Abstract
To maintain water and electrolyte balance, nectar-feeding vertebrates oscillate between two extremes: avoiding overhydration when feeding and preventing dehydration during fasts. Several studies have examined how birds resolve this osmoregulatory dilemma, but no data are available for nectar-feeding mammals. In this article, we 1) estimated the ability of Pallas's long-tongued bats (Glossophaga soricina; Phyllostomidae) to dilute and concentrate urine and 2) examined how water intake affected the processes that these bats use to maintain water balance. Total urine osmolality in water- and salt-loaded bats ranged between 31 ± 37 mosmol/kgH2O (n = 6) and 578 ± 56 mosmol/kgH2O (n = 2), respectively. Fractional water absorption in the gastrointestinal tract was not affected by water intake rate. As a result, water flux, body water turnover, and renal water load all increased with increasing water intake. Despite these relationships, glomerular filtration rate (GFR) was not responsive to water loading. To eliminate excess water, Pallas's long-tongued bats increased water excretion rate by reducing fractional renal water reabsorption. We also found that rates of total evaporative water loss increased with increasing water intake. During their natural daytime fast, mean GFR in Pallas's long-tongued bats was 0.37 ml/h (n = 10). This is ∼90% lower than the GFR we measured in fed bats. Our findings 1) suggest that Pallas's long-tongued bats do not have an exceptional urine-diluting or -concentrating ability and 2) demonstrate that the bats eliminate excess ingested water by reducing renal water reabsorption and limit urinary water loss during fasting periods by reducing GFR.
Keywords: glomerular filtration rate, Glossophaga soricina, urine dilution, water balance, water flux
osmoregulation in nectar-feeding vertebrates demands that two diametric challenges be met. When feeding, both nectarivorous birds and mammals ingest multiples of their body mass (mB) each day in water (4, 26, 34). Consequently, to avoid overhydration (1, 12), they must be efficient at eliminating the excess water they ingest (4, 26, 34) and recovering filtered electrolytes (2, 41). However, when these nectar-feeding vertebrates are fasting, their high mass-specific rates of evaporative water loss (46, 50) and poor urine-concentrating abilities (14, 16, 31, 51) appear to place them at risk of dehydrating (22). Curiously, even though a large number of mammals eat nectar (8, 40), no studies have sought to identify the physiological processes that nectarivorous mammals use to osmoregulate. Understanding these processes in nectar-feeding mammals is of interest for two reasons. First, although the mechanisms underlying both water elimination and conservation are reasonably well understood (10), relatively little is known with respect to how these mechanisms adjust to meet oscillating demands. Second, several of the physiological processes that nectar-feeding birds use to osmoregulate, such as interrupted renal filtration (20, 21) and regulated water absorption (36), are thought to cause harm and/or not exist in mammals (10, 24, 45). Here, we report the results of two experiments designed to understand how osmoregulatory processes in a specialized nectar-feeding mammal, the Pallas's long-tongued bat (Glossophaga soricina; Phyllostomidae), respond to both water excess and stress.
Our first experiment examined the electrolyte aspect of osmoregulation. When feeding, specialized nectar-feeding birds appear to be exceptionally good at recovering electrolytes filtered in the kidney (6, 14, 31). Hummingbirds (Trochilidae), for example, have been shown to void urine with Na+ and K+ concentrations as low as 0.4 and 0.2 mM/l, respectively (31). Despite the need to limit urinary water loss during fasting periods (23), nectar-feeding vertebrates have a limited urine-concentrating capacity; among both birds and mammals, maximum urine concentrations range from approximately isotonic to roughly three times greater than plasma concentration (6, 7, 14, 16, 31, 37, 51). In this article, we measured urinary osmolyte and electrolyte concentrations in water- and salt-loaded Pallas's long-tongued bats. Although we expected them to excrete hyposmotic urine when water loaded, we did not expect values to be as low as those observed in specialized nectar-feeding birds (14, 31). When salt loaded, we expected Pallas's long-tongued bats to excrete hyperosmotic urine (51). However, because the renal medullae in these bats are relatively undeveloped (9, 47), we did not expect them to produce urine that was more concentrated than that observed in other nectar-feeding vertebrates.
To eliminate excess ingested water, nectar-feeding birds have been shown to reduce renal water reabsorption (17, 20, 21, 37). Under some circumstances, nectar-feeding birds also appear to reduce water absorption in the gastrointestinal tract (GIT) and increase glomerular filtration rate (GFR) when water intake rates are high (20, 36, 37). During fasting periods, hummingbirds have been shown to reduce, even cease, GFR to limit urinary water loss (20, 21). In our second experiment, we measured the response of water-handling processes in the GIT and kidney of Pallas's long-tongued bats to varying rates of water intake. We expected these bats to reduce renal water reabsorption as water intake rate increased. We did not, however, expect the GIT or GFR to have an osmoregulatory role during water loading; that is, no mechanism has been identified that would decouple dietary water absorption from nutrient assimilation (30, 44, 45), and water loading does not appear to overwhelm the autoregulatory control of GFR in mammals (56). During fasting periods, however, we did expect GFR in Pallas's long-tongued bats to be reduced. However, because these bats appear capable of producing hyperosmotic urine (51) and mammals cannot tolerate interruptions in renal filtration (10, 24), we did not expect GFR reductions to be as dramatic as those reported in hummingbirds.
MATERIALS AND METHODS
When conducting the research described in this article, we adhered to the principles and guidelines articulated by the Institute of Laboratory Animal Resources (27). Our protocols were approved by the University of Wyoming's Institutional Animal Care and Use Committee.
Bat Capture and Maintenance
Pallas's long-tongued bats (mB = 10.78 ± 0.97 g, n = 16; 9 males, 7 females) were caught with mist nets in the state of Colima, Mexico (19°1′N, 103°47′W) and were transported to the University of Wyoming. We housed bats at 28 ± 2°C and 45 ± 7% relative humidity inside an irregularly shaped, wire-mesh cage (1.73 m3) on a 12-12 h light-dark photoperiod (photophase: 0700–1859 MST). Bats were maintained on an aqueous diet of 16.9% ripe banana (mass%), 2.6% mixed grain baby cereal (Gerber, Fremont, MI), 1.9% full cream powdered milk (Nido Clásica; Nestlé South America, Vevey, Switzerland), and 1.3% sucrose with a 0.3% vitamin supplement (Nekton-S; Guenter Enderle, Tarpon Springs, FL). Except as described in the experiments below, bats fed ad libitum on this maintenance diet during the scotophase and always had access to drinking water. For the experiments described below, ambient temperature, relative humidity, and photoperiod were the same as the maintenance conditions.
