysis of BAC or WB following in vitro stimulation of the cells with PPD and live M.tb. Two × 106 BAC in 2mL complete culture media, or 1mL WB were either left unstimulated (culture medium), or were stimulated with 10μg PPD (Staten Serum Institute, Copenhagen, Denmark) or live M.tb at MOI 10 and incubated at 37°C in 5% CO2 for a period of 12 hours. Brefeldin A (Sigma Chemical Co.) was added during the last 4 hrs of the incubation. Two x 105 BAC or 100μl WB were surface-stained with PerCP-labeled anti-human CD3, CD4, and CD8, and FITC-labeled CD4, CD8, CD56, γδ TCR and CD45RO monoclonal antibodies. After 15 min of incubation, red blood cells were lysed in WB samples (1x lysis solution, BD Biosciences). BAC and remaining white blood cells were then permeabilized (1x permeabilizing solution, BD Biosciences) and incubated for 30 min with anti-human IFN-γPE and CD69FITC and their respective γ1PerCp, γ1FITC, γ2aFITC and γ2aPE -labeled isotype antibodies (BD Biosciences). Cells were subsequently washed, fixed and the proportions of IFN-γ-producing CD4+, CD8+, γδ TCR+, CD56+ and CD45RO+ cells determined within a CD3 gate. Proportions of IFN-γ producing NK cells were determined in a lymphocyte gate (FSC vs. SSC).
IFN-γ Production by ELISA in Culture Supernatants from BAC and PBMC
IFN-γ production was measured in culture supernatants from in vitro stimulated BAC and PBMC. BAC or PBMC were plated at a concentration of 106/mL in complete culture medium in 24-well culture plates. BAC and PBMC were then either left unstimulated (culture medium), or were stimulated with PPD (10μg/mL), live M.tb at MOI 10, rhIL-12 (5ng/mL, R&D System Co. Palo Alto, CA), PHA (5μg/mL) plus rhIL-12 (5ng/mL) (PHA stimulates expression of IL-12R), or PHA (5μg/mL). Culture supernatants were collected after a 24-hour incubation period, and stored frozen at −70°C until cytokine determination. IFN-γ concentrations were assessed by enzyme linked immunosorbent assay (ELISA) using anti-human IFN-γ Abs (Endogen Woburn, MA). Absorbance was read on a microplate reader (Labsystems Multiskan MCC/340, Labsystems, Finland) at 450nm. Following removal of supernatants, cultured cells were lysed in Trizol (Invitrogen Life Technologies, Carlsbad, CA) and lysates stored frozen at −70°C for qRT-PCR.
IL-4, IL-9, IL-10 and IL-12p70 Determination in Culture Supernatants from PBMC and BAC by Multiplex Bead Array Assay
Cytokines were measured in 24-hour culture supernatants from 106/mL BAC and PBMC of TB patients and CC, respectively, in unstimulated cells (culture medium) and following stimulation with live M.tb MOI 10 and/or LPS (5μg/mL, Sigma Chemical Co). Cytokine production was determined in duplicate wells using Beadlyte Multiplex Immunoassay Kits (Upstate, Temecula, CA). Plates were read using a Luminex 100TM reader. The lower limits of detection using this method were 1.84, <1.0, 0.34 and 0.76 pg/mL for IL-4, IL-9, IL-10 and IL-12p70 respectively.
Cytokine Determination in BAL fluid
Concentrations of IL-10 and TGF-β were determined by ELISA in unconcentrated BAL fluid samples that were recovered from the first 60mL of saline fluid that were instilled during the BAL procedure. ELISA assays were performed according to the manufacturer’s recommendations (R&D Systems, Minneapolis, MN).
Total RNA Extraction and cDNA Synthesis
To determine the expression of the IFN-γ IL-12Rβ1, IL-12Rβ2, TNF-α and IFN-γR1 genes in BAC and PBMC from patients with TB and CC, cells were stimulated for 24 hours with PPD (10μg/mL), live M.tb at MOI 10, rhIL-12 (5μg/mL, Sigma Chemical Co), rhIL-12 plus PHA (5 μg/mL), PHA alone (5μg/mL), rhIFN-γ alone (100U), LPS alone (5μg/mL, Sigma Chemical Co) or left unstimulated (culture medium). Stimulated and unstimulated BAC and PBMC were then lysed in Trizol (Invitrogen) and lysates kept frozen (−70°C) until extraction of RNA. RNA was extracted from lysates according to standard methods 51. Briefly, cell lysates were mixed with chloroform and centrifuged. Following removal of the aqueous phase, RNA was precipitated with cold isopropanol, washed with 75% cold ethanol, dried and re-suspended in DEPC-water (Biowhittaker, Walkersville, MD). RNA concentrations were determined at 260nm in a NanoDrop Spectrophotometer (NanoDrop Technologies, Inc., Wilmington, DE) and purity was defined by measurement at 260nm and 280nm with a ratio >1.8 considered acceptable. For the cDNA synthesis RNA was mixed with 10mM dNTP’s and random hexamers, incubated at 65°C for 5 min and mixed with Reverse Transcriptase Mix (10x Reverse Transcriptase buffer, 25mM MgCl2 and RNaseOUT recombinant, Ribonuclease inhibitor). The cDNA was synthesized using SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen) according to the manufacturer’s instructions with SuperScript II RT enzyme at 25°C for 10 min, 42°C for 50 min and 70°C for 10 min. cDNAs were then treated with RNAse H, and their concentrations determined at 260 nm prior to preparing 20ng/μl dilutions for qRT-PCR.
Gene Expression Analysis by qRT-PCR
Pre-Developed TaqMan assay kits (Applied Biosystems, Foster City, CA) were used to determine the expression of IFN-γ and TNF-α genes according to the manufacturer’s instructions. The endogenous control (housekeeping) gene 18S rRNA was identified as the most stable gene in preliminary experiments comparing stimulated and unstimulated BAC and PBMC using a commercially available TaqMan Human Endogenous Control Plate (Applied Biosystems). IL-12Rβ1, IL-12Rβ2, and IFN-γR1 primers and probes were designed using Primer Express software (Applied Biosystems) and the probes were labeled (Applied Biosystems). qRT-PCR was performed following standard procedures. Briefly, universal master mix, target gene primers and probes (IFN-γ, IL-12Rβ1, IL-12Rβ2, IFN-γR1, TNF-α and 18S rRNA) and DEPC water were mixed and added to each well within 96-well plates together with sample cDNA (60ng/well). Seven serial dilutions (range from 1 to 10−7pg) of each target gene plasmid were included to obtain a standard curve permitting assessment of target gene mRNA copy numbers. A 18S rRNA standard curve was added to each plate and run simultaneously with each cell type, for each experimental condition, and each donor sample. Amplification reactions were performed using an ABI Prisma 7700 Sequence Detection System (Applied Biosystems) starting with 2 min incubations at 50°C, followed by 10 min at 95°C and 40 cycles of 30 s at 95°C, and 60 s at 60°C. To control for intra, and inter experimental variations in mRNA expression, median values of 18S rRNA copy numbers were generated as baseline values for each cell type, stimulus and study group (TB patients and CC) respectively. These median 18S rRNA copy values were then used to normalize the target gene mRNA copy numbers for the respective cell types, stimuli and study groups.
