Abstract
Some patients with ascites due to liver cirrhosis become no longer responsive to diuretics. Once other causes of ascites such as portal vein thrombosis, malignancy or infection and non-compliance with medications and low sodium diet have been excluded, the diagnosis of refractory ascites can be made based on strict criteria. Patients with refractory ascites have very poor prognosis and therefore referral for consideration for liver transplantation should be initiated. Search for reversible components of the underlying liver pathology should be undertaken and targeted therapy, when available, should be considered. Currently, serial large volume paracentesis (LVP) and transjugular intrahepatic portasystemic stent-shunt (TIPS) are the two mainstay treatment options for refractory ascites. Other treatment options are available but not widely used either because they carry high morbidity and mortality (most surgical options) rates, or are new interventions that have shown promise but still need further evaluation. In this comprehensive review, we describe the evaluation and management of patients with refractory ascites from the prospective of the practicing physician.
Keywords: Refractory ascites, Aquaretics, Albumin infusion, Transjugular intrahepatic portosystemic stent-shunt, Large volume paracentesis
INTRODUCTION
Ascites means pathological fluid accumulation within the abdominal cavity. The word “ascites” itself is derived from the Greek word “askos,” which means a bag or sack[1]. Cirrhosis accounts for over 75% of patients who present with ascites[2]. Ascites is the most common of the three major complications of cirrhosis; the other complications are hepatic encephalopathy and variceal hemorrhage[3]. Approximately 50%-60% of patients with compensated cirrhosis will develop ascites during 10 years of observation[3,4].
Patients with ascites can be divided into the following categories based on their response to treatment. (1) Less than 10% have natural sodium excretion (i.e. without diuretics) more than 78 meq/d. These patients, have relatively preserved liver functions and will respond to dietary salt restriction [88 meq (2000 mg) per day] alone[5]. (2) As liver function deteriorates, patients excrete less sodium in the urine and sodium restriction alone is no longer enough to create a negative sodium balance and control ascites[6]. Most patients will need diuretics combined with sodium-restricted diet[7]. This regimen is effective in about 90% of the patients[8]. Over time, up to 20% of patients that were initially diuretic-responsive will become diuretic-resistant[9]. (3) 5%-10% of patients never respond to this regimen and have refractory ascites[4,10].
Development of ascites is associated with a poor quality of life, increased risks of infections and renal failure, and a poor long-term outcome[11]. Furthermore, patients with refractory ascites have worse prognosis and shortened survival.
Cirrhotic patients who develop ascites have a probability of survival of 85% at 1 year and 56% at 5 years without liver transplantation[12]. In patients who become resistant to diuretic therapy, the prognosis decreases to 50% survival at 2 years[13].
Patients with refractory ascites have lower sodium excretion compared to sensitive patients. It has been shown that patients with ascites and urinary sodium excretion below 10 meq/d had a mean survival rate of 5-6 mo compared to > 2 years in those with ascites and a higher rate of sodium excretion[14].
DEFINITIONS
For the correct diagnosis of true refractory ascites, the patient’s condition should fulfill the following criteria[2,15].
Diuretic-resistant ascites
Failure of mobilization or the early recurrence of ascites which cannot be prevented because of a lack of response to sodium restriction and diuretic treatment is called diuretic-resistant ascites.
Diuretic-intractable ascites
Failure of mobilization or the early recurrence of ascites which cannot be prevented because of the development of diuretic-induced complications that prevent the use of an effective diuretic dosage is called diuretic-intractable ascites.
Treatment duration
Patients must be on intensive diuretic therapy (spironolactone 400 mg/d and furosemide 160 mg/d) for at least 1 wk and on a salt-restricted diet of less than 90 mmol/d.
Lack of response
Mean weight loss of less than 0.8 kg over 4 d and urinary sodium output less than the sodium intake.
Early ascites recurrence
There is an reappearance of grade 2 or 3 ascites (clinically detectable) within 4 wk of initial mobilization. However, it is important to notice that in patients with severe peripheral edema, reaccumulation of ascites within 2-3 d of paracentesis must not be considered as early ascites recurrence because it represents a shift of interstitial fluid to the intraperitoneal space[16].
Diuretic-induced complications
Diuretic-induced hepatic encephalopathy is the development of encephalopathy in the absence of any other precipitating factor. Diuretic-induced renal impairment is indicated by an increase of serum creatinine by > 100% to a value of > 2 mg/dL in patients with ascites otherwise responding to treatment. Diuretic-induced hyponatremia is defined as a decrease of serum sodium by > 10 mEq/L to a serum sodium of < 125 mEq/L. Diuretic-induced hypo- or hyperkalemia is defined as a change in serum potassium to < 3 mEq/L or > 6 mEq/L despite appropriate measures.
In addition to this, we should exclude dietary non-compliance (patient taking excess sodium in diet) and exclude the use of nonsteroidal antiinflammatory drugs (NSAIDs), which can induce renal vasoconstriction and diminish diuretic responsiveness[17,18].
EVALUATION OF PATIENT WITH REFRACTORY ASCITES
The aim of the work-up is to confirm the diagnosis and exclude other conditions that can be misdiagnosed as refractory ascites.
Exclude causes of ascites other than cirrhosis that are not responsive to diuretic therapy. This includes malignant ascites due to peritoneal carcinomatosis (but not due to massive hepatic metastasis)[19] and nephrogenic ascites, which develop in patients with end-stage renal disease[20]. This is important because about 5% of patients with ascites have more than one underlying etiology (mixed ascites)[21]; for example, a patient may have cirrhosis in addition to peritoneal carcinomatosis and be misdiagnosed as having true refractory ascites. The importance of this is that lines of therapy are different.
Patient should have ultrasound with portal vein Doppler and serum alpha fetoprotein level to exclude the presence of hepatocellular carcinoma or portal vein thrombosis[4], because these conditions are associated with lack of response to diuretics in patients with cirrhosis, while true refractory ascites represents actual progression of the liver disease[3] (discussed below).
Confirm compliance to dietary sodium restriction because patient may not be responding to diuretics simply because of the lack of dietary compliance. Therefore, the diagnosis of refractory ascites is not complete until it is proven that the patient has low urinary sodium excretion on the diuretic doses mentioned before[17]. This can be done through the following.
Twenty-four-hour urinary sodium: Patients who gain weight despite excreting more than 78 meq sodium per day are not compliant with the diet. The value of 78 meq sodium per day is derived from the difference between sodium intake (2 gm/d = 88 mEq) and non-urinary loss (10 mEq/d)[22]. The drawback of the 24-h urinary collections is that they are labor-intensive for patients and staff alike. Verbal and written instructions should be given to the patient in order to assure accurate collection. Completeness of collection can be assessed by measurement of urinary creatinine. Urinary creatinine excretion per day should be more than 15 mg of creatinine per kg of body weight for men and more than 10 mg/kg for women[23]. Samples with less creatinine indicate incomplete collection that may affect the results. However, this may not be very accurate because patients with advanced cirrhosis have muscle wasting and therefore lower creatinine excretion in urine even with complete collection[24,25].
Sodium in spot urine specimen: Measuring sodium in a spot urine specimen should be easier and more convenient for the patient but lack of accuracy is the problem as excretion of sodium is not uniform throughout the day. Random urinary sodium concentrations are of value when they are 0 mmol/L (meaning low sodium excretion and lack of diuretic response) or greater than 100 mmol/L (means either adequate response to diuretics or diet non compliance) but are not helpful when they are intermediate[22].
Random urinary Na/K ratio: Random urinary Na/K ratio may be as helpful as 24-h urinary sodium collection, with accuracy rates of 86% according to one study and 90% according to another. A ratio of more than 1 is equivalent to 24 h sodium more than 78 mmol Na/d. This test is easier for the patient as it does not involve collection of 24-h urine.
Furosemide-induced natriuresis: Furosemide-induced natriuresis is another alternative where a single intravenous 80-mg dose of furosemide is given and urinary sodium is measured in the next 8 h. Patients with diuretic-resistant ascites have sodium excretion less than 50 mEq/8 h[26,27]. Another advantage of this test is that it allows more rapid identification of diuretic-resistant patients without the need to follow them up for weeks with increasing doses of diuretics.
PATHOGENESIS OF ASCITES
Currently, the most widely accepted theory of ascites formation is the forward theory which is based on the peripheral arterial vasodilation hypothesis of renal dysfunction in cirrhosis[28]. According to this theory, the initial step is the development of sinusoidal portal hypertension[29,30]. This leads to systemic vasodilation and reduction in systemic vascular resistance, which is most evident in splanchnic blood vessels[31,32]. Portal hypertension causes vasodilation through increased release of local vasodilators such as nitric oxide (which seems to be the primary mediator[33]), glucagon[34], prostacyclins[35], vasoactive intestinal peptide, substance P and platelet activating factor[29]. Splanchnic vasodilation leads to a forward increase in filtration across splanchnic capillaries[36]. In patients with decompensated cirrhosis, the lymphatic system is not capable of returning back all the filtered fluid and causes accumulation of ascites fluid[37]. Besides this, systemic vasodilation causes systemic vascular underfilling, which stimulates the sodium-retaining neurohumoral mechanisms in order to refill the dilated vascular bed; these mechanisms include mainly the renin-angiotensin-aldosterone system, sympathetic nervous system, and antidiuretic hormone[38]. This leads to sodium retention, water retention (with dilutional hyonatremia) and renal vasoconstriction, which may later lead to hepatorenal syndrome[28]. This causes retention of more fluid, which is not effective in filling the systemic vascular bed because of the continuous leakage into the peritoneal cavity leading to more ascites formation[28]. These changes become more severe with the progression of liver disease, which is why the degree of sodium retention (measured as urinary sodium excretion)[14] and hyponatremia[39,40] correlate with worsening survival in cirrhotic patients.
Patients with more advanced cirrhosis have a marked degree of circulatory dysfunction and marked neurohumoral activation. This results in renal vasoconstriction and enhanced sodium reabsorption in the renal tubule and very low urinary excretion of sodium (even with high doses of diuretics), which is how refractory ascites develops[17]. Hepatorenal syndrome has a pathogenesis similar to that of ascites[41]; refractory ascites is considered a pre-hepatorenal state and actually refractory ascites is a usual manifestation of type 2-hepatorenal syndrome[4].
TREATMENT OF REFRACTORY ASCITES
The ideal treatment of ascites should be effective in mobilization of ascites and prevention of recurrence, should improves patient’s quality of life and survival, and should be acting directly on one or more steps in the pathogenesis of ascites and not just the mechanical removal of the fluid[42].
Currently, the main lines of treatment for refractory ascites are serial large volume paracentesis (LVP), transjugular intrahepatic portasystemic stent-shunt (TIPS), liver transplantation and peritoneovenous shunt[22]. We will also discuss promising new therapies that are currently being evaluated.
LVP
LVP with administration of intravenous albumin represents the standard therapy for refractory ascites[43]. Several studies have shown its effectiveness and safety[44–46]. Beside rapid control of ascites, it may decrease the risk of variceal bleeding because it is associated with reductions in the hepatic venous pressure gradient and intravariceal pressure[47,48].
Frequency of LVP: Therapeutic paracentesis is a local therapy that does not modify the mechanisms that lead to ascites formation. Therefore ascites will always recur in patients with refractory ascites unless there is an improvement in liver disease, as in alcoholic liver disease when patients stop drinking, or after liver transplantation[49,50]. Two weeks are considered a reasonable interval between paracentesis sessions in patients with refractory ascites[22,51]. Less frequent sessions are needed in the patient with some sodium excretion and more frequent sessions are required in patients who are not compliant with dietary sodium restriction. The explanation requires knowing some details related to sodium balance in patients with ascites. The sodium concentration of ascitic fluid is approximately equivalent to that of plasma in these patients: 130 mmol/L. A 10-L paracentesis removes 1300 mmol (130 × 10). If the patient is adherent to the diet, he/she will consume 88 mmol sodium every day, and excrete 10 mmol/d in non-urinary loss and excrete nothing in the urine if there is no urinary sodium excretion at all. Therefore, the net gain every day will be 78 mmol. Therefore, a 6-L paracentesis removes 10 d (780 mmol/78 mmol per day) of retained sodium, and a 10-L paracentesis removes approximately 17 d of retained sodium (1300 mmol/78 mmol per day = 16.7 d) in patients with no urinary sodium excretion[22].