Experiment 1: Urine Concentrations During Water and Salt Loading
Design.
To estimate the ability of Pallas's long-tongued bats (mB = 10.83 ± 0.65 g, n = 6; 4 males, 2 females) to dilute and concentrate urine, we fed them 292 mmol/l (mM) sucrose solutions containing 0, 75, 150, 225, and 300 mM NaCl. Although the salt-free diet will lead to measurements that closely approximate the urine-diluting capacity of these bats, our salt-loading design precludes an accurate assessment of maximum urine-concentrating ability (13). We made all experimental diets with deionized water (Milli-Q; Millipore, Billerica, MA), and the order of feeding trials was determined randomly. Bats spent a minimum of 2 days feeding on the maintenance diet between trials.
Protocol.
Before lights off (∼1830), we transferred bats from the maintenance cage to individual experiment cages (0.3 × 0.3 × 0.3 m). The front panel of these cages was Mylar-coated glass; the remaining panels were opaque plexiglas. We equipped each cage with one perch, and bats accessed food while perching. We calculated the rate of NaCl ingestion (mg/h) on the basis of 1) the concentration of NaCl in the diet and 2) the rate at which bats consumed the experimental diet (ml/h) after accounting for evaporation and spillage. We collected urine immediately after it was voided onto the wax paper that lined the bottom of each cage using glass microcapillary tubes (100 μl; Drummond Scientific, Broomall, PA). We did not analyze urine that came into contact with either the perch or the bat.
Beginning at lights off (1900), bats fed for ∼8 h on one of the experimental diets. After this acclimation period, we weighed the bats (±0.01 g) and then began collecting urine. Urine collection occurred between ∼0400 and ∼0645. We recorded ad libitum food consumption (±0.1 ml) throughout the diet acclimation and urine-collection periods. At ∼0645, we weighed the bats again and then returned them to the maintenance cage. If, during the diet-acclimation phase, a bat did not eat for a continuous 4 h span, we removed it from the experiment and returned it to the maintenance cage/diet.
To ensure that the bats were acclimated to the experimental diet, we pooled urine samples from ∼0400 to 0530 for individual bats and compared their total osmolality (mosmol/kgH2O) to the samples we collected between 0531 and ∼0645. The urine samples from this latter period were similarly pooled for individual bats. The total osmolality of urine between these two periods was similar (paired t-test: t17 = −1.41, n = 18, P = 0.1759), and we only analyzed the urine osmolality (Osmette II; Precision Systems, Natick, MA) and NaCl ingestion data from the latter period (0531 to ∼0645). We measured the concentration of Na+ (±1 mosmol/kgH2O), Cl− (±1 mosmol/kgH2O), and K+ (±0.1 mosmol/kgH2O) in urine using a Starlyte III electrolyte analyzer (Alpha Wassermann Diagnostics, West Caldwell, NJ).
Experiment 2: Water-Handling Processes in the GIT and Kidney
Design.
We randomly assigned Pallas's long-tongued bats (mB = 10.65 ± 1.04 g, n = 10; 5 males, 5 females) to one salt-free diet containing either 146, 292, 438, or 584 mM sucrose. We then closely followed the two-marker technique of Hartman Bakken and Sabat (21). Their approach combines mass-balance (35) and slope-intercept models (15, 19) to measure GIT and renal responses to water intake simultaneously in nonanesthetized animals feeding naturally. However, to use the two-marker technique in Pallas's long-tongued bats, we modified 1) how l-glucose distribution space was determined and 2) how we determined the 14C concentration in urine to estimate fractional renal water reabsorption. We describe these modifications below.
The two-marker technique (21) requires information on the following parameters: 1) Q, the quantity of marker injected [disintegrations/min (dpm)]; 2) I, the time 0 intercept concentration of marker in body fluid (dpm/ml); and 3) K, the hourly fractional rate of marker elimination. Throughout this article, parameter subscripts specify whether the marker is 3H2O (denoted by 3H) or l-[14C]-labeled glucose (denoted by 14C). We calculated total body water (TBW; ml) as
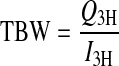 |
where K3H is used to extrapolate to I3H from a single blood sample taken ∼2 h after injection. The rate at which dietary water is incorporated into body water, or water flux (W; ml/h), is then
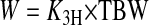 |
and the hourly fractional turnover rate of body water (fT) is
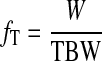 |
To estimate the rate of metabolic water production (VM; ml/h), we made four assumptions: 1) that Pallas's long-tongued bats fuel their metabolism solely with dietary sucrose (53, 54), 2) that sucrose assimilation is independent of sucrose intake rate (SI; g/h; see Ref. 26), 3) that sucrose assimilation efficiency is 99% (55), and 4) that the catabolism of 1 g of sucrose liberates 0.56 ml of water (35, 36). Thus VM in Pallas's long-tongued bats is
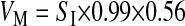 |
and fractional water absorption in the GIT (fA) equals
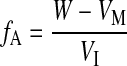 |
where VI is the rate of dietary water intake (ml/h). We calculated both SI and VI on the basis of volumetric food intake (±0.1 ml) after correcting for evaporation and spillage. The rate of renal water loading (VR; ml/h) can then be estimated as
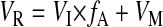 |
Because handling stress may cause reductions in renal filtration (11), we estimated GFR during feeding periods as
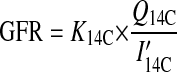 |
where I14C is the concentration of 14C in the first urine sample after injection, and the quotient of Q14C over I′14C is equal to the l-glucose distribution space. We then estimated fractional renal water reabsorption (fR) as
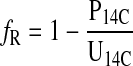 |
where P14C and U14C are the 14C concentrations in plasma and urine (dpm/ml), respectively. We estimated U14C by extrapolating (Fig. 1, A and C) or interpolating (Fig. 1B) K14C to the time that we drew the blood sample. To estimate total evaporative water loss (TEWL′; ml/h), we first estimated the rate of water excretion (VE; ml/h) as
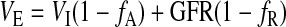 |
and then calculated TEWL′ as
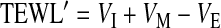 |
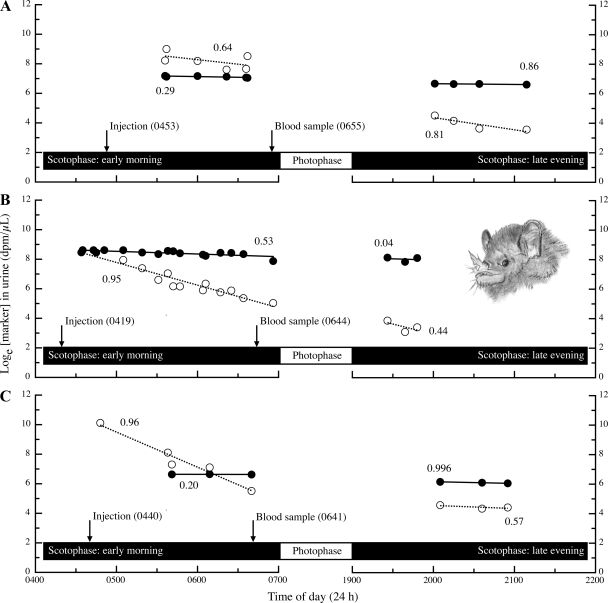
Fig. 1.