Normalization Procedure of Gene Expression Data
BAC from TB patients contained 3-fold more alveolar CD3+ T cells (see % CD3+ BAC, Table 1 and Results page 18) and a relatively smaller proportion of alveolar macrophages than BAC from CC. IL-12Rβ1 and IL-12Rβ2 copy numbers were therefore normalized by dividing the proportion of alveolar CD3+ T cells from each individual TB patient with the median proportion of alveolar CD3+ T cells from the CC group obtained by FACS. Similarly, IFN-γ receptor 1 copy numbers were normalized by dividing the proportion of CD3− alveolar cells from each TB patient with the median proportion of CD3- alveolar cells from the CC group obtained by FACs.
TABLE 1. Cell Subpopulations in BAC and PBMC.
BAC or PBMC (2 × 105 each) were surface stained with PerCp (CD3), and FITC (CD4, CD8, CD56, γδ TCR)-labeled anti-human monoclonal antibodies and analyzed by FACS analysis. Lymphocytes in BAC and PBMC were assessed in a CD3+ vs. SSC gate and NK cells in a FSC vs. SSC gate using Cell Quest software. Except for BAC numbers in BALF, values represent median percentages of CD3+ T cells or NK cells within BAC and PBMC (bold numbers) and 25 and 75 percentiles (in parentheses).
| CC (n=13) | TB (n=8) | |||
|---|---|---|---|---|
| BAC | PBMC | BAC | PBMC | |
| # BAC/10 ml BALF [106] | 1.4 (0.9–1.8) | - | 2.3 (1.4–2.7) | - |
| % CD3+ BAC | 5 (2–15) * | - | 15 (8–26) | - |
| % CD3+ CD4+ | 48 (45–51)* | 59 (56–66) ** | 70 (57–72) | 62 (53–67) |
| % CD3+ CD8+ | 27 (17–38) | 23 (15–27) | 20 (15–28) | 26 (18–27) |
| % CD3+ CD56+ | 10 (6–13) | 3 (2–8) | 7 (5–9) | 7 (5–8) |
| % CD3− CD56+ | 4 (3–6) | 14 (10–21) ** | 3 (3–4) | 16 (13–20) ** |
| % CD3+ γδ TCR+ | 7 (3–10) * | 3 (2–5) ** | 5 (4–7) | 5 (4–7) |
Significances: p ≤0.05, CC vs. TB in BAC
p ≤0.05, BAC vs. autologous PBMC (Mann-Whitney U test).
Statistical analysis
Comparisons between subject groups were made using the nonparametric Mann-Whitney Rank Sum Test. Comparisons between different stimulation conditions within subject groups were made with the Wilcoxon Signed Rank Test. The analyses were made with the SPSS Statistical Software package, version 13.0 (2004). Statistical significance was considered at p ≤ 0.05.
RESULTS
Cytospin Analysis
By cytospin analysis (Wrights and Peroxidase stain) median (25/75 percentiles) proportions of subpopulations from TB patients were 67% (54/83) AM, 16% (9/22) AL, 3% (2/17) neutrophils and 0% eosinophils. BAC subpopulations from CC were 95% (88/97) AM, 5% (3/12) AL, 0% neutrophils and 0% eosinophils.
CD4+ T Cells are the Principal T Cell Subpopulation in the BAC of TB Patients
One of the aims of the current study was to assess cellularity, phenotype and activation status of BAC during active TB (Table 1). Using FACS analysis in the current study, we confirmed earlier findings 16 of a lymphocytic alveolitis with a three-fold increased proportion and 4.5-fold increased absolute number of T cells in BAC from TB patients compared with BAC from CC (p ≤ 0.05, Table 1). A novel finding was that proportions of alveolar CD4+ T cells were significantly higher in BAC from TB patients than in BAC from CC (median % 70 vs. 48; p ≤ 0.05). Proportions of alveolar and peripheral CD8+ T cells were similar in both study groups (median % BAC vs. PBMC: TB, 20 vs. 26 and CC, 27 vs. 23, respectively), and significantly lower than that of alveolar and peripheral CD4+ T cells (p ≤ 0.001). Other T cell subpopulations and NK (CD3−CD56+) cells are presented in Table 1.
CD4+ T Cells with Memory Phenotype are the Principal Source of IFN-γ in the Bronchoalveolar Compartment during active TB
Next the phenotype of IFN-γ-producing alveolar T cell subpopulations was assessed by FACS analysis (Figure 1). Proportions of constitutively (unstimulated) IFN-γ-producing alveolar T cells were low and comparable in BAC from TB patients (median 4%) and CC (median 1%). However, upon in vitro stimulation with PPD and M.tb, pulmonary compartmentalization of antigen specific T-cell responses was clearly demonstrated in TB patients in whom proportions of antigen-specific IFN-γ-producing alveolar CD4+ T cells were significantly larger than in CC (median % TB vs. CC: PPD, 46 vs. 6 and M.tb, 37 vs. 4 p ≤ 0.05) (Figure 1A). We also assessed the contribution of CD45RO+ alveolar T cells to the production of IFN-γ as the prominent PPD and M.tb-specificity in TB patients suggested presence of increased local memory immunity. Indeed, proportions of both PPD and M.tb-specific CD4+CD45RO+ and CD8+CD45RO+ IFN-γ-producing alveolar T cells were significantly (p ≤ 0.05) increased in TB patients compared with CC (Figure 1F) despite similar proportions of CD4+ and CD8+ alveolar T cells with memory phenotype (CD45RO+) in TB patients and CC (data not shown).
Figure 1. IFN-γ-Producing BAC Subpopulations.
BAC (2 × 106) were stimulated with 10μg PPD or with M.tb H37Ra (MOI 10) for 12hrs in presence of Brefeldin A, and subsequently surface-stained with PerCp (CD3, CD4, CD8) and FITC (CD4, CD8, CD56, γδ TCR, CD45RO)-labeled anti-human monoclonal antibodies. Intracellular staining was done using an anti-human IFN-γPE monoclonal antibody. IFN-γ-producing cells were assessed in CD3+ vs. SSC (A-D, F) or FSC vs. SSC (E) gates using Cell Quest software. Panels (A) – (F) represent median percentages (horizontal black and white lines) and 25 and 75 percentiles (vertical bars) of IFN-γ-producing BAC subpopulations. Panel (A) CD4+ [CC n=10, TB n=7]; (B) CD8+, [CC n=10, TB n=7]; (C) γδ TCR+ [CC n=8, TB n=7]; (D) CD56+ [CC n=8, TB n=7], (E) CD3−CD56+.[CC n=8, TB n=7] and (F) memory (CD45RO+) CD4+ and CD8+ [CC n=4, TB n= 4]. Significance: *p ≤ 0.05, CC vs. TB (Mann-Whitney U test).
Alveolar CD8+, γδ TCR+, CD3+CD56+ and NK cells also contributed to the local production of IFN-γ and were increased in proportion (CD8+ [PPD] and NK [M.tb], p ≤ 0.05) in TB patients compared with CC (Figures 1B, 1C, 1D and 1E). The proportions of peripheral IFN-γ-producing T cell subpopulations and NK cells (median <10%) were significantly lower (p<0.05) in both study groups than within their respective autologous BAC.