Patients who are not compliant need education regarding their diet rather than more frequent LVP sessions. This is important because although the patients are no longer responding to diuretics, diet is still very important. One should not think about a more restricted diet as a solution for diuretic resistance, as it is not more effective and makes food less palatable therefore, malnutrition may result[52]. Fluid restriction is indicated in patients with ascites and serum sodium lower than 130 mEq/L[53].
At the time of LVP measurement of the white cell count with differential should be performed on the acidic fluid sample as a screening for spontaneous bacterial peritonitis even if the patient is asymptomatic, while if symptomatic, cultures should be added[50].
Most authors prefer total LVP than repeated LVP (removing 4-6 L daily until ascites completely disappears) because it is faster and can be done as an outpatient procedure; also, it is associated with lower incidences of complications that may be related to needle insertion and associated with no fluid leakage after paracentesis because no fluid stays in the abdominal cavity[38]. Another measure to reduce leakage is using the ‘‘Z’’ track where skin is penetrated perpendicularly, then the needle is advanced obliquely in subcutaneous tissue before the peritoneal cavity is punctured, so that the puncture site on the skin and the peritoneum are not overlying. Also asking the patient to recline for 2 h on the side opposite to the paracentesis site will prevent the leakage of ascitic fluid. If there is significant leakage that is not controlled with these measures, a suture or purse string may be inserted around the site of drainage[54].
Complication associated with LVP: It is considered a safe procedure associated with very low incidence of serious complications even in patients with coagulopathy[55,56]. The risk of developing a large hematoma is about 1% and the risk of hemoperitoneum or iatrogenic infection is only about 1 per 1000[57]. There is no evidence in clinical trials that transfusion of plasma or platelets before the procedure decreases the risk of bleeding[58]. However, one should avoid puncture of the visible dilated abdominal wall veins in order to avoid severe bleeding. Also, there is no coagulation profile cut-off value that paracentesis should be avoided beyond it. According to one study, patients tolerated the procedures with INR up to 8.7 and platelet counts as low as 19 000[59]. It may be that the only condition when the procedure should be avoided due to high bleeding risk is the presence of disseminated intravascular coagulation with clinically evident fibrinolysis[60].
One problem with repeated LVPs is ascitic fluid protein and complement depletion, which may predispose to ascitic fluid infections[61], in comparison with diuretic therapy, but this is of special concern in diuretic-sensitive patients while in refractory ascites, diuretics are no longer an option.
Another problem with large volume paracentesis is post-paracentesis circulatory dysfunction (PCD). Circulatory changes after LVP can be described as follows. (1) Immediately after paracentesis, there is an improvement in circulatory function in regard to increased cardiac output and suppression of the renin-angiotensin and sympathetic nervous systems. This effect is mostly due to mechanical factors that mainly increase venous return due to reduced intraabdominal pressure[62]. (2) After about 12 h, there are opposite hemodynamic changes, including a reduction in cardiac output to baseline values and marked activation of the renin-angiotensin and sympathetic nervous systems over the levels before paracentesis[63]. These changes are not spontaneously reversed as once plasma renin activity and plasma norepinephrine concentration increase, this elevation persists[38]. PCD has been defined as a 50% increase in plasma renin activity over baseline on the sixth day after treatment, up to a value greater than 4 ng/mL per hour[38,62,64]. Despite being asymptomatic, PCD adversely affects the clinical course of the disease with higher incidences of hyponatremia, and renal impairment. In patients who develop PCD, it is severity correlates inversely with patient survival[65]. Severity of the circulatory dysfunction correlates with the amount of fluid removed in paracentesis being most significant when it exceeds 5L[65].
A number of measures can be applied to prevent PCD.
Albumin infusion: Albumin infusion has been studied for the prevention of PCD[45,66]. Incidence of PCD following LVP reaches 80% when albumin is not used and albumin infusion reduces the incidence to 15%-20%[45]. However, some controversy still exists related to albumin infusion. The reasons behind this are: lack of direct survival advantage with albumin infusion[67]; albumin is very expensive and some studies state that albumin infusion inhibits synthesis of albumin[58] and stimulates albumin degradation inside the body[68,69]. Another reason for the controversy is that the circulatory changes that can follow LVP may not be related to a decreased intravascular volume due to rapid accumulation of ascitic fluid as was thought before[70], but it is actually due to accentuation of the arterial vasodilatation already present in these patients[38].
The current American Association for the Study of Liver Diseases (AASLD) guidelines state that post-paracentesis albumin infusion may not be necessary for a single paracentesis of less than 4-5 L. For LVP, an albumin infusion of 8-10 g per liter of fluid removed can be considered[22].
Albumin should be given once the session is completed[54]. Some authors recommend giving one half of the plasma expander immediately after the paracentesis and the other half 6 h later[65,71]. Others say this is unwarranted and converts an otherwise simple outpatient procedure into an all-day clinic visit[67].
It has been suggested that reducing the flow rate of ascites extraction may help prevent PCD[72], however, this may need further evaluation before being applied in practice.
Other alternatives to albumin for prevention of PCD: Many trials were carried out to find less expensive alternatives to albumin therapy, however none is accepted currently to replace albumin. Some of the alternatives to albumin infusion include synthetic colloids, extracorporeal ultrafiltration and reinfusion, and vasoconstrictors.
(I) Synthetic colloids. Studies comparing replacement of albumin with dextran 70 or polygeline showed no survival advantage[71] and PCD was much less common with albumin administration in patients where >= 5 L of fluid were removed[65]. Saline also has been tried but without showing any survival advantage[73]. This is mostly related to the half-life of the colloid used, being highest with albumin (21 d), this may explain its effectiveness in prevention of PCD[74]. Some authors state that paracentesis of < 5 L can be followed by synthetic plasma expander and albumin is not required in this setting[54].
One important point is that hydroxyethyl starch can increase portal pressure as it fills Kupffer cells (lysosomal storage) and this may increase the risk of variceal bleeding[75].
(II) Extracorporeal ultrafiltration and reinfusion. This procedure involves ultrafiltration of ascitic fluid and intraperitoneal or intravascular reperfusion[76]. Advantages compared to albumin are the reduced expenses and avoidance of depletion of complement lost with paracentesis[77]. The main problem reported is the development of disseminated intravascular coagulation (DIC) in some patients, and this may be why it is not approved for use now; however, one simplified method is suggested that limits the incidence of DIC[78]. One point is that only a few patients have been studied until now therefore, better evaluation with larger studies is needed.
(III) Vasoconstrictors. Administration of vasoconstrictors may decrease the development of PCD and may prevent complications associated with a decrease in effective arterial blood volume as with the plasma volume expander albumin[64]. This may be due to the fact mentioned before, as the pathogenesis of PCD is accentuation of splanchnic vasodilation rather than depletion of intravascular volume.
Terlipressin: More than one study showed that terlipressin may be as effective as intravenous albumin and well tolerated in preventing paracentesis-induced circulatory dysfunction in patients with cirrhosis after therapeutic paracentesis[79–82]. One study recommended a dose of terlipressin (1 mg every 4 h for 48 h)[79], while another study suggested that a total dose of 3 mg terlipressin should be administered as an intravenous bolus of 1 mg terlipressin at the onset of paracentesis and then 8 and 16 h after the first bolus[80]. A problem with using terlipressin is that it requires hospital admission for a simple outpatient procedure, as it is given as intravenous injections for up to 48 h after the procedure.
Midodrine: One study carried out on 40 patients showed that midodrine may be as effective as albumin[83], while another one carried out on 24 patients showed that PCD developed in six patients of the midodrine group (60%) and in only four patients (31%) of the albumin group[64]. The dose given was 12.5 mg every 8 h post-paracentesis for 2 d. Being much cheaper than albumin and terlipressin and much easier to administer, midodrine may be worth more trials to assess its use instead of albumin.
Noradrenaline: Another study showed noradrenaline to be as effective as albumin in the prevention of PCD[84]. Noradrenaline was suggested as a less expensive alternative to albumin but no further studies were done to confirm this.
TIPS
TIPS is a side-to-side portacaval shunt by which an intrahepatic communication between the portal and the hepatic vein is created[85]. It is a non-surgical procedure performed under local anesthesia by an interventional radiologist. A catheter is advanced through the jugular vein into a hepatic vein and into a main branch of the portal vein. There an expandable stent is introduced connecting hepatic and portal systems, which allows shunting of blood from the high-pressure portal circulation (splanchnic and sinusoidal beds) to the low-pressure systemic circulation (hepatic vein)[42].
The mechanism by which TIPS helps control ascites is decompression of the portal circulation and reduction in the portacaval gradient and the portal venous pressure[38]. As mentioned before, portal hypertension is essential in ascites formation so that cirrhotic patients with portal venous pressure less than 12 mm Hg do not develop ascites[29,30], and ascites in these patients disappears if portal venous pressure drops below
12 mm Hg[86,87]. Another mechanism is that the blood volume pooled in the dilated splanchnic vascular bed is transferred to the systemic circulation through the shunt, therefore, it corrects the systemic vascular underfilling and causes a decrease in the renin-angiotensin-aldosterone system and thereby improves renal sodium excretion[42,88].
Several studies showed that TIPS is highly effective in controlling ascites[54]. According to these studies, ascites was controlled in 27%-92%[89,90], with 75% of cases showing complete resolution[91]. It takes about 1 -3 mo for ascites to resolve after TIPS procedure[38]. One important point is that diuretic therapy will still be required in about 95% of patients. The explanation is that TIPS produces partial resolution of ascites pathogenesis, so portal pressure and renin and aldosterone levels, although they are markedly reduced after TIPS, they are not back to normal as in healthy subjects[38].
In patients with cirrhosis, TIPS may have some advantages beside ascites control. Improvement in renal function is seen in these patients in the form of increased urine volume, increased sodium excretion[91,92] and even a reduction in serum creatinine level which is a delayed effect seen after 6 mo according to one study[93]. Another advantage is the improvement in the nutritional status (in the form of an increase in dry weight and total body nitrogen)[88,94] and improvements in quality of life[93]; however, these effects (nutrition and quality of life) may be simply due to improved eating when ascites is controlled[54].