Data from Pallas's long-tongued bats (Glossophaga soricina) illustrating: 1) our protocol in experiment 2 and 2) that the appearance of 3H2O (•, solid lines) and l-[14C]-labeled glucose (○, dashed lines) in urine over time follows single-compartment, first-order kinetics. Each panel (A–C) shows data for an individual bat. For each marker elimination curve, numerical values represent the coefficient of determination (r2). 3H and 14C concentrations in urine are loge transformed here for clarity; however, we determined r2 values and performed our analyses on nontransformed data (38). dpm, disintegrations per minute.
During fasting periods we estimated mean GFR (GFR′; ml/h) as
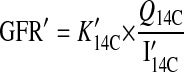 |
where K′14C is the difference between the 14C concentration in the last urine sample before the fasting period and the first urine sample after the fasting period divided by the length of the fasting period (20).
Protocol.
On the day of a trial, ∼10 h before the scotophase, we removed bats from the maintenance cage and placed them into individual experiment cages. These cages are the same ones we used in experiment 1 except we suspended the perch from an electronic balance (± 0.01 g) so that mB data could be obtained without disturbing the bat. At lights off (1900), each bat began feeding ad libitum on one of the randomly assigned experimental diets.
Approximately 2.25 h before lights on (∼0445), we injected ∼2.5 × 105 Bq of 3H2O (lot no. 824–167-001; Moravek Biochemicals, Brea, CA) and ∼2.0 × 105 Bq of l-[14C]-labeled glucose (lot no. 150–149-050, Moravek Biochemicals) into the pectoralis muscle. Both markers were dissolved in deionized water, and the total volume injected was 24.2 ± 7.3 μl (n = 10). Promptly after injection, we began collecting cleanly voided urine. Roughly 15 min before lights on (∼0645), we removed each bat from its cage and collected a blood sample from the brachial artery (range: 6.0–85.5 μl). We then returned each bat to its cage and removed the experimental diet. At lights off the following evening (1900), bats resumed feeding ad libitum on the same experimental diet, and we resumed collecting cleanly voided urine. An illustration of this protocol is provided in Fig. 1.
All injection aliquots, 3H and 14C background, urine, and plasma samples were placed in individual borosilicate glass scintillation vials immediately after they were collected. We added EcoLume scintillation cocktail (ICN Biomedicals, Costa Mesa, CA) to all samples before measuring counts with a liquid scintillation counter (LS 6000IC; Beckman Coulter, Fullerton, CA). All counts were corrected for 3H and 14C background, quench, chemiluminescence, and 14C spillover.
Statistical Analyses
Experiment 1: urine concentrations during water and salt loading.
During the diet acclimation period, we used ANOVA models to determine the effect of subject and diet NaCl concentration on the rate of food intake. To assess urine-diluting ability, we used descriptive statistics on data obtained from bats that were feeding on the salt-free diet. To determine how dietary salt loading affected urine concentration, we used data from bats that were feeding on the salt-containing diets in ANOVA models. Specifically, we evaluated the effect of subject and diet NaCl concentration on measurements of total, Na+, Cl−, and K+ osmolality in urine.
Experiment 2: water-handling processes in the GIT and kidney.
To determine the effect of both sucrose concentration and subject on water intake rate and GFR, we used repeated-measures ANOVA (R-M ANOVA) models. Afterward, we evaluated differences among means with Tukey's honest significant difference (Tukey's HSD) tests. We also used R-M ANOVA models to compare measurements between the early morning and late evening. In all other cases, we used least-squares linear regression (LR) to analyze data.
For both experiments, we used paired t-tests to evaluate changes in mB over the course of a trial. We assessed significance at α = 0.05, and all data are reported as means ± SD.
RESULTS
Experiment 1: Urine Concentrations During Water and Salt Loading
Diet acclimation.
During the diet acclimation phase, not all bats consumed the full range of NaCl solutions. As noted previously, bats that did not eat the experimental diet for a continuous 4-h period during the acclimation phase were returned to the maintenance cage/diet and did not participate in this experiment. Of the bats that fed on the experimental diets during the acclimation phase, food intake rate was not influenced by the diet's NaCl concentration (ANOVA: F1,16 = 3.13, n = 18, P = 0.0959). Subject had no effect on food intake rate (P = 0.0786), and we excluded this parameter from the above analysis. Food intake rates among the bats that qualified for experiment 1 were 1.58 ± 0.74 ml/h (n = 6), 1.67 ± 0.65 ml/h (n = 6), 1.34 ± 0.27 ml/h (n = 4), and 0.64 ± 0.27 ml/h (n = 2) on the 0, 75, 150, and 225 mM NaCl solutions, respectively. No bats consumed the 300 mM NaCl solution.
NaCl intake.
In the analysis below, subject was not a significant parameter (P = 0.3562), and we removed it from our model. During the experiment, NaCl intake rate increased with increasing NaCl concentration (ANOVA: F1,10 = 22.20, n = 12, P < 0.0001). NaCl intakes rates are reported in Table 1. Bats maintained mB on both the salt-free (paired t-test: t5 = −1.13, n = 6, P = 0.3113) and salt-containing diets (paired t-test: t11 = −0.58, n = 12, P = 0.5715).
Table 1.