Increased IFN-γ-Production in the Bronchoalveolar Compartment during active TB
To assess antigen-specific and IL-12R-mediated IFN-γ production in the bronchoalveolar and systemic compartments during active TB, BAC and PBMC were stimulated with PPD, live M.tb (MOI 10), rhIL-12, and rhIL-12 plus PHA (Figure 2). IFN-γ protein and IFN-γ mRNA expression were determined by ELISA and qRT-PCR, respectively. To account for differences in alveolar T cell numbers within the BAC from TB patients and CC subjects (TB patients BAC were characterized by a lymphocytic alveolitis), IFN-γ mRNA amounts in BAC were normalized as described in the “Materials and Methods” section. Constitutive (medium) IFN-γ-production was significantly increased in BAC from TB patients (median ng/mL: TB 0.9 and CC 0, p ≤ 0.05). Upon stimulation with PPD and live M.tb, IFN-γ-production from BAC of TB patients increased to levels that were significantly higher than that from BAC of CC (median ng/mL TB vs. CC: PPD, 7.4 vs. 0.04; M.tb 10, 4.6 vs. 0.1, p ≤ 0.05) (Figure 2A). In contrast to the findings in BAC from TB patients, constitutive IFN-γ-production levels were lower in autologous PBMC (median ng/mL BAC vs. PBMC: 0.9 vs. 0.015, p ≤ 0.05) (Figure 2C).
Figure 2. IFN-γ Protein Production and mRNA Expression of BAC and PBMC.
BAC or PBMC (106 each) were stimulated with 10μg PPD, 5ng rhIL-12, 5μg PHA plus 5ng rhIL-12, or 5μg PHA (per mL final concentration) or with M.tb H37Ra (MOI 10) for 24hrs. IFN-γ protein production was determined in culture supernatants by ELISA (ng/mL): panel (A) BAC and (C) PBMC [(A, C): CC n=7 (TST+ n=4; TST − n= 3), TB n= 6]. IFN-γ mRNA expression was assessed by qRT-PCR (copy numbers/60ng of cDNA): panels (B) BAC and (D) PBMC [(B, D): CC n=5 (TST+ n=3; TST − n= 2), TB n= 6]. IFN-γ mRNA expression data are normalized to account for differences in alveolar T cell proportions in BAC from TB patients and CC. Significance: *p ≤ 0.05 (Mann-Whitney U test).
To assess induction of IFN-γ-production through direct engagement of the IL-12R, BAC and PBMC from TB patients and CC were in vitro stimulated with rhIL-12 or a combination of rhIL-12 plus PHA. Stimulation with rhIL-12 and rhIL-12+PHA induced significantly higher IFN-γ levels in BAC from TB patients than in BAC from CC (median ng/mL TB vs. CC: rhIL-12, 1.3 vs. 0.06, rhIL-12+PHA 16.5 vs. 2; p ≤ 0.05).
We also assessed IFN-γ mRNA levels by qRT-PCR in the BAC samples that were studied for IFN-γ-protein production. Constitutive IFN-γ mRNA copy numbers in BAC from TB patients were significantly increased compared with that in BAC from CC (median copy number/60ng cDNA TB vs. CC: 80 vs. 2, p ≤ 0.05) (Figure 2B). Similar to our findings of increased antigen-specific IFN-γ protein production in patients with TB, stimulation of BAC with PPD or live M.tb, induced a significant increase of IFN-γ mRNA copy numbers in the TB group (median copy number/60ng cDNA TB vs. CC: PPD, 806 vs. 6; M.tb 10, 488 vs. 10). Stimulation with rhIL-12 or rhIL-12 plus PHA resulted in comparable IFN-γ mRNA copy numbers in BAC of both groups (Figure 2B).
In contrast to the observed study group’s differences in BAC, constitutive and PPD-induced IFN-γ levels were similar in autologous PBMC from both study groups. IFN-γ copy numbers in PBMC from CC were however significantly higher in response to live M.tb and PHA plus rhIL-12 than that in TB patients (Figure 2D).
In summary, the data demonstrates local accumulation in TB patients of live M.tb-responsive cells with the capacity to produce IFN-γ.
IL-12p70-Production in Response to Live M.tb
To further explore the observed increased local production of IFN-γ in TB patients, we determined IL-12p70 concentrations in culture supernatants from BAC and PBMC of TB patients and CC. IL-12p70 protein production from BAC of TB patients was significantly increased in response to live M.tb in BAC from TB patients compared with CC (Figure 3 A). In contrast, in culture supernatants from PBMC IL-12p70 concentrations were similar between both study groups (Figure 3B).
Figure 3. IL-12p70 Protein Production in BAC and PBMC.
BAC or PBMC (106 each) were stimulated with M.tb (MOI 10) or with 5μg LPS (per mL final concentration) for 24hrs. IL-12p70 production was determined in culture supernatants by Multiplex Bead Array Assay. Panels A and B represent median (horizontal black and white lines) and 25 and 75 percentiles (vertical bars). IL-12p70 concentrations (pg/mL): panel (A) BAC [CC n=5, TB n= 6] and (B) PBMC [CC n=7, TB n= 6]. Significance: *p ≤ 0.05 (Mann-Whitney U test).
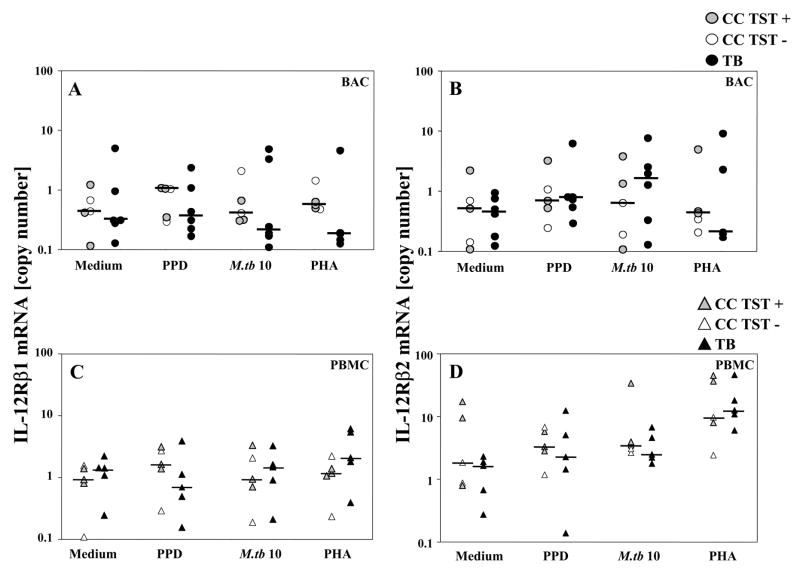
IL-12Rβ1 and IL-12Rβ2 Expression in BAC and PBMC During Active TB
IL-12Rβ1 and IL-12Rβ2 mRNA expression was assessed by qRT-PCR and found to be similar in the study groups both constitutively and following stimulation with PPD and live M.tb (Figure 4).
Figure 4. IL-12Rβ1 and IL-12Rβ2 mRNA Expression in BAC and PBMC.
