Abstract
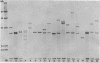
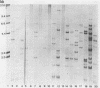
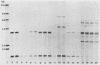
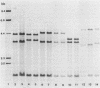
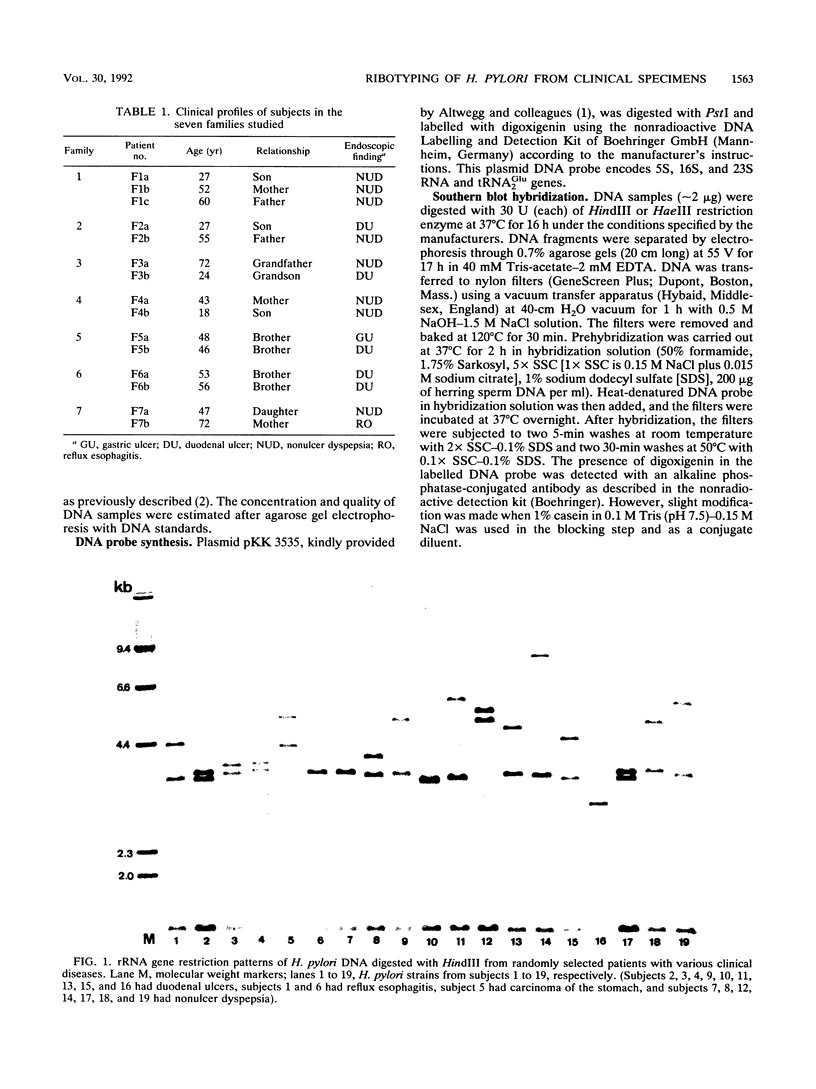
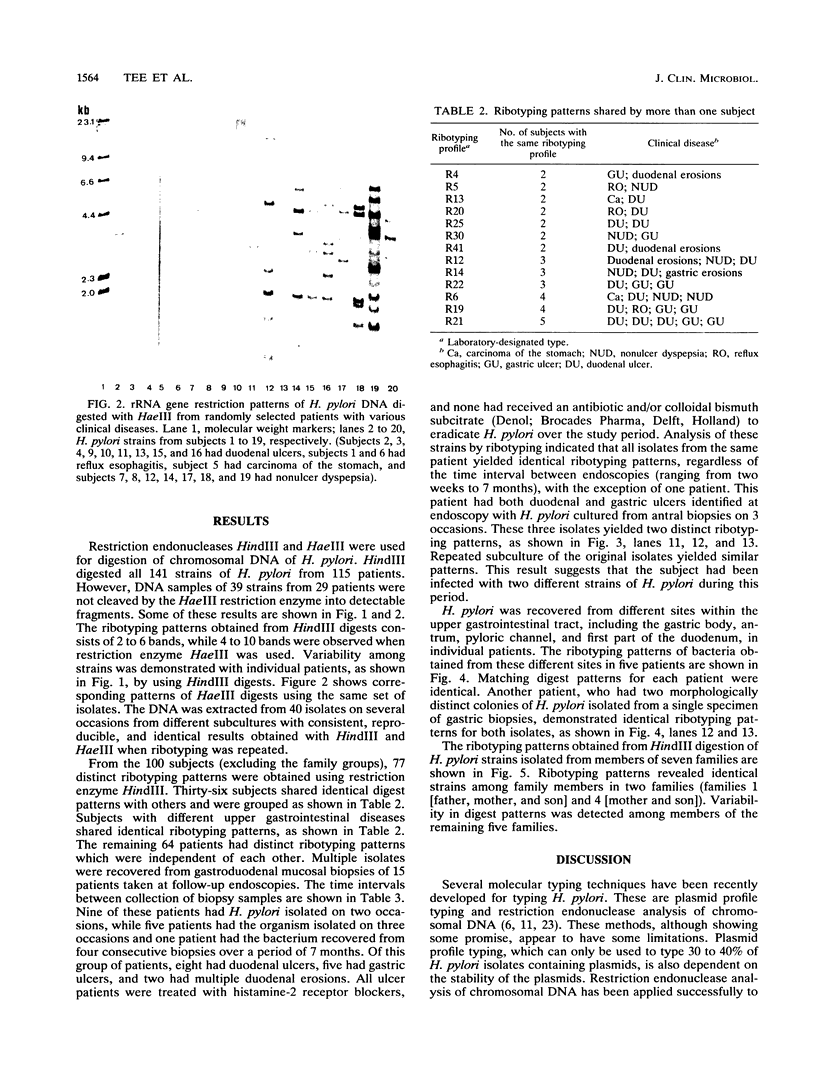
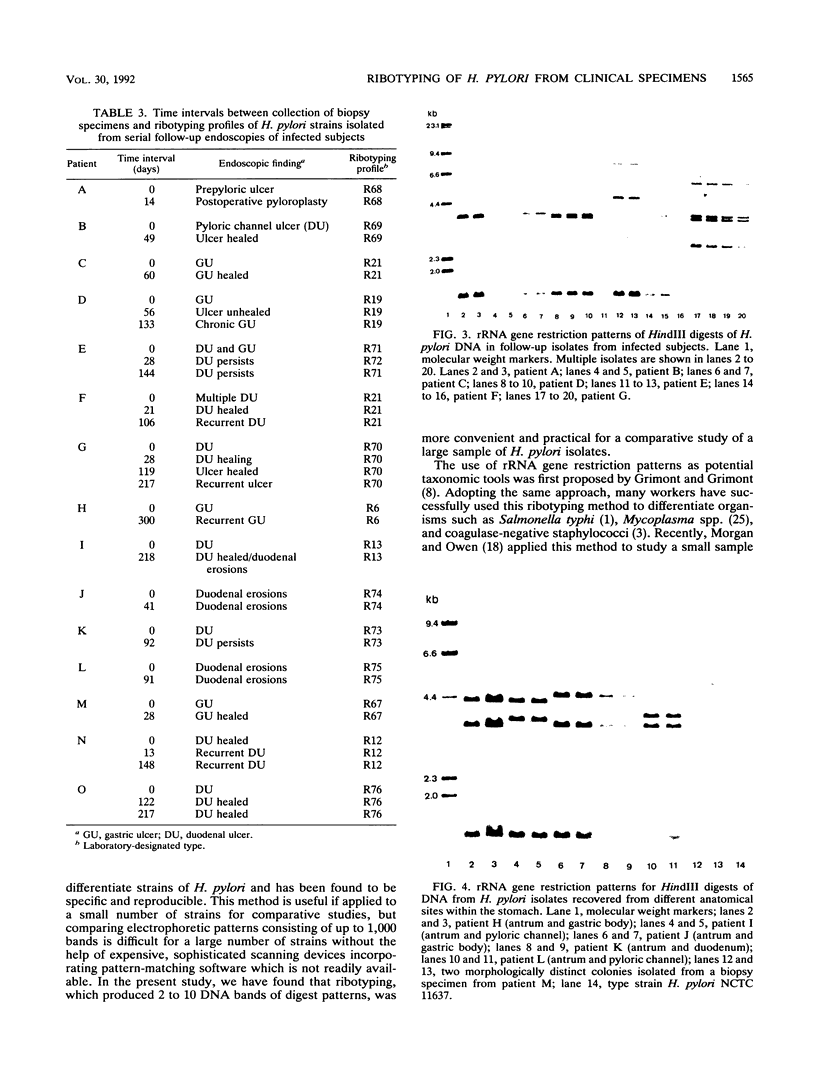
Ribotyping is a method used to type strains of bacteria by analyzing the restriction enzyme digestion patterns of the rRNA genes. This method was applied to 126 strains of Helicobacter pylori from 100 unrelated symptomatic patients who had endoscopies done and to 15 strains from 15 infected subjects from seven families. Analysis of the rRNA gene patterns revealed 77 distinct ribotypes from the 100 patients. From 15 of these subjects, isolates were recovered from antral mucosal biopsies at follow-up endoscopy. All follow-up isolates from the same patient, with one exception, yielded identical digest patterns. This patient had strains with two distinct digest patterns obtained from a set of three isolates cultured from biopsy specimens taken at different times. Five patients who had isolates recovered from different sites in the stomach (antrum, gastric body, duodenum, and pyloric channel) showed ribotyping patterns which were identical for each patient yet distinct between patients. In seven family groups studied, identical digest patterns were detected in members of two families, with variability in strains detected among members of the remaining families. This study demonstrates that ribotyping provides a useful, reliable, reproducible, and highly discriminatory typing scheme for the study of H. pylori infection.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altwegg M., Hickman-Brenner F. W., Farmer J. J., 3rd Ribosomal RNA gene restriction patterns provide increased sensitivity for typing Salmonella typhi strains. J Infect Dis. 1989 Jul;160(1):145–149. doi: 10.1093/infdis/160.1.145. [DOI] [PubMed] [Google Scholar]