Complications associated with TIPS: (1) Technical complications. Estimated technical success rate is reported in the range of 93%-100%[90,91]. Procedure-related mortality is very low (1%-2%) according to one study[95], and it was due to hemoperitoneum, hemobilia, hemolysis, and sepsis. The procedure-related complication rate is around 9%, with intraperitoneal hemorrhage and acute renal failure (mostly due to contrast media) being the most frequent[42]. Complications also include those of sedation and arrhythmia if the catheter enters the right atrium or right ventricle[96], and transient right bundle branch block, which may be significant in patients already with left bundle branch block as it may lead to complete heart block. The liver capsule is frequently punctured (reported frequency around 33%[97]), especially if the liver is shrunken but intraperitoneal bleed only occurs in 1%-2% of the cases[98]. (2) Hepatic encephalopathy occurs in about 30% of patients after TIPS[99,100]. Factors associated with the development of encephalopathy that can be used for patient selection are increasing age, advanced liver failure, and a history of encephalopathy before TIPS insertion[101,102]. Encephalopathy usually becomes clinically apparent 2-3 wk after TIPS insertion[99]. According to one study[100], encephalopathy starts to develop about 10 d after insertion, and then begins to decline (as measured by the portosystemic encephalopathy index) at 6 mo, however, it remained significantly higher than the baseline values. A possible explanation for this decline may be shunt stenosis with time. Treatment is medical in most of the cases and consists of controlling any precipitating factor, lactulose and non-absorbable antibiotics (neomycin or rifaximin)[100]. In case of medical therapy failure, the TIPS can be occluded[103] or the diameter of the shunt can be narrowed in some types with a “wasp waist” constrictor[104]. (3) Shunt occlusion. These problems occur in 22% to 50 % of patients[91,105,106]. This occurs due to growth of collagenous fibrils and endothelial cells (pseudointima) inside the stent[107]. It can diffuse all over the whole length (type 1) or localized to the hepatic venous end (type 2); however, both have the same management[108]. The incidence of this complication increases with time, according to one study, all patients surviving more than 2 years had shunt stenosis[108]. Shunt stenosis or occlusion presents as a recurrence of portal hypertension or variceal bleeding[109]. TIPS patency should be followed up after insertion, however, the best strategy for follow-up is not yet defined. Methods used to monitor patency include venography (which is the best test but not usually used because is invasive and caries a high cost), but Doppler sonography is the most frequently used (although it is less sensitive than venography)[110]. Helical CT angiography also can be used; one study showed a 92% correlation with venography[111]. One recommended approach for surveillance is duplex ultrasonography every 3 mo and venography annually. Venography can be done earlier if shunt obstruction is suspected clinically[110]. Treatment is redilation of the shunt done by interventional radiology[105]. New stents covered with polytetrafluoroethylene (Goretex) showed lower rates of occlusion and stenosis[112,113]. Using antiplatelet therapy was evaluated in the prevention of shunt stenosis[114] and it showed some efficacy, but it is not used in practice because of the increased risk of bleeding, as those patients already have bleeding tendencies and thrombocytopenia. (4) Haemolysis occurs in about 10% of patients and it is believed to be due to direct mechanical trauma to the red blood cells when they pass through the metallic stent[115]. This may be why spontaneous resolution is seen in most patients after 8-12 wk with covering of the metallic stent by pseudointima[116]. In most patients, the anemia is mild with less than 2 g/dL reduction in hemoglobin level starting 1-2 wk after placement. Blood smears may show schistocytes in patients who develop severe anemia[115]. (5) Infection. According to one study, infection occurred weeks to months after placement and presented with fever, continuous bacteremia and presence of vegetations or thrombi in the stent. It was treated with intravenous antibiotics[117]. (6) Portosystemic myelopathy (PSM, also called shunt myelopathy) is a rare syndrome that includes spastic paraparesis with intact sensation occurring in patients with surgical portosytemic shunts and also described after TIPS placement[118,119]. A possible explanation is accumulation of ammoniacal substances (that bypass the liver through the stent), leading to loss of motor neurons in the spinal cord. According to one study[118] carried out on 212 patients, four patients (1.89%) had this progressive spastic paresis starting to appear between 5 wk and 5 mo after stent placement. (7) Deterioration of cardiac function. TIPS increases the cardiac preload, and hence it may precipitate heart failure in those with pre-existing heart disease[120]. Echocardiography is usually done before the procedure to exclude patients with subtle heart failure; usually, patients with ejection fraction less than 60 are excluded.
TIPS versus paracentesis
Several studies have compared TIPS to repeated LVP plus albumin, but the detailed discussion of these studies is beyond the scope of this article. However, the conclusion from these studies is: (1) TIPS controls ascites effectively and is associated with a lower rate of ascites recurrence[106,121–123]; (2) patients with ascites who undergo TIPS improve their nutritional status as mentioned before; (3) there is a higher incidence of side effects, mainly hepatic encephalopathy and shunt dysfunction in the group treated with TIPS; and (4) there is no proven effect for TIPS on survival. In one study, TIPS had no effect on survival[123,124], while others have reported both reduced[106] as well as improved survival[106,121,122] compared with therapeutic paracentesis. For these reasons, we believe repeated LVP plus albumin should be considered the first-line therapy for refractory ascites, and TIPS should be used as a second line of management[15,125,126]. TIPS should be considered in appropriately selected patients who meet the following criteria.
Patients with very rapid recurrence of ascites (those who require paracentesis > 3 times/mo) and preserved liver function [bilirubin < 3 mg/dL, serum sodium level >130 mEq/L, Child-Pugh score < 12, model for end-stage liver disease (MELD) score < 18], aged < 70 years, without hepatic encephalopathy, central hepatocellular carcinoma, or cardiopulmonary disease[15,127].
SURGICAL OPTIONS
Peritoneo-venous shunt is a surgically inserted shunt that drains ascitic fluid from the peritoneal cavity into the internal jugular vein. It has limited indications because there is no survival advantage in addition to frequent complications including bacteremia, small bowel obstruction and volume overload leading to variceal bleeding[10]. The use of the peritoneo-venous shunt is limited to patients with refractory ascites who are not candidate for TIPS or liver transplantation, and has a lot of abdominal scars that makes frequent paracentesis unsafe[22,67]. One study described percutaneous placement of a peritoneovenous shunt by interventional radiology which may carry less complications than surgery however further studies are needed to confirm this[128].
A more simple method of peritoneovenous drainage was described, the sapheno-peritoneal anastomosis[129–131]. Advantages over the ordinary peritoneovenous shunt are simpler and less expensive and use a biological shunt instead of a prosthetic one. Also, one study described the possibility of doing the procedure under local anesthesia[129], which is an advantage in cirrhotic patients. Patients had reduction in admissions and paracentesis, however, no survival advantage was noted.
Portosystemic shunt works, similar to TIPS, through decompression of the portal circulation; however, mortality is higher ranging from 12% to 39% and encephalopathy rates are more than 50%[132].
One study described a technique of peritoneal-urinary drainage of the fluid using a surgically implanted pump[133].
MEASURES THAT MAY IMPROVE THE RESPONSE TO DIURETICS
Several medications have been suggested that attack certain step(s) in the pathogenesis of ascites.
Aquaretics
Aquaretics are vasopressin receptor antagonists that act on the distal tubule of the kidney so as to increase the excretion of solute-free water[15]. They are already approved for management of hyponatremia due to syndrome of inappropriate anti-diuretic hormone secretion (SIADH), and are being evaluated for management of hyponatremia in cirrhosis and in refractory ascites to be combined with diuretics to improve response[134].
Vasopressin receptors are V1a, V1b and V2. The oral forms of aquaretics (e.g. lixivaptan and satavaptan) are selective for V2 receptors, which mediate the antidiuretic response of vasopressin. While, the intravenous forms (conivaptan) works on V2 and V1a receptors and V1a mediates the vasoconstrictor response of vasopressin[135]. Although conivaptan is the only approved one right now (used in SIADH), it cannot be used in ascites as it may cause variceal bleeding when it blocks the vasoconstrictor effect of the anti diuretic hormone[136].
One study carried out in 110 patients with cirrhosis and ascites receiving satavaptan or placebo in addition to diuretics[137]. Those receiving satavaptan had significant decrease in abdominal girth and more weight reduction without significant side effects. However, because of the short follow-up duration in this study (14 d), more studies are needed to evaluate the use of this drug in patients with ascites before they are approved for use in practice.
Vasoconstrictors
Vasoconstrictors may theoretically improve the action of diuretics as they improve the systemic vasodilation, so as to reduce the antinaturetic factors described before[15]. They are already used in hepatorenal syndrome based on a similar mechanism of action[138].
Terlipressin: A potent vasoconstrictor approved for use in the acute control of variceal hemorrhage and hepatorenal syndrome. In one study in 15 patients with cirrhosis and ascites without hepatorenal syndrome[139], eight of them had refractory ascites and received terlipressin. This group had significant decreases in plasma norepinephrine and renin in addition to an increase in urinary sodium.
Octerotide: According to a case report[140], octreotide treatment improved renal function and diuretic response in two patients with refractory ascites. Octreotide administration has been associated with arterial splanchnic vasoconstriction, which is mediated by a reduction in glucagon secretion. In addition, octreotide inhibits the release of renin and aldosterone in both normal humans and cirrhotic patients, possibly through a direct effect on renin-producing cells and adrenals.
Midodrine: One study carried out in 39 cirrhotic patients[141] evaluated the effects of a 7-d treatment with midodrine. It showed a significant increase in mean arterial blood pressure and urine volume and decrease in plasma renin and aldosterone activity in those with ascites treated with midodrine.
One study evaluated the combination of midodrine and octerotide[142], another one evaluated both drugs given with albumen[143].
Clonidine
Several studies evaluated the response of diuretics in cirrhotic patients with ascites[144–147]. It is a centrally acting α2-agonist, therefore, it decreases sympathetic over activity which increases renal sodium reabsorption and stimulates the renin-angiotensin-aldosterone system[148]. One study was carried out in 32 alcoholic cirrhotic patients with ascites to compare the effect of spironolactone, clonidine and the combination of both in control of ascites[147]. After 10 d of spironolactone and clonidine, patients had a significant decrease in plasma renin and aldosterone, decrease in body weight and increase in natriuresis without adverse effects.
Chronic albumin infusion
Some studies showed better diuretic response when combined with albumin; this is noticed as decreased recurrence and shorter hospital admissions[149–151]. One study showed improved survival in patients receiving chronic albumin infusion[152]. Because of cost and lack of definite survival benefit, albumin infusion is not routinely recommended in patients with ascites. Currently, the accepted indications of albumin infusion in liver patients with ascites are with LVP to prevent PCD (discussed before), patients with spontaneous bacterial peritonitis[153,154] and those with hepatorenal syndrome[155,156].
Splenic artery embolization
One case report described the successful control of refractory ascites with splenic artery embolization[157]. This was a 32-year-old female who developed refractory ascites due to portal vein thrombosis after liver transplantation due to Budd Chiari syndrome. With further evaluation, this can be an alternative for patients with refractory ascites who cannot tolerate TIPS or surgical shunts.
Mannitol
In one study[158], a dose of 100 mL 20% mannitol was given as infusion followed by the usual dose of diuretics taken by the patient. Increase in urine volume and urinary sodium was noticed. Therefore, mannitol may be used in refractory ascites to improve response to diuretics.
The measures described above target mainly mobilization of ascites. In addition, as part of comprehensive strategy of managing refractory ascites, one can aim to prevent other complications of cirrhosis and also improve liver function by ether treating the underlying liver disease or liver transplantation. (1) Prevention of other complication of cirrhosis as patients with ascites due to liver cirrhosis are liable to other complications[7]. This includes portal hypertensive bleeding (prevention using either prophylactic banding or propranolol[159]), spontaneous bacterial peritonitis (using prophylactic antibiotics in patients with acute variceal bleeding or ascitic fluid protein less than
1 gm/dL[67]) and hepatorenal syndrome (using albumin infusion in patients with spontaneous bacterial peritonitis[154] and using pentoxyfillin in patients with severe alcoholic hepatitis[160]). (2) Correction of liver function through either liver transplantation or treatment of the underlying liver pathology: some causes of liver cirrhosis have a reversible element; this is most evident in patients with alcoholic liver disease in which stopping alcohol consumption can lead to improvement of portal hypertension and ascites control[161,162]. There is also some evidence of similar improvement in patients with cirrhosis due to hepatitis B (treated with antiviral therapy)[163] and patients with autoimmune hepatitis treated with steroids or azathioprine[164,165].
LIVER TRANSPLANTATION
As mentioned above, patients with ascites have a poor long-term outcome and survival is shortened in those who become refractory to diuretic therapy. The 12-mo survival rate for patients with ascites refractory to medical therapy is only 25%[166]. The survival rate for liver transplantation is much higher[67]. Therefore, those who develop refractory ascites ideally should be on the transplantation list already.
After liver transplantation, portal hypertension reversed immediately and completely; however, ascites disappearance may take 3 to 6 mo[13]. The reason for this is not fully understood, but some studies showed that the systemic vasodilation and hyperdynamic circulation persist for months after transplantation[167,168].
Priority of receiving liver transplant is based upon the MELD score. Some authors suggested that it may not be accurate enough for all patients with ascites, particularly for those with persistent or refractory ascites, who may have a poor prognosis despite low MELD scores[7,15]. One possible explanation is that the MELD score includes serum bilirubin, serum creatinine, and the INR, and some patients with refractory ascites might have a near-normal serum creatinine (as a result of low endogenous production), despite a low glomerular filtration rate and this may affect the accuracy of MELD score in this setting[4].
Therefore, some studies suggested that addition of serum sodium to the MELD score may improve its accuracy[169–171], but one point is that serum sodium is a not as steady as other parameters of the MELD score; it can change rapidly with diuretics or fluid administration, so this change may not reflect an actual change in the prognosis[15]. Further evaluation is needed before modified MELD (with serum sodium added to it) replaces the current MELD score in allocation of liver transplantation.
CONCLUSION
Refractory ascites is a relatively common condition in patients with liver cirrhosis. Wrong diagnosis may occur sometimes therefore, certain criteria should be fulfilled with exclusion of dietary non-compliance, which can be done through a variety of tests. Different treatment options are available, although definitive treatment is liver transplantation. An algorithmic approach to patients with refractory ascites is available (Figure 1). Other treatment options are not listed in the algorithm because they are still being evaluated and include mainly vasoconstrictor agents.
Figure 1.
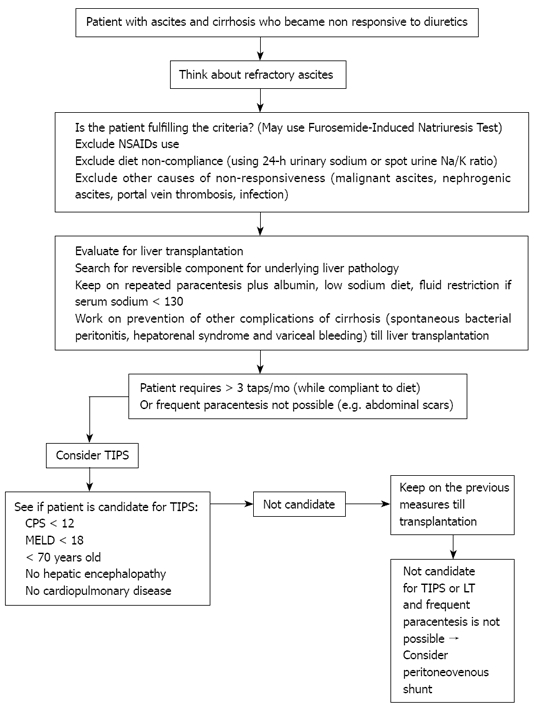
Suggested approach to the patient with refractory ascites. NSAIDs: Non-steroidal anti-inflammatory drugs; LT: Liver transplantation; CPS: Child-pugh score; MELD: Model for end-sage liver disease; TIPS: Transjugular intrahepatic portosystemic shunt.
Peer reviewer: Oliviero Riggio, Professor, Dipartimento di Medicina Clinica, Sapienza Universitá di Roma,Viale dell’ Università 37, 00185 Roma, Italy
S- Editor Tian L L- Editor Alpini GD E- Editor Lin YP
References
- 1.Reynolds TB. Ascites. Clin Liver Dis. 2000;4:151–168, vii. doi: 10.1016/s1089-3261(05)70101-x. [DOI] [PubMed] [Google Scholar]
- 2.Moore KP, Wong F, Gines P, Bernardi M, Ochs A, Salerno F, Angeli P, Porayko M, Moreau R, Garcia-Tsao G, et al. The management of ascites in cirrhosis: report on the consensus conference of the International Ascites Club. Hepatology. 2003;38:258–266. doi: 10.1053/jhep.2003.50315. [DOI] [PubMed] [Google Scholar]
- 3.Gines P, Quintero E, Arroyo V, Teres J, Bruguera M, Rimola A, Caballeria J, Rodes J, Rozman C. Compensated cirrhosis: natural history and prognostic factors. Hepatology. 1987;7:122–128. doi: 10.1002/hep.1840070124. [DOI] [PubMed] [Google Scholar]
- 4.Cardenas A, Gines P. Management of refractory ascites. Clin Gastroenterol Hepatol. 2005;3:1187–1191. doi: 10.1016/s1542-3565(05)00861-x. [DOI] [PubMed] [Google Scholar]
- 5.Wongcharatrawee S, Garcia-Tsao G. Clinical management of ascites and its complications. Clin Liver Dis. 2001;5:833–850. doi: 10.1016/s1089-3261(05)70194-x. [DOI] [PubMed] [Google Scholar]
- 6.Wensing G, Lotterer E, Link I, Hahn EG, Fleig WE. Urinary sodium balance in patients with cirrhosis: relationship to quantitative parameters of liver function. Hepatology. 1997;26:1149–1155. doi: 10.1002/hep.510260510. [DOI] [PubMed] [Google Scholar]
- 7.Gines P, Cardenas A, Arroyo V, Rodes J. Management of cirrhosis and ascites. N Engl J Med. 2004;350:1646–1654. doi: 10.1056/NEJMra035021. [DOI] [PubMed] [Google Scholar]
- 8.Bernardi M, Laffi G, Salvagnini M, Azzena G, Bonato S, Marra F, Trevisani F, Gasbarrini G, Naccarato R, Gentilini P. Efficacy and safety of the stepped care medical treatment of ascites in liver cirrhosis: a randomized controlled clinical trial comparing two diets with different sodium content. Liver. 1993;13:156–162. doi: 10.1111/j.1600-0676.1993.tb00624.x. [DOI] [PubMed] [Google Scholar]
- 9.Arroyo V, Rodes J. A rational approach to the treatment of ascites. Postgrad Med J. 1975;51:558–562. doi: 10.1136/pgmj.51.598.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stanley MM, Ochi S, Lee KK, Nemchausky BA, Greenlee HB, Allen JI, Allen MJ, Baum RA, Gadacz TR, Camara DS. Peritoneovenous shunting as compared with medical treatment in patients with alcoholic cirrhosis and massive ascites. Veterans Administration Cooperative Study on Treatment of Alcoholic Cirrhosis with Ascites. N Engl J Med. 1989;321:1632–1638. doi: 10.1056/NEJM198912143212403. [DOI] [PubMed] [Google Scholar]
- 11.Guevara M, Cardenas A, Uriz J, Gines P. Prognosis of Patients with Cirrhosis and Ascites. In: P Gines, V Arroyo, J Rodes, RW Schrier., editors. Ascites and renal dysfunction in liver disease: pathogenesis, diagnosis, and treatment. Malden, Mass: Blackwell Science; 2005. pp. 260–271. [Google Scholar]
- 12.Planas R, Montoliu S, Balleste B, Rivera M, Miquel M, Masnou H, Galeras JA, Gimenez MD, Santos J, Cirera I, et al. Natural history of patients hospitalized for management of cirrhotic ascites. Clin Gastroenterol Hepatol. 2006;4:1385–1394. doi: 10.1016/j.cgh.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Wong F, Blendis L. The pathophysiologic basis for the treatment of cirrhotic ascites. Clin Liver Dis. 2001;5:819–832. doi: 10.1016/s1089-3261(05)70193-8. [DOI] [PubMed] [Google Scholar]
- 14.Arroyo V, Bosch J, Gaya-Beltran J, Kravetz D, Estrada L, Rivera F, Rodes J. Plasma renin activity and urinary sodium excretion as prognostic indicators in nonazotemic cirrhosis with ascites. Ann Intern Med. 1981;94:198–201. doi: 10.7326/0003-4819-94-2-198. [DOI] [PubMed] [Google Scholar]
- 15.Gines P, Cardenas A. The management of ascites and hyponatremia in cirrhosis. Semin Liver Dis. 2008;28:43–58. doi: 10.1055/s-2008-1040320. [DOI] [PubMed] [Google Scholar]
- 16.Arroyo V, Gines P, Gerbes AL, Dudley FJ, Gentilini P, Laffi G, Reynolds TB, Ring-Larsen H, Scholmerich J. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology. 1996;23:164–176. doi: 10.1002/hep.510230122. [DOI] [PubMed] [Google Scholar]
- 17.Runyon BA. Refractory ascites. Semin Liver Dis. 1993;13:343–351. doi: 10.1055/s-2007-1007362. [DOI] [PubMed] [Google Scholar]
- 18.Wong F, Massie D, Hsu P, Dudley F. Indomethacin-induced renal dysfunction in patients with well-compensated cirrhosis. Gastroenterology. 1993;104:869–876. doi: 10.1016/0016-5085(93)91024-c. [DOI] [PubMed] [Google Scholar]
- 19.Pockros PJ, Esrason KT, Nguyen C, Duque J, Woods S. Mobilization of malignant ascites with diuretics is dependent on ascitic fluid characteristics. Gastroenterology. 1992;103:1302–1306. doi: 10.1016/0016-5085(92)91520-e. [DOI] [PubMed] [Google Scholar]
- 20.Han SH, Reynolds TB, Fong TL. Nephrogenic ascites. Analysis of 16 cases and review of the literature. Medicine (Baltimore) 1998;77:233–245. doi: 10.1097/00005792-199807000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Runyon BA, Montano AA, Akriviadis EA, Antillon MR, Irving MA, McHutchison JG. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med. 1992;117:215–220. doi: 10.7326/0003-4819-117-3-215. [DOI] [PubMed] [Google Scholar]
- 22.Runyon BA. Management of adult patients with ascites due to cirrhosis. Hepatology. 2004;39:841–856. doi: 10.1002/hep.20066. [DOI] [PubMed] [Google Scholar]
- 23.Pirlich M, Selberg O, Boker K, Schwarze M, Muller MJ. The creatinine approach to estimate skeletal muscle mass in patients with cirrhosis. Hepatology. 1996;24:1422–1427. doi: 10.1002/hep.510240620. [DOI] [PubMed] [Google Scholar]
- 24.Caregaro L, Menon F, Angeli P, Amodio P, Merkel C, Bortoluzzi A, Alberino F, Gatta A. Limitations of serum creatinine level and creatinine clearance as filtration markers in cirrhosis. Arch Intern Med. 1994;154:201–205. [PubMed] [Google Scholar]
- 25.Papadakis MA, Arieff AI. Unpredictability of clinical evaluation of renal function in cirrhosis. Prospective study. Am J Med. 1987;82:945–952. doi: 10.1016/0002-9343(87)90156-2. [DOI] [PubMed] [Google Scholar]
- 26.Spahr L, Villeneuve JP, Tran HK, Pomier-Layrargues G. Furosemide-induced natriuresis as a test to identify cirrhotic patients with refractory ascites. Hepatology. 2001;33:28–31. doi: 10.1053/jhep.2001.20646. [DOI] [PubMed] [Google Scholar]
- 27.Cho HS, Park GT, Kim YH, Shim SG, Kim JB, Lee OY, Choi HS, Hahm JS, Lee MH. [The significance of urine sodium measurement after furosemide administration in diuretics-unresponsive patients with liver cirrhosis] Taehan Kan Hakhoe Chi. 2003;9:324–331. [PubMed] [Google Scholar]
- 28.Cardenas A, Bataller R, Arroyo V. Mechanisms of ascites formation. Clin Liver Dis. 2000;4:447–465. doi: 10.1016/s1089-3261(05)70118-5. [DOI] [PubMed] [Google Scholar]
- 29.Gines P, Fernandez-Esparrach G, Arroyo V, Rodes J. Pathogenesis of ascites in cirrhosis. Semin Liver Dis. 1997;17:175–189. doi: 10.1055/s-2007-1007196. [DOI] [PubMed] [Google Scholar]
- 30.Morali GA, Sniderman KW, Deitel KM, Tobe S, Witt-Sullivan H, Simon M, Heathcote J, Blendis LM. Is sinusoidal portal hypertension a necessary factor for the development of hepatic ascites? J Hepatol. 1992;16:249–250. doi: 10.1016/s0168-8278(05)80128-x. [DOI] [PubMed] [Google Scholar]
- 31.Fernandez-Seara J, Prieto J, Quiroga J, Zozaya JM, Cobos MA, Rodriguez-Eire JL, Garcia-Plaza A, Leal J. Systemic and regional hemodynamics in patients with liver cirrhosis and ascites with and without functional renal failure. Gastroenterology. 1989;97:1304–1312. doi: 10.1016/0016-5085(89)91704-6. [DOI] [PubMed] [Google Scholar]
- 32.Schrier RW, Arroyo V, Bernardi M, Epstein M, Henriksen JH, Rodes J. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988;8:1151–1157. doi: 10.1002/hep.1840080532. [DOI] [PubMed] [Google Scholar]
- 33.Vallance P, Moncada S. Hyperdynamic circulation in cirrhosis: a role for nitric oxide? Lancet. 1991;337:776–778. doi: 10.1016/0140-6736(91)91384-7. [DOI] [PubMed] [Google Scholar]
- 34.Kravetz D, Arderiu M, Bosch J, Fuster J, Visa J, Casamitjana R, Rodes J. Hyperglucagonemia and hyperkinetic circulation after portocaval shunt in the rat. Am J Physiol. 1987;252:G257–G261. doi: 10.1152/ajpgi.1987.252.2.G257. [DOI] [PubMed] [Google Scholar]
- 35.Guarner C, Soriano G, Such J, Teixido M, Ramis I, Bulbena O, Rosello J, Guarner F, Gelpi E, Balanzo J. Systemic prostacyclin in cirrhotic patients. Relationship with portal hypertension and changes after intestinal decontamination. Gastroenterology. 1992;102:303–309. [PubMed] [Google Scholar]
- 36.Korthuis RJ, Kinden DA, Brimer GE, Slattery KA, Stogsdill P, Granger DN. Intestinal capillary filtration in acute and chronic portal hypertension. Am J Physiol. 1988;254:G339–G345. doi: 10.1152/ajpgi.1988.254.3.G339. [DOI] [PubMed] [Google Scholar]
- 37.Dudley FJ. Pathophysiology of ascites formation. Gastroenterol Clin North Am. 1992;21:215–235. [PubMed] [Google Scholar]
- 38.Arroyo V, Navasa M. Ascites and Spontaneous Bacterial Peritonitis. In: ER Schiff, MF Sorrel, WS Maddrey., editors. Schiff's diseases of the liver. 10th ed. Philadelphia: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 39.Rafael Valdivia L, Ferrandiz Quiroz J. [Hyponatremia as a possible mortality factor in cirrhotic patients hospitalised in the Guillermo Almenara Irigoyen State Hospital, 2003-2005] Rev Gastroenterol Peru. 2007;27:37–46. [PubMed] [Google Scholar]
- 40.Biggins SW, Rodriguez HJ, Bacchetti P, Bass NM, Roberts JP, Terrault NA. Serum sodium predicts mortality in patients listed for liver transplantation. Hepatology. 2005;41:32–39. doi: 10.1002/hep.20517. [DOI] [PubMed] [Google Scholar]
- 41.Bataller R, Gines P, Guevara M, Arroyo V. Hepatorenal syndrome. Semin Liver Dis. 1997;17:233–247. doi: 10.1055/s-2007-1007201. [DOI] [PubMed] [Google Scholar]
- 42.Garcia-Tsao G. Transjugular Intrahepatic Portosystemic Shunt (TIPS) for the Management of Refractory Ascites in Cirrhosis. In: P Gines, V Arroyo, J Rodes, RW Schrier., editors. Ascites and renal dysfunction in liver disease: pathogenesis, diagnosis, and treatment. Blackwell Science: Malden, Mass; 2005. pp. 251–260. [Google Scholar]
- 43.Cardenas A, Gines P. A Practical Approach to Treatment of Patients with Cirrhosis and Ascites. In: P Gines, V Arroyo, J Rodes, RW Schrier., editors. Ascites and renal dysfunction in liver disease: pathogenesis, diagnosis, and treatment. Malden, Mass: Blackwell Science; 2005. pp. 286–293. [Google Scholar]
- 44.Gines P, Arroyo V, Quintero E, Planas R, Bory F, Cabrera J, Rimola A, Viver J, Camps J, Jimenez W. Comparison of paracentesis and diuretics in the treatment of cirrhotics with tense ascites. Results of a randomized study. Gastroenterology. 1987;93:234–241. doi: 10.1016/0016-5085(87)91007-9. [DOI] [PubMed] [Google Scholar]
- 45.Gines P, Tito L, Arroyo V, Planas R, Panes J, Viver J, Torres M, Humbert P, Rimola A, Llach J. Randomized comparative study of therapeutic paracentesis with and without intravenous albumin in cirrhosis. Gastroenterology. 1988;94:1493–502. doi: 10.1016/0016-5085(88)90691-9. [DOI] [PubMed] [Google Scholar]
- 46.Salerno F, Badalamenti S, Incerti P, Tempini S, Restelli B, Bruno S, Bellati G, Roffi L. Repeated paracentesis and i.v. albumin infusion to treat 'tense' ascites in cirrhotic patients. A safe alternative therapy. J Hepatol. 1987;5:102–108. doi: 10.1016/s0168-8278(87)80067-3. [DOI] [PubMed] [Google Scholar]
- 47.Kravetz D, Romero G, Argonz J, Guevara M, Suarez A, Abecasis R, Bildozola M, Valero J, Terg R. Total volume paracentesis decreases variceal pressure, size, and variceal wall tension in cirrhotic patients. Hepatology. 1997;25:59–62. doi: 10.1053/jhep.1997.v25.pm0008985265. [DOI] [PubMed] [Google Scholar]
- 48.Nevens F, Bustami R, Scheys I, Lesaffre E, Fevery J. Variceal pressure is a factor predicting the risk of a first variceal bleeding: a prospective cohort study in cirrhotic patients. Hepatology. 1998;27:15–19. doi: 10.1002/hep.510270104. [DOI] [PubMed] [Google Scholar]
- 49.Fernandez-Esparrach G, Guevara M, Sort P, Pardo A, Jimenez W, Gines P, Planas R, Lebrec D, Geuvel A, Elewaut A, et al. Diuretic requirements after therapeutic paracentesis in non-azotemic patients with cirrhosis. A randomized double-blind trial of spironolactone versus placebo. J Hepatol. 1997;26:614–620. doi: 10.1016/s0168-8278(97)80427-8. [DOI] [PubMed] [Google Scholar]
- 50.Morillas RM, Santos J, Montoliu S, Planas R. Paracentesis for Cirrhotic Ascites. In: P Gines, V Arroyo, J Rodes, RW Schrier., editors. Ascites and renal dysfunction in liver disease: pathogenesis, diagnosis, and treatment. Malden, Mass: Blackwell Science; 2005. pp. 241–250. [Google Scholar]
- 51.Runyon BA. Care of patients with ascites. N Engl J Med. 1994;330:337–342. doi: 10.1056/NEJM199402033300508. [DOI] [PubMed] [Google Scholar]
- 52.Soulsby CT, Morgan MY. Dietary management of hepatic encephalopathy in cirrhotic patients: survey of current practice in United Kingdom. BMJ. 1999;318:1391. doi: 10.1136/bmj.318.7195.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gines P, Berl T, Bernardi M, Bichet DG, Hamon G, Jimenez W, Liard JF, Martin PY, Schrier RW. Hyponatremia in cirrhosis: from pathogenesis to treatment. Hepatology. 1998;28:851–864. doi: 10.1002/hep.510280337. [DOI] [PubMed] [Google Scholar]
- 54.Moore KP, Aithal GP. Guidelines on the management of ascites in cirrhosis. Gut. 2006;55 Suppl 6:vi1–vi12. doi: 10.1136/gut.2006.099580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grabau CM, Crago SF, Hoff LK, Simon JA, Melton CA, Ott BJ, Kamath PS. Performance standards for therapeutic abdominal paracentesis. Hepatology. 2004;40:484–488. doi: 10.1002/hep.20317. [DOI] [PubMed] [Google Scholar]
- 56.McVay PA, Toy PT. Lack of increased bleeding after paracentesis and thoracentesis in patients with mild coagulation abnormalities. Transfusion. 1991;31:164–171. doi: 10.1046/j.1537-2995.1991.31291142949.x. [DOI] [PubMed] [Google Scholar]
- 57.Runyon BA. Paracentesis of ascitic fluid. A safe procedure. Arch Intern Med. 1986;146:2259–2261. [PubMed] [Google Scholar]
- 58.Runyon BA. Management of adult patients with ascites caused by cirrhosis. Hepatology. 1998;27:264–272. doi: 10.1002/hep.510270139. [DOI] [PubMed] [Google Scholar]
- 59.Pache I, Bilodeau M. Severe haemorrhage following abdominal paracentesis for ascites in patients with liver disease. Aliment Pharmacol Ther. 2005;21:525–529. doi: 10.1111/j.1365-2036.2005.02387.x. [DOI] [PubMed] [Google Scholar]
- 60.Hu KQ, Yu AS, Tiyyagura L, Redeker AG, Reynolds TB. Hyperfibrinolytic activity in hospitalized cirrhotic patients in a referral liver unit. Am J Gastroenterol. 2001;96:1581–1586. doi: 10.1111/j.1572-0241.2001.03781.x. [DOI] [PubMed] [Google Scholar]
- 61.Runyon BA, Antillon MR, Montano AA. Effect of diuresis versus therapeutic paracentesis on ascitic fluid opsonic activity and serum complement. Gastroenterology. 1989;97:158–162. doi: 10.1016/0016-5085(89)91430-3. [DOI] [PubMed] [Google Scholar]
- 62.Pozzi M, Redaelli E, Ratti L, Poli G, Guidi C, Milanese M, Calchera I, Mancia G. Time-course of diastolic dysfunction in different stages of chronic HCV related liver diseases. Minerva Gastroenterol Dietol. 2005;51:179–186. [PubMed] [Google Scholar]
- 63.Ruiz-del-Arbol L, Monescillo A, Jimenez W, Garcia-Plaza A, Arroyo V, Rodes J. Paracentesis-induced circulatory dysfunction: mechanism and effect on hepatic hemodynamics in cirrhosis. Gastroenterology. 1997;113:579–586. doi: 10.1053/gast.1997.v113.pm9247479. [DOI] [PubMed] [Google Scholar]
- 64.Appenrodt B, Wolf A, Grunhage F, Trebicka J, Schepke M, Rabe C, Lammert F, Sauerbruch T, Heller J. Prevention of paracentesis-induced circulatory dysfunction: midodrine vs albumin. A randomized pilot study. Liver Int. 2008;28:1019–1025. doi: 10.1111/j.1478-3231.2008.01734.x. [DOI] [PubMed] [Google Scholar]
- 65.Gines A, Fernandez-Esparrach G, Monescillo A, Vila C, Domenech E, Abecasis R, Angeli P, Ruiz-Del-Arbol L, Planas R, Sola R, et al. Randomized trial comparing albumin, dextran 70, and polygeline in cirrhotic patients with ascites treated by paracentesis. Gastroenterology. 1996;111:1002–1010. doi: 10.1016/s0016-5085(96)70068-9. [DOI] [PubMed] [Google Scholar]
- 66.Moreau R, Valla DC, Durand-Zaleski I, Bronowicki JP, Durand F, Chaput JC, Dadamessi I, Silvain C, Bonny C, Oberti F, et al. Comparison of outcome in patients with cirrhosis and ascites following treatment with albumin or a synthetic colloid: a randomised controlled pilot trail. Liver Int. 2006;26:46–54. doi: 10.1111/j.1478-3231.2005.01188.x. [DOI] [PubMed] [Google Scholar]
- 67.Runyon BA. Ascites and spontaneous bacterial peritonitis. In: M Feldman, L Friedman, LJ Brandt., editors. Sleisenger and Fordtran’s Gastrointestinal & Liver Disease: pathophysiology, diagnosis, management. 8th ed. Philadephia: Saunders; 2006. pp. 1935–1964. [Google Scholar]
- 68.Wilkinson P, Sherlock S. The effect of repeated albumin infusions in patients with cirrhosis. Lancet. 1962;2:1125–1129. doi: 10.1016/s0140-6736(62)90895-4. [DOI] [PubMed] [Google Scholar]
- 69.Rothschild MA, Oratz M, Evans C, Schreiber SS. Alterations in Albumin Metabolism after Serum and Albumin Infusions. J Clin Invest. 1964;43:1874–1880. doi: 10.1172/JCI105061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Salo J, Gines A, Gines P, Piera C, Jimenez W, Guevara M, Fernandez-Esparrach G, Sort P, Bataller R, Arroyo V, et al. Effect of therapeutic paracentesis on plasma volume and transvascular escape rate of albumin in patients with cirrhosis. J Hepatol. 1997;27:645–653. doi: 10.1016/s0168-8278(97)80081-5. [DOI] [PubMed] [Google Scholar]
- 71.Planas R, Gines P, Arroyo V, Llach J, Panes J, Vargas V, Salmeron JM, Gines A, Toledo C, Rimola A. Dextran-70 versus albumin as plasma expanders in cirrhotic patients with tense ascites treated with total paracentesis. Results of a randomized study. Gastroenterology. 1990;99:1736–1744. doi: 10.1016/0016-5085(90)90481-f. [DOI] [PubMed] [Google Scholar]
- 72.Coll S, Vila MC, Molina L, Gimenez MD, Guarner C, Sola R. Mechanisms of early decrease in systemic vascular resistance after total paracentesis: influence of flow rate of ascites extraction. Eur J Gastroenterol Hepatol. 2004;16:347–353. doi: 10.1097/00042737-200403000-00016. [DOI] [PubMed] [Google Scholar]
- 73.Cabrera J, Inglada L, Quintero E, Jimenez W, Losada A, Mayor J, Guerra C. Large-volume paracentesis and intravenous saline: effects on the renin-angiotensin system. Hepatology. 1991;14:1025–1028. [PubMed] [Google Scholar]
- 74.Sola-Vera J, Such J. Understanding the mechanisms of paracentesis-induced crculatory dysfunction. Eur J Gastroenterol Hepatol. 2004;16:295–298. doi: 10.1097/00042737-200403000-00008. [DOI] [PubMed] [Google Scholar]
- 75.Christidis C, Mal F, Ramos J, Senejoux A, Callard P, Navarro R, Trinchet JC, Larrey D, Beaugrand M, Guettier C. Worsening of hepatic dysfunction as a consequence of repeated hydroxyethylstarch infusions. J Hepatol. 2001;35:726–732. doi: 10.1016/s0168-8278(01)00200-8. [DOI] [PubMed] [Google Scholar]
- 76.Cadranel JF, Gargot D, Grippon P, Lunel F, Bernard B, Valla D, Opolon P. Spontaneous dialytic ultrafiltration with intraperitoneal reinfusion of the concentrate versus large paracentesis in cirrhotic patients with intractable ascites: a randomized study. Int J Artif Organs. 1992;15:432–435. [PubMed] [Google Scholar]
- 77.Bernardi M, Rimondi A, Gasbarrini A, Trevisani F, Caraceni P, Legnani C, Palareti G, Gasbarrini G. Ascites apheresis, concentration and reinfusion for the treatment of massive or refractory ascites in cirrhosis. J Hepatol. 1994;20:289–295. doi: 10.1016/s0168-8278(05)80071-6. [DOI] [PubMed] [Google Scholar]
- 78.Albalate M, Lopez Garcia MD, Vazquez A, De Sequera P, Marriott E, Tan D, Ortiz A, Casado S, Carreno V, Caramelo C. Concentrated ascitic fluid reinfusion in cirrhotic patients: a simplified method. Am J Kidney Dis. 1997;29:392–398. doi: 10.1016/s0272-6386(97)90200-6. [DOI] [PubMed] [Google Scholar]
- 79.Lata J, Marecek Z, Fejfar T, Zdenek P, Bruha R, Safka V, Hulek P, Hejda V, Dolina J, Stehlik J, et al. The efficacy of terlipressin in comparison with albumin in the prevention of circulatory changes after the paracentesis of tense ascites--a randomized multicentric study. Hepatogastroenterology. 2007;54:1930–1933. [PubMed] [Google Scholar]
- 80.Moreau R, Asselah T, Condat B, de Kerguenec C, Pessione F, Bernard B, Poynard T, Binn M, Grange JD, Valla D, et al. Comparison of the effect of terlipressin and albumin on arterial blood volume in patients with cirrhosis and tense ascites treated by paracentesis: a randomised pilot study. Gut. 2002;50:90–94. doi: 10.1136/gut.50.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Singh Ranger G. Terlipressin and arterial blood volume after paracentesis for tense ascites in cirrhosis. Gut. 2002;51:755. doi: 10.1136/gut.51.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Singh V, Kumar R, Nain CK, Singh B, Sharma AK. Terlipressin versus albumin in paracentesis-induced circulatory dysfunction in cirrhosis: a randomized study. J Gastroenterol Hepatol. 2006;21:303–307. doi: 10.1111/j.1440-1746.2006.04182.x. [DOI] [PubMed] [Google Scholar]
- 83.Singh V, Dheerendra PC, Singh B, Nain CK, Chawla D, Sharma N, Bhalla A, Mahi SK. Midodrine versus albumin in the prevention of paracentesis-induced circulatory dysfunction in cirrhotics: a randomized pilot study. Am J Gastroenterol. 2008;103:1399–1405. doi: 10.1111/j.1572-0241.2008.01787.x. [DOI] [PubMed] [Google Scholar]
- 84.Singh V, Kumar B, Nain CK, Singh B, Sharma N, Bhalla A, Sharma AK. Noradrenaline and albumin in paracentesis-induced circulatory dysfunction in cirrhosis: a randomized pilot study. J Intern Med. 2006;260:62–68. doi: 10.1111/j.1365-2796.2006.01654.x. [DOI] [PubMed] [Google Scholar]
- 85.Rosch J, Uchida BT, Putnam JS, Buschman RW, Law RD, Hershey AL. Experimental intrahepatic portacaval anastomosis: use of expandable Gianturco stents. Radiology. 1987;162:481–485. doi: 10.1148/radiology.162.2.3797662. [DOI] [PubMed] [Google Scholar]
- 86.Casado M, Bosch J, Garcia-Pagan JC, Bru C, Banares R, Bandi JC, Escorsell A, Rodriguez-Laiz JM, Gilabert R, Feu F, et al. Clinical events after transjugular intrahepatic portosystemic shunt: correlation with hemodynamic findings. Gastroenterology. 1998;114:1296–1303. doi: 10.1016/s0016-5085(98)70436-6. [DOI] [PubMed] [Google Scholar]
- 87.Reichle FA, Owen OE. Hemodynamic patterns in human hepatic cirrhosis: a prospective randomized study of the hemodynamic sequelae of distal splenorenal (Warren) and mesocaval shunts. Ann Surg. 1979;190:523–534. doi: 10.1097/00000658-197910000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rossle M, Siegerstetter V, Huber M, Ochs A. The first decade of the transjugular intrahepatic portosystemic shunt (TIPS): state of the art. Liver. 1998;18:73–89. doi: 10.1111/j.1600-0676.1998.tb00132.x. [DOI] [PubMed] [Google Scholar]
- 89.Jalan R, Lui HF, Redhead DN, Hayes PC. TIPSS 10 years on. Gut. 2000;46:578–581. doi: 10.1136/gut.46.4.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Forrest EH, Stanley AJ, Redhead DN, McGilchrist AJ, Hayes PC. Clinical response after transjugular intrahepatic portosystemic stent shunt insertion for refractory ascites in cirrhosis. Aliment Pharmacol Ther. 1996;10:801–806. doi: 10.1046/j.1365-2036.1996.60202000.x. [DOI] [PubMed] [Google Scholar]
- 91.Ochs A, Rossle M, Haag K, Hauenstein KH, Deibert P, Siegerstetter V, Huonker M, Langer M, Blum HE. The transjugular intrahepatic portosystemic stent-shunt procedure for refractory ascites. N Engl J Med. 1995;332:1192–1197. doi: 10.1056/NEJM199505043321803. [DOI] [PubMed] [Google Scholar]
- 92.Wong W, Liu P, Blendis L, Wong F. Long-term renal sodium handling in patients with cirrhosis treated with transjugular intrahepatic portosystemic shunts for refractory ascites. Am J Med. 1999;106:315–322. [PubMed] [Google Scholar]
- 93.Nazarian GK, Ferral H, Bjarnason H, Castaneda-Zuniga WR, Rank JM, Bernadas CA, Hunter DW. Effect of transjugular intrahepatic portosystemic shunt on quality of life. AJR Am J Roentgenol. 1996;167:963–969. doi: 10.2214/ajr.167.4.8819395. [DOI] [PubMed] [Google Scholar]
- 94.Allard JP, Chau J, Sandokji K, Blendis LM, Wong F. Effects of ascites resolution after successful TIPS on nutrition in cirrhotic patients with refractory ascites. Am J Gastroenterol. 2001;96:2442–2447. doi: 10.1111/j.1572-0241.2001.04051.x. [DOI] [PubMed] [Google Scholar]
- 95.Boyer TD. Transjugular intrahepatic portosystemic shunt: current status. Gastroenterology. 2003;124:1700–1710. doi: 10.1016/s0016-5085(03)00377-9. [DOI] [PubMed] [Google Scholar]
- 96.Pidlich J, Peck-Radosavljevic M, Kranz A, Wildling R, Winkelbauer FW, Lammer J, Mayer C, Muller C, Stix G, Gangl A, et al. Transjugular intrahepatic portosystemic shunt and cardiac arrhythmias. J Clin Gastroenterol. 1998;26:39–43. doi: 10.1097/00004836-199801000-00011. [DOI] [PubMed] [Google Scholar]
- 97.Freedman AM, Sanyal AJ, Tisnado J, Cole PE, Shiffman ML, Luketic VA, Purdum PP, Darcy MD, Posner MP. Complications of transjugular intrahepatic portosystemic shunt: a comprehensive review. Radiographics. 1993;13:1185–1210. doi: 10.1148/radiographics.13.6.8290720. [DOI] [PubMed] [Google Scholar]
- 98.LaBerge JM, Ring EJ, Gordon RL, Lake JR, Doherty MM, Somberg KA, Roberts JP, Ascher NL. Creation of transjugular intrahepatic portosystemic shunts with the wallstent endoprosthesis: results in 100 patients. Radiology. 1993;187:413–420. doi: 10.1148/radiology.187.2.8475283. [DOI] [PubMed] [Google Scholar]
- 99.Riggio O, Merlli M, Pedretti G, Servi R, Meddi P, Lionetti R, Rossi P, Bezzi M, Salvatori F, Ugolotti U, et al. Hepatic encephalopathy after transjugular intrahepatic portosystemic shunt. Incidence and risk factors. Dig Dis Sci. 1996;41:578–584. doi: 10.1007/BF02282344. [DOI] [PubMed] [Google Scholar]
- 100.Sanyal AJ, Freedman AM, Shiffman ML, Purdum PP 3rd, Luketic VA, Cheatham AK. Portosystemic encephalopathy after transjugular intrahepatic portosystemic shunt: results of a prospective controlled study. Hepatology. 1994;20:46–55. doi: 10.1016/0270-9139(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 101.Rossle M, Piotraschke J. Transjugular intrahepatic portosystemic shunt and hepatic encephalopathy. Dig Dis. 1996;14 Suppl 1:12–19. doi: 10.1159/000171579. [DOI] [PubMed] [Google Scholar]
- 102.Somberg KA, Riegler JL, LaBerge JM, Doherty-Simor MM, Bachetti P, Roberts JP, Lake JR. Hepatic encephalopathy after transjugular intrahepatic portosystemic shunts: incidence and risk factors. Am J Gastroenterol. 1995;90:549–555. [PubMed] [Google Scholar]
- 103.Kerlan RK Jr, LaBerge JM, Baker EL, Wack JP, Marx M, Somberg KA, Gordon RL, Ring EJ. Successful reversal of hepatic encephalopathy with intentional occlusion of transjugular intrahepatic portosystemic shunts. J Vasc Interv Radiol. 1995;6:917–921. doi: 10.1016/s1051-0443(95)71212-x. [DOI] [PubMed] [Google Scholar]
- 104.Hauenstein KH, Haag K, Ochs A, Langer M, Rossle M. The reducing stent: treatment for transjugular intrahepatic portosystemic shunt-induced refractory hepatic encephalopathy and liver failure. Radiology. 1995;194:175–179. doi: 10.1148/radiology.194.1.7997547. [DOI] [PubMed] [Google Scholar]
- 105.Martinet JP, Fenyves D, Legault L, Roy L, Dufresne MP, Spahr L, Lafortune M, Pomier-Layrargues G. Treatment of refractory ascites using transjugular intrahepatic portosystemic shunt (TIPS): a caution. Dig Dis Sci. 1997;42:161–166. doi: 10.1023/a:1018861827399. [DOI] [PubMed] [Google Scholar]
- 106.Lebrec D, Giuily N, Hadengue A, Vilgrain V, Moreau R, Poynard T, Gadano A, Lassen C, Benhamou JP, Erlinger S. Transjugular intrahepatic portosystemic shunts: comparison with paracentesis in patients with cirrhosis and refractory ascites: a randomized trial. French Group of Clinicians and a Group of Biologists. J Hepatol. 1996;25:135–144. doi: 10.1016/s0168-8278(96)80065-1. [DOI] [PubMed] [Google Scholar]
- 107.LaBerge JM, Ferrell LD, Ring EJ, Gordon RL. Histopathologic study of stenotic and occluded transjugular intrahepatic portosystemic shunts. J Vasc Interv Radiol. 1993;4:779–786. doi: 10.1016/s1051-0443(93)71972-7. [DOI] [PubMed] [Google Scholar]
- 108.Sanyal AJ, Freedman AM, Luketic VA, Purdum PP 3rd, Shiffman ML, DeMeo J, Cole PE, Tisnado J. The natural history of portal hypertension after transjugular intrahepatic portosystemic shunts. Gastroenterology. 1997;112:889–898. doi: 10.1053/gast.1997.v112.pm9041251. [DOI] [PubMed] [Google Scholar]
- 109.LaBerge JM, Somberg KA, Lake JR, Gordon RL, Kerlan RK Jr, Ascher NL, Roberts JP, Simor MM, Doherty CA, Hahn J. Two-year outcome following transjugular intrahepatic portosystemic shunt for variceal bleeding: results in 90 patients. Gastroenterology. 1995;108:1143–1151. doi: 10.1016/0016-5085(95)90213-9. [DOI] [PubMed] [Google Scholar]
- 110.Rosado B, Kamath PS. Transjugular intrahepatic portosystemic shunts: an update. Liver Transpl. 2003;9:207–217. doi: 10.1053/jlts.2003.50045. [DOI] [PubMed] [Google Scholar]
- 111.Chopra S, Dodd GD 3rd, Chintapalli KN, Rhim H, Encarnacion CE, Palmaz JC, Esola CC, Ghiatas AA. Transjugular intrahepatic portosystemic shunt: accuracy of helical CT angiography in the detection of shunt abnormalities. Radiology. 2000;215:115–122. doi: 10.1148/radiology.215.1.r00ap51115. [DOI] [PubMed] [Google Scholar]
- 112.Angermayr B, Cejna M, Koenig F, Karnel F, Hackl F, Gangl A, Peck-Radosavljevic M. Survival in patients undergoing transjugular intrahepatic portosystemic shunt: ePTFE-covered stentgrafts versus bare stents. Hepatology. 2003;38:1043–1050. doi: 10.1053/jhep.2003.50423. [DOI] [PubMed] [Google Scholar]
- 113.Bureau C, Garcia-Pagan JC, Otal P, Pomier-Layrargues G, Chabbert V, Cortez C, Perreault P, Peron JM, Abraldes JG, Bouchard L, Bilbao JI, Bosch J, Rousseau H, Vinel JP. Improved clinical outcome using polytetrafluoroethylene-coated stents for TIPS: results of a randomized study. Gastroenterology. 2004;126:469–475. doi: 10.1053/j.gastro.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 114.Siegerstetter V, Huber M, Ochs A, Blum HE, Rossle M. Platelet aggregation and platelet-derived growth factor inhibition for prevention of insufficiency of the transjugular intrahepatic portosystemic shunt: a randomized study comparing trapidil plus ticlopidine with heparin treatment. Hepatology. 1999;29:33–38. doi: 10.1002/hep.510290139. [DOI] [PubMed] [Google Scholar]
- 115.Sanyal AJ, Freedman AM, Purdum PP, Shiffman ML, Luketic VA. The hematologic consequences of transjugular intrahepatic portosystemic shunts. Hepatology. 1996;23:32–39. doi: 10.1002/hep.510230105. [DOI] [PubMed] [Google Scholar]
- 116.Conn HO. Hemolysis after transjugular intrahepatic portosystemic shunting: the naked stent syndrome. Hepatology. 1996;23:177–181. doi: 10.1002/hep.510230123. [DOI] [PubMed] [Google Scholar]
- 117.Sanyal AJ, Reddy KR. Vegetative infection of transjugular intrahepatic portosystemic shunts. Gastroenterology. 1998;115:110–115. doi: 10.1016/s0016-5085(98)70371-3. [DOI] [PubMed] [Google Scholar]
- 118.Wang MQ, Dake MD, Cui ZP, Wang ZQ, Gao YA. Portal-systemic myelopathy after transjugular intrahepatic portosystemic shunt creation: report of four cases. J Vasc Interv Radiol. 2001;12:879–881. doi: 10.1016/s1051-0443(07)61514-0. [DOI] [PubMed] [Google Scholar]
- 119.Conn HO, Rossle M, Levy L, Glocker FX. Portosystemic myelopathy: spastic paraparesis after portosystemic shunting. Scand J Gastroenterol. 2006;41:619–625. doi: 10.1080/00365520500318932. [DOI] [PubMed] [Google Scholar]
- 120.Huonker M, Schumacher YO, Ochs A, Sorichter S, Keul J, Rossle M. Cardiac function and haemodynamics in alcoholic cirrhosis and effects of the transjugular intrahepatic portosystemic stent shunt. Gut. 1999;44:743–748. doi: 10.1136/gut.44.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rossle M, Ochs A, Gulberg V, Siegerstetter V, Holl J, Deibert P, Olschewski M, Reiser M, Gerbes AL. A comparison of paracentesis and transjugular intrahepatic portosystemic shunting in patients with ascites. N Engl J Med. 2000;342:1701–1707. doi: 10.1056/NEJM200006083422303. [DOI] [PubMed] [Google Scholar]
- 122.Salerno F, Merli M, Riggio O, Cazzaniga M, Valeriano V, Pozzi M, Nicolini A, Salvatori F. Randomized controlled study of TIPS versus paracentesis plus albumin in cirrhosis with severe ascites. Hepatology. 2004;40:629–635. doi: 10.1002/hep.20364. [DOI] [PubMed] [Google Scholar]
- 123.Gines P, Uriz J, Calahorra B, Garcia-Tsao G, Kamath PS, Del Arbol LR, Planas R, Bosch J, Arroyo V, Rodes J. Transjugular intrahepatic portosystemic shunting versus paracentesis plus albumin for refractory ascites in cirrhosis. Gastroenterology. 2002;123:1839–1847. doi: 10.1053/gast.2002.37073. [DOI] [PubMed] [Google Scholar]
- 124.Sanyal AJ, Genning C, Reddy KR, Wong F, Kowdley KV, Benner K, McCashland T. The North American Study for the Treatment of Refractory Ascites. Gastroenterology. 2003;124:634–641. doi: 10.1053/gast.2003.50088. [DOI] [PubMed] [Google Scholar]
- 125.Albillos A, Banares R, Gonzalez M, Catalina MV, Molinero LM. A meta-analysis of transjugular intrahepatic portosystemic shunt versus paracentesis for refractory ascites. J Hepatol. 2005;43:990–996. doi: 10.1016/j.jhep.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 126.Deltenre P, Mathurin P, Dharancy S, Moreau R, Bulois P, Henrion J, Pruvot FR, Ernst O, Paris JC, Lebrec D. Transjugular intrahepatic portosystemic shunt in refractory ascites: a meta-analysis. Liver Int. 2005;25:349–356. doi: 10.1111/j.1478-3231.2005.01095.x. [DOI] [PubMed] [Google Scholar]
- 127.Salerno F, Camma C, Enea M, Rossle M, Wong F. Transjugular intrahepatic portosystemic shunt for refractory ascites: a meta-analysis of individual patient data. Gastroenterology. 2007;133:825–834. doi: 10.1053/j.gastro.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 128.Park JS, Won JY, Park SI, Park SJ, Lee DY. Percutaneous peritoneovenous shunt creation for the treatment of benign and malignant refractory ascites. J Vasc Interv Radiol. 2001;12:1445–1448. doi: 10.1016/s1051-0443(07)61707-2. [DOI] [PubMed] [Google Scholar]
- 129.Lasheen AE, Elzeftawy A, Ibrahim S, Attia M, Emam M. Implantation of a skin graft tube to create a saphenoperitoneal shunt for refractory ascites. Surg Today. 2007;37:622–625. doi: 10.1007/s00595-006-3471-7. [DOI] [PubMed] [Google Scholar]
- 130.Deen KI, de Silva AP, Jayakody M, de Silva HJ. Saphenoperitoneal anastomosis for resistant ascites in patients with cirrhosis. Am J Surg. 2001;181:145–148. doi: 10.1016/s0002-9610(00)00565-1. [DOI] [PubMed] [Google Scholar]
- 131.Vadeyar HJ, Doran JD, Charnley R, Ryder SD. Saphenoperitoneal shunts for patients with intractable ascites associated with chronic liver disease. Br J Surg. 1999;86:882–885. doi: 10.1046/j.1365-2168.1999.01156.x. [DOI] [PubMed] [Google Scholar]
- 132.Zervos EE, Rosemurgy AS. Management of medically refractory ascites. Am J Surg. 2001;181:256–264. doi: 10.1016/s0002-9610(01)00565-7. [DOI] [PubMed] [Google Scholar]
- 133.Rozenblit GN, Del Guercio LR, Rundback JH, Poplausky MR, Lebovics E. Peritoneal-urinary drainage for treatment of refractory ascites: a pilot study. J Vasc Interv Radiol. 1998;9:998–1005. doi: 10.1016/s1051-0443(98)70440-3. [DOI] [PubMed] [Google Scholar]
- 134.Ali F, Guglin M, Vaitkevicius P, Ghali JK. Therapeutic potential of vasopressin receptor antagonists. Drugs. 2007;67:847–858. doi: 10.2165/00003495-200767060-00002. [DOI] [PubMed] [Google Scholar]
- 135.Pham PC, Pham PM, Pham PT. Vasopressin excess and hyponatremia. Am J Kidney Dis. 2006;47:727–737. doi: 10.1053/j.ajkd.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 136.Greenberg A, Verbalis JG. Vasopressin receptor antagonists. Kidney Int. 2006;69:2124–2130. doi: 10.1038/sj.ki.5000432. [DOI] [PubMed] [Google Scholar]
- 137.Gines P, Wong F, Watson H, Milutinovic S, del Arbol LR, Olteanu D. Effects of satavaptan, a selective vasopressin V(2) receptor antagonist, on ascites and serum sodium in cirrhosis with hyponatremia: a randomized trial. Hepatology. 2008;48:204–213. doi: 10.1002/hep.22293. [DOI] [PubMed] [Google Scholar]
- 138.Angeli P, Volpin R, Gerunda G, Craighero R, Roner P, Merenda R, Amodio P, Sticca A, Caregaro L, Maffei-Faccioli A, et al. Reversal of type 1 hepatorenal syndrome with the administration of midodrine and octreotide. Hepatology. 1999;29:1690–1697. doi: 10.1002/hep.510290629. [DOI] [PubMed] [Google Scholar]
- 139.Krag A, Moller S, Henriksen JH, Holstein-Rathlou NH, Larsen FS, Bendtsen F. Terlipressin improves renal function in patients with cirrhosis and ascites without hepatorenal syndrome. Hepatology. 2007;46:1863–1871. doi: 10.1002/hep.21901. [DOI] [PubMed] [Google Scholar]
- 140.Kalambokis G, Fotopoulos A, Economou M, Tsianos EV. Octreotide in the treatment of refractory ascites of cirrhosis. Scand J Gastroenterol. 2006;41:118–121. doi: 10.1080/00365520510024043. [DOI] [PubMed] [Google Scholar]
- 141.Kalambokis G, Fotopoulos A, Economou M, Pappas K, Tsianos EV. Effects of a 7-day treatment with midodrine in non-azotemic cirrhotic patients with and without ascites. J Hepatol. 2007;46:213–221. doi: 10.1016/j.jhep.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 142.Kalambokis G, Economou M, Fotopoulos A, Al Bokharhii J, Pappas C, Katsaraki A, Tsianos EV. The effects of chronic treatment with octreotide versus octreotide plus midodrine on systemic hemodynamics and renal hemodynamics and function in nonazotemic cirrhotic patients with ascites. Am J Gastroenterol. 2005;100:879–885. doi: 10.1111/j.1572-0241.2005.40899.x. [DOI] [PubMed] [Google Scholar]
- 143.Tandon P, Tsuyuki RT, Mitchell L, Hoskinson M, Ma MM, Wong WW, Mason AL, Gutfreund K, Bain VG. The effect of 1 month of therapy with midodrine, octreotide-LAR and albumin in refractory ascites: a pilot study. Liver Int. 2008;100 doi: 10.1111/j.1478-3231.2008.01778.x. [DOI] [PubMed] [Google Scholar]
- 144.Lenaerts A, Van Cauter J, Moukaiber H, Meunier JC, Ligny G. [Treatment of refractory ascites with clonidine and spironolactone] Gastroenterol Clin Biol. 1997;21:524–525. [PubMed] [Google Scholar]
- 145.Lenaerts A, Codden T, Meunier JC, Henry JP, Ligny G. Effects of clonidine on diuretic response in ascitic patients with cirrhosis and activation of sympathetic nervous system. Hepatology. 2006;44:844–849. doi: 10.1002/hep.21355. [DOI] [PubMed] [Google Scholar]
- 146.Rai RR, Jain P. Combination of clonidine and diuretic in cirrhotic ascites with activated sympathetic nervous system: Would it be a magical bullet? Hepatology. 2008;47:360. doi: 10.1002/hep.21771. [DOI] [PubMed] [Google Scholar]
- 147.Lenaerts A, Codden T, Van Cauter J, Meunier JC, Henry JP, Ligny G. Interest of the association clonidine-spironolactone in cirrhotic patients with ascites and activation of sympathetic nervous system. Acta Gastroenterol Belg. 2002;65:1–5. [PubMed] [Google Scholar]
- 148.Henriksen JH, Ring-Larsen H. Hepatorenal disorders: role of the sympathetic nervous system. Semin Liver Dis. 1994;14:35–43. doi: 10.1055/s-2007-1007296. [DOI] [PubMed] [Google Scholar]
- 149.Laffi G, Gentilini P, Romanelli RG, La Villa G. Is the use of albumin of value in the treatment of ascites in cirrhosis? The case in favour. Dig Liver Dis. 2003;35:660–663. doi: 10.1016/s1590-8658(03)00384-0. [DOI] [PubMed] [Google Scholar]
- 150.Gentilini P, Casini-Raggi V, Di Fiore G, Romanelli RG, Buzzelli G, Pinzani M, La Villa G, Laffi G. Albumin improves the response to diuretics in patients with cirrhosis and ascites: results of a randomized, controlled trial. J Hepatol. 1999;30:639–645. doi: 10.1016/s0168-8278(99)80194-9. [DOI] [PubMed] [Google Scholar]
- 151.Trotter J, Pieramici E, Everson GT. Chronic albumin infusions to achieve diuresis in patients with ascites who are not candidates for transjugular intrahepatic portosystemic shunt (TIPS) Dig Dis Sci. 2005;50:1356–1360. doi: 10.1007/s10620-005-2787-2. [DOI] [PubMed] [Google Scholar]
- 152.Romanelli RG, La Villa G, Barletta G, Vizzutti F, Lanini F, Arena U, Boddi V, Tarquini R, Pantaleo P, Gentilini P, et al. Long-term albumin infusion improves survival in patients with cirrhosis and ascites: an unblinded randomized trial. World J Gastroenterol. 2006;12:1403–1407. doi: 10.3748/wjg.v12.i9.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fernandez J, Monteagudo J, Bargallo X, Jimenez W, Bosch J, Arroyo V, Navasa M. A randomized unblinded pilot study comparing albumin versus hydroxyethyl starch in spontaneous bacterial peritonitis. Hepatology. 2005;42:627–634. doi: 10.1002/hep.20829. [DOI] [PubMed] [Google Scholar]
- 154.Sort P, Navasa M, Arroyo V, Aldeguer X, Planas R, Ruiz-del-Arbol L, Castells L, Vargas V, Soriano G, Guevara M, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341:403–409. doi: 10.1056/NEJM199908053410603. [DOI] [PubMed] [Google Scholar]
- 155.Gines P, Guevara M, De Las Heras D, Arroyo V. Review article: albumin for circulatory support in patients with cirrhosis. Aliment Pharmacol Ther. 2002;16 Suppl 5:24–31. doi: 10.1046/j.1365-2036.16.s5.4.x. [DOI] [PubMed] [Google Scholar]
- 156.Gines P, Torre A, Terra C, Guevara M. Review article: pharmacological treatment of hepatorenal syndrome. Aliment Pharmacol Ther. 2004;20 Suppl 3:57–62; discussion 63-64. doi: 10.1111/j.1365-2036.2004.02115.x. [DOI] [PubMed] [Google Scholar]
- 157.Chang CY, Singal AK, Ganeshan SV, Schiano TD, Lookstein R, Emre S. Use of splenic artery embolization to relieve tense ascites following liver transplantation in a patient with paroxysmal nocturnal hemoglobinuria. Liver Transpl. 2007;13:1532–1537. doi: 10.1002/lt.21317. [DOI] [PubMed] [Google Scholar]
- 158.Pamuk ON, Sonsuz A. The effect of mannitol infusion on the response to diuretic therapy in cirrhotic patients with ascites. J Clin Gastroenterol. 2002;35:403–405. doi: 10.1097/00004836-200211000-00009. [DOI] [PubMed] [Google Scholar]
- 159.Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922–938. doi: 10.1002/hep.21907. [DOI] [PubMed] [Google Scholar]
- 160.Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:1637–1648. doi: 10.1053/gast.2000.20189. [DOI] [PubMed] [Google Scholar]
- 161.Runyon BA. Historical aspects of treatment of patients with cirrhosis and ascites. Semin Liver Dis. 1997;17:163–173. doi: 10.1055/s-2007-1007195. [DOI] [PubMed] [Google Scholar]
- 162.Veldt BJ, Laine F, Guillygomarc'h A, Lauvin L, Boudjema K, Messner M, Brissot P, Deugnier Y, Moirand R. Indication of liver transplantation in severe alcoholic liver cirrhosis: quantitative evaluation and optimal timing. J Hepatol. 2002;36:93–98. doi: 10.1016/s0168-8278(01)00228-8. [DOI] [PubMed] [Google Scholar]
- 163.Malekzadeh R, Mohamadnejad M, Rakhshani N, Nasseri-Moghaddam S, Merat S, Tavangar SM, Sohrabpour AA. Reversibility of cirrhosis in chronic hepatitis B. Clin Gastroenterol Hepatol. 2004;2:344–347. doi: 10.1016/s1542-3565(04)00066-7. [DOI] [PubMed] [Google Scholar]
- 164.Malekzadeh R, Mohamadnejad M, Nasseri-Moghaddam S, Rakhshani N, Tavangar SM, Sohrabpour AA, Tahaghoghi S. Reversibility of cirrhosis in autoimmune hepatitis. Am J Med. 2004;117:125–129. doi: 10.1016/j.amjmed.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 165.Dufour JF, DeLellis R, Kaplan MM. Reversibility of hepatic fibrosis in autoimmune hepatitis. Ann Intern Med. 1997;127:981–985. doi: 10.7326/0003-4819-127-11-199712010-00006. [DOI] [PubMed] [Google Scholar]
- 166.Bories P, Garcia Compean D, Michel H, Bourel M, Capron JP, Gauthier A, Lafon J, Levy VG, Pascal JP, Quinton A. The treatment of refractory ascites by the LeVeen shunt. A multi-centre controlled trial (57 patients) J Hepatol. 1986;3:212–218. doi: 10.1016/s0168-8278(86)80028-9. [DOI] [PubMed] [Google Scholar]
- 167.Henderson JM, Mackay GJ, Hooks M, Chezmar JL, Galloway JR, Dodson TF, Kutner MH. High cardiac output of advanced liver disease persists after orthotopic liver transplantation. Hepatology. 1992;15:258–262. doi: 10.1002/hep.1840150214. [DOI] [PubMed] [Google Scholar]
- 168.Hadengue A, Lebrec D, Moreau R, Sogni P, Durand F, Gaudin C, Bernuau J, Belghiti J, Gayet B, Erlinger S. Persistence of systemic and splanchnic hyperkinetic circulation in liver transplant patients. Hepatology. 1993;17:175–178. [PubMed] [Google Scholar]
- 169.Ruf AE, Kremers WK, Chavez LL, Descalzi VI, Podesta LG, Villamil FG. Addition of serum sodium into the MELD score predicts waiting list mortality better than MELD alone. Liver Transpl. 2005;11:336–343. doi: 10.1002/lt.20329. [DOI] [PubMed] [Google Scholar]
- 170.Biggins SW, Kim WR, Terrault NA, Saab S, Balan V, Schiano T, Benson J, Therneau T, Kremers W, Wiesner R, et al. Evidence-based incorporation of serum sodium concentration into MELD. Gastroenterology. 2006;130:1652–1660. doi: 10.1053/j.gastro.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 171.Luca A, Angermayr B, Bertolini G, Koenig F, Vizzini G, Ploner M, Peck-Radosavljevic M, Gridelli B, Bosch J. An integrated MELD model including serum sodium and age improves the prediction of early mortality in patients with cirrhosis. Liver Transpl. 2007;13:1174–1180. doi: 10.1002/lt.21197. [DOI] [PubMed] [Google Scholar]