BAC or PBMC (106 each) were stimulated with 10μg PPD, or 2.5μg PHA (per mL final concentration) or with M.tb (MOI 10) for 24 hrs. Expression of IL-12Rβ1 and IL-12Rβ2 was assessed by qRT-PCR. IL-12Rβ1 mRNA expression (copy numbers/60ng cDNA): panel (A) BAC and (C) PBMC [(A, C): CC n=5 (TST+ n=3, TST − n=2), TB n= 6]. IL-12Rβ2 mRNA expression (copy numbers/60ng cDNA): panel (B) BAC and (D) PBMC [(B, D): CC n=5 (TST+ n=3, TST− n=2); (B) TB n= 6, (D) TB n=5]. IL-12Rβ1 and IL-12Rβ2 mRNA expression data are normalized to account for differences in alveolar T cell proportions in BAC from TB patients and CC.
IFN-γR1 Expression in BAC and PBMC
To assess if antigen-specific IFN-γ-production during TB could mediate macrophage activation, we assessed the expression of IFN-γR1 mRNA (one of the two subunits of the IFN-γR) in BAC and PBMC. Expression levels of IFN-γR1 mRNA were similar in BAC from TB patients and CC both constitutively and after stimulation with PPD, rhIFN-γ, LPS or with live M.tb (MOI 10) (Figure 5A). In PBMC from TB patients, constitutive IFN-γR1 mRNA expression was significantly higher than in CC (p<0.05). After stimulation with PPD or live M.tb, IFN-γR1 mRNA expression appeared to be down-regulated compared to constitutive levels in TB patients. In CC, no change of IFN-γR1 mRNA expression levels was seen following stimulation with PPD, M.tb or rhIFN-γ (Figure 5B). We also assessed TNF-α expression (protein and mRNA) in BAC and PBMC from the study groups (data not shown). There were no significant differences between TNF-α concentration in stimulated and unstimulated BAC and neither were there significant differences in PBMC from both TB patients and CC.
Figure 5. IFN-γR1 mRNA Expression in BAC and PBMC.
BAC or PBMC (106 each) were stimulated with 10μg PPD, M.tb H37Ra (MOI 10), 100U rhIFN-γ, and 5μg LPS (per mL final concentration) for 24hrs. Expression of IFN-γR1 mRNA was assessed by qRT-PCR. IFN-γR1 mRNA expression (copy numbers/60ng of cDNA): panels (A) BAC and (B) PBMC, [(A, B): CC n=5 (TST+ n=3, TST− n=2); TB n= 6]. IFN-γR1 mRNA expression data are normalized to account for differences in alveolar macrophages proportions in BAC from TB patients and CC. Significance: *p ≤ 0.05 (Mann-Whitney U test).
Immunosuppressive Cytokines in BAC and PBMC Culture Supernatants and BAL Fluid
To assess cytokines described to be involved in suppression of M.tb specific immune responses we analyzed concentrations of IL-4, IL-9 and IL-10 in 24-hour culture supernatants of BAC and PBMC. M.tb stimulation (MOI 10) induced significantly increased IL-4 levels in supernatants from BAC of TB patients compared to that in CC (p<0.05) (Figure 6A). There were no significant differences between IL-9 and IL-10 concentrations in BAC and PBMC of patients and controls (data not shown). Higher levels of TGF-β were observed in BAL fluids from TB patients in comparison with CC although differences were not significant (data not shown).
Figure 6. IL-4 Protein Production in BAC and PBMC.
BAC or PBMC (106 each) were stimulated with M.tb (MOI 10) for 24hrs. IL-4 production was determined in culture supernatants by Multiplex Bead Array Assay. Panels A and B represent IL-4 concentrations (pg/mL): panel (A) BAC [CC n=5, TB n= 6] and (B) PBMC [CC n=7, TB n= 6]. Significance: *p ≤ 0.05 (Mann-Whitney U test).
DISCUSSION
To further analyze the constituents of local Th1 immunity and expression of immunosuppressive cytokines during pulmonary TB, this study assessed the phenotype of IFN-γ producing alveolar T cells, the expression of IFN-γ, IL-12Rβ1, IL-12Rβ2, IFN-γR1 and TNF-α mRNA and of IL-4, IL-9, IL-10 and TGF-β in BAC from the bronchoalveolar spaces of radiographically affected lung segments of patients with active pulmonary TB. Our data provide evidence that compartmentalized bronchoalveolar immune responses during TB do not result solely from sequestration of cells to the infection site and increased local T cell numbers, but also from an increased responsiveness of these resident alveolar T cells during active TB disease.
Seventy percent (median) of the alveolar T cells of TB patients are CD4+ and more than 46 % (median) of these cells secrete IFN-γ upon in vitro stimulation with PPD or live M.tb demonstrating that alveolar CD4+ T cells are the principal source of local bronchoalveolar antigen-specific IFN-γ-production during TB. Alveolar CD8+, γδ T, CD3+CD56+ as well as CD3−CD56+ (NK) cells contribute to the local production of M.tb-induced IFN-γ, although to a much lesser degree than CD4+ T cells. Our findings in TB patients coincide with a report in which PPD-induced IFN-γ production by CD4+ T cells in BAL fluid of TB patients was found to be higher than in autologous blood cells 52 and contrast those from Taha et al. 21 who reported increased proportions of alveolar CD8+ T cells that expressed IFN-γ mRNA constitutively in TB patients. It is possible that this difference is based on dissimilarities in the stages of TB disease in the study populations and the experimental approaches used to determine the cellular source of IFN-γ (FACS analysis versus in situ hybrization 21).
We also assessed the proportions of PPD,- and M.tb-specific IFN-γ-producing alveolar memory T cells and found that CD4+CD45RO+ and CD8+CD45RO+ BAC are significantly increased in TB patients. This finding is consistent with the observed enhanced antigen-specific IFN-γ recall responses in TB patient’s BAC.
Our findings in BAC of TB patients provide evidence for a highly increased compartmentalized constitutive and M.tb induced/specific production of IFN-γ that is paralleled by significantly increased proportions of activated alveolar T cells (median 40% CD69+, data not shown) at the infection site.
The increased IL-12p70 levels in response to stimulation with live M.tb in BAC from TB patients in our study positively correlate with the observed highly increased local IFN-γ production, and suggest M.tb-induced macrophage and/or dendritic cell activation in the bronchoalveolar spaces. This observation coincides with high constitutive levels of IL-12p70 in BAL fluid from pulmonary TB patients 53 and a report of increased levels of IL-12p40 compared to autologous serum in pleural fluid of patients with TB pleurisy, which however is a self limiting disease and thus rather a model of protective immunity 54.
