Abstract
A compatible interaction between a plant and a pathogen is the result of a complex interplay between many factors of both plant and pathogen origin. Our objective was to identify host factors involved in this interaction. These factors may include susceptibility factors required for pathogen growth, factors manipulated by the pathogen to inactivate or avoid host defenses, or negative regulators of defense responses. To this end, we identified 20 recessive Arabidopsis mutants that do not support normal growth of the powdery mildew pathogen, Erysiphe cichoracearum. Complementation analyses indicated that four loci, designated powdery mildew resistant 1–4 (pmr1–4), are defined by this collection. These mutants do not constitutively accumulate elevated levels of PR1 or PDF1.2 mRNA, indicating that resistance is not simply due to constitutive activation of the salicylic acid- or ethylene- and jasmonic acid-dependent defense pathways. Further Northern blot analyses revealed that some mutants accumulate higher levels of PR1 mRNA than wild type in response to infection by powdery mildew. To test the specificity of the resistance, the pmr mutants were challenged with other pathogens including Pseudomonas syringae, Peronospora parasitica, and Erysiphe orontii. Surprisingly, one mutant, pmr1, was susceptible to E. orontii, a very closely related powdery mildew, suggesting that a very specific resistance mechanism is operating in this case. Another mutant, pmr4, was resistant to P. parasitica, indicating that this resistance is more generalized. Thus, we have identified a novel collection of mutants affecting genes required for a compatible interaction between a plant and a biotrophic pathogen.
In nature, plants interact with many different microbes. Some of these relationships are very complex, requiring the formation of unique structures composed of both microbe and plant gene products at the plant–microbe interface. Nitrogen-fixing bacteria and mycorrhizal fungi are two examples of microorganisms that have complex interactions with plants that greatly enhance plant growth. In recent years, a wealth of knowledge has been discovered about the microbial components essential to plant–microbe interactions (e.g., nodulation genes in Rhizobium and virulence genes from numerous bacterial pathogens). Likewise, our understanding of the plant genes necessary for resistance to various pathogens has taken tremendous strides with the cloning of resistance genes from several plant species (1). The majority of these resistance genes are involved in race-specific resistance and are thought to encode receptors that specifically recognize an avirulence gene product of the pathogen. After binding the avirulence gene product, the resistance gene product is thought to activate host defenses, commonly resulting in the hypersensitive response (1).
In stark contrast to our knowledge of microbial genes involved in plant–microbe interactions and plant genes involved in disease resistance, little is known about the plant components of compatible plant–microbe interactions. The best-characterized examples of host susceptibility factors are plant metabolites that induce bacterial genes and/or serve as chemoattractants. For example, flavonoids produced by plant roots induce nodulation genes in Rhizobium spp., the first step in the formation of a nitrogen-fixing root nodule (2). Another susceptibility factor, acetosyringone, is produced by wounded plant cells and plays an important role in the development of crown gall disease caused by Agrobacterium tumefaciens (3).
As biotrophic pathogens, powdery mildews require living plant tissue to grow and reproduce. Therefore, these pathogens must strike a delicate balance between extracting sufficient resources from the plant to complete their life cycle, but not so much that the plant is killed prematurely. Biotrophic pathogens must also evade or resist host defenses until their life cycle is complete. In addition, powdery mildews form an intricate, intracellular feeding structure, the haustorium, which is completely surrounded by an invagination of the plant cell membrane (4). Because everything required for fungal growth (water, carbon, nitrogen, etc.) must pass through a plant membrane before being taken up by the fungus, there are likely to be plant gene products that are necessary for nutrient export from plant cells and for establishing the infection site as a metabolic sink. Given the intricacies of the powdery mildew life cycle, we reasoned that a screen for Arabidopsis mutants that cannot support growth of a powdery mildew pathogen would be successful.
Other groups have identified powdery mildew-resistant mutants; however, these mutants appear to affect host defenses rather than susceptibility factors. Among large collections of powdery mildew-resistant barley mutants, only one locus, MLO, was identified (5). Based on genetic experiments, MLO is postulated to act as a negative regulator of plant defenses. Therefore, when MLO is inactivated by mutation, plant defenses are partially activated or are more sensitive to activation signals. Consistent with this hypothesis, mlo plants spontaneously form papillae (cell wall appositions thought to play a role in defense) and form larger papillae faster than wild-type plants at penetration sites (6). Also supporting the interpretation of MLO as a negative regulator of host defenses, mlo plants form spontaneous lesions reminiscent of a hypersensitive necrosis response, even under axenic conditions. Unfortunately, the sequence of the cloned MLO gene reveals little about its specific function (7). Recently, a disease-resistant Arabidopsis mutant, edr1, was identified, based on its resistance to Pseudomonas syringae (8). Further characterization of edr1 revealed that it was also resistant to the powdery mildew Erysiphe cichoracearum. The resistance displayed by edr1 is associated with massive cell death at infection sites, possibly an uncontrolled hypersensitive necrosis response, and a dramatic increase in PR1 mRNA levels. Because both MLO and EDR1 seem to be regulators of defense responses, they tell us little about the compatible interaction between plants and powdery mildew.
