Abstract
The last five years have been an exciting time in the study of esophageal motor disorders due to the recent advances in esophageal function testing. New technologies have emerged, such as intraluminal impedance, while conventional techniques, such as manometry, have enjoyed many improvements due to advances in transducer technology, computerization and graphic data presentation. While these techniques provide more detailed information regarding esophageal function, our understanding of whether they can improve our ability to diagnose and treat patients more effectively is evolving. These techniques are also excellent research tools and they have added substantially to our understanding of esophageal motor function in dysphagia. This review describes the potential benefits that these new technologies may have over conventional techniques for the evaluation of dysphagia.
Keywords: Dysphagia, Multichannel intraluminal Impedance, Bolus transit, High-resolution manometry, Esophagogastric junction, Achalasia
INTRODUCTION
The evaluation of a patient with dysphagia should begin with a careful evaluation for structural causes for obstruction, such as webs, rings, strictures and mass lesions. Once these entities are ruled out with either endoscopy or fluoroscopy, the work-up should shift its focus to defining esophageal motor function and bolus transit abnormalities. Although fluoroscopy can provide information on bolus transit, it is burdened by the requirement of radiation, and the inability to detect the pressure profile of the peristaltic event. Currently, only manometry can define the pressure profile of both peristalsis and LES relaxation. However, this technology is limited by an inability to provide information on bolus transit and emptying. Furthermore, conventional manometry has been hindered by a lack of standardization of methodology and conflicting notions of how to analyze the data. Direct evidence of this predicament can be found in a recent publication highlighting the poor inter-observer agreement in the analysis of clinical manometry; even amongst expert practitioners[1].
Given the above observations, two technologies have been developed that have the potential to improve the management of dysphagia: high-resolution manometry and intraluminal impedance monitoring. While high-resolution manometry is an evolution of current manometric techniques, impedance monitoring is a new technology that complements manometric data by providing information on bolus transit without the need for radiation or an additional test. These two techniques have advanced our understanding of esophageal motility and it is very likely that they will also improve clinical management.
MULTICHANNEL INTRALUMINAL IMPEDANCE (MII)
MII: Technical aspects
As mentioned previously, fluoroscopic evaluation of the esophagus is an excellent tool to assess the intraluminal anatomy of the esophagus as well as bolus transit. However, it requires radiation and also lacks the detail to define and classify esophageal motor function. Intraluminal impedance monitoring was devised to circumvent the requirement of radiation in assessing bolus transit by monitoring intraluminal resistance. Impedance monitoring works by using an alternating current generator to apply an electrical potential between pairs of metal electrode rings separated by an isolator. The electrical current loop can only be closed through the conduction of electrical charges by the surrounding material bridging the two metal electrode rings. Air, liquid (saline/refluxate), and the esophageal mucosa each have unique impedance characteristics, thereby allowing definition of which material resides between each pair of electrodes. Air is highly resistant to current flow and, thus, has a high impedance value, while saline and gastric juice have relatively low resistance to flow and, thus, have a low impedance value. Esophageal mucosa has an intermediate impedance range and, thus, serves as a baseline during monitoring.
By dispersing the impedance electrodes along a catheter, and defining impedance changes at adjacent pairs of rings, one can determine the direction of bolus transit within the esophagus and also document whether complete bolus clearance has occurred (Figure 1)[2–4]. Studies using combined fluoroscopy and impedance have validated the convention that liquid bolus entry is characterized by a 50% drop in impedance at the recording site while bolus exit is characterized by a return to at least 50% of baseline[5,6]. Studies assessing the correlation between simultaneous barium videoesophagram and impedance revealed agreement in over 97% of swallows for determining normal bolus transit or retrograde escape and stasis[7]. Thus, this technique provides qualitative evidence of esophageal emptying. However, it does not provide quantitative data regarding volume.
Figure 1.
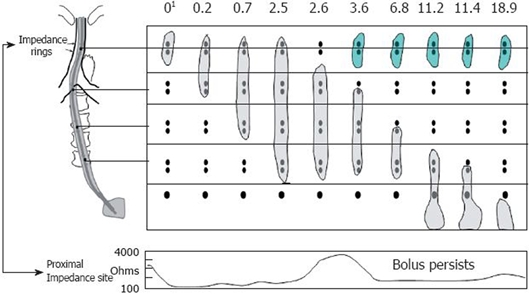
Example of retrograde escape and proximal stasis on simultaneous multichannel intraluminal impedance and fluoroscopy. The fluoroscopic bolus is represented by the gray bolus at each time interval during the swallow and the green bolus during retrograde escape. The bottom panel is a single impedance tracing at the proximal location. The bolus is present at the proximal impedance site until 2.6 s where the bolus tails moves distal to the two rings at that level and the impedance tracing returns to baseline. At 3.6 s, the bolus (green) reenters the recording site and once again the impedance drops consistent with bolus retention. Modified from Imam et al[7]. 1Time in seconds.
MII: Investigations into dysphagia pathogenesis
An important first observation using combined MII and conventional manometry was focused on describing bolus transit patterns associated with various esophageal motor patterns. Tutuian et al[8] described an experience in 350 patients presenting for evaluation of esophageal function. Bolus transit was assessed based on conventional manometric criteria of swallowing function. All patients with manometrically defined achalasia and scleroderma had abnormal bolus transit. In contrast, only half of the patients with ineffective esophageal motility (IEM) and spasm had abnormal bolus transit. The majority of patients with intact peristalsis and various LES abnormalities had normal bolus transit. The authors theorized that impedance monitoring could potentially categorize esophageal motor abnormalities into more clinically relevant groups based on abnormalities of both bolus transit and pressure as opposed to pressure alone.
Looking to provide more focused information regarding a clinical role for impedance, Tutuian et al[9] analyzed 71 subjects with DES and characterized them based on both their motor function and ability to obtain complete bolus transit[9]. Their findings suggested that DES patients with dysphagia were more likely to have abnormal bolus transit than DES patients who presented with predominant chest pain. Furthermore, they also noted that DES patients presenting with chest pain were not only more likely to have normal bolus transit, but also exhibited higher contraction amplitudes. These observations are intriguing as it appears that impedance may help define treatment strategies for various patient groups: chest pain patients with extremely high contractile amplitudes may be a sub-population amenable to treatments with nitrates and calcium channel blockers; while patients with impaired bolus transit may require alternative therapies.
MII: Clinical aspects
The clinical protocol for combined impedance/manometry is similar to the standard conventional manometric protocol with the exception that normal saline is used for the 10 liquid swallows and there is an additional portion of the study that utilizes viscous swallows. Saline is used because its high conductivity provides a contrast in impedance between the liquid bolus and the esophageal wall, while the viscous bolus is typically a gel provided by the manufacturer or another food, such as yogurt or apple sauce. Impedance parameters calculated for the evaluation of bolus transit using liquid and viscous swallows are: (1) total bolus transit time (between bolus entry at 20 cm above the LES and bolus exit at 5 cm above the LES); (2) bolus presence time (the interval between bolus entry and bolus exit at each impedance-measuring site); and (3) segmental transit times (the interval between bolus entry at a given level above the LES and bolus exit at the next most distal level)[10]. Swallows are classified as having: (1) complete bolus transit if bolus exit is recorded in all three distal impedance-measuring sites or (2) incomplete bolus transit if bolus exit is not identified at one or more of the three distal impedance-measuring sites.
Normal values for both liquid and viscous swallows have been reported by different groups using slightly different techniques. Tutuian et al[10]. performed combined impedance/manometry on 43 normal healthy subjects and analyzed 10 liquid and 10 viscous swallows to determine normative ranges for total bolus transit time and the percent of swallows associated with complete bolus transit. Their results revealed that total bolus transit time for both liquid and viscous swallows were 12.5 s and greater than 90% of individuals had > 80% of the liquid swallows associated with complete bolus transit and > 70% of the viscous swallows associated with complete bolus transit[10]. Two other studies in normal controls revealed similar bolus transit parameters and thus, it appears that this technology is reproducible in normal subjects[11,12].
MII: Future directions
Although available data suggests that abnormal bolus transit occurs in many patients presenting with dysphagia, there is no clear data supporting an association of abnormal bolus transit, and the perception of dysphagia. Thus, future research should focus on the mechanisms by which abnormal bolus transit elicits symptoms. Such studies should consider the role of sensitivity, anxiety state and other potential confounders that may modulate symptoms. In addition, studies focused on whether or not abnormal bolus transit can predict clinical outcomes in dysphagia patients are also needed to support the role of impedance in clinical practice.
New technologies incorporating impedance techniques are also evolving that can provide more detail regarding anatomy and mechanical properties of the esophageal body[13,14]. The functional lumen imaging probe (FLIP) was created by McMahon et al[13,14] to measure cross-sectional area of the esophagus at extremely small intervals. This technique applies computer software that converts the high-resolution planimetry data into a computer animation that recreates the geometry of the anatomic zone being studied. In addition, a pressure/distention relationship can be measured to define elasticity and compliance of the lumen wall and, thus, represents the first dynamic technique to profile both anatomy and function of the esophagus with a single device. Although these devices are currently only available for research purposes, it is likely that they will be incorporated into clinical devices along with high-resolution impedance and topographic display methods.
HIGH-RESOLUTION MANOMETRY
Esophageal manometry is considered the “gold standard” for assessing esophageal motor function[15]. Accordingly, the current diagnostic classification of esophageal motor disorders is based almost entirely on manometric patterns of abnormal peristalsis and LES function[16]. Conventional manometry typically utilizes 3-8 pressure sensors positioned within the esophageal lumen to assess the contractile pattern during water swallows. A variety of sensor technologies exist, including solid-state transducers, circumferentially sensitive transducers, perfused ports, and the Dentsleeve device. The heterogeneity of the sensor types and lack of consensus regarding the optimal spacing of sensors may be partially responsible for the poor intra- and inter-observer reproducibility reported in the literature[1]. Thus, refinements in manometry are needed to improve reproducibility and accuracy of this “gold standard”.
HRM: Technical aspects
High-resolution manometry is not a new technology and represents a modification of existing technology to provide greater detail by utilizing a vastly increased number of sensors spaced closely together. Thus, not only time, but also the spatial domain of the pressure profile within the esophagus can be captured as a continuum after interpolation between the sensors. The ideal system for esophageal studies should span from the pharynx to the stomach with sensor separation of no more than a centimeter apart within and around the sphincters and a temporal frequency response matched to the zone of the esophagus in which the sensors reside. High-resolution manometry can be adapted to work with any transducer technology, as long as the recording fidelity of the sensor is adequate. The frequency response required to reproduce esophageal pressure waves with 98% accuracy is 0-4 Hz, while that required for reproducing pharyngeal pressure waves is 0-56 Hz[17]. Expressed in terms of maximal recordable ΔP/Δt, 300 mmHg/s is sufficient for studying the esophageal body while the pharynx will require a ΔP/Δt of 4000 mmHg/s for the pharynx.
Early studies incorporating high-resolution manometry utilized water perfused systems due to availability of appropriate multilumen extrusions, the flexibility of the sensor spacing, and the cost of solid-state pressure sensors. However, the response characteristics of the water perfused sensors were technically limited for studying the pharynx or for measuring detailed pressure gradients through both the upper and lower esophageal sphincters. Thus, the ideal system would incorporate solid-state sensors, which have become clinically available. The advantages of this type of high-resolution system are: (1) a simplified procedural set up with improved sphincter localization; (2) elimination of movement artifact; (3) simplified data interpretation; and (4) ability to perform more sophisticated analysis of esophageal function. These attributes alone make this technology more user-friendly and efficient. Thus, it has the potential to replace conventional manometry utilizing a line tracing format.
The vastly increased quantity of data and the confusing presentation of multiple overlapping tracings spaced closely together presents new challenges for interpreting high-resolution manometry. Thus, new algorithms have been devised to provide a seamless dynamic representation of pressure at every axial position from the hypopharynx through the EGJ. Advances in computerization and graphic data presentation have been adapted so that esophageal contractile activity following a swallow can be portrayed in the format of color isobaric contour topographic plots (Figure 2). Clouse and Staino were the first to apply this technique in the esophagus and developed a methodology to interpolate and convert this information into topographic plots[18]. This data presentation format is more akin to an imaging technique, as opposed to conventional manometric tracings, and has the potential to greatly simplify and standardize the clinical manometric study.
Figure 2.
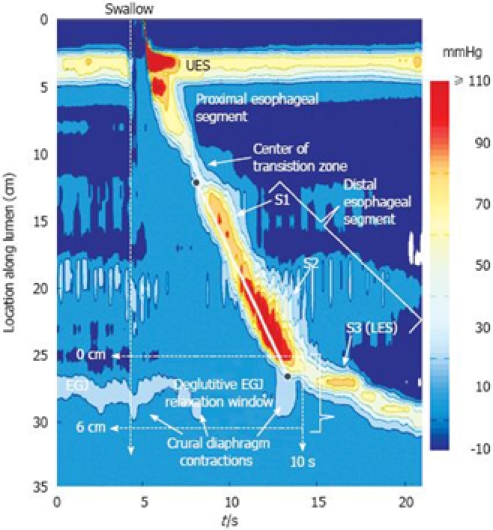
Typical swallow pressure topography spanning from the pharynx (locations 0-2 cm) to stomach (locations 29-35 cm) of a normal subject with normal peristalsis and normal EGJ relaxation. The transition zone, demarcating the end of the proximal esophageal segment (striated muscle) and the beginning of the distal esophageal segment (smooth muscle), is readily identified as a pressure minimum. Note that the distal segment, in fact, has three sub-segments (S1, S2, S3) within it, each with an identifiable pressure peak. Sub-segment 3, the LES, contracts at the termination of peristalsis and then descends back to the level of the CD as the period of swallow-related esophageal shortening ends. The onset of the deglutitive relaxation window is at the onset of upper sphincter relaxation while the offset is 10 s later. The spatial domain within which EGJ relaxation is assessed (the eSleeve™ range) is user defined, spanning at least 6 cm, depending on the extent of esophageal shortening after the swallow. The contractile front velocity (CFV) is the slope of the line connecting the black circle-points on the 30 mmHg isobaric contour at the proximal margin of S1 and the distal margin of S2.
HRM: Investigations into dysphagia pathogenesis
Topographic analysis of HRM data has clearly advanced our knowledge of esophageal motor function. The recent application of topographic analysis to esophageal peristalsis demonstrated that progression through the esophageal body is not seamless. Rather, it is comprised of a sequence of contractile events occurring in four discrete pressure segments (Figure 2). The first segment represents the striated muscle component of the proximal esophagus, and extends from the UES to the first pressure trough in the region of the aortic arch. This trough, representing the transition zone is usually easily identified. The distal portion of the esophagus is considered the smooth muscle dominant portion, and can be separated into two overlapping neuromuscular segments. The fourth contractile segment encompasses the LES. This segmental configuration was not appreciated by conventional manometry and underscores the strength of topographic analysis of manometric data[18–20].
Within the esophageal body one of the early achievements of HRM was the understanding of the transition zone in the mid esophagus, not just as the locus of the nadir in peristaltic pressure amplitude, but also as a physiological transition between propagated contractions of completely distinct physiology[21]. The proximal segment is that dominated by striated muscle, while the distal segment is smooth muscle. The proximal contraction is attributable to sequenced activation of motor neurons in the medulla, while the distal contraction in the smooth muscle is sequenced as a function of the balance between the excitatory and inhibitory interneurons of the myenteric plexus. This enhanced understanding of the transition zone can also account for the distinct pathology in which there is an abnormal delay between the termination of the proximal contraction and the origination of the distal contraction or a spatial gap between the two, as an explanation for dysphagia[22].
One of the first important investigations that took advantage of the detail HRM provides in defining simultaneous pressure across anatomic zones, was performed by Pal et al to define pathologic constriction across the UES[23]. Their findings suggested that intrabolus pressure was an important marker of impaired bolus transit, and represented constriction at the UES. Our group built upon this work, utilizing combined HRM and fluoroscopy at the upper esophageal sphincter to determine if manometry alone could be leveraged to accurately predict bolus transit across the EGJ in patients with a spectrum of pathological conditions[24]. Using the normative data for esophago-gastric flow permissive time in 20 controls and mathematical modeling data of antegrade esophageal emptying[25], we performed a ROC analysis for the predictive value of flow permissive time that is optimal to predict complete clearance. Our findings suggest that a cut-off value of ≤ 2.5 s had the best predictive value for incomplete clearance with a sensitivity of 86% and specificity of 92%. We speculate that intrabolus pressure elevations above the EGJ may also be useful in defining pathologic constriction or impaired EGJ opening and future work should be focused on defining normative values for intrabolus pressure.
HRM: Clinical aspects
Given the fact that high-resolution manometry is a refinement of an already existing clinical tool, it can be easily substituted for conventional manometry in our standard evaluation of esophageal motor disorders. The clinical protocol for high-resolution manometry is almost identical to standard conventional manometry with the obvious exclusion of the initial pull-through protocol to localize the LES. This portion of the initial intubation procedure and positioning of the catheter is not required due to the increased number and close proximity of the sensors making the sphincters easily identifiable on the topographic pressure plots.
With the adoption of HRM technology and pressure topography display methodology, the classification of esophageal motility that was developed for conventional manometric systems needs to be reconsidered. Although conventional metrics can be easily measured using simple software programs to convert the topographic data back to conventional line tracings and then applying a conventional analysis to a selected set of the line tracings, this method ignores the incremental gain in information obtained from the patterns presented by the pressure topographic plots. The alternative approach is to build an analysis and classification scheme that parallels conventional manometric classification, but also enhances it based on the strengths of the technology. To that end, we recently completed a comprehensive characterization of esophageal HRM data using novel analysis paradigms devised for pressure topography interpretation. We analyzed 75 normal subjects to develop normative ranges and applied this analysis system to 400 patients[26]. The major conclusions from that work, along with relevant contributions from other research groups, has allowed the initial formulation of an analysis and classification system for clinical practice and will be summarized in the following sections and tables.
The approach to analyzing and classifying HRM studies parallels conventional manometric technique in that it is focused on defining sphincteric and esophageal body function. However, new analysis paradigms have been created to assess deglutitive EGJ relaxation and peristaltic integrity and vigor with higher accuracy and detail. Basal EGJ relaxation pressure is measured using similar methodology to that used in conventional manometry; by assessing the mean end-expiratory pressure during a sufficient baseline period at the beginning of the study. Defining EGJ relaxation, however, has been modified to quantify EGJ relaxation pressure during the entire deglutitive period. Although a single nadir pressure measurement can be easily measured, bolus emptying and flow through the EGJ is not instantaneous and may take up to 4 to 5 s based on the volume swallowed[24]. Thus, deglutitive relaxation pressure is now quantified by measuring the 4 s integrated relaxation pressure (IRP), which represents the lowest 4 s cumulative pressure values for the deglutitive time period through the anatomic zone defining the EGJ. Normative values for this parameter were derived form 75 normal controls (Table 1) and this measurement was shown to be superior to a single nadir measurement and the continuous 3 s nadir pressure[27].
Table 1.
Classification of individual swallows based on pressure topography criteria
| Classification | Criteria |
| EGJ Deglutitive Relaxation (referenced to gastric pressure) | |
| Normal relaxation | 4 s Integrated Relaxation Pressure (IRP) < 15 mmHg |
| Impaired relaxation | 4 s IRP >= 15 mmHg |
| Distal Segment Contraction (referenced to gastric pressure) | |
| Normal | <= 2 cm defect in the 30 mmHg isobaric contour, Contractile Front Velocity (CFV) < 8 cm/s, Intrabolus Pressure (IBP) < 15 mmHg, and Distal Contractile Integral (DCI) < 5000 mmHg × s × cm |
| Mild peristaltic defect | Normal appearing wavefront propagation with a 2-5 cm defect in the 30 mmHg isobaric contour |
| Severe peristaltic defect | Evidence of wavefront propagation with a >= 5 cm defect in the 30 mmHg isobaric contour |
| Absent peristalsis | No propagating contractile wavefront and minimal (< 3 cm) contractile activity or pressurization greater than the 30 mmHg IBC |
| Nutcracker | DCI > 5000 and < 8000 mmHg × s × cm |
| Spastic nutcracker | DCI > 8000 mmHg × s × cm |
| Spasm | Simultaneous contraction (CFV > 7.5 cm/s) |
| Elevated intrabolus pressure | IBP > 15 mmHg compartmentalized between the EGJ and the peristaltic wavefront |
| Panesophageal pressurization | Esophageal pressurization UES to EGJ with > 30 mmHg IBP |
Recognizing that esophageal bolus transport is effected by the interaction of resistance through the EGJ, intrabolus pressure, and esophageal closure pressure behind the bolus[28], the second step of the analysis focuses on defining peristaltic integrity. Given previous data suggesting that pressures greater than 30 mmHg are almost uniformly associated with complete bolus transit[29,30], this threshold value was applied to define an intact peristaltic wavefront. This analysis is facilitated by the generation of 30 mmHg isobaric contour plots that delineate a pressure domain such that all pressures above this threshold value are circumscribed by a dark line. Normal swallows should have a seamless intact 30 mmHg isobaric contour with a contractile front velocity (CFV) below 7.5 cm/s. Abnormalities of single swallows are defined by the defects in the 30 mmHg isobaric contour and the contractile front velocity (Table 1).
The vigor of the smooth muscle contraction is another component that can be quantified in detail with HRM using a parameter defined as the distal contractile integral (DCI). This parameter quantifies the contractile activity in a space-time box by multiplying the length of the smooth muscle esophagus by the duration of propagation of the contractile wave front, and the mean pressure value over the entire box excluding pressure signal below 20 mmHg[26]. The DCI provides much more detail than 3 isolated measurements of maximal contractile amplitude at 3-5 cm intervals, as it incorporates duration of contraction into the measurement. Although a DCI value > 5000 mmHg × s × cm exceeded the 95th percentile of normal thereby meeting the usual criterion for nutcracker esophagus, a threshold value of 8000 mmHg × s × cm distinguished a spastic nutcracker subgroup (n = 12) characterized by repetitive high amplitude contractions that was uniformly associated with dysphagia or chest pain[26]. Intrabolus pressure is another feature that can easily be defined by HRM using the isobaric contour tool to assess patterns of pressurization (Figure 3).
Figure 3.
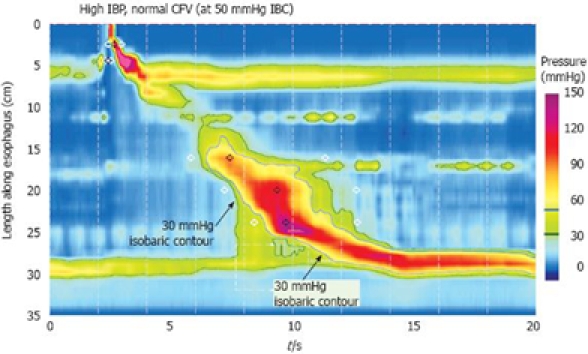
Defining increased intrabolus pressure using high-resolution manometry. The figure illustrates a swallow with functional obstruction at the EGJ. Note that the 30 mmHg isobaric contour line (black) deviates quickly from the 50 mmHg isobaric contour line (blue). In this case, the contractile front velocity is normal, reflecting the propagation velocity of 50 mmHg isobaric contour rather than the 30 mmHg isobaric contour. The intrabolus pressure domain is defined by the compartmentalized pressurization between the propagating contraction and the EGJ. Modified from: Pandolfino et al[26].
These measurement parameters for single swallows can be utilized to classify esophageal motor disorders[26,31]. Although HRM classification schemes have not been rigorously validated, they once again parallel conventional classification, and have the added benefit of much greater detail and an ability to characterize intrabolus pressure. Thus, it should not be difficult to incorporate these classification schemes into current clinical practice. Table 2 represents our motility laboratories version of an evolving classification scheme. Apart from changing the paradigm of categorizing peristalsis based on an intact isobaric contour, the classification of peristaltic dysfunction is very similar to conventional classification with the extra detail of distinguishing spastic nutcracker. The fundamental differences between the HRM classification system and previous conventional classification systems focuses on the sub-classification of achalasia and the category of EGJ obstruction defined by elevated IBP and impaired EGJ relaxation (Figure 3). In our analysis of 400 consecutive patients, we found that 44 patients met this criteria and these patients were a heterogeneous group composed of post-fundoplication surgery, esophageal stricture, eosinophilic esophagitis and an idiopathic group that could represent an achalasia variant or pathology that could not be defined by conventional methodology[26].
Table 2.
Distal esophageal motility disorders based on pressure topography criteria
| Disorder | Criteria |
| With Normal EGJ Relaxation (mean IRP < 15 mmHg) | |
| Peristaltic Weakness | |
| Intermediate | More than 30% of swallows with mild or severe peristaltic defects, but numerically insufficient to constitute severe peristaltic weakness |
| Severe | >= 70% of swallows with severe peristaltic defects |
| Aperistalsis | 100% swallows with absent peristalsis |
| Nutcracker Esophagus | Normal CFV, Mean DCI > 5000 and < 8000 mmHg × s × cm, can be localized to either distal subsegment or LES |
| Spastic Nutcracker | Normal CFV, Mean DCI > 8000 mmHg × s × cm |
| Distal Esophageal Spasm | Normal EGJ relaxation and spasm (CFV > 8 cm/s) with >= 20% of swallows |
| Esophageal Obstruction | Increased IBP or panesophageal pressurization not associated with EGJ obstruction |
| With Impaired EGJ Relaxation (mean IRP >= 15 mmHg) | |
| Achalasia | |
| Classic achalasia | Impaired EGJ relaxation and aperistalsis |
| Achalasia with esophageal compression | Impaired EGJ relaxation, aperistalsis, and panesophageal pressurization with >= 20% of swallows |
| Spastic achalasia | Impaired EGJ relaxation, aperistalsis, and spasm with >= 20% of swallows |
| EGJ Obstruction | |
| Mild | Elevated IBP (15-30 mmHg) that is compartmentalized between the peristaltic wavefront (normal, weak, or nutcracker) and EGJ |
| Severe | IBP > 30 mmHg that is compartmentalized between the peristaltic wavefront (normal or nutcracker) and EGJ |
A diagnosis of achalasia requires both aperistalsis and impaired deglutitive EGJ relaxation. However, there are specific subtypes that can be identified using HRM that can predict clinical outcome (Figure 4). In its most obvious form, achalasia occurs in the setting of esophageal dilatation with negligible pressurization within the esophagus (Type 1). However, despite there being no peristalsis, substantial pressurization within the esophagus can also occur. In fact, a very common pattern encountered in achalasia is of pan-esophageal pressurization (Type 2). These patients generally have a non-dilated esophagus with no obvious endoscopic or radiographic abnormalities. The other, much less common, pattern is of spastic achalasia in which there is a spastic contraction within the distal esophageal segment (Type 3). In a series of 73 consecutive achalasics, 40 (54.8%) had aperistalsis, 29 (39.7%) had pan-esophageal pressurization, and only 4 (5.5%) had spastic achalasia (Pandolfino et al[26]). The importance of this classification scheme was highlighted when logistic regression analysis found Type 2 to be a predictor of positive treatment response while Type 3 was predictive of negative treatment response regardless of whether treatment was medical, endoscopic or surgical.
Figure 4.
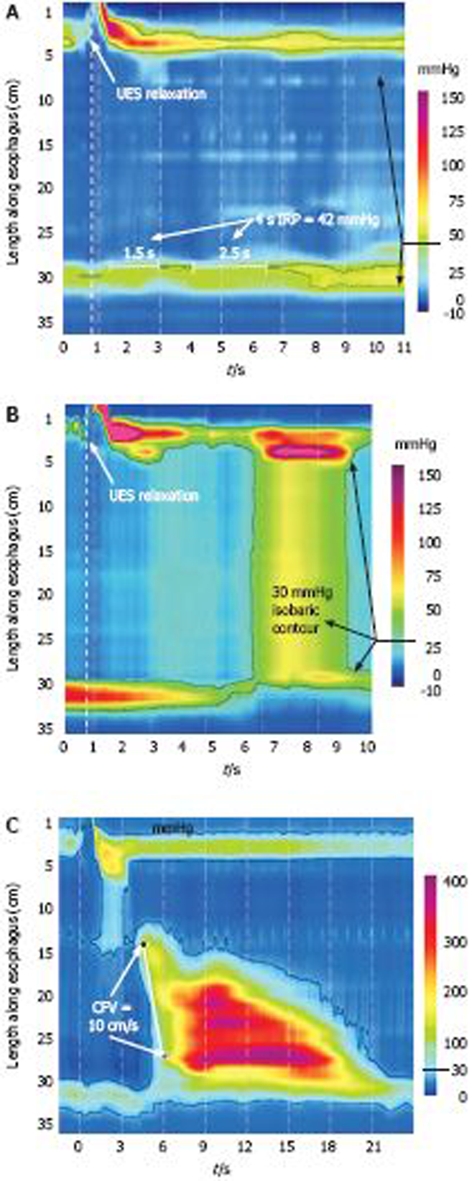
Achalasia subtypes based on manometric patterns of esophageal body contractility. A: Classic achalasia. There is no significant pressurization within the body of the esophagus and there is concurrent impaired EGJ relaxation (IRP of 42 mmHg in this example);Achalasia subtypes based on manometric patterns of esophageal body contractility. B: Achalasia with compression. This subtype exhibits a rapid pan-esophageal pressurization;Achalasia subtypes based on manometric patterns of esophageal body contractility. C: Spastic Achalasia. Although this swallow is associated with rapidly propagated pressurization, the pressurization in this case is attributable to an abnormal lumen obliterating contraction. Modified from: Pandolfino et al[26].
CONCLUSION
Multichannel intraluminal impedance and HRM are new tools that can improve the accuracy and detail in describing esophageal function. These technologies should not be viewed as competing technologies, as each provides distinct information. Rather, efforts should be focused on combining these techniques, as they are largely complementary. For instance, high-resolution manometry is the best method to analyze the pressure profile of the esophagus, and certainly it should be combined with impedance monitoring if bolus transit abnormalities are shown to help define disease states and direct clinical treatment.
Footnotes
Supported by RO1 DC00646 (PJK and JEP) from the Public Health Service
Peer reviewers: Asbjørn M Drewes, Professor, Department of Medical Gastroenterology, Center for Visceral Biomechanics and Pain, Aalborg Hospital, Aalborg 9000, Denmark; Ian D Wallace, MD, Shakespeare Specialist Group, 181 Shakesperare Rd, Milford, Auckland 1309, New Zealand
S- Editor Li LF L- Editor Stewart GJ E- Editor Ma WH
References
- 1.Nayar DS, Khandwala F, Achkar E, Shay SS, Richter JE, Falk GW, Soffer EE, Vaezi MF. Esophageal manometry: assessment of interpreter consistency. Clin Gastroenterol Hepatol. 2005;3:218–224. doi: 10.1016/s1542-3565(04)00617-2. [DOI] [PubMed] [Google Scholar]
- 2.Fass J, Silny J, Braun J, Heindrichs U, Dreuw B, Schumpelick V, Rau G. Measuring esophageal motility with a new intraluminal impedance device. First clinical results in reflux patients. Scand J Gastroenterol. 1994;29:693–702. doi: 10.3109/00365529409092496. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen HN, Silny J, Albers D, Roeb E, Gartung C, Rau G, Matern S. Dynamics of esophageal bolus transport in healthy subjects studied using multiple intraluminal impedancometry. Am J Physiol. 1997;273:G958–G964. doi: 10.1152/ajpgi.1997.273.4.G958. [DOI] [PubMed] [Google Scholar]
- 4.Srinivasan R, Vela MF, Katz PO, Tutuian R, Castell JA, Castell DO. Esophageal function testing using multichannel intraluminal impedance. Am J Physiol Gastrointest Liver Physiol. 2001;280:G457–G462. doi: 10.1152/ajpgi.2001.280.3.G457. [DOI] [PubMed] [Google Scholar]
- 5.Simren M, Silny J, Holloway R, Tack J, Janssens J, Sifrim D. Relevance of ineffective oesophageal motility during oesophageal acid clearance. Gut. 2003;52:784–790. doi: 10.1136/gut.52.6.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sifrim D, Castell D, Dent J, Kahrilas PJ. Gastro-oesophageal reflux monitoring: review and consensus report on detection and definitions of acid, non-acid, and gas reflux. Gut. 2004;53:1024–1031. doi: 10.1136/gut.2003.033290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imam H, Shay S, Ali A, Baker M. Bolus transit patterns in healthy subjects: a study using simultaneous impedance monitoring, videoesophagram, and esophageal manometry. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1000–G1006. doi: 10.1152/ajpgi.00372.2004. [DOI] [PubMed] [Google Scholar]
- 8.Tutuian R, Castell DO. Combined multichannel intraluminal impedance and manometry clarifies esophageal function abnormalities: study in 350 patients. Am J Gastroenterol. 2004;99:1011–1019. doi: 10.1111/j.1572-0241.2004.30035.x. [DOI] [PubMed] [Google Scholar]
- 9.Tutuian R, Mainie I, Agrawal A, Gideon RM, Katz PO, Castell DO. Symptom and function heterogenicity among patients with distal esophageal spasm: studies using combined impedance-manometry. Am J Gastroenterol. 2006;101:464–469. doi: 10.1111/j.1572-0241.2006.00408.x. [DOI] [PubMed] [Google Scholar]
- 10.Tutuian R, Vela MF, Balaji NS, Wise JL, Murray JA, Peters JH, Shay SS, Castell DO. Esophageal function testing with combined multichannel intraluminal impedance and manometry: multicenter study in healthy volunteers. Clin Gastroenterol Hepatol. 2003;1:174–182. doi: 10.1053/cgh.2003.50026. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen HN, Domingues GR, Winograd R, Koppitz P, Lammert F, Silny J, Matern S. Impedance characteristics of normal oesophageal motor function. Eur J Gastroenterol Hepatol. 2003;15:773–780. doi: 10.1097/01.meg.0000059161.46867.2b. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen NQ, Rigda R, Tippett M, Conchillo J, Smout AJ, Holloway RH. Assessment of oesophageal motor function using combined perfusion manometry and multi-channel intra-luminal impedance measurement in normal subjects. Neurogastroenterol Motil. 2005;17:458–465. doi: 10.1111/j.1365-2982.2005.00646.x. [DOI] [PubMed] [Google Scholar]
- 13.McMahon BP, Frokjaer JB, Drewes AM, Gregersen H. A new measurement of oesophago-gastric junction competence. Neurogastroenterol Motil. 2004;16:543–546. doi: 10.1111/j.1365-2982.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- 14.McMahon BP, Frokjaer JB, Liao D, Kunwald P, Drewes AM, Gregersen H. A new technique for evaluating sphincter function in visceral organs: application of the functional lumen imaging probe (FLIP) for the evaluation of the oesophago-gastric junction. Physiol Meas. 2005;26:823–836. doi: 10.1088/0967-3334/26/5/019. [DOI] [PubMed] [Google Scholar]
- 15.Pandolfino JE, Kahrilas PJ. AGA technical review on the clinical use of esophageal manometry. Gastroenterology. 2005;128:209–224. doi: 10.1053/j.gastro.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Spechler SJ, Castell DO. Classification of oesophageal motility abnormalities. Gut. 2001;49:145–151. doi: 10.1136/gut.49.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orlowski J, Dodds WJ, Linehan JH, Dent J, Hogan WJ, Arndorfer RC. Requirements for accurate manometric recording of pharyngeal and esophageal peristaltic pressure waves. Invest Radiol. 1982;17:567–572. doi: 10.1097/00004424-198211000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Clouse RE, Staiano A. Topography of the esophageal peristaltic pressure wave. Am J Physiol. 1991;261:G677–G684. doi: 10.1152/ajpgi.1991.261.4.G677. [DOI] [PubMed] [Google Scholar]
- 19.Clouse RE, Staiano A. Topography of normal and high-amplitude esophageal peristalsis. Am J Physiol. 1993;265:G1098–G1107. doi: 10.1152/ajpgi.1993.265.6.G1098. [DOI] [PubMed] [Google Scholar]
- 20.Clouse RE, Prakash C. Topographic esophageal manometry: an emerging clinical and investigative approach. Dig Dis. 2000;18:64–74. doi: 10.1159/000016967. [DOI] [PubMed] [Google Scholar]
- 21.Ghosh SK, Janiak P, Schwizer W, Hebbard GS, Brasseur JG. Physiology of the esophageal pressure transition zone: separate contraction waves above and below. Am J Physiol Gastrointest Liver Physiol. 2006;290:G568–G576. doi: 10.1152/ajpgi.00280.2005. [DOI] [PubMed] [Google Scholar]
- 22.Fox M, Hebbard G, Janiak P, Brasseur JG, Ghosh S, Thumshirn M, Fried M, Schwizer W. High-resolution manometry predicts the success of oesophageal bolus transport and identifies clinically important abnormalities not detected by conventional manometry. Neurogastroenterol Motil. 2004;16:533–542. doi: 10.1111/j.1365-2982.2004.00539.x. [DOI] [PubMed] [Google Scholar]
- 23.Pal A, Williams RB, Cook IJ, Brasseur JG. Intrabolus pressure gradient identifies pathological constriction in the upper esophageal sphincter during flow. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1037–G1048. doi: 10.1152/ajpgi.00030.2003. [DOI] [PubMed] [Google Scholar]
- 24.Ghosh SK, Kahrilas PJ, Lodhia N, Pandolfino JE. Utilizing intraluminal pressure differences to predict esophageal bolus flow dynamics. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1023–G1028. doi: 10.1152/ajpgi.00384.2007. [DOI] [PubMed] [Google Scholar]
- 25.Ghosh SK, Kahrilas PJ, Zaki T, Pandolfino JE, Joehl RJ, Brasseur JG. The mechanical basis of impaired esophageal emptying postfundoplication. Am J Physiol Gastrointest Liver Physiol. 2005;289:G21–G35. doi: 10.1152/ajpgi.00235.2004. [DOI] [PubMed] [Google Scholar]
- 26.Pandolfino JE, Ghosh SK, Rice J, Clarke JO, Kwiatek MA, Kahrilas PJ. Classifying esophageal motility by pressure topography characteristics: a study of 400 patients and 75 controls. Am J Gastroenterol. 2008;103:27–37. doi: 10.1111/j.1572-0241.2007.01532.x. [DOI] [PubMed] [Google Scholar]
- 27.Ghosh SK, Pandolfino JE, Rice J, Clarke JO, Kwiatek M, Kahrilas PJ. Impaired deglutitive EGJ relaxation in clinical esophageal manometry: a quantitative analysis of 400 patients and 75 controls. Am J Physiol Gastrointest Liver Physiol. 2007;293:G878–G885. doi: 10.1152/ajpgi.00252.2007. [DOI] [PubMed] [Google Scholar]
- 28.Massey BT, Dodds WJ, Hogan WJ, Brasseur JG, Helm JF. Abnormal esophageal motility. An analysis of concurrent radiographic and manometric findings. Gastroenterology. 1991;101:344–354. [PubMed] [Google Scholar]
- 29.Kahrilas PJ, Dodds WJ, Hogan WJ. Effect of peristaltic dysfunction on esophageal volume clearance. Gastroenterology. 1988;94:73–80. doi: 10.1016/0016-5085(88)90612-9. [DOI] [PubMed] [Google Scholar]
- 30.Tutuian R, Castell DO. Clarification of the esophageal function defect in patients with manometric ineffective esophageal motility: studies using combined impedance-manometry. Clin Gastroenterol Hepatol. 2004;2:230–236. doi: 10.1016/s1542-3565(04)00010-2. [DOI] [PubMed] [Google Scholar]
- 31.Fox MR, Bredenoord AJ. Oesophageal high-resolution manometry: moving from research into clinical practice. Gut. 2008;57:405–423. doi: 10.1136/gut.2007.127993. [DOI] [PubMed] [Google Scholar]