- Bialkowska-Hobrzanska H., Harry V., Jaskot D., Hammerberg O. Typing of coagulase-negative staphylococci by Southern hybridization of chromosomal DNA fingerprints using a ribosomal RNA probe. Eur J Clin Microbiol Infect Dis. 1990 Aug;9(8):588–594. doi: 10.1007/BF01967213. [DOI] [PubMed] [Google Scholar]
- Burnie J. P., Lee W., Dent J. C., McNulty C. A. Immunoblot fingerprinting of Campylobacter pylori. J Med Microbiol. 1988 Oct;27(2):153–159. doi: 10.1099/00222615-27-2-153. [DOI] [PubMed] [Google Scholar]
- Drumm B., Perez-Perez G. I., Blaser M. J., Sherman P. M. Intrafamilial clustering of Helicobacter pylori infection. N Engl J Med. 1990 Feb 8;322(6):359–363. doi: 10.1056/NEJM199002083220603. [DOI] [PubMed] [Google Scholar]
- Dworkin B. M., Chodos J. E., Fernandez M. E., Van Horn K., Cabello F., Wormser G. P. Use of plasmid profiles in the investigation of a patient with Helicobacter pylori infection and peptic ulcer disease. Am J Gastroenterol. 1991 Mar;86(3):354–356. [PubMed] [Google Scholar]
- Graham D. Y. Campylobacter pylori and peptic ulcer disease. Gastroenterology. 1989 Feb;96(2 Pt 2 Suppl):615–625. doi: 10.1016/s0016-5085(89)80057-5. [DOI] [PubMed] [Google Scholar]
- Grimont F., Grimont P. A. Ribosomal ribonucleic acid gene restriction patterns as potential taxonomic tools. Ann Inst Pasteur Microbiol. 1986 Sep-Oct;137B(2):165–175. doi: 10.1016/s0769-2609(86)80105-3. [DOI] [PubMed] [Google Scholar]
- Hornick R. B. Campylobacter pylori: its role in gastritis and peptic ulcer disease. Curr Clin Top Infect Dis. 1989;10:157–173. [PubMed] [Google Scholar]