The eds mutants, including pad4, npr1, eds5, eds10, and eds13, exhibit enhanced susceptibility to both P. syringae and Erysiphe orontii (9, 10). Although, PAD4, NPR1, and EDS5 play roles in plant defense, it is possible that EDS10 and EDS13 are negative regulators of susceptibility.
Here we present the analysis of mutations at four novel loci required for a compatible interaction between Arabidopsis and E. cichoracearum. These mutants potentially identify susceptibility factors required by a biotrophic pathogen. Some of these mutants also may identify novel defense pathways or new control points for known defense pathways.
Materials and Methods
Plant Lines and Growth Conditions.
Arabidopsis thaliana Columbia (Col) and Wassilewskija were used in this study. Plants were grown in ProMix HP (Premier Horticulture, Red Hill, PA). Plants to be infected with powdery mildew were grown in growth chambers at 22°C with a 14-hr photoperiod. Plants to be infected with Peronospora parasitica were grown at 16°C with a 10-hr photoperiod. Light intensity was >150 μE/m2 per sec for all experiments, excluding testing pmr3 light sensitivity.
Growth of Powdery Mildews.
Squash, variety Kuta (Park Seed, Greenwood, SC), was used as a host for the production of E. cichoracearum UCSC1 inoculum (11). Inoculum was prepared by touching squash plants with infected squash leaves 10–12 days before use. Arabidopsis plants were inoculated by placing 1.3-m-tall settling towers over two flats and tapping one to four squash leaves over the top of the settling towers. For measuring fungal growth, the squash leaves were tapped over a 3–41/33 Nitex nylon monofilament screen (Sefar America, Kansas City, MO) and the resulting powder was brushed back and forth to allow single conidia to fall through. After 15 min, the plants were placed in a dew chamber for 45 min (11).
E. orontii MGH1 (12) was maintained on Capsella bursa-pastoris by placing uninfected plants in a growth chamber with infected plants and allowing them to be inoculated by air movement in the growth chamber. Arabidopsis plants were inoculated by placing a 65-cm-tall settling tower over a 13.5-cm pot and tapping six heavily infected Capsella leaves over the top of the tower. After 5 min, the pot was returned to the growth chamber.
Isolation of Mutants.
M2 seeds (Col) derived from M1 seeds mutagenized with methanesulfonic acid ethyl ester were sown in flats, placed at 4°C for 4 days, and then moved to a greenhouse. Two weeks after germination, seedlings were thinned to approximately one plant per 6 cm2. Plants were inoculated, as described above, when they had at least four true leaves. Seven days postinoculation (dpi), plants with reduced powdery mildew growth were selected and allowed to set seed.
Genetic Mapping.
The pmr mutants were crossed to Wassilewskija to generate a mapping population. F2 seeds were planted and scored for disease resistance as described above. DNA was extracted from homozygous-resistant F2 plants as described (13).
Microscopy.
To visualize fungal hyphae, infected leaves were cleared in 95% ethanol and stained with 250 μg/ml trypan blue in a solution of lactic acid, glycerol, and water (1:1:1) for 15 min, rinsed with the same solution, and mounted (11). Individual colonies were photographed by using a Pixera (Los Gatos, CA) digital camera attached to a Leica microscope. Total hyphal length was measured by using nih image software (http://rsb.info.nih.gov/nih-image/).
To visualize microscopic lesions, leaves were stained by using a modification of a previously described method (14). Leaves were vacuum-infiltrated twice in a solution of phenol, lactic acid, glycerol, and water (1:1:1:1) plus 250 μg/ml trypan blue. The tubes with the samples were placed in a boiling water bath for 2 min and allowed to cool for 1 hr. The leaves were destained in the 1:1:1:1 solution for 1 hr and examined under bright-field illumination.
To visualize callose, leaves were cleared in 95% ethanol, stained with aniline blue, and examined for fluorescence as described (11).
To detect autofluorescent compounds, leaves were cleared in 95% ethanol, equilibrated in a solution of lactic acid, glycerol, and water (1:1:1), mounted, and examined under epifluorescent illumination (11).
Growth and Inoculation of Other Pathogens.