NaCl intake rates and urine osmolalities in Pallas's long-tongued bats (Glossophaga soricina) feeding voluntarily on 292 mM sucrose solutions with varying NaCl concentrations
| Diet [NaCl], mM | NaCl IntakeRate, mg/h | Total, mOsm/kg | Na+, mosmol/kg H2O | Cl−, mosmol/kg H2O | K+, mosmol/kg H2O |
|---|---|---|---|---|---|
| 0 | 31±37 (6) | 4±8 (5) | 11±12 (5) | 1.2±1.2 (5) | |
| 75 | 5.6±1.9 (6) | 210±33 (6) | 95±19 (6) | 105±17 (6) | 3.8±1.7 (6) |
| 150 | 14.0±1.0 (4) | 403±65 (4) | 168±41 (4) | 183±44 (4) | 7.7±4.1 (4) |
| 225 | 13.5±1.7 (2) | 578±56 (2) | 272±11 (2) | 287±16 (2) | 8.7±3.5 (2) |
Values are means ± SD; n values are in parentheses.
Urine concentrations in water-loaded bats.
Total osmolality of urine from bats feeding on the salt-free experimental diet ranged from 11 to 104 mosmol/kgH2O. Table 1 summarizes these data. Table 1 also contains the Na+, Cl−, and K+ osmolalities of urine from bats fed the salt-free diet. We were unable to obtain individual ion measurements for one bat; total urine osmolality for this individual was 13 mosmol/kgH2O.
Urine concentrations in salt-loaded bats.
Subject had no influence on the total osmolality of urine (P = 0.6889), so we removed this parameter from our analysis. When bats were feeding on the salt-containing experimental diets, total urine osmolality increased with increasing NaCl concentration (ANOVA: F1,10 = 107.70, n = 12, P < 0.0001). Total urine osmolality values are reported in Table 1. The greatest total osmolality value we observed was 617 mosmol/kgH2O from a bat feeding on the 225 mM NaCl solution. The concentration of Na+ and Cl− in urine of Pallas's long-tongued bats increased with increasing NaCl concentration (ANOVA: Na+, F1,10 = 64.46, n = 12, P < 0.001; Cl−, F1,10 = 64.95, n = 12, P < 0.0001). The same was true for K+; however, the concentration of K+ in urine was also influenced by subject (ANOVA: F2,9 = 11.00, n = 12, P = 0.0038). Subject did not affect the concentration of Na+ or Cl− in urine (P = 0.9096 and 0.4962, respectively), and we removed this parameter from our analyses above. Individual ion osmolalities are reported in Table 1.
Experiment 2: Water-Handling Processes in the GIT and Kidney
Marker equilibration and elimination.
Equilibration times for 3H and 14C were 0.96 ± 0.47 (n = 10) and 0.76 ± 0.44 h (n = 10), respectively. The appearance of both 3H and 14C in urine over time was described by negative exponential functions: during the early morning, coefficient of determination (r2) values were 0.54 ± 0.28 for 3H (n = 10; range: 0.14–0.98) and 0.63 ± 0.30 for 14C (n = 10; range: 0.27–0.96); during late evening, r2 values were 0.70 ± 0.29 for 3H (n = 10; range: 0.04–0.996) and 0.64 ± 0.16 for 14C (n = 10; range: 0.44–0.90). Despite wide-ranging r2 values, there is no indication that the elimination of both 3H and 14C followed a pattern other than that expected from single-compartment, first-order kinetics. In Fig. 1, we show marker elimination data for three of the 10 individuals to substantiate this conclusion.
Were Pallas's long-tongued bats in neutral water balance?
During the early morning, mB after injection (10.65 ± 1.04 g, n = 10) did not differ from that at lights on (10.71 ± 1.21 g, n = 10; paired t-test: t9 = 0.77, n = 10, P = 0.4589). This indicates that the assumption of neutral water balance during the early morning period was reasonable. Over the course of the 12-h photophase, bats lost ∼6% (0.68 ± 0.25 g, n = 10) of their lights-on mB. During the late evening, MB at lights off (10.02 ± 1.05 g, n = 10) was significantly less than mB at the end of this measurement period (10.35 ± 1.06 g, n = 10; paired t-test: t9 = 4.43, n = 10, P = 0.0016). Consequently, the neutral water balance assumption was not satisfied for this period.
Body fluid spaces.
TBW in Pallas's long-tongued bats was 6.93 ± 0.90 ml (n = 10), which represents 65.5 ± 9.8% of mB (n = 10). Using our modified approach, l-glucose distribution space was 2.04 ± 0.62 ml (n = 10). This volume corresponds to 19.1 ± 5.3% of mB (n = 10).
Water intake.
Subject did not influence water intake rate (P = 0.4955), so we removed this parameter from the analyses in this section. During both the early morning and late evening, the rate of water intake by bats increased as the sucrose concentration in their food decreased (R-M ANOVA: F1,8 = 20.19, n = 10, P = 0.0020). Water intake rates were greater during the late evening compared with the early morning (R-M ANOVA: F1,8 = 9.03, n = 10, P = 0.0170). We report water intake rates for each experimental diet during both the early morning and late evening in Table 2. Table 2 also contains water intake data for individual bats.
Table 2.
Water intake rates in Pallas's long-tongued bats (Glossophaga soricina) feeding voluntarily on solutions that differed in sucrose concentration during the early morning and late evening
| Diet/Bat ID | Early Morning, ml/h | Late Evening, ml/h |
|---|---|---|
| 146 mM sucrose | 1.39±0.45 (2) | 2.96±0.23 (2) |
| F | 1.71 | 3.13 |
| H | 1.08 | 2.80 |
| 292 mM sucrose | 1.02±0.09 (3) | 1.92±1.34 (3) |
| A | 1.10 | 2.02 |
| I | 0.92 | 0.53 |
| J | 1.04 | 3.21 |
| 438 mM sucrose | 1.10±0.74 (2) | 1.12±0.29 (2) |
| B | 1.62 | 1.32 |
| G | 0.58 | 0.91 |
| 534 mM sucrose | 0.40±0.31 (3) | 0.58±0.31 (3) |
| C | 0.43 | 0.44 |
| D | 0.69 | 0.37 |
| E | 0.07 | 0.93 |
For each diet, data obtained from individual bats are summarized as means ± SD; n values are in parentheses.
Water flux.
During both the early morning and late evening, water flux increased linearly as the rate of water intake increased (LR: early morning, y = −0.02 + 0.92x, r2 = 0.94, n = 10, P < 0.0001; late evening, y = −0.14 + 0.96x, r2 = 0.98, n = 10, P < 0.0001; Fig. 2A). Water flux did not differ between these two periods (R-M ANOVA: F1,9 = 4.44, n = 10, P = 0.0643).