To further examine the apparent lack of IFN-γ-mediated growth control of M.tb in the bronchoalveolar spaces during active disease (demonstrated by the shedding of viable M.tb into the airways of untreated pulmonary TB patients), we also assessed the constitutive and stimulated IL-12Rβ1, IL-12Rβ2 and IFN-γR1 expression, i.e. additional components of Th1 immunity. There were no significant differences found or patterns recognized in regards to the expression of these receptors after normalization for alveolar lymphocyte numbers when BAC and PBMC were compared between the study groups. Prior to lymphocyte normalization however, total constitutive IL-12Rβ1 and IL-12Rβ2 mRNA expression in BAC were significantly increased in the TB patient group. Earlier studies by Taha demonstrated ex vivo increased IL-12Rβ1 and IL-12Rβ2 mRNA expression in alveolar CD8+ T cells from active pulmonary TB patients; however the data were not normalized to account for lymphocytic alveolitis in TB patients 55. The increased IFN-γR1 mRNA expression in PBMC from our TB patients contradicts a study by Singal et al 56 which showed reduced surface expression of IFN-γR1 on PBMC from TB patients. Post-translational regulatory mechanisms that interfere with IFN-γR1 protein expression and differences in the IFN-γR1 study time points however, may explain this difference.
Altogether, the induction of IFN-γ production and the release of IFN-γ from BAC of TB patients appear to occur unimpeded when compared with autologous PBMC and BAC from CC. Lack of M.tb growth control in the bronchoalveolar spaces during TB may therefore not be due to lack of IFN-γ-production or impaired IL-12R-mediated IFN-γ induction. Our group’s earlier findings in aerogenically exposed healthy household contacts of patients with TB support this conclusion in that IFN-γ levels in cocultures of AM with CD4+ and CD8+ T cells did not correlate with T cell-mediated M.tb growth control 57. Additionally, treatment of patients with multidrug resistant TB with aerosolized rhIFN-γ does not lead to sputum sterilization and results in transient effects on M.tb growth control only 58. Thus, although IFN-γ is an indispensable activator of macrophage-mediated killing of M.tb, IFN-γ-induced protective antimycobacterial effector mechanisms appear to be impaired in patients with TB.
The apparent failure of M.tb growth control during active TB disease may have several reasons including the expression of locally active immunosuppressive mechanisms or M.tb-induced immune evasion mechanisms. Immunosuppressive cytokines production may modulate and subvert the antimycobacterial effects of IFN-γ on macrophages. The observation of significantly increased IL-4 levels in supernatants of BAC from TB patients in the current study supports the notion of local immunosuppressive processes and coincides with earlier findings in BAL material from TB patients 18;59. In addition the current study shows a trend towards increased levels in patients with TB of IL-9 and IL-10 in BAC supernatants and for TGF-β in unconcentrated BAL fluid compared to CC (data not shown).
Further, in a recent study of untreated patients with TB, surface expression of IFN-γR1 was decreased on fresh PBMC (and could be restored following antituberculous therapy) 56. In rare cases have interferences with IFN-γ-induced protective effects been shown to derive from autoantibodies directed against IFN-γ 60 or increased systemic IL-9 levels 61. Other immunosuppressive (and thus M.tb growth-promoting) mechanisms may be operative such as immunoregulation by regulatory T cells in the bronchoalveolar compartment during M.tb infection 62.
Efficient macrophage function may also be impaired directly by M.tb or its products. M.tb reduces CD1 expression 63 and induction of IFN-γ-induced genes such as CD64 (FcγR1) in THP1 cells 64 and inhibits IL-12 production and MHC class II presentation pathways in human macrophages 65–69.
The current study has limitations that are associated with the complex subject recruitment processes for research in the lung compartment of human volunteers. This limitation accounts for the relatively small study subject numbers and may affect variability and comparisons and perhaps underestimate phenotypic and functional differences between BAC and PBMC from TB patients and CC. Utilization of virulent M.tb H37Rv or of clinical M.tb isolates for in vitro stimulation studies may produce different results from those obtained here with M.tb H37Ra.
In conclusion, functional responses to M.tb of immune cells in the bronchoalveolar compartment of TB patients appear to be preserved fully as regards to induction, production and release of IFN-γ and IL-12 and differ significantly from those in the autologous systemic compartment. The uncontrolled M.tb infection despite enhanced Th1 immunity suggests that that local IFN-γ release from CD4+ and CD8+ T cells and IL-12 production are insufficient to confer protective immunity and that additional molecules, cell types and/or molecular mechanisms are required for immunological protection.
Our finding of increased IL-4 production from BAC of TB patients together with earlier studies from Fletcher, Dheda, and Seah 59;70–73 warrant efforts to further decipher if suppressive and immunoregulatory mechanisms that interfere locally with M.tb growth are the cause or consequence of TB disease. Unraveling such mechanisms will have important implications for the assessment of new antituberculous vaccine candidates, as these ought to generate protection at the entry site of M.tb; the human lung. Whereas the assessments of IFN-γ-induction in vitro and of immune responses in the systemic compartment may be useful for the evaluation of vaccine immunogenicity, they do not correlate with local pulmonary protective antimycobacterial immunity. The identification of new vaccine candidates on the basis of their ability to induce M.tb specific IFN-γ responses may thus not be a prudent approach. Induction of protective lung immunity may require new application routes and antituberculous vaccines that differ from those conventionally assessed through the study of systemic immunity. Lung immune responses may provide unique read outs of TB vaccine efficacy and immunogenicity that may be missed interrogating systemic immunity alone.
Acknowledgments
This work was supported by grant 2R01HL51630-10 from National Heart Lung and Blood Institute (NHLBI)
We are grateful to Padmini Salgame PhD for careful review of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bates JH. Transmission and pathogenesis of tuberculosis. Clin Chest Med. 1980;1:167–174. [PubMed] [Google Scholar]
- 2.Sodhi A, Gong J, Silva C, Qian D, Barnes PF. Clinical correlates of interferon gamma production in patients with tuberculosis. Clin Infect Dis. 1997;25:617–620. doi: 10.1086/513769. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch CS, Toossi Z, Othieno C, Johnson JL, Schwander SK, Robertson S, Wallis RS, Edmonds K, Okwera A, Mugerwa R, Peters P, Ellner JJ. Depressed T-cell interferon-gamma responses in pulmonary tuberculosis: analysis of underlying mechanisms and modulation with therapy. J Infect Dis. 1999;180:2069–2073. doi: 10.1086/315114. [DOI] [PubMed] [Google Scholar]
- 4.Torres M, Herrera T, Villareal H, Rich EA, Sada E. Cytokine profiles for peripheral blood lymphocytes from patients with active pulmonary tuberculosis and healthy household contacts in response to the 30-kilodalton antigen of Mycobacterium tuberculosis. Infect Immun. 1998;66:176–180. doi: 10.1128/iai.66.1.176-180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellner JJ. Suppressor adherent cells in human tuberculosis. J Immunol. 1978;121:2573–2579. [PubMed] [Google Scholar]
- 6.Ellner JJ. Regulation of the human immune response during tuberculosis. J Lab Clin Med. 1997;130:469–475. doi: 10.1016/s0022-2143(97)90123-2. [DOI] [PubMed] [Google Scholar]
- 7.Fujiwara H, Kleinhenz ME, Wallis RS, Ellner JJ. Increased interleukin-1 production and monocyte suppressor cell activity associated with human tuberculosis. Am Rev Respir Dis. 1986;133:73–77. doi: 10.1164/arrd.1986.133.1.73. [DOI] [PubMed] [Google Scholar]
- 8.Toossi Z, Kleinhenz ME, Ellner JJ. Defective interleukin 2 production and responsiveness in human pulmonary tuberculosis. J Exp Med. 1986;163:1162–1172. doi: 10.1084/jem.163.5.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirsch CS, Hussain R, Toossi Z, Dawood G, Shahid F, Ellner JJ. Cross-modulation by transforming growth factor beta in human tuberculosis: suppression of antigen-driven blastogenesis and interferon gamma production. Proc Natl Acad Sci USA. 1996;93:3193–3198. doi: 10.1073/pnas.93.8.3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang M, Lin Y, Iyer DV, Gong J, Abrams JS, Barnes PF. T-cell cytokine responses in human infection with Mycobacterium tuberculosis. Infect Immun. 1995;63:3231–3234. doi: 10.1128/iai.63.8.3231-3234.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirsch CS, Yoneda T, Averill L, Ellner JJ, Toossi Z. Enhancement of intracellular growth of Mycobacterium tuberculosis in human monocytes by transforming growth factor-beta 1. J Infect Dis. 1994;170:1229–1237. doi: 10.1093/infdis/170.5.1229. [DOI] [PubMed] [Google Scholar]
- 12.Othieno C, Hirsch CS, Hamilton BD, Wilkinson K, Ellner JJ, Toossi Z. Interaction of Mycobacterium tuberculosis-induced transforming growth factor beta1 and interleukin-10. Infect Immun. 1999;67:5730–5735. doi: 10.1128/iai.67.11.5730-5735.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirsch CS, Ellner JJ, Blinkhorn R, Toossi Z. In vitro restoration of T cell responses in tuberculosis and augmentation of monocyte effector function against Mycobacterium tuberculosis by natural inhibitors of transforming growth factor beta. Proc Natl Acad Sci USA. 1997;94:3926–3931. doi: 10.1073/pnas.94.8.3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toossi Z, Gogate P, Shiratsuchi H, Young T, Ellner JJ. Enhanced production of TGF-beta by blood monocytes from patients with active tuberculosis and presence of TGF-beta in tuberculous granulomatous lung lesions. Journal of Immunology (Baltimore, Md: 1950) 1995;154:465–473. [PubMed] [Google Scholar]
- 15.Schwander SK, Sada E, Torres M, Escobedo D, Sierra JG, Alt S, Rich EA. T lymphocytic and immature macrophage alveolitis in active pulmonary tuberculosis. J Infect Dis. 1996;173:1267–1272. doi: 10.1093/infdis/173.5.1267. [DOI] [PubMed] [Google Scholar]
- 16.Schwander SK, Torres M, Sada E, Carranza C, Ramos E, Tary-Lehmann M, Wallis RS, Sierra J, Rich EA. Enhanced responses to Mycobacterium tuberculosis antigens by human alveolar lymphocytes during active pulmonary tuberculosis. J Infect Dis. 1998;178:1434–1445. doi: 10.1086/314454. [DOI] [PubMed] [Google Scholar]
- 17.Condos R, Rom WN, Liu YM, Schluger NW. Local immune responses correlate with presentation and outcome in tuberculosis. Am J Respir Crit Care Med. 1998;157:729–735. doi: 10.1164/ajrccm.157.3.9705044. [DOI] [PubMed] [Google Scholar]
- 18.Mazzarella G, Bianco A, Perna F, D'Auria D, Grella E, Moscariello E, Sanduzzi A. T lymphocyte phenotypic profile in lung segments affected by cavitary and non-cavitary tuberculosis. Clin Exp Immunol. 2003;132:283–288. doi: 10.1046/j.1365-2249.2003.02121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ribeiro-Rodrigues R, Resende CT, Johnson JL, Ribeiro F, Palaci M, Sa RT, Maciel EL, Pereira Lima FE, Dettoni V, Toossi Z, Boom WH, Dietze R, Ellner JJ, Hirsch CS. Sputum cytokine levels in patients with pulmonary tuberculosis as early markers of mycobacterial clearance. Clin Diagn Lab Immunol. 2002;9:818–823. doi: 10.1128/CDLI.9.4.818-823.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aubert-Pivert EM, Chedevergne FM, Lopez-Ramirez GM, Colle JH, Scheinmann PL, Gicquel BM, de Blic JM. Cytokine transcripts in pediatric tuberculosis: a study with bronchoalveolar cells. Tuber Lung Dis. 2000;80:249–258. doi: 10.1054/tuld.2000.0259. [DOI] [PubMed] [Google Scholar]
- 21.Taha RA, Kotsimbos TC, Song YL, Menzies D, Hamid Q. IFN-gamma and IL-12 are increased in active compared with inactive tuberculosis. Am J Respir Crit Care Med. 1997;155:1135–1139. doi: 10.1164/ajrccm.155.3.9116999. [DOI] [PubMed] [Google Scholar]
- 22.Giacomini E, Iona E, Ferroni L, Miettinen M, Fattorini L, Orefici G, Julkunen I, Coccia EM. Infection of human macrophages and dendritic cells with Mycobacterium tuberculosis induces a differential cytokine gene expression that modulates T cell response. J Immunol. 2001;166:7033–7041. doi: 10.4049/jimmunol.166.12.7033. [DOI] [PubMed] [Google Scholar]
- 23.Yin XJ, Schafer R, Ma JY, Antonini JM, Weissman DD, Siegel PD, Barger MW, Roberts JR, Ma JK. Alteration of pulmonary immunity to Listeria monocytogenes by diesel exhaust particles (DEPs). I. Effects of DEPs on early pulmonary responses. Environ Health Perspect. 2002;110:1105–1111. doi: 10.1289/ehp.021101105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yin XJ, Schafer R, Ma JY, Antonini JM, Roberts JR, Weissman DN, Siegel PD, Ma JK. Alteration of pulmonary immunity to Listeria monocytogenes by diesel exhaust particles (DEPs). II. Effects of DEPs on T-cell-mediated immune responses in rats. Environ Health Perspect. 2003;111:524–530. doi: 10.1289/ehp.5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rich EA, Panuska JR, Wallis RS, Wolf CB, Leonard ML, Ellner JJ. Dyscoordinate expression of tumor necrosis factor-alpha by human blood monocytes and alveolar macrophages. Am Rev Respir Dis. 1989;139:1010–1016. doi: 10.1164/ajrccm/139.4.1010. [DOI] [PubMed] [Google Scholar]