Leaves were pressure-infiltrated with P. syringae pv. tomato DC3000 (pLAFR3) (15) by using a 1-ml syringe without a needle. Three different concentrations of bacteria were used: 106, 107, and 108 cfu/ml. Also, plants were inoculated by dipping in a 108 cfu/ml suspension of bacteria (8). Symptoms were scored 4–6 days after inoculation.
P. parasitica Emco5 inoculum was maintained on Wassilewskija (16). Seven-day-old seedlings were inoculated with a suspension of 1 × 105 conidiosporangia per ml by using an atomizer, covered with plastic wrap, and returned to the growth chamber. Six days later, sporangiophores were counted.
Northern Blot Analysis.
Plants were heavily inoculated with E. cichoracearum, and six plants per genotype were harvested daily from 0 to 4 dpi. Total RNA was prepared by using Trizol reagent and following the manufacturer's instructions (Life Technologies, Gaithersburg, MD). Northern blot analysis was performed as described, using 15 μg of total RNA (17). Signals were collected by using a PhosphorImager (Molecular Dynamics) and quantified by using nih image software.
Results
Isolation of Mutants.
From a screen of approximately 26,000 M2 Arabidopsis plants, 32 confirmed powdery mildew-resistant mutants that did not exhibit spontaneous macroscopic lesions or constitutively express PR1 were identified. Twelve of these mutants had obvious lesions after inoculation, reminiscent of edr1, and were set aside. The remaining 20 mutants showed no macroscopic lesions after inoculation and are the subject of this paper.
Genetic Analysis.
Crosses between the 20 mutants defined 4 complementation groups, designated powdery mildew resistant 1–4 (pmr1–4) (Fig. 1A). Multiple, independent alleles were identified for pmr1 (7), pmr2 (6), and pmr4 (2). No obvious phenotypic differences were noted among the alleles at each locus. The number of alleles is less than 20 because multiple mutants from the same lot of mutagenized seed were counted as one allele.
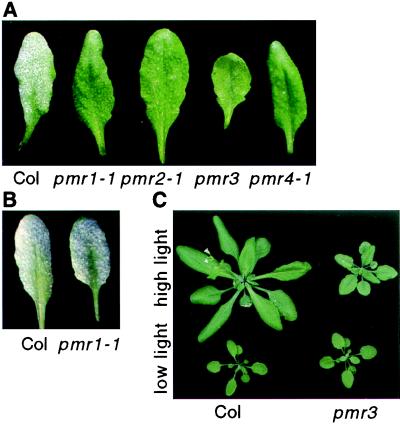
Figure 1.
Phenotype of pmr mutants. (A) Plants were inoculated with E. cichoracearum 8 days before being photographed. Note the extensive fungal growth on Col. (B) Plants were inoculated with E. orontii 10 days before being photographed. (C) Effect of light levels on pmr3. Twenty-two-day-old plants grown under high, 150 μE/m2 per sec, or low, 45 μE/m2 per sec, light conditions.
The mutants were backcrossed to the wild type, Col, two times before characterization, excluding mapping. Segregation of powdery mildew resistance in the F1 and F2 generations indicated that all mutations are recessive and that pmr2, pmr3, and pmr4 segregate in a simple 3:1 Mendelian fashion (Table 1). By contrast, all seven alleles of pmr1 had a more complex segregation pattern (Table 1). Reciprocal crosses between plants heterozygous and homozygous for pmr1 indicated that transmission of pmr1 through the pollen is reduced (Table 1). To further characterize the decreased transmission of pmr1, a pollen competition experiment was conducted. Pollen from heterozygous plants was placed on stigmas of homozygous pmr1 plants. When the siliques were fully ripe, but not yet brittle, they were cut into three sections: top, middle, and bottom. The segregation of powdery mildew resistance among the progeny and, by inference, the genotype of the pollen fertilizing the ovules derived from the different sections were determined. Plants originating from the top sections segregated in a 2.9:1 ratio (55 susceptible; 19 resistant), plants from middle sections segregated in a 9.3:1 ratio (93 susceptible; 10 resistant), and plants from the bottom sections segregated 52:1 (103 susceptible; 2 resistant). Thus, pmr1 pollen fertilizes ovules close to the stigmatic surface more efficiently than distal ovules. Slower pollen tube growth or an inability of the pollen tubes to sense and grow toward unfertilized ovules could explain these results. It is important to note that homozygous pmr1 plants are fertile, indicating that pmr1 pollen can fertilize all ovules; it is just less efficient than wild-type pollen.
Table 1.