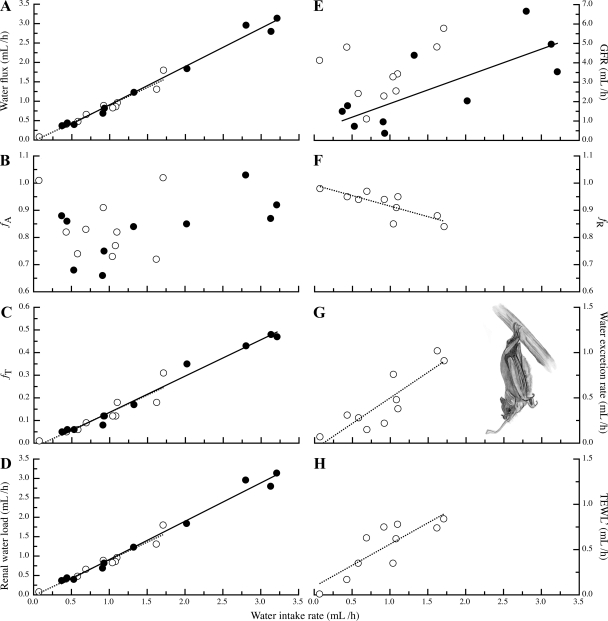
Fig. 2.
The influence of water intake rate on water budget components in Pallas's long-tongued bats (Glossophaga soricina) during both the early morning (○, dashed lines) and late evening (•, solid lines). Water flux increased linearly with water intake (A). Fractional water absorption in the gastrointestinal tract (fA) was independent of water intake (B). Hourly rates of both fractional body water turnover (fT; C) and renal water load (D) increased linearly with water intake. During the early morning, glomerular filtration rate (GFR) was independent of water intake; during the late evening, however, GFR increased linearly with water intake (E). Fractional water reabsorption in the kidney (fR) decreased linearly with water intake during the early morning (F). Also during the early morning, both the rate of water excretion (G) and our indirect estimate of total evaporative water loss (TEWL′; H) increased linearly with water intake. The assumption of neutral water balance was not met for bats during the late evening measurement period.
Water absorption in the GIT.
Fractional water absorption was independent of water intake rate during both the early morning (LR: P = 0.7108; Fig. 2B) and late evening (LR: P = 0.0729; Fig. 2B). Fractional water absorption was 0.84 ± 0.11 during the early morning (n = 10) and 0.83 ± 0.11 in the late evening (n = 10; Fig. 2B). Measurement period had no influence on fractional water absorption (R-M ANOVA: F1,9 = 0.003, n = 10, P = 0.9546; Fig. 2B).
Body water turnover.
The fractional turnover of body water increased linearly as water intake rates increased (LR: early morning, y = −0.02 + 0.15x, r2 = 0.86, n = 10, P < 0.0001; late evening, y = −0.03 + 0.16x, r2 = 0.98, n = 10, P < 0.0001; Fig. 2C). Fractional body water turnover rates were similar between the early morning and late evening (R-M ANOVA: F1,9 = 4.87, n = 10, P = 0.0547; Fig. 2C).
Metabolic water production.
The rate of metabolic water production was independent of water intake rate during both the early morning (LR: P = 0.1054) and late evening (LR: P = 0.0529). Metabolic water production rates were 0.06 ± 0.04 (n = 10) and 0.10 ± 0.05 ml/h (n = 10) during the early morning and late evening, respectively. Metabolic water production rates were not different between these two periods (R-M ANOVA: F1,9 = 3.30, n = 10, P = 0.1028).
Renal water load.
We found a linear increase in renal water load with increasing water intake during both the early morning (LR: y = −0.02 + 0.92x, r2 = 0.94, n = 10, P < 0.0001) and late evening (LR: y = −0.08 + 0.99x, r2 = 0.99, n = 10, P < 0.0001; Fig. 2D). Renal water loading rates, however, were not different between these periods (R-M ANOVA: F1,9 = 4.44, n = 10, P = 0.0643; Fig. 2D).
Glomerular filtration rate.
Both subject and sucrose concentration were nonsignificant parameters in our model (P = 0.2010 and 0.0824, respectively), and we removed them from the analyses in this section. There were significant differences among our GFR estimates (R-M ANOVA: F2,8 = 19.56, n = 10, P = 0.0008), and Tukey's HSD tests revealed that these differences were between daytime GFR' and the GFRs we measured during the early morning and late evening (Fig. 3). During the early morning, GFR was 3.46 ± 1.43 ml/h (n = 10; Fig. 3) and independent of water intake rate (LR: P = 0.3236; Fig. 2E). During the day, when Pallas's long-tongued bats are fasting, GFR' was 0.37 ± 0.20 ml/h (n = 10; Fig. 3). During the late evening, when bats resumed feeding, GFR was 2.69 ± 2.09 ml/h (n = 10; Fig. 3). GFR during this period was responsive to the rate of water intake (LR: y = 0.49 + 1.40x, r2 = 0.58, n = 10, P = 0.0108; Fig. 2E).
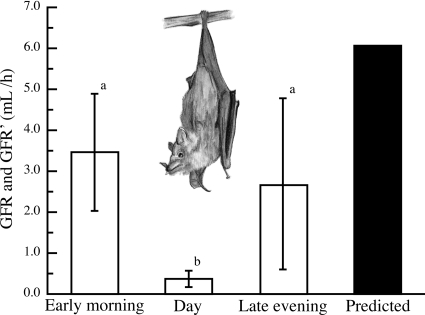
Fig. 3.
GFR during dissimilar times of day in Pallas's long-tongued bats (Glossophaga soricina). Our GFR and mean GFR (GFR′) estimates (open bars) were all lower than the allometric prediction of 6.06 ml/h (solid bar; see Ref. 45). GFR during the early morning and late evening feeding periods was 3.46 ± 1.43 (n = 10) and 2.69 ± 2.09 ml/h (n = 10), respectively. Some of the variability in late evening GFR is explained by the positive and linear relationship between GFR and water intake during this period (Fig. 2E). GFR′ during the day, when bats were naturally fasted, was 0.37 ± 0.20 ml/h (n = 10). Values are means ± SD; lowercase letters denote statistical differences.
Renal water reabsorption.
During the early morning, fractional water reabsorption decreased linearly as water intake rate increased (LR: y = 0.997 − 0.08x, r2 = 0.68, n = 10, P = 0.0034; Fig. 2F). A similar relationship was observed when we regressed fractional water reabsorption against the rate of renal water loading (LR: y = 0.99 − 0.08x, r2 = 0.64, n = 10, P = 0.0053).
Water excretion.
The rate of water excretion increased linearly with water intake during the early morning (LR: y = −0.07 + 0.57x, r2 = 0.77, n = 10, P = 0.0009; Fig. 2G). We observed a similar relationship between the rate of renal water loading and the water excretion rate during this period (LR: y = −0.003 + 0.56x, r2 = 0.66, n = 10, P = 0.0043).
TEWL.
During the early morning, TEWL' was 0.53 ± 0.29 ml/h (n = 10). Some of the variance in this estimate is explained by the linear increase in TEWL′ we observed as water intake rates increased (LR: y = 0.09 + 0.47x, r2 = 0.69, n = 10, P = 0.0028; Fig. 2H).
DISCUSSION
Our research sought to understand how water- and electrolyte-handling processes in a nectar-feeding mammal adjust to meet contrasting osmoregulatory demands. Accordingly, our discussion focuses on the physiological processes that Pallas's long-tongued bats use to osmoregulate during times of water excess and stress. We adopt a comparative outlook throughout this discussion, paying particular attention to previous findings in nectar-feeding birds.
Overhydration Avoidance
Water elimination.
Given that water is known to accompany nutrient absorption in the GIT (30, 44, 45), our finding that fractional water absorption was independent of water intake is not surprising (Fig. 2B). This nonregulated water absorption in the GIT means that Pallas's long-tongued bats can experience very high rates of water flux (Fig. 2A) and body water turnover (Fig. 2C) when water intake rates are high. Interestingly, if the hourly water fluxes we observed here (0.08–3.14 ml/h; Fig. 2C) are extrapolated to a 12-h day (0.96–37.68 ml/12 h), Pallas's long-tongued bats can, when water intake rates are high, have daily water fluxes greater than those experienced by equal-sized marine fishes (39). A similar conclusion has also been reached for hummingbirds when their water intake rates are high (34).
In terms of maintaining water balance, nonregulated water absorption in the GIT means that the kidneys of Pallas's long-tongued bats must process potentially large water volumes (Fig. 2D). Despite this, early morning GFR was insensitive to water loading (Fig. 2E) and 57% lower than the allometric expectation of 6.06 ml/h (Fig. 3; see Ref. 48). Although we found that GFR increases with water intake during the late evening (Fig. 2E), this observation needs to be reproduced before such a response can be considered a water elimination mechanism. With this caveat in mind, our findings suggest that Pallas's long-tongued bats eliminate excess water by reducing fractional water reabsorption in the kidney (Fig. 2F). Such a response explains our observation that water excretion rate increased with water loading (Fig. 2G). Interestingly, our indirect estimate of TEWL suggests that it and excretory water loss are of comparable importance for eliminating excess water (Figs. 2, G and H). However, we note that, whereas water excretion rates are likely to be determined by hormonally controlled water reabsorption processes in phyllostomid bats (42, 43), it is unlikely that TEWL is upregulated when water intake is high. In nectar-feeding birds, metabolic rate increases when feeding on energetically dilute sugar solutions (32, 33). Our finding that TEWL′ increased with water intake in nectar-feeding bats may be explained by a similar increase in metabolic rate. In general, our findings show that the processes Pallas's long-tongued bats use to eliminate water are similar to those used by both hummingbirds (23) and nectar-feeding passerines (17, 37). The one exception to this conclusion is that Palestine sunbirds (Nectarinia osea) also decrease water absorption in the GIT as water intake rates increase (36).
Urine dilution.
Although Pallas's long-tongued bats excreted highly dilute urine when they were water loaded (Table 1), their capacity to recover filtered electrolytes does not appear exceptional among mammals (10, 28). Although specialized nectar-feeding hummingbirds and passerines may be able to subsist on a strict diet of nectar (6, 14), the same may not be true for Pallas's long-tongued bats. Although this species relies heavily on nectar, they are known to consume insects in nature (25, 29).
Dehydration Avoidance
Urine concentration.
We found that salt-loaded Pallas's long-tongued bats produce urine with a total osmolality of 578 ± 56 mosmol/kgH2O (n = 2; Table 1). Although this value is approximately twice as concentrated as plasma, it is lower than Studier and Wilson's (51) measurement for this species (832 mosmol/kgH2O). Compared with other terrestrial mammals, both measurements suggest that Pallas's long-tongued bats have a weak urine-concentrating ability (3); however, this capacity does not appear to be different from that observed in other nectar-feeding vertebrates; nectar-feeding bats (excluding the species we studied; see Ref. 7), hummingbirds (31), and passerines (14) are reported to have urine-concentrating capacities no greater than 342, 600, and 461 mosmol/kgH2O, respectively. In terms of avoiding dehydration, these weak urine-concentrating capacities indicate that nectarivorous vertebrates must rely on other renal processes to limit their urinary water loss during periods of water stress.
Water conservation.
We found that GFR in Pallas's long-tongued bats was very responsive to water deprivation. During the 12-h day, when bats were naturally fasting, mean GFR was reduced by roughly 90% compared with the GFR we measured in fed bats during the early morning (Fig. 3). Although GFR has not been measured in fasted nectar-feeding passerines, drastically reduced and arrested GFRs were previously observed in fasted hummingbirds (23). Interestingly though, among mammals, GFR reductions of this magnitude have only been observed during hibernation (56, 57). In lieu of producing highly concentrated urine, nectar-feeding vertebrates appear to limit urinary water loss during times of water stress by reducing the volume of body water filtered in the kidney (20, 21; Fig. 3). An interesting difference between nectar-feeding mammals and birds, however, may be their renal water reabsorption capacity. During the early morning, the intercept of the relationship between fractional renal water reabsorption and water intake rate in Pallas's long-tongued bats suggests that they reabsorb 99.7% of the water that enters the renal tubules (intercept = 0.997; Fig. 2F). These same relationships in specialized nectar-feeding birds suggest that they are only capable of reabsorbing roughly 75 to 91% of filtered water (23, 37). We suspect that nectar-feeding mammals may show a greater renal water reabsorption capacity because 1) unlike birds, mammals cannot tolerate interruptions in renal filtration (10, 24) and 2) compared with birds, mammals appear to have less control over the composition of urine after it leaves the kidney (5, 18, 49).
GRANTS
This work was supported by grants from the National Institutes of Health INBRE Program of the National Center for Research Resources (5P20RR016474-07 awarded to B. Hartman Bakken and C. Martinez del Rio), Consejo Nacional de Ciencia y Tecnología (43343 awarded to L. G. Herrera M.), and Coordinación de la Investigación Científica Universidad Nacional Autónoma de México (47-07 awarded to L. G. Herrera M.).
Acknowledgments
We could not have conducted this work without Patricia L. Bakken. We thank Jazmín Osorio M. for helping us capture the bats, Katherine Bardsley at the Wyoming State Veterinary Laboratory for analyzing the ion species, and Annie Hartman Bakken for the bat illustrations. Kenneth C. Welch, Jr. and Raul K. Suarez were also a big help. This article benefited from the insightful criticisms of Alyeska Hayduke, Graham Mitchell, Edie Snekkermoen, and two anonymous reviewers.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Almond CSD, Shin AY, Fortescue EB, Mannix RC, Wypij D, Binstadt BA, Duncan CN, Olson DP, Salerno AE, Newburger JW, Greenes DS. Hyponatremia among runners in the Boston Marathon. N Engl J Med 352: 1550–1556, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Baker HG, Baker I, Hodges SA. Sugar composition of nectars and fruits consumed by birds and bats in the tropics and subtropics. Biotropica 30: 559–586, 1998. [Google Scholar]
- 3.Beuchat CA Body size, medullary thickness, and urine concentrating ability in mammals. Am J Physiol Regul Integr Comp Physiol 258: R298–R308, 1990. [DOI] [PubMed] [Google Scholar]
- 4.Beuchat CA, Calder WA, Braun EJ. The integration of osmoregulation and energy balance in hummingbirds. Physiol Zool 63: 1059–1081, 1990. [Google Scholar]
- 5.Braun EJ Regulation of renal and lower gastrointestinal function: role in fluid and electrolyte balance. Comp Biochem Physiol A Mol Integr Physiol 136: 499–505, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Calder WA, Hiebert SA. Nectar feeding, diuresis, and electrolyte replacement of hummingbirds. Physiol Zool 56: 325–334, 1983. [Google Scholar]
- 7.Carpenter RE Structure and function of the kidney and the water balance of desert bats. Physiol Zool 42: 288–302, 1969. [Google Scholar]
- 8.Carthew SM, Goldingay RL. Non-flying mammals as pollinators. Trends Ecol Evol 12: 104–108, 1997. [DOI] [PubMed] [Google Scholar]
- 9.Casotti G, Herrera M LG, Flores JJ, Mancina CA. Relationships between renal morphology and diet in 26 species of New World bats (suborder Microchiroptera). Zoology 109: 196–207, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Dantzler WH Comparative Physiology of the Vertebrate Kidney. Berlin, Germany: Springer-Verlag, 1989.
- 11.DiBona GF Physiology in perspective: the wisdom of the body. Neural control of the kidney. Am J Physiol Regul Integr Comp Physiol 289: R633–R641, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Fanestil DD Hyposmolar syndromes. In: Disturbances in Body Fluid Osmolality, edited by Andreoli TE, Grantham JJ, and Rector FC Jr. Bethesda, MD: Am Physiol Soc, 1977, chapt. 13, p. 267–284.
- 13.Fenton RA, Chou CL, Sowersby H, Smith CP, Knepper MA. Gamble's “economy of water” revisited: studies in urea transporter knockout mice. Am J Physiol Renal Physiol 291: F148–F154, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Fleming PA, Nicolson SW. Osmoregulation in an avian nectarivore, the whitebellied sunbird Nectarinia talatala: response to extremes of diet concentration. J Exp Biol 206: 1845–1854, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Florijn KW, Barendregt JNM, Lentjes EGWM, van Dam W, Prodjosudjadi W, van Saase JLCM, van Es LA, Chang PC. Glomerular filtration rate measured by a “single-shot” injection of inulin. Kidney Int 46: 252–259, 1994. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein DL, Bradshaw SD. Regulation of water and sodium balance in the field by Australian honeyeaters (Meliphagidae). Physiol Zool 71: 214–225, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein DL, Bradshaw SD. Renal function in red wattlebirds in response to varying fluid intake. J Comp Physiol [B] 168: 265–272, 1998. [Google Scholar]
- 18.Goldstein DL, Skadhauge E. Renal and extrarenal regulation of body fluid composition. In: Sturkie's Avian Physiology (5th edition), edited by Whittow GC. San Diego, CA: Academic, 2000, p. 265–297.
- 19.Hall JE, Guyton AC, Farr BM. A single-injection method for measuring glomerular filtration rate. Am J Physiol Renal Fluid Electrolyte Physiol 232: F72–F76, 1977. [DOI] [PubMed] [Google Scholar]
- 20.Hartman Bakken B, McWhorter TJ, Tsahar E, Martínez del Rio C. Hummingbirds arrest their kidneys at night: diel variation in glomerular filtration rate in Selasphorus platycercus. J Exp Biol 207: 4383–4391, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Hartman Bakken B, Sabat P. Gastrointestinal and renal responses to water intake in the green-backed firecrown (Sephanoides sephanoides), a South American hummingbird. Am J Physiol Regul Integr Comp Physiol 291: R830–R836, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Hartman Bakken B, Sabat P. Evaporative water loss and dehydration during the night in hummingbirds. Rev Chil Hist Nat 80: 267–273, 2007. [Google Scholar]
- 23.Hartman Bakken B, Sabat P. The mechanisms and ecology of water balance in hummingbirds. Ornitol Neotrop 19: 501–509, 2008. [Google Scholar]
- 24.Hays SR Ischemic acute renal failure. Am J Med Sci 304: 93–108, 1992. [DOI] [PubMed] [Google Scholar]
- 25.Heithaus ER, Fleming TH, Opler PA. Foraging patterns and resource utilization in seven species of bats in a seasonal tropical forest. Ecology 56: 841–854, 1975. [Google Scholar]
- 26.Herrera M LG, Mancina CA. Sucrose hydrolysis does not limit food intake by Pallas's long-tongued bats. Physiol Biochem Zool 81: 119–124, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Institute of Laboratory Animal Resources. Guide for the Care and Use of Laboratory Animals, National Research Council. Washington, DC: National Academy Press, 1996.
- 28.Kellogg RH, Burack WR, Isselbacher KJ. Comparison of diuresis produced by isotonic saline solutions and water in rats studied by a 'steady state' method. Am J Physiol 177: 27–37, 1954. [DOI] [PubMed] [Google Scholar]
- 29.Lemke TO Foraging ecology of the long-nosed bat, Glossophaga soricina, with respect to resource availability. Ecology 65: 538–548, 1984. [Google Scholar]
- 30.Loo DDF, Zeuthen T, Chandy G, Wright EM. Cotransport of water by the Na+/glucose cotransporter. Proc Natl Acad Sci USA 93: 13367–13370, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lotz CN, Martínez del Rio C. The ability of rufous hummingbirds Selasphorus rufus to dilute and concentrate urine. J Avian Biol 35: 54–62, 2004. [Google Scholar]
- 32.Lotz CN, Martínez del Rio C, Nicolson SW. Hummingbirds pay a high cost for a warm drink. J Comp Physiol [B] 173: 455–462, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Lotz CN, Nicolson SW. Nectar dilution increases metabolic rate in the lesser double-collared sunbird. Condor 104: 672–675, 2002. [Google Scholar]
- 34.Martínez del Rio C, Schondube JE, McWhorter TJ, Herrera M LG. Intake responses in nectar feeding birds: digestive and metabolic causes, osmoregulatory consequences, and coevolutionary effects. Am Zool 41: 902–915, 2001. [Google Scholar]
- 35.McWhorter TJ, and Martínez del Rio C. Food ingestion and water turnover in hummingbirds: how much dietary water is absorbed? J Exp Biol 202: 2851–2858, 1999. [DOI] [PubMed] [Google Scholar]
- 36.McWhorter TJ, Martínez del Rio C, Pinshow B. Modulation of ingested water absorption by Palestine sunbirds: evidence for adaptive regulation. J Exp Biol 206: 659–666, 2003. [DOI] [PubMed] [Google Scholar]
- 37.McWhorter TJ, Martínez del Rio C, Pinshow B, Roxburgh L. Renal function in Palestine sunbirds: elimination of excess water does not constrain energy intake. J Exp Biol 207: 3391–3398, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Motulsky HJ, Ransnas LA. Fitting curves to data using nonlinear regression: a practical and nonmathematical review. FASEB J 1: 365–374, 1987. [PubMed] [Google Scholar]
- 39.Nagy KA, Peterson CC. Scaling of Water Flux Rate in Animals. Berkeley and Los Angeles, CA: University of California, 1988.
- 40.Nicolson SW Nectar consumers. In: Nectar and Nectaries, edited by Nicolson SW, Nepi M, and Pacini E. Dordrecht, The Netherlands: Springer, 2007, p. 289–342.
- 41.Nicolson SW, Thornburg RW. Nectar chemistry. In: Nectar and Nectaries, edited by Nicolson SW, Nepi M, and Pacini E. Dordrecht, The Netherlands: Springer, 2007, p. 215–264.
- 42.Nielsen S, Frøkiær J, Marples D, Kwon TH, Agre P, Knepper MA. Aquaporins in the kidney: from molecules to medicine. Physiol Rev 82: 205–244, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Nishimura H, Fan Z. Regulation of water movement across vertebrate renal tubules. Comp Biochem Physiol A Mol Integr Physiol 136: 479–498, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Pappenheimer JR, Reiss KZ. Contribution of solvent drag through intercellular junctions to absorption of nutrients by the small intestine of the rat. J Membr Biol 100: 123–126, 1987. [DOI] [PubMed] [Google Scholar]
- 45.Powell DW Intestinal water and electrolyte transport. In: Physiology of the Gastrointestinal Tract, edited by Johnson LR. New York: Raven, 1987, p. 1267–1305.
- 46.Powers DR Effect of temperature and humidity on evaporative water loss in Anna's hummingbird (Calypte anna). J Comp Physiol [B] 162: 74–84, 1992. [Google Scholar]
- 47.Schondube JE, Herrera M LG, Martínez del Rio C. Diet and the evolution of digestion and renal function in phyllostomid bats. Zoology 104: 59–73, 2001. [DOI] [PubMed] [Google Scholar]
- 48.Singer MA Of mice and men and elephants: metabolic rate sets glomerular filtration rate. Am J Kidney Dis 37: 164–178, 2001. [DOI] [PubMed] [Google Scholar]
- 49.Spector DA, Wade JB, Dillow R, Steplock DA, Weinman EJ. Expression, localization, and regulation of aquaporin-1 to -3 in rat urothelia. Am J Physiol Renal Physiol 282: F1034–F1042, 2002. [DOI] [PubMed] [Google Scholar]
- 50.Studier EH Evaporative water loss in bats. Comp Biochem Physiol 35: 935–943, 1970. [Google Scholar]
- 51.Studier EH, Wilson DE. Natural urine concentrations and composition in neotropical bats. Comp Biochem Physiol Comp Physiol 75: 509–515, 1983. [Google Scholar]
- 52.Voigt CC, Kelm DH, Visser GH. Field metabolic rates of phytophagous bats: do pollinator strategies make life of nectar-feeders spin faster? J Comp Physiol [B] 176: 213–222, 2006. [DOI] [PubMed] [Google Scholar]
- 53.Voigt CC, Speakman JR. Nectar-feeding bats fuel their high metabolism directly with exogenous carbohydrates. Funct Ecol 21: 913–921, 2007. [Google Scholar]
- 54.Welch KC Jr, Herrera M LG, Suarez RK. Dietary sugar as a direct fuel for flight in the nectarivorous bat Glossophaga soricina. J Exp Biol 211: 310–316, 2008. [DOI] [PubMed] [Google Scholar]
- 55.Winter Y In vivo measurement of near maximal rates of nutrient absorption in a mammal. Comp Biochem Physiol A Mol Integr Physiol 119: 853–859, 1998. [DOI] [PubMed] [Google Scholar]
- 56.Yokota SD, Benyajati S, Dantzler WH. Comparative aspects of glomerular filtration in vertebrates. Ren Physiol 8: 193–221, 1985. [DOI] [PubMed] [Google Scholar]
- 57.Zatzman ML, South FE. Renal function of the awake and hibernating marmot Marmota flaviventris. Am J Physiol 22: 1035–1039, 1972. [DOI] [PubMed] [Google Scholar]