- 26.Law K, Weiden M, Harkin T, Tchou-Wong K, Chi C, Rom WN. Increased release of interleukin-1 beta, interleukin-6, and tumor necrosis factor-alpha by bronchoalveolar cells lavaged from involved sites in pulmonary tuberculosis. Am J Respir Crit Care Med. 1996;153:799–804. doi: 10.1164/ajrccm.153.2.8564135. [DOI] [PubMed] [Google Scholar]
- 27.Vankayalapati R, Wizel B, Lakey DL, Zhang Y, Coffee KA, Griffith DE, Barnes PF. T cells enhance production of IL-18 by monocytes in response to an intracellular pathogen. J Immunol. 2001;166:6749–6753. doi: 10.4049/jimmunol.166.11.6749. [DOI] [PubMed] [Google Scholar]
- 28.Zissel G, Baumer I, Schlaak M, Muller-Quernheim J. In vitro release of interleukin-15 by bronchoalveolar lavage cells and peripheral blood mononuclear cells from patients with different lung diseases. Eur Cytokine Netw. 2000;11:105–112. [PubMed] [Google Scholar]
- 29.Gately MK, Renzetti LM, Magram J, Stern AS, Adorini L, Gubler U, Presky DH. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 30.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 31.Watford WT, Moriguchi M, Morinobu A, O'Shea JJ. The biology of IL-12: coordinating innate and adaptive immune responses. Cytokine Growth Factor Rev. 2003;14:361–368. doi: 10.1016/s1359-6101(03)00043-1. [DOI] [PubMed] [Google Scholar]
- 32.Desai BB, Quinn PM, Wolitzky AG, Mongini PK, Chizzonite R, Gately MK. IL-12 receptor. II. Distribution and regulation of receptor expression. J Immunol. 1992;148:3125–3132. [PubMed] [Google Scholar]
- 33.Presky DH, Minetti LJ, Gillessen S, Wilkinson VL, Wu CY, Gubler U, Chizzonite R, Gately MK. Analysis of the multiple interactions between IL-12 and the high affinity IL-12 receptor complex. J Immunol. 1998;160:2174–2179. [PubMed] [Google Scholar]
- 34.Presky DH, Yang H, Minetti LJ, Chua AO, Nabavi N, Wu CY, Gately MK, Gubler U. A functional interleukin 12 receptor complex is composed of two beta-type cytokine receptor subunits. Proc Natl Acad Sci USA. 1996;93:14002–14007. doi: 10.1073/pnas.93.24.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rogge L, Barberis-Maino L, Biffi M, Passini N, Presky DH, Gubler U, Sinigaglia F. Selective expression of an interleukin-12 receptor component by human T helper 1 cells. J Exp Med. 1997;185:825–831. doi: 10.1084/jem.185.5.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rogge L, Papi A, Presky DH, Biffi M, Minetti LJ, Miotto D, Agostini C, Semenzato G, Fabbri LM, Sinigaglia F. Antibodies to the IL-12 receptor beta 2 chain mark human Th1 but not Th2 cells in vitro and in vivo. J Immunol. 1999;162:3926–3932. [PubMed] [Google Scholar]
- 37.Szabo SJ, Dighe AS, Gubler U, Murphy KM. Regulation of the interleukin (IL)-12R beta 2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med. 1997;185:817–824. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 39.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Altare F, Durandy A, Lammas D, Emile JF, Lamhamedi S, Le Deist F, Drysdale P, Jouanguy E, Doffinger R, Bernaudin F, Jeppsson O, Gollob JA, Meinl E, Segal AW, Fischer A, Kumararatne D, Casanova JL. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science. 1998;280:1432–1435. doi: 10.1126/science.280.5368.1432. [DOI] [PubMed] [Google Scholar]
- 42.Altare F, Ensser A, Breiman A, Reichenbach J, Baghdadi JE, Fischer A, Emile JF, Gaillard JL, Meinl E, Casanova JL. Interleukin-12 receptor beta1 deficiency in a patient with abdominal tuberculosis. J Infect Dis. 2001;184:231–236. doi: 10.1086/321999. [DOI] [PubMed] [Google Scholar]
- 43.Fieschi C, Dupuis S, Catherinot E, Feinberg J, Bustamante J, Breiman A, Altare F, Baretto R, Le Deist F, Kayal S, Koch H, Richter D, Brezina M, Aksu G, Wood P, Al Jumaah S, Raspall M, Silva Duarte AJ, Tuerlinckx D, Virelizier JL, Fischer A, Enright A, Bernhoft J, Cleary AM, Vermylen C, Rodriguez-Gallego C, Davies G, Blutters-Sawatzki R, Siegrist CA, Ehlayel MS, Novelli V, Haas WH, Levy J, Freihorst J, Al Hajjar S, Nadal D, De M, Jeppsson VO, Kutukculer N, Frecerova K, Caragol I, Lammas D, Kumararatne DS, Abel L, Casanova JL. Low penetrance, broad resistance, and favorable outcome of interleukin 12 receptor beta1 deficiency: medical and immunological implications. J Exp Med. 2003;197:527–535. doi: 10.1084/jem.20021769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van de Vosse E, Hoeve MA, Ottenhoff TH. Human genetics of intracellular infectious diseases: molecular and cellular immunity against mycobacteria and salmonellae. Lancet Infect Dis. 2004;4:739–749. doi: 10.1016/S1473-3099(04)01203-4. [DOI] [PubMed] [Google Scholar]
- 45.Jouanguy E, Lamhamedi-Cherradi S, Altare F, Fondaneche MC, Tuerlinckx D, Blanche S, Emile JF, Gaillard JL, Schreiber R, Levin M, Fischer A, Hivroz C, Casanova JL. Partial interferon-gamma receptor 1 deficiency in a child with tuberculoid bacillus Calmette-Guerin infection and a sibling with clinical tuberculosis. J Clin Invest. 1997;100:2658–2664. doi: 10.1172/JCI119810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jouanguy E, Altare F, Lamhamedi-Cherradi S, Casanova JL. Infections in IFNGR-1-deficient children. J Interferon Cytokine Res. 1997;17:583–587. doi: 10.1089/jir.1997.17.583. [DOI] [PubMed] [Google Scholar]
- 47.Caragol I, Raspall M, Fieschi C, Feinberg J, Larrosa MN, Hernandez M, Figueras C, Bertran JM, Casanova JL, Espanol T. Clinical tuberculosis in 2 of 3 siblings with interleukin-12 receptor beta1 deficiency. Clin Infect Dis. 2003;37:302–306. doi: 10.1086/375587. [DOI] [PubMed] [Google Scholar]
- 48.Picard C, Fieschi C, Altare F, Al Jumaah S, Al Hajjar S, Feinberg J, Dupuis S, Soudais C, Al Mohsen IZ, Genin E, Lammas D, Kumararatne DS, Leclerc T, Rafii A, Frayha H, Murugasu B, Wah LB, Sinniah R, Loubser M, Okamoto E, Al Ghonaium A, Tufenkeji H, Abel L, Casanova JL. Inherited interleukin-12 deficiency: IL12B genotype and clinical phenotype of 13 patients from six kindreds. Am J Hum Genet. 2002;70:336–348. doi: 10.1086/338625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Classification of Pulmonary Tuberculosis. Diagnostic Standards and Classification of Tuberculosis. New York: National Tuberculosis and Respiratory Disease Association; 1969. pp. 68–74. [Google Scholar]
- 50.Boyum A. Isolation of lymphocytes, granulocytes and macrophages. Scand J Immunol. 1976;(Suppl 5):9–15. [PubMed] [Google Scholar]
- 51.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 52.Barry SM, Lipman MC, Bannister B, Johnson MA, Janossy G. Purified protein derivative-activated type 1 cytokine-producing CD4+ T lymphocytes in the lung: a characteristic feature of active pulmonary and nonpulmonary tuberculosis. J Infect Dis. 2003;187:243–250. doi: 10.1086/346112. [DOI] [PubMed] [Google Scholar]
- 53.Morosini M, Meloni F, Marone BA, Paschetto E, Uccelli M, Pozzi E, Fietta A. The assessment of IFN-gamma and its regulatory cytokines in the plasma and bronchoalveolar lavage fluid of patients with active pulmonary tuberculosis. Int J Tuberc Lung Dis. 2003;7:994–1000. [PubMed] [Google Scholar]
- 54.Zhang M, Gately MK, Wang E, Gong J, Wolf SF, Lu S, Modlin RL, Barnes PF. Interleukin 12 at the site of disease in tuberculosis. J Clin Invest. 1994;93:1733–1739. doi: 10.1172/JCI117157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taha RA, Minshall EM, Olivenstein R, Ihaku D, Wallaert B, Tsicopoulos A, Tonnel AB, Damia R, Menzies D, Hamid QA. Increased expression of IL-12 receptor mRNA in active pulmonary tuberculosis and sarcoidosis. Am J Respir Crit Care Med. 1999;160:1119–1123. doi: 10.1164/ajrccm.160.4.9807120. [DOI] [PubMed] [Google Scholar]
- 56.Singhal A, Jaiswal A, Arora VK, Prasad HK. Modulation of Interferon Gamma Receptor 1 By Mycobacterium tuberculosis: A potential immune evasive mechanism. Infect Immun. 2007 doi: 10.1128/IAI.01743-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carranza C, Juarez E, Torres M, Ellner JJ, Sada E, Schwander SK. Myobacterium tuberculosis Growth Control by Lung Macrophages and CD8 Cells from Patient Contacts. Am J Respir Crit Care Med. 2005;173:238–245. doi: 10.1164/rccm.200503-411OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Condos R, Rom WN, Schluger NW. Treatment of multidrug-resistant pulmonary tuberculosis with interferon-gamma via aerosol. Lancet. 1997;349:1513–1515. doi: 10.1016/S0140-6736(96)12273-X. [DOI] [PubMed] [Google Scholar]
- 59.Dheda K, Chang JS, Breen RA, Kim LU, Haddock JA, Huggett JF, Johnson MA, Rook GA, Zumla A. In vivo and in vitro studies of a novel cytokine, interleukin 4delta2, in pulmonary tuberculosis. Am J Respir Crit Care Med. 2005;172:501–508. doi: 10.1164/rccm.200502-278OC. [DOI] [PubMed] [Google Scholar]
- 60.Kampmann B, Hemingway C, Stephens A, Davidson R, Goodsall A, Anderson S, Nicol M, Scholvinck E, Relman D, Waddell S, Langford P, Sheehan B, Semple L, Wilkinson KA, Wilkinson RJ, Ress S, Hibberd M, Levin M. Acquired predisposition to mycobacterial disease due to autoantibodies to IFN-gamma. J Clin Invest. 2005;115:2480–2488. doi: 10.1172/JCI19316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu B, Huang C, Kato-Maeda M, Hopewell PC, Daley CL, Krensky AM, Clayberger C. IL-9 is associated with an impaired Th1 immune response in patients with tuberculosis. Clin Immunol. 2007 doi: 10.1016/j.clim.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 62.Ribeiro-Rodrigues R, Resende CT, Rojas R, Toossi Z, Dietze R, Boom WH, Maciel E, Hirsch CS. A role for CD4+CD25+ T cells in regulation of the immune response during human tuberculosis. Clin Exp Immunol. 2006;144:25–34. doi: 10.1111/j.1365-2249.2006.03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stenger S, Niazi KR, Modlin RL. Down-regulation of CD1 on antigen-presenting cells by infection with Mycobacterium tuberculosis. J Immunol. 1998;161:3582–3588. [PubMed] [Google Scholar]
- 64.Kincaid EZ, Ernst JD. Mycobacterium tuberculosis exerts gene-selective inhibition of transcriptional responses to IFN-gamma without inhibiting STAT1 function. J Immunol. 2003;171:2042–2049. doi: 10.4049/jimmunol.171.4.2042. [DOI] [PubMed] [Google Scholar]
- 65.Noss EH, Pai RK, Sellati TJ, Radolf JD, Belisle J, Golenbock DT, Boom WH, Harding CV. Toll-like receptor 2-dependent inhibition of macrophage class II MHC expression and antigen processing by 19-kDa lipoprotein of Mycobacterium tuberculosis. J Immunol. 2001;167:910–918. doi: 10.4049/jimmunol.167.2.910. [DOI] [PubMed] [Google Scholar]
- 66.Pai RK, Pennini ME, Tobian AA, Canaday DH, Boom WH, Harding CV. Prolonged Toll-Like Receptor Signaling by Mycobacterium tuberculosis and Its 19-Kilodalton Lipoprotein Inhibits Gamma Interferon-Induced Regulation of Selected Genes in Macrophages. Infect Immun. 2004;72:6603–6614. doi: 10.1128/IAI.72.11.6603-6614.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pennini ME, Pai RK, Schultz DC, Boom WH, Harding CV. Mycobacterium tuberculosis 19-kDa lipoprotein inhibits IFN-gamma-induced chromatin remodeling of MHC2TA by TLR2 and MAPK signaling. J Immunol. 2006;176:4323–4330. doi: 10.4049/jimmunol.176.7.4323. [DOI] [PubMed] [Google Scholar]
- 68.Pai RK, Convery M, Hamilton TA, Boom WH, Harding CV. Inhibition of IFN-gamma-induced class II transactivator expression by a 19-kDa lipoprotein from Mycobacterium tuberculosis: a potential mechanism for immune evasion. J Immunol. 2003;171:175–184. doi: 10.4049/jimmunol.171.1.175. [DOI] [PubMed] [Google Scholar]
- 69.Gehring AJ, Rojas RE, Canaday DH, Lakey DL, Harding CV, Boom WH. The Mycobacterium tuberculosis 19-kilodalton lipoprotein inhibits gamma interferon-regulated HLA-DR and Fc gamma R1 on human macrophages through Toll-like receptor 2. Infect Immun. 2003;71:4487–4497. doi: 10.1128/IAI.71.8.4487-4497.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fletcher HA, Owiafe P, Jeffries D, Hill P, Rook GA, Zumla A, Doherty TM, Brookes RH. Increased expression of mRNA encoding interleukin (IL)-4 and its splice variant IL-4delta2 in cells from contacts of Mycobacterium tuberculosis, in the absence of in vitro stimulation. Immunology. 2004;112:669–673. doi: 10.1111/j.1365-2567.2004.01922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dheda K, Chang JS, Huggett JF, Kim LU, Johnson MA, Zumla A, Rook GA. The stability of mRNA encoding IL-4 is increased in pulmonary tuberculosis, while stability of mRNA encoding the antagonistic splice variant, IL-4delta2, is not. Tuberculosis(Edinb) 2007;87:237–241. doi: 10.1016/j.tube.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 72.Seah GT, Rook GA. IL-4 influences apoptosis of mycobacterium-reactive lymphocytes in the presence of TNF-alpha. J Immunol. 2001;167:1230–1237. doi: 10.4049/jimmunol.167.3.1230. [DOI] [PubMed] [Google Scholar]
- 73.Seah GT, Scott GM, Rook GA. Type 2 cytokine gene activation and its relationship to extent of disease in patients with tuberculosis. J Infect Dis. 2000;181:385–389. doi: 10.1086/315200. [DOI] [PubMed] [Google Scholar]