Genetic analysis of powdery mildew-resistant mutants
| Cross (female × male) | Type | Total | Disease response
|
χ2 | |
|---|---|---|---|---|---|
| Susceptible | Resistant | ||||
| PMR1/PMR1 × pmr1/pmr1 | F1 | 28 | 28 | 0 | |
| PMR1/PMR1 × pmr1/pmr1 | F2 | 1,841 | 1,742 | 99 | 378*; P < 0.05 |
| PMR1/pmr1 × pmr1/pmr1 | Testcross | 124 | 66 | 58 | 0.47†; P > 0.05 |
| pmr1/pmr1 × PMR1/pmr1 | Testcross | 199 | 182 | 17 | 137†; P < 0.05 |
| PMR2/PMR2 × pmr2/pmr2 | F1 | 71 | 71 | 0 | |
| PMR2/PMR2 × pmr2/pmr2 | F2 | 193 | 140 | 53 | 0.43*; P > 0.05 |
| PMR3/PMR3 × pmr3/pmr3 | F1 | 11 | 11 | 0 | |
| PMR3/PMR3 × pmr3/pmr3 | F2 | 98 | 71 | 27 | 0.56*; P > 0.05 |
| PMR4/PMR4 × pmr4/pmr4 | F1 | 22 | 22 | 0 | |
| PMR4/PMR4 × pmr4/pmr4 | F2 | 443 | 340 | 103 | 0.4*; P > 0.05 |
*χ2 calculated for an expected 3:1, wild type to mutant ratio.
†χ2 calculated for an expected 1:1, wild type to mutant ratio.
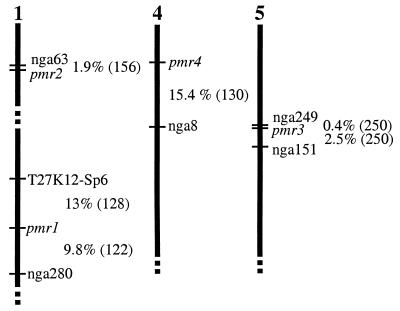
pmr1 and pmr2 mapped to chromosome 1 and pmr4 mapped to chromosome 4 (Fig. 2). pmr3 mapped to chromosome 5, close to the position of the dwarf mutant lu-1 (lutescens). Because the description of the lu-1 phenotype was similar to pmr3, described below, the mutants were crossed. The F1 progeny were not dwarf, indicating that pmr3 and lu-1 complement one another and presumably affect different genes.
Figure 2.
Map positions of pmr mutants. Vertical bars represent chromosomes. Percent recombination between markers and the pmr mutants is indicated. The number of chromosomes scored is in parentheses.
Pleiotropic Phenotypes.
In addition to powdery mildew resistance, pmr3 plants are much smaller and paler than wild type at light intensities >150 μE/m2 per sec (Fig. 1C). However, at light intensities <75 μE/m2 per sec, pmr3 plants are nearly wild type in stature and are susceptible to powdery mildew. A careful analysis reveals that the size of pmr3 plants under high- and low-light conditions is similar, but wild-type plants are much larger under high light (Fig. 1C). The dwarf phenotype cosegregated 100% with powdery mildew resistance through two backcrosses and among 598 F2 plants. In addition, the fact that both the dwarf phenotype and the resistance phenotype are dependent on light intensity strongly suggests that the same mutation is responsible for both phenotypes.
Leaves from pmr4 plants are epinastic, especially when grown under short-day conditions. Both alleles of pmr4 display this phenotype, indicating that the same mutations are responsible for both the powdery mildew resistance and the epinasty. Adult pmr1 and pmr2 plants are indistinguishable from wild type.
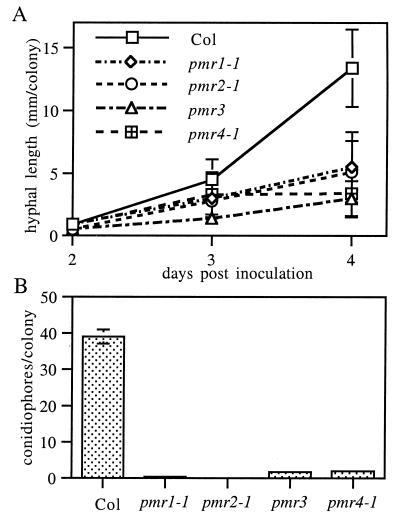
Time Course of Fungal Growth.
To determine whether fungal growth on the mutants was blocked at a defined step in the infection cycle, the growth of hyphae and level of conidiation was measured over time. The same general trend was observed for all mutants. Conidia germinate and begin growth in a normal fashion, but colonies on mutant plants grow more slowly than colonies on wild-type plants. By 3 dpi, hyphal length was significantly (ANOVA, P < 0.05) less on all the mutants than on wild-type plants (Fig. 3A). Eventually, most colonies on the mutants appeared to be dead or dying and produced few, if any, conidiophores, the aerial stalks bearing asexual conidia. In contrast, colonies on wild-type plants appeared healthy and produced many conidiophores (Fig. 3B). However, on all mutants a small subset of colonies do produce some conidiophores, indicating that none of the mutations result in a complete block at any stage of fungal development.
Figure 3.
Quantification of E. cichoracearum growth on pmr mutants. (A) Hyphal length per colony. (B) Conidiophores per colony at 6 dpi. The means ± SD based on at least 15 replicates are plotted in A and B. The entire experiment was repeated once with similar results.
Cytological Characterization.
To ascertain whether microscopic lesions, increased callose accumulation, or the formation of autofluorescent compounds was correlated with resistance, leaves were examined microscopically at various times after inoculation. Approximately 30 colonies per leaf on eight leaves from four infected plants were examined for each time point, and all experiments were repeated at least twice, with similar results. No lesions were associated with fungal colonies on mutant or wild-type plants at 1 dpi. By 5 dpi, occasional dead epidermal cells beneath a small percentage of colonies were observed on both wild-type and mutant plants. Because the majority of colonies on the mutants were not associated with dead epidermal cells, but were stunted, cell death is not the primary mechanism of resistance in any of the mutants. Interestingly, uninfected leaves from pmr3 plants occasionally had single, dead mesophyll cells (Fig. 4A). It is important to note that these microlesions did not grow or increase in frequency after infection with powdery mildew and were always limited to individual mesophyll cells.
Figure 4.
Cytological description of pmr3. (A) Uninfected leaves stained with trypan blue. Dead cells are blue. Note that individual mesophyll cells are stained in pmr3. (B) Uninfected leaves stained with aniline blue to highlight callose. Note the bright-yellow spot of callose in pmr3. (C) Accumulation of autofluorescent compounds, as indicated by the bright-blue spots, in uninfected pmr3 leaves. Colocalization of callose, autofluorescence, and dead cells could not be determined because different leaves were used for each stain.
Plants reinforce the cell wall directly beneath sites of fungal penetration by depositing new cell wall material, creating papillae. Enhanced papillae formation is correlated with the resistance of some barley cultivars and is proposed to be a mechanism for the resistance (4). To address the possibility that some of the mutants may have enhanced papillae formation, we stained infected leaves for callose, a major component of papillae. Wild-type plants had brightly staining papillae beneath the majority of colonies at both 1 and 3 dpi. pmr1, pmr2, and pmr3 formed papillae that were indistinguishable from wild type, suggesting that increased papillae formation is not responsible for the resistance. Surprisingly, pmr4 showed an almost complete lack of callose even after 5 days of fungal growth (Fig. 5A). To determine whether pmr4 plants could make callose under other circumstances, we stained wounded leaves and pollen tubes for callose. No callose was detected in wounded pmr4 leaves (Fig. 5B). By contrast, pmr4 pollen tubes had abundant callose (not shown). While staining for callose in papillae, we noticed that a small percentage of pmr3 leaves constitutively displayed small, brightly staining spots scattered over the leaf (Fig. 4B). As with the microlesions, this staining did not increase noticeably after infection.
Figure 5.
Lack of callose accumulation by pmr4. (A) Infected leaves were stained with aniline blue at 1 dpi. Note the brightly stained papilla, adjacent to the conidiospore, in Col and the lack of staining in pmr4. (B) Leaves were stained with aniline blue 1 day after wounding. Note the accumulation of callose along the wound edge in Col and the lack of staining in pmr4.
The deposition of autofluorescent compounds is correlated with the limitation of pathogen growth (4). No autofluorescence was detected in wild-type, pmr1, pmr2, or pmr4 plants. pmr3 plants had small spots of fluorescent material, even in uninfected leaves (Fig. 4C). As with the microlesions, autofluorescence did not increase noticeably after infection.
Responses to Other Pathogens.
To determine the specificity of the resistance observed in the mutants, they were challenged with a battery of pathogens. First, they were challenged with a closely related powdery mildew, E. orontii (12). Surprisingly, pmr1 was susceptible to E. orontii, indicating that this resistance is very specific (Fig. 1B). The other mutants were resistant to E. orontii (not shown).
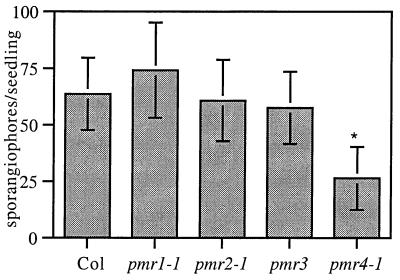
Next, the mutants were challenged with a completely unrelated pathogen, P. parasitica, which shares the biotrophic lifestyle with E. cichoracearum. Interestingly, pmr4 was resistant to P. parasitica (Fig. 6), indicating that PMR4 also is required for a compatible interaction with P. parasitica. The other mutants were fully susceptible to P. parasitica (Fig. 6).
Figure 6.
Growth of P. parasitica on pmr mutants. The means ± SD based on 20 seedlings are plotted. Bars marked with * were significantly different than Col. (ANOVA, P < 10−12, followed by Student's t test, P < 10−9, for the Col/pmr4 comparison). Results shown are from one of two experiments with similar results.
The mutants also were challenged with P. syringae. Yellow, water-soaked lesions, indistinguishable from wild type, were evident on all of the mutants by 4 dpi (not shown). Thus, none of the mutants was resistant to P. syringae, indicating that the resistances are not broad spectrum.
Kinetics of PR1 and PDF1.2 Expression.
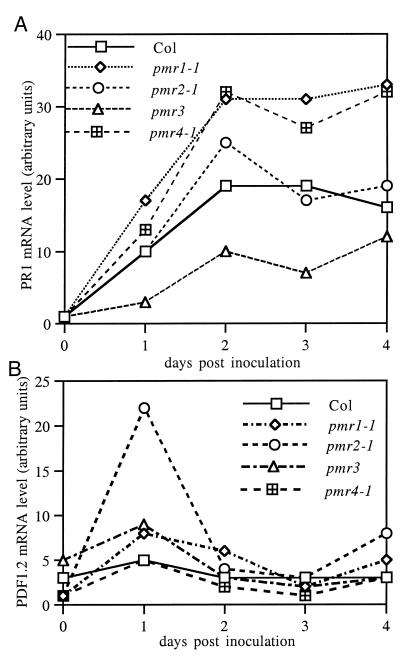
To determine whether the resistance observed in the mutants was correlated with increased expression of known defense genes, the levels of PR1 and PDF1.2 mRNA were determined from 0 to 4 dpi (Fig. 7). PR1 was chosen as a marker for the salicylic acid-dependent pathway (18) and PDF1.2 was chosen as a marker for the ethylene- and jasmonic acid-dependent pathway (19). Wild-type plants displayed a gradual increase in PR1 mRNA from no detectable signal on day 0 to a strong signal that peaked 2–3 days after inoculation (Fig. 7A). None of the mutants had detectable PR1 mRNA on day 0. Two mutants, pmr1 and pmr4, displayed higher levels of PR1 mRNA than wild type from day 1 onward. pmr2 displayed essentially the same kinetics as wild type. Surprisingly, pmr3 plants accumulated lower levels of PR1 mRNA than wild-type plants at all time points.
Figure 7.
Time course of PR1 and PDF1.2 mRNA levels. The levels of PR1 (A) and PDF1.2 (B) mRNA are shown as determined by Northern blot analysis and normalized for loading by using rRNA levels. Similar trends were observed in two other experiments by using tissues harvested at 0, 1, 3, and 5 dpi.
The kinetics of PDF1.2 expression was quite different than PR1. Wild-type plants had a very weak PDF1.2 signal on day 0, which did not increase after infection (Fig. 7B). pmr1, pmr3, and pmr4 displayed essentially the same kinetics as wild type. pmr2 exhibited a transient 4-fold increase in PDF1.2 message on day 1. as the wild-type level is close to the limit of detection. However, as the wild-type level is close to the limit of detection, the absolute level of PDF1.2 message was quite small.
Discussion
We have identified a novel and varied collection of Arabidopsis mutants that are unable to support normal growth of E. cichoracearum. Unlike previously identified Arabidopsis disease-resistant mutants, cpr, lsd, and acd (20–22), pmr1–pmr4 do not constitutively express PR1. Similarly, unlike edr1 (8), pmr1–pmr4 do not form lesions in response to pathogen attack. Also, the resistance observed in pmr1–pmr3 is highly specific for powdery mildew. Indeed, pmr1 was susceptible even to a closely related powdery mildew. Although pmr4 was resistant to the biotrophic pathogen P. parasitica, it was not resistant to the bacterial pathogen P. syringae, indicating that, even in this case, the resistance has a narrow spectrum. This differs markedly from the broad spectrum resistance observed in the lsd, cpr, and edr1 mutants (8, 21, 22). Indeed, edr1 was isolated based on its resistance to P. syringae. These distinctions set the pmr mutants apart from previously identified, defense-related mutants. In addition, pmr1, pmr3, and pmr4 have pleiotropic phenotypes, indicating that the corresponding genes play roles in plant growth and development.
The pmr mutants can be divided into three classes based on the possible role of host defenses in mediating resistance. In the first class, consisting of pmr2, resistance is not correlated with dramatically increased activation of host defenses as measured by PR1 expression, papilla formation, cell death, or the accumulation of autofluorescent compounds. Although pmr2 plants do show a transient induction of PDF1.2 at 1 dpi, the most dramatic reduction in fungal growth occurs well after PDF1.2 mRNA levels return to baseline. Therefore, it seems unlikely that the modest increase in PDF1.2 is responsible for the resistance. The PDF1.2 increase may be a result of failed infection attempts rather than the cause of resistance. Thus, the failure of E. cichoracearum to grow on pmr2 may be due to the loss of a susceptibility factor. However, the possibility that the PDF1.2 burst may be an indicator of a larger response of unknown defense genes or that PDF1.2 is a very long-lived protein cannot be ruled out.
In the second class, consisting of pmr1 and pmr4, resistance is correlated with approximately 1.7-fold more PR1 message than wild-type, suggesting that the salicylic acid pathway may play a role in mediating resistance. It is interesting to note that pmr1 resistance is ineffective against the closely related powdery mildew E. orontii. In this regard, the resistance resembles a classic, race-specific natural resistance. However, the recessive nature, pollen phenotype, and high frequency of pmr1 mutations make this hypothesis untenable because natural resistance genes are typically dominant and do not affect pollen and a mutation that precisely alters the specificity of a resistance gene would be a very rare event. Thus, pmr1 is a mutant that mounts a successful defense response in a very specific, but nonclassical, fashion. One possibility is that in a compatible interaction, E. cichoracearum actively suppresses host defenses via PMR1. Thus, when PMR1 is inactivated, host defenses are not suppressed and the plant mounts a successful defense. In this scenario, PMR1 normally may not be part of any defense pathway in wild-type plants. Indeed, the segregation distortion observed in pmr1 crosses indicates PMR1 is required for pollen tube growth or guidance. Alternatively, the increased PR1 message may be the result of failed infection attempts rather than the cause of the resistance, suggesting that PMR1 is a true susceptibility factor.
The contradictory phenotypes of pmr4 lend themselves to speculation. On the one hand, pmr4 plants exhibit an exaggerated PR1 response, suggesting an active defense mechanism. On the other hand, pmr4 plants accumulate very little callose in response to fungal penetration, suggesting that the mutants actually should be more susceptible. One hypothesis that could reconcile these observations is that the decrease in callose accumulation allows the plant to recognize the invading fungus and mount a successful defense response. In this scenario, because papillae do not form normally, a secondary defense pathway is activated that is, in fact, more effective than the wild-type response. Another intriguing possibility is that the fungus normally uses callose as a cue for development (e.g., a trigger for forming haustoria), and when callose is absent, fungal development is retarded. In this case, the increased PR1 response is simply a side effect of failed infections and not the cause of resistance.
Resistance in the third class, represented by pmr3, may be mediated by a novel defense pathway because, although some hallmarks of defense responses, microlesions, autofluorescent compounds, and callose, are constitutively present, PR1 and PDF1.2 mRNA levels are not elevated. In fact, pmr3 accumulates lower levels of PR1 mRNA than wild type, indicating that the PR1 pathway is suppressed. This raises the possibility that an unknown pathway is activated in pmr3. The lack of resistance toward unrelated pathogens suggests that the subset of defenses activated in pmr3 plants is not broad-spectrum. Alternatively, the microlesions, callose, and autofluorescent compounds found in pmr3 plants may be pleiotropic phenotypes unrelated to defense or resistance. Another possibility is that pmr3 is simply too sickly to support normal powdery mildew growth. However, this is unlikely because many dwarf, pale, sickly mutants were seen in the screen and these supported luxuriant fungal growth.
Whatever the basis for resistance, the pmr mutants are very different from each other and from previously identified defense-related mutants. The recessive nature of the pmr mutants indicates that all the genes affected are host factors required for a compatible interaction. Furthermore, the pleiotropic nature of the pmr1, pmr3, and pmr4 mutations indicates that the corresponding genes play a role in plant growth and development. PMR2 has the potential to be a true susceptibility factor (e.g., PMR2 may be a component of the haustorial complex) because the resistance observed in pmr2 is not associated with a dramatic increase in any of the defense responses measured. Also, the lack of broad spectrum resistance to other pathogens indicates that the resistance observed in the mutants employs a specific mechanism, suggesting an interaction between E. cichoracearum and the PMR gene products. However, the possibility that the pmr mutants affect negative regulators of defense responses cannot be ruled out.
Acknowledgments
We thank F. Ausubel, L. Reuber, and J. Plotnikova for supplying E. orontii and C. bursa-pastoris; J. Dangl and J. McDowell for supplying P. parasitica; B. Staskawicz for supplying P. syringae; J. Ogas and S. Turner for supplying mutagenized seeds; and the reviewers for helpful suggestions. This work was supported by National Institutes of Health Fellowship F32 GN19499-01 and by the U.S. Department of Energy, Biological Energy Research Program. This article is publication no. 1426 from the Carnegie Institution of Washington.
Abbreviations
- Col
Columbia
- dpi
days postinoculation
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.030531997.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.030531997
References
- 1.Hammond-Kosack K E, Jones J D G. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:575–607. doi: 10.1146/annurev.arplant.48.1.575. [DOI] [PubMed] [Google Scholar]
- 2.Denarié J, Debelle F, Rosenberg C. Annu Rev Microbiol. 1992;46:497–531. doi: 10.1146/annurev.mi.46.100192.002433. [DOI] [PubMed] [Google Scholar]
- 3.Stachel S E, Messens E, VanMontagu M, Zambryski P. Nature (London) 1985;318:624–629. [Google Scholar]
- 4.Giese H, Hippe-Sanwald S, Somerville S, Weller J. In: The Mycota. Carroll G C, Tudzynski P, editors. Berlin: Springer; 1997. pp. 55–78. [Google Scholar]
- 5.Jørgensen J. Euphytica. 1992;63:141–152. [Google Scholar]
- 6.Wolter M, Hollricher K, Salamini F, Schulze-Lefert P. Mol Gen Genet. 1993;239:122–128. doi: 10.1007/BF00281610. [DOI] [PubMed] [Google Scholar]
- 7.Büschges R, Hollricher K, Panstruga R, Simons G, Wolter M, Frijters A, Vandaelen R, Vanderlee T, Diergaarde P, Groenendijk J, et al. Cell. 1997;88:695–705. doi: 10.1016/s0092-8674(00)81912-1. [DOI] [PubMed] [Google Scholar]
- 8.Frye C A, Innes R W. Plant Cell. 1998;10:947–956. doi: 10.1105/tpc.10.6.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reuber T L, Plotnikova J M, Dewdney J, Rogers E E, Wood W, Ausubel F M. Plant J. 1998;16:473–485. doi: 10.1046/j.1365-313x.1998.00319.x. [DOI] [PubMed] [Google Scholar]
- 10.Volko S M, Boller T, Ausubel F M. Genetics. 1998;149:537–548. doi: 10.1093/genetics/149.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adam L, Somerville S C. Plant J. 1996;9:341–356. doi: 10.1046/j.1365-313x.1996.09030341.x. [DOI] [PubMed] [Google Scholar]
- 12.Plotnikova J M, Reuber T L, Ausubel F M. Mycologia. 1998;90:1009–1016. [Google Scholar]
- 13.Klimyuk V I, Carroll B J, Thomas C M, Jones J D G. Plant J. 1993;3:493–494. doi: 10.1111/j.1365-313x.1993.tb00169.x. [DOI] [PubMed] [Google Scholar]
- 14.Bowling S A, Clarke J D, Liu Y, Klessig D F, Dong X. Plant Cell. 1997;9:1573–1584. doi: 10.1105/tpc.9.9.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whalen M C, Innes R W, Bent A F, Staskawicz B J. Plant Cell. 1991;3:49–59. doi: 10.1105/tpc.3.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dangl J L, Holub E B, Debener T, Lehnackers H, Ritter C, Crute I R. In: Methods in Arabidopsis Research. Koncz C, Chua N H, Schell J, editors. Singapore: World Scientific; 1992. pp. 393–418. [Google Scholar]
- 17.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current Protocols in Molecular Biology. New York: Wiley; 1996. [Google Scholar]
- 18.Ryals J A, Neuenschwander U H, Willits M G, Molina A, Steiner H Y, Hunt M D. Plant Cell. 1996;8:1809–1819. doi: 10.1105/tpc.8.10.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Penninckx I, Eggermont K, Terras F R G, Thomma B, Desamblanx G W, Buchala A, Metraux J P, Manners J M, Broekaert W F. Plant Cell. 1996;8:2309–2323. doi: 10.1105/tpc.8.12.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenberg J T, Ausubel F M. Plant J. 1993;4:327–341. doi: 10.1046/j.1365-313x.1993.04020327.x. [DOI] [PubMed] [Google Scholar]
- 21.Bowling S A, Guo A, Cao H, Gordon A S, Klessig D F, Dong X. Plant Cell. 1994;6:1845–1857. doi: 10.1105/tpc.6.12.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dietrich R A, Delaney T P, Uknes S J, Ward E R, Ryals J A, Dangl J L. Cell. 1994;77:565–577. doi: 10.1016/0092-8674(94)90218-6. [DOI] [PubMed] [Google Scholar]