- Langenberg W., Rauws E. A., Widjojokusumo A., Tytgat G. N., Zanen H. C. Identification of Campylobacter pyloridis isolates by restriction endonuclease DNA analysis. J Clin Microbiol. 1986 Sep;24(3):414–417. doi: 10.1128/jcm.24.3.414-417.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddocks A. C. Helicobacter pylori (formerly Campylobacter pyloridis/pylori) 1986-1989: a review. J Clin Pathol. 1990 May;43(5):353–356. doi: 10.1136/jcp.43.5.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewski S. I., Goodwin C. S. Restriction endonuclease analysis of the genome of Campylobacter pylori with a rapid extraction method: evidence for considerable genomic variation. J Infect Dis. 1988 Mar;157(3):465–471. doi: 10.1093/infdis/157.3.465. [DOI] [PubMed] [Google Scholar]
- Marshall B. J. Campylobacter pylori: its link to gastritis and peptic ulcer disease. Rev Infect Dis. 1990 Jan-Feb;12 (Suppl 1):S87–S93. doi: 10.1093/clinids/12.supplement_1.s87. [DOI] [PubMed] [Google Scholar]
- Mitchell H. M., Bohane T. D., Berkowicz J., Hazell S. L., Lee A. Antibody to Campylobacter pylori in families of index children with gastrointestinal illness due to C pylori. Lancet. 1987 Sep 19;2(8560):681–682. doi: 10.1016/s0140-6736(87)92459-7. [DOI] [PubMed] [Google Scholar]
- Mitchell H. M., Lee A., Carrick J. Increased incidence of Campylobacter pylori infection in gastroenterologists: further evidence to support person-to-person transmission of C. pylori. Scand J Gastroenterol. 1989 May;24(4):396–400. doi: 10.3109/00365528909093065. [DOI] [PubMed] [Google Scholar]
- Morgan D. D., Owen R. J. Use of DNA restriction endonuclease digest and ribosomal RNA gene probe patterns to fingerprint Helicobacter pylori and Helicobacter mustelae isolated from human and animal hosts. Mol Cell Probes. 1990 Aug;4(4):321–334. doi: 10.1016/0890-8508(90)90023-s. [DOI] [PubMed] [Google Scholar]
- Nomura A., Stemmermann G. N., Chyou P. H., Kato I., Perez-Perez G. I., Blaser M. J. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991 Oct 17;325(16):1132–1136. doi: 10.1056/NEJM199110173251604. [DOI] [PubMed] [Google Scholar]
- Parsonnet J., Friedman G. D., Vandersteen D. P., Chang Y., Vogelman J. H., Orentreich N., Sibley R. K. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991 Oct 17;325(16):1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- Rauws E. A., Langenberg W., Houthoff H. J., Zanen H. C., Tytgat G. N. Campylobacter pyloridis-associated chronic active antral gastritis. A prospective study of its prevalence and the effects of antibacterial and antiulcer treatment. Gastroenterology. 1988 Jan;94(1):33–40. [PubMed] [Google Scholar]
- Simor A. E., Shames B., Drumm B., Sherman P., Low D. E., Penner J. L. Typing of Campylobacter pylori by bacterial DNA restriction endonuclease analysis and determination of plasmid profile. J Clin Microbiol. 1990 Jan;28(1):83–86. doi: 10.1128/jcm.28.1.83-86.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tytgat G. N., Rauws E. A. Campylobacter pylori and its role in peptic ulcer disease. Gastroenterol Clin North Am. 1990 Mar;19(1):183–196. [PubMed] [Google Scholar]
- Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983 Jun 4;1(8336):1273–1275. [PubMed] [Google Scholar]
- Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983 Jun 4;1(8336):1273–1275. [PubMed] [Google Scholar]
- Yogev D., Halachmi D., Kenny G. E., Razin S. Distinction of species and strains of mycoplasmas (mollicutes) by genomic DNA fingerprints with an rRNA gene probe. J Clin Microbiol. 1988 Jun;26(6):1198–1201. doi: 10.1128/jcm.26.6.1198-1201